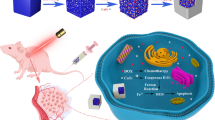
Abstract
Inorganic nanoparticles play important roles in cancer treatment, but the issues of biodegradable properties, and tumor penetration restricted further clinical application. Aiming at the low efficiency and toxic side effects problems existing in the current clinical treatment of cancer, a biodegradable mesoporous silica nanoparticle (MSN) platform integrating pH-responsive drug release and synergistic chemo-radiotherapy was developed. The doxorubicin hydrochloride (DOX) was loaded in MSNs via electrostatic interactions, with ultra-small hafnium sulfide (HfS2) nanodots were anchored to the surface of MSN. At physiological pH, HfS₂ reinforces DOX retention through steric and electrostatic effects, while in acidic tumor microenvironments, it triggers DOX release. Concurrently, dissociated HfS2 acts as a radiosensitizer, enhancing X-ray-induced cytotoxicity and promoting deep tumor penetration. Both the in vitro cellular and in vivo animal level results confirmed synergistically enhanced chemoradiotherapy efficiency. Therefore, this research, we believed, will bring a new train of thought and scientific support for more safety and more efficiency in cancer therapy.
Similar content being viewed by others
Introduction
Nowadays, cancer still remains a leading cause of death worldwide, with traditional therapies such as radiotherapy and chemotherapy facing critical challenges including limited tumor specificity, drug resistance, and severe systemic toxicity1. Radiotherapy (RT), a cornerstone of cancer treatment, relies on ionizing radiation to damage tumor DNA, but its efficacy is constrained by the radioresistance of hypoxic tumor cells and the vulnerability of adjacent normal tissues2,3. Chemotherapy, while systemic, often suffers from poor tumor accumulation, rapid drug clearance, and the emergence of multidrug resistance (MDR) due to insufficient intracellular drug concentrations4. To address these issues, nanotechnology has emerged as a promising strategy to enhance therapeutic precision.
Various nano-carriers have been used for cancer treatment and achieved good results. Micelles, liposomes, prodrugs, biodegradable polymers, and inorganic material nano drug delivery systems have been exhibited to have better therapeutic efficacy and less toxic side effects than free chemotherapeutic agents5,6,7. Especially, the sol-gel based mesoporous silica nanoparticles (MSNs) have attracted great attention in the field of drug delivery due to their homogeneous size distribution, adjustable mesoporous aperture, amorphous Si-O-Si framework that is easy to be functionalized and degraded8,9. Meanwhile, the MSNs possess a large specific surface area used for loading considerable cargo and modifiable surface features for targeted unit modification10,11,12. Therefore, the MSNs have been proven to be a great application prospect as a drug delivery system. particularly through MSNs that offer high drug-loading capacity, tunable surface chemistry, and biocompatibility.
In recent years, radiation sensitizers have emerged as a promising strategy to address tumor radioresistance, leveraging high-atomic-number elements to enhance X-ray-induced cytotoxicity13,14. Among various candidates, hafnium-based materials, such as hafnium sulfide (HfS₂), have drawn increasing attention due to their unique advantages over traditional sensitizers (e.g., gold nanoparticles or gemcitabine). HfS₂ nanodots, characterized by an ultra-small size and high atomic number (Hf, Z = 72), exhibit superior radiation absorption capabilities, generating abundant secondary electrons (e.g., Auger electrons) upon X-ray irradiation to induce intensified DNA double-strand breaks in tumor cells15,16,17. Compared to HfO2 (e.g., NBTXR3), the two-dimensional layered structure of HfS₂ not only boosts the specific surface area for potential functionalization but also enables deep tumor tissue penetration, overcoming the poor infiltration issue of larger nanoparticles (50–200 nm) that often accumulate at tumor peripheries18,19,20. Due to the ultra-small size (below the 6–8 nm renal filtration threshold), the biodegradable and renal clearance ability of HfS₂ have great potential in clinical application. These attributes HfS₂ as an ideal candidate for synergistic radiotherapeutic strategies, particularly when integrated into multifunctional nanocarriers to achieve spatiotemporally coordinated chemoradiotherapy.
Existing mesoporous silica-based composites for combined therapy typically adopt simple physical mixing of radiosensitizers and chemotherapeutics, leading to suboptimal synergism21,22. However, traditional systems suffer from a lack of responsiveness to tumor microenvironmental (TME) cues23. The acidic pH (pH 6.5–6.8) and overexpressed enzymes in tumors are rarely harnessed for controlled drug release, leading to premature dissociation of DOX in the bloodstream or normal tissues24,25. This not only reduces the therapeutic window due to systemic exposure but also diminishes tumor accumulation, as unbound drugs are rapidly cleared by the reticuloendothelial system. Additionally, the static physical mixing of components often results in poor stability during circulation, with radiosensitizers potentially dissociating from the MSNs surface and accumulating in off-target organs like the liver or kidneys. Therefore, there is urgent to develop advanced nano-platforms that integrate dynamic release mechanisms and collaborative treatment.
In this work, we report an advanced biodegradable MSN platform that integrates dynamic dual-responsive drug release and synergistic chemo-radiotherapy for enhanced cancer treatment. The MSNs were engineered to load doxorubicin hydrochloride (DOX) through electrostatic interactions between the protonated amine groups of DOX and the negatively charged silanol groups on the MSN surface, achieving stable encapsulation at physiological pH. The ultra-small HfS₂ nanodots anchored to the mesoporous channel surfaces exhibit dual functionalities. It reinforced DOX retention via steric hindrance and electrostatic interactions at neutral pH. While in acidic microenvironment, protonation of the MSN surface disrupts electrostatic binding, triggering rapid DOX dissociation, while the concurrently released HfS₂ nanodots leverage their high atomic number (Hf, Z = 72) to enhance X-ray-induced photoelectric interactions, amplifying radiation cytotoxicity. Different from conventional static physical mixtures, this dynamic design ensures spatiotemporal synchronization of chemotherapy and radiosensitization. This nanoplatform establishes a versatile blueprint for intelligent nanotheranostics, combining biodegradability, microenvironment responsiveness, and multi-modal therapy to improve treatment precision while minimizing off-target toxicity.
Experimental section
Experimental materials
Anhydrous ethanol (C2H5OH), tetraethyl orthosilicate (C8H20O4Si, TEOS), triethanolamine (C6H15NO3), sodium sulfide (Na2S) and sodium hydroxide (NaOH) were bought from Sinopharmaceutical Chemical Reagents Co., LTD. Cetane trimethyl ammonium bromide (CH3(CH2)15(CH3)3NBr, CTAB) and doxorubicin (DOX, C27H29NO11, 99.9%) were obtained from Aladdin Reagent (Shanghai) Co., LTD. Polyvinylpyrrolidone (PVP, Mw = 10 kDa) from Sigma-Aldrich.
Preparation of SiO2-HfS2 nanocomposites
In a typical procedure, 5 mL of ethanol was mixed with 25 mL of ultrapure water and then 0.2 g of CTAB was added and stirred to form a uniform solution. Subsequently, 50 µL of triethanolamine (TEA) was added and stir at 60 °C for 0.5 h. Subsequently, 2 mL of TEOS solution was added and stirred for another 2 h. Finally, the white turbid liquid was cooled down, and the precipitate was collected by centrifugal process. After the drying process, the powder was calcined at 550 °C for 5 h to obtain the silica nanoparticles (SiO2 NPs). Then, the SiO2 NPs were modified with NaOH solution to increase the dispersion performance. Specifically, 100 mg of SiO2 NPs were dispersed in 50 mL of 5 mmol/L NaOH solution under ultrasound for 30 min and stirred at room temperature overnight. Finally, the as-obtained nanoparticles were collected by centrifugation and washed with ultrapure water for three times to remove the unreacted NaOH ingredient.
Subsequently, 0.032 g of HfCl4 (0.1 mmol) was dissolved in the appropriate amount of ultrapure water. Then, the above HfCl4 solution was added to 100 mL of PVP solution (100 mg/mL) slowly and stirred for a period. After the above solution is thoroughly mixed, add 0.2 mL of 1 M Na2S solution slowly and continue stirring to form the HfS2 ultra-small nanodots. Then, the above NaOH-modified SiO2 NPs were ultrasonically dispersed in HfS2 aqueous solution, followed by centrifugation, washing, and vacuum drying at 37 °C to form SiO2-HfS2 composite nanosystem.
Loading and releasing of the DOX
An appropriate amount of doxorubicin hydrochloride was first dispersed in ultra-pure water and then added with the NaOH-modified SiO2 NPs. To be specific, the NaOH-modified SiO2 NPs (10 mg) were immersed in DOX solution (5 mL, 2 mg/mL), and sonicated for 10 min. After the ultrasonic process, the above solution continues to stir at room temperature under dark conditions. And then, centrifuge, and wash the redundant DOX molecules to get the DOX-loaded SiO2 NPs. Subsequently, the DOX-loaded SiO2 NPs were dispersed in HfS2 aqueous solution and stirred, centrifugated, and washed to obtain DOX loaded SiO2-HfS2 composite system (DOX-SiO2-HfS2). The DOX encapsulation rate can be determined by the Thermogravimetric (TG) method.
For the releasing process, the phosphate buffered solution (PBS) with different pH values (5.8 and 7.4) were selected to investigate the pH-triggered drug release behavior at room temperature. The absorbance values of all the samples were measured by a UV-visible spectrophotometer (UV-2600, Shimadzu Co., Ltd.) at 478 nm. The release percentage of the drug molecule was calculated at different pH conditions and at several time points.
In vitro study of radio-sensitization of SiO2-HfS2 nanoparticles
Radio-sensitization experiment of SiO2-HfS2 was further performed. The 4T1 cancer cells were cultured first. After cell adhesion, the original medium was replaced with a culture medium containing SiO2-HfS2. After culturing for 2 h, the cell culture plates were irradiated with X-rays with different intensities. The survival rate of 4T1 cells was then investigated. Subsequently, the 4T1 cells were under different treatments (control, SiO2-HfS2 treatment, RT treatment, and SiO2-HfS2 plus RT treatment) to study the sensitization effect of SiO2-HfS2.
An assessment of 4T1 cell migration was performed by using a scratch assay. Briefly, 1 × 105 cells were seeded onto dishes and incubated for 6 h. The supernatant was removed, and the cells were washed with fresh phosphate-buffered saline (PBS) solution. The cell layer was scraped off to form a gap, and cell migration was calculated by the width of the gap.
In a Colony formation assay, 4T1 cells were used as a tumor cell model to evaluate the cell proliferation capacity after treatment with SiO2-HfS2 under X-ray irradiation. A total of 5 × 104 cells per well were plated in 6 cm plates and incubated at 37 °C for 24 h. The culture medium was then removed, and fresh DMEM containing 10% FBS with/without SiO2-HfS2 was added. Later, the cells were exposed to different doses (0–8 Gy) of X-ray radiation. After 24 h of incubation at 37 °C, the medium was replaced with DMEM containing 10% FBS. Incubation at 37 °C for another 5 days, cells were washed twice with PBS, fixed in 4% paraformaldehyde, stained with 0.1% crystal violet, and washed three times with water. The colony formation was observed by staining with Giemsa (YEASEN, Shanghai, China) and quantified by counting the cell colonies.
Biodistribution of DOX-loaded SiO2-HfS2 in vivo
All male Balb/c mice used in this study were purchased from Shanghai Silex Experimental Animal Co., Ltd. All mice were 6 weeks old and were housed in the Experimental Animal Center of Sir Run Run Shaw Hospital. The laboratory environment was maintained at a room temperature of 22 ± 1 °C and a humidity of 50%±10%, with artificial light–dark cycles of 12 h of light and 12 h of darkness. All mice had free access to food and water. Our animal experimental protocol was approved by the Animal Ethics Committee of Sir Run Run Shaw Hospital (Approval Number: SRRSH2025-0011), and the procedures for anesthesia and euthanasia were conducted in accordance with the ARRIVE Guidelines.
Balb/c mice were subcutaneously transplanted with 5 × 106 4T1 tumor cells. When the tumor volume reached about 100 mm3mice were intravenously injected with DOX or DOX-loaded SiO2-HfS2 samples, separately. After 24 h, experimental mice were anesthetized via intraperitoneal injection of pentobarbital (80 mg/kg). Anesthesia depth was confirmed by the absence of the righting reflex and lack of response to pain stimuli.the mice were dissected and each organ of the mice was then photographed by IVIS Lumina LT Series III (Perkin Elmer, Massachusetts, USA). The total radiant efficiency of each photograph was quantified by using Living Image 4.5 software (Perkin Elmer, Massachusetts, USA). Euthanasia was performed using a CO₂ asphyxiation method at a rate of 30% chamber volume per minute.
Anti-tumor effect of DOX-loaded SiO2-HfS2 in vivo
The mouse model of breast cancer was established. When the tumor diameter grew to 5.0 mm (subcutaneous), the mice were divided into six groups: control; SiO2-HfS2 treatment; RT treatment; SiO2-HfS2 plus RT treatment; DOX- SiO2-HfS2 treatment; DOX- SiO2-HfS2 plus RT treatment. The nanoparticles were injected through the tail vein. The mice in each group were observed for about 30 consecutive days, and the changes in tumor volume and body weight of nude mice in each group were counted (volume V = tumor length × (tumor width) 2/2). Subsequently, the tumor and main organ were removed, weighed, sectioned, and stained with H&E for tumor biologic histological study after the different treatments mentioned above (n = 5). The anesthesia and euthanasia procedures for mice in the experiment were performed as described in “Biodistribution of DOX-loaded SiO2-HfS2 in vivo” section.
All methods were carried out in accordance with relevant guidelines and regulations.
Results and discussion
Characteristics of SiO2-HfS2 nanocomposites
The design and synthesis of SiO2-HfS2 nanocomposite have been illustrated in Scheme 1.
The procedure of preparation can be divided into three steps. Firstly, SiO2 NPs were prepared. And then, the DOX molecule was loaded into the pores of the SiO2 NPs. Finally, ultrasmall HfS2 nanodots were in situ attached to the surface of SiO2 NPs to control the DOX drug release and simultaneously endow the radiotherapy sensitization functionalities. The representative transmission electron microscope (TEM) images of the as-prepared SiO2-HfS2 nanocomposite are exhibited in Fig. 1a–d.
The SiO2-HfS2 nanocomposite exhibited uniform spherical morphology and present a spherical morphology with a uniform size of 70 nm. Meanwhile, many tiny nanodots were covered on the surface of SiO2 NPs. Moreover, the high-resolution TEM images showed that the size of the ultrasmall HfS2 nanodots was 6 nm. Additionally, the element mapping images further indicated that the Si, O, Hf, and S elements were distributed within the SiO2 NPs matrix (Fig. 1d). In addition, the EDS spectrum in Fig. 1e also confirms the element composition, demonstrating the successful preparation of SiO2-HfS2 nanocomposite. Furthermore, the X-ray photoelectron spectroscopy (XPS) of SiO2-HfS2 nanocomposites revealed existing Si, O, Hf, and S elements, suggesting that HfS2 nanodots were successfully attached on the surface of SiO2 NPs (Fig. 1f). The HfS2 nanodots may connect to the SiO2 NPs by electrostatic bond effect. In addition, SiO2 usually has an amorphous structure, so a wide peak at 2θ ranging from 15° to 35° has been shown in Figure S1. While HfS2 exists in the form of nanodots rather than in a non-crystalline state, the quantum size effect of HfS2, or the increased degree of disorder caused by its interfacial interaction with SiO2, leads to the broadening of the diffraction peaks of SiO2-HfS2.
Subsequently, dynamic light scattering (DLS) was used to analyze the size variation before and after the HfS2 nanodot attached process. As demonstrated in Fig. 2a, the size increased by 10–20 nm after the HfS2 nanodot was attached. Meanwhile, the zeta potential changed from − 23.6 to − 3.3 mV after the attached process (Fig. 2b). The nitrogen adsorption-desorption isotherm and corresponding textural parameters of SiO2 NPs and SiO2-HfS2 are provided in Fig. 2c-e, respectively.
(a) Size change of SiO2 surface pores and HfS2 nanodots in aqueous solution, (b) Zeta potential change in aqueous solution before and after SiO2 surface pores and HfS2 nanodots, (c, d) Nitrogen adsorption desorption curve and pore size change before and after SiO2 surface pores and HfS2 nanodots. (e) Changes of structural parameters before and after SiO2 surface channels composite HfS2 nanodots.
The specific surface area of SiO2 NPs was 863 m2/g. However, the specific surface area was reduced to 237 m2/g after the HfS2 nanodots were attached. The pore volume also decreased from 1.2 cm3/g to 0.2cm3/g (Fig. 2e). The reduced nitrogen adsorption may be caused by the attached HfS2 nanodots in the channels and surfaces of SiO2 NPs. Therefore, the above experimental results together prove that HfS2 nanodots were attached to the SiO2 NPs surface successfully.
In vitro DOX loading and pH-responsive release behavior
In this work, DOX molecules were loaded within SiO2 NPs channels by immersion method, and then the HfS2 nanodots were attached. As exhibited in Fig. 3a, DOX aqueous solution has an obvious characteristic absorption peak at 478 nm, while SiO2-HfS2 has no absorption peak around 478 nm. However, SiO2-HfS2 demonstrated an absorption peak at 478 nm after the DOX loading process, which proves that DOX was loaded within SiO2-HfS2, successfully. The drug loading capacity of the SiO2-HfS2 sample was calculated by thermogravimetric analysis (TGA), as exhibited in Fig. 3b. The 15% weight loss of pure SiO₂ was attributed to the physical adsorption of water, surface hydroxyl groups, or impurities. In the case of the SiO₂-HfS₂-DOX ternary system, the overall thermal weight loss reached 71%, comprising the inherent weight loss of SiO₂ (15%), the weight loss of HfS₂ (19%), and the decomposition weight loss of DOX. The mass proportion of DOX in the composite was calculated to be 37%. Then DOX loaded SiO2-HfS2 was immersed in PBS solutions with two different pH values to investigate the drug release performance. As demonstrated in Fig. 3c, the DOX release rate was faster in the acidic environment. At pH = 7.4, the release rate of DOX is very slow, only 35% of the DOX drug is released within the first 24 h, and only 48% after 72 h. With the decrease in pH value, the release rate of DOX drug in an acidic environment was accelerated. When pH was reduced to 5.8, the drug release reached 52% within the first 24 h. After 72 h of drug release, the drug release amount was 75%. The main reason may be that the ultra-small HfS2 nanodots on the SiO2 NPs surface are liberated from the mesoporous surface and thus the DOX drug can be released quickly under acidic conditions26,27. At the same time, it can be observed that HfS2 nanodots were almost liberated from the SiO2-HfS2 and exhibited a certain degradation behavior in solution with pH = 5.8 (Fig. 3d). The distribution of DOX fluorescence in the 4T1 tumor cryosphere was observed by confocal laser microscopy. As exhibited in Fig. 3e, acidic conditions promote more drug release and more DOX infiltration into tumor cell spheres. Under acidic pH, DOX fluorescence intensity is 70 μm from the bottom of the cell sphere, which was significantly higher than that under neutral pH conditions.
(a) DOX molecules loaded with SiO2-HfS2 composite nanoparticles and UV-vis spectra of DOX molecules in aqueous solution, (b) TG curves of DOX molecules loaded with SiO2-HfS2 composite nanoparticles, (c) The corresponding diagram of the cumulative release curve and time of DOX molecules under different pH conditions after loading SiO2-HfS2 composite nanoparticles, (d) TEM diagram of DOX loaded SiO2-HfS2 composite nanoparticles after releasing 120 h, (e) The penetration of DOX loaded SiO2-HfS2 composite nanoparticles in 4T1 cell spheres under different pH conditions. DOX fluorescence distribution was scanned by Z-axis confocal laser microscope. The scale is 100 μm.
The cytocompatibility and anti-tumor effect of SiO2-HfS2 nanocomposites in vitro
The toxicity of SiO2-HfS2 nanocomposites was first evaluated. MTT assay was used to detect the toxicity of SiO2-HfS2, SiO2-HfS2+RT, SiO2-HfS2-DOX and SiO2-HfS2-DOX + RT. As demonstrated in Figure S2, the relative cell viability of 4T1 cells remained above 80%, when the concentration of SiO2-HfS2 increased from 0 to 1000 µg/mL. The fluorescence images of live-dead cells assay were consistent with the results of MTT cell activity (Figure S3). Therefore, these results indicate that pure SiO2-HfS2 nanocomposites possess low cytotoxicity.
In this study, 4T1 cells were used as model tumor cells, and the radio sensitization effect of SiO2-HfS2 nanocomposites on 4T1 cells was evaluated through cell cloning formation and scratch test. As shown in Fig. 4a, the scratch test exhibited that the SiO2-HfS2 + RT group had the largest scratch area after 24 h co-culture, indicating that SiO2-HfS2 with X-ray irradiation could significantly inhibit the migration of 4T1 cells, while the SiO2-HfS2 treatment group reveal no significant change on cell mobility compared with the control group. Cloning experiments revealed that, in contrast to the cell group solely treated with X-ray, the clone formation and cell viability of cells in the SiO₂ - HfS₂ treatment group were markedly suppressed when exposed to varying X-ray doses ranging from 0 to 8 Gy (Figs. 4b, c). Moreover, as the X-ray dose reaches 8 Gy, the cell survival rate dropped below 1%, and cell cloning was almost negligible. The SER value of SiO2-HfS2 calculated is about 1.32 (Fig. 4d). Due to the high X-ray attenuation of the Hf element, and thus DNA damage is caused by reactive oxygen species (ROS) produced by X-rays irradiation, which is the radiotherapy performance. Therefore, this study took 4T1 cells as an example to study the ability of SiO2-HfS2 to induce ROS generation after X-ray irradiation in vitro. Reactive oxygen species (ROS) produced in 4T1 cells were detected by using DCFH-DA fluorescent dye. As exhibited in Fig. 4e, the fluorescence intensity of intracellular DCFH-DA in the RT + SiO2-HfS2 treatment group was significantly higher than that of only the RT treatment group, and the effect of radiotherapy was improved by increasing the production of ROS.
(a) Scratch experiment of 4T1 cells after coculture of SiO2-HfS2 composite nanoparticles with 4T1 cells 24 h after different treatment, (b) Cloning experiment of SiO2-HfS2 composite nanoparticles with 4T1 cells, and X-ray radiation intensity from 0 to 8 Gy, (c) Survival rate of SiO2-HfS2 composite nanoparticles co-cultured with 4T1 cells under different X-ray irradiation (n = 3), (d) radiation sensitivity ratio of SiO2-HfS2 composite nanoparticles. (e) Representative fluorescence image (DCFH-DA) of oxidative stress degree of cells.
The in vivo distribution of DOX-loaded SiO2-HfS2 in 4T1 tumor-cells-bearing mice
A variety of fluorescence imaging techniques have been developed in the small animal imaging field to track the distribution of dye-labeled nanoparticle ex vivo and in vivo28,29. In this study, DOX molecules were used as fluorescence signal molecules for imaging. A small animal imaging system was used to explore the DOX-loaded SiO2-HfS2 and DOX fluorescence signal in tumor-bearing mice after intravenous injection. Tumor-bearing mice were intravenously injected with DOX or DOX-SiO2-HfS2 for 24 h. Subsequently, the mice were dissected and each organ of the mice was used for fluorescence imaging. As shown in Fig. 5a, the fluorescent signal at the tumor site of mice in the DOX-loaded SiO2-HfS2 group was stronger. The quantitative results were also consistent with those of fluorescence imaging (Fig. 5b). These results indicate that SiO2-HfS2 can efficiently transport DOX molecules to the tumor site.
In vivo anti-tumor and preliminary biosafety exploration
4T1 tumor model mice were intravenously injected with DOX-loaded SiO2-HfS2 to systematically study the efficacy of combined radiotherapy and chemotherapy. As demonstrated in Figs. 6a&b, the tumor growth rate of mice treated with DOX-loaded SiO2-HfS2 + RT was significantly lower than that of the SiO2-HfS2 + RT or DOX-loaded SiO2-HfS2 groups, and the treatment effects of SiO2-HfS2 + RT and DOX loaded SiO2-HfS2 groups were basically at the same level. However, the effect of the SiO2-HfS2 + RT group was better than that of only RT treated group, indicating that SiO2-HfS2 + RT has promoted radiation effect on the tumor. The SiO2-HfS2 treatment group had a similar tumor growth rate as the control group. The mean tumor weight of the SiO2-HfS2-DOX + RT group was lower than that of all the other groups (Fig. 6c). These results indicate that radiotherapy/chemotherapy combined therapy has obvious advantages in tumor destruction. The changes in body weight in each group were small (Fig. 6d). The H&E staining was used to observe the antitumor effect of different treatment methods. The histological analysis showed that control and SiO2-HfS2 treatment groups showed the typical herringbone appearance of fibrosarcoma, but tumors in the SiO2-HfS2 + RT and SiO2-HfS2-DOX + RT demonstrated necrosis (Fig. 6e).
(a) different treatment group mice tumor volume growth curve (n = 7), (b) different treatment group mice tumor physical photos, (c) collected from mice tumor at the end of radiotherapy the average weight (1: Control, 2: SiO2-HfS2, 3: RT, 4: SiO2-HfS2 + RT, 5: SiO2-HfS2-DOX, 6: SiO2-HfS2-DOX + RT), (d) the weight of the mice in different treatment processes changes over time (e) at the end of the different treatment mice tumor biopsies of H&E staining. Scale = 100 μm. Control group represents the cell without treatment. *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001.
Meanwhile, DOX-loaded SiO2-HfS2 + RT group treatment exhibited the most destructive tumor effect. Finally, the heart, liver, spleen, lung, and kidney were histologically stained with H&E staining to investigate toxic side effects (Fig. 7). No significant abnormal signs were observed, indicating negligible toxicity with SiO2-HfS2-DOX + RT.
Conclusion
In this work, a multistage structure SiO2-HfS2 composite nanocarrier with the function of combined radiotherapy and chemotherapy was synthesized by a simple sol-gel method. Firstly, SiO2 NPs were synthesized as drug carrier materials, and chemotherapy drug (DOX) was loaded into the mesoporous structure. And then the HfS2 nano-dots were attached to the surface as pore-sealing agents to achieve a controlled release of chemotherapy drugs. Meanwhile, in an acidic environment, HfS2 nano-dots can slowly fall off to release chemotherapy drugs. Meanwhile, the HfS2 nano-dots can be used as radiotherapy sensitization to improve the therapeutic effect combined with chemotherapy drugs. Therefore, SiO2-HfS2 multistage composite nanocarriers synthesized possess great enlightening significance for synergistically enhanced chemoradiotherapy application.
Data availability
The data that support the results presented in this paper are available from the corresponding author upon reasonable request.
References
Zhang, S. et al. Tumor initiation and early tumorigenesis: molecular mechanisms and interventional targets. Signal. Transduct. Target. Therapy. 9, 149. https://doi.org/10.1038/s41392-024-01848-7 (2024).
McLaughlin, M. et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer. 20, 203–217. https://doi.org/10.1038/s41568-020-0246-1 (2020).
Wu, Y., Song, Y., Wang, R. & Wang, T. Molecular mechanisms of tumor resistance to radiotherapy. Mol. Cancer. 22, 96. https://doi.org/10.1186/s12943-023-01801-2 (2023).
Cornen, S. & Vivier, E. Chemotherapy and tumor immunity. Science 362, 1355–1356. https://doi.org/10.1126/science.aav7871 (2018).
Dykman, L., Khlebtsov, B. & Khlebtsov, N. Drug delivery using gold nanoparticles. Adv. Drug Deliv. Rev. 216, 115481. https://doi.org/10.1016/j.addr.2024.115481 (2025).
Liu, T. et al. Biomimetic drug delivery for anticancer: Focusing on the relationship between drugs and biomimetic carriers. Coord. Chem. Rev. 524, 216328. https://doi.org/10.1016/j.ccr.2024.216328 (2025).
Zhang, Q., Kuang, G., Wang, L., Fan, L. & Zhao, Y. Tailoring drug delivery systems by microfluidics for tumor therapy. Mater. Today. 73, 151–178. https://doi.org/10.1016/j.mattod.2024.01.004 (2024).
Yang, B., Chen, Y. & Shi, J. Mesoporous silica/organosilica nanoparticles: Synthesis, biological effect and biomedical application. Mater. Sci. Engineering: R: Rep. 137, 66–105. https://doi.org/10.1016/j.mser.2019.01.001 (2019).
Hu, Y., Bai, S., Wu, X., Tan, S. & He, Y. Biodegradability of mesoporous silica nanoparticles. Ceram. Int. 47, 31031–31041. https://doi.org/10.1016/j.ceramint.2021.08.129 (2021).
Liu, Y. et al. Constructing activatable photosensitizers using covalently modified mesoporous silica. Adv. Sci. 12, 2406887. https://doi.org/10.1002/advs.202406887 (2025).
Liu, G. et al. Turn Hood into good: Recycling silicon from mesoporous silica nanoparticles through magnesium modification to lower toxicity and promote tissue regeneration. ACS Nano. 18, 32932–32949. https://doi.org/10.1021/acsnano.4c12519 (2024).
Fu, Y. et al. pH-responsive drug-targeted delivery system: Dual-drug loading Hollow mesoporous silica for synergistic therapy bacterial enteritis of micropterus salmoides. Chem. Eng. J. 515, 163819. https://doi.org/10.1016/j.cej.2025.163819 (2025).
Salehiabar, M. et al. Targeted CuFe2O4 hybrid nanoradiosensitizers for synchronous chemoradiotherapy. J. Controlled Release 353, 850–863. https://doi.org/10.1016/j.jconrel.2022.12.004 (2023).
Shin, S. et al. Dual-functional hafnium oxide nanoplatform combining high-Z radiosensitization with Bcl-2 gene silencing for enhanced cancer radiotherapy. Adv. Healthc. Mater. 2404819 https://doi.org/10.1002/adhm.202404819 (2025).
Li, J. et al. Hafnium (Hf)-chelating porphyrin-decorated gold nanosensitizers for enhanced radio–radiodynamic therapy of Colon carcinoma. ACS Nano 17, 25147–25156. https://doi.org/10.1021/acsnano.3c08068 (2023).
Li, Q. R. et al. Bioinspired Hf-based metal-organic framework radiosensitizer for nitric oxide-assisted radio-immunotherapy. Nano Today. 58, 102447. https://doi.org/10.1016/j.nantod.2024.102447 (2024).
Liu, N. et al. X-ray-induced release of nitric oxide from hafnium-based nanoradiosensitizers for enhanced radio-immunotherapy. Adv. Mater. 35, 2302220. https://doi.org/10.1002/adma.202302220 (2023).
Wang, X. et al. Hafnium oxide-based sensitizer with radiation-triggered cuproptosis for radiotherapy. Nano Today. 61, 102626. https://doi.org/10.1016/j.nantod.2024.102626 (2025).
Stergioula, A., Pantelis, E., Kontogeorgakos, V., Lazaris, A. C. & Agrogiannis, G. Understanding the role of Radio-Sensitizing nanoparticles in enhancing pathologic response in soft tissue sarcomas. Cancers 15 (2023).
Ding, S. et al. Harnessing hafnium-based nanomaterials for cancer diagnosis and therapy. Small 19, 2300341. https://doi.org/10.1002/smll.202300341 (2023).
Zhang, J. et al. Custom-design of multi-stimuli-responsive degradable silica nanoparticles for advanced cancer-specific chemotherapy. Small 20, 2400353. https://doi.org/10.1002/smll.202400353 (2024).
Zhang, J. et al. Recent advances in silica-based nanomaterials for enhanced tumor imaging and therapy. ACS Appl. Bio Mater. 7, 7133–7169. https://doi.org/10.1021/acsabm.4c01318 (2024).
Singleton, D. C., Macann, A. & Wilson, W. R. Therapeutic targeting of the hypoxic tumour microenvironment. Nat. Reviews Clin. Oncol. 18, 751–772. https://doi.org/10.1038/s41571-021-00539-4 (2021).
Feng, Q. et al. Severely polarized extracellular acidity around tumour cells. Nat. Biomed. Eng. 8, 787–799. https://doi.org/10.1038/s41551-024-01178-7 (2024).
He, Q. et al. Tumor microenvironment responsive drug delivery systems. Asian J. Pharm. Sci. 15, 416–448. https://doi.org/10.1016/j.ajps.2019.08.003 (2020).
Fu, Y. et al. pH-triggered SrTiO3: Er nanofibers with optically monitored and controlled drug delivery functionality. ACS Appl. Mater. Interfaces 7, 25514–25521. https://doi.org/10.1021/acsami.5b08953 (2015).
Zeng, L. et al. Doxorubicin-loaded NaYF4:Yb/Tm–TiO2 inorganic photosensitizers for NIR-triggered photodynamic therapy and enhanced chemotherapy in drug-resistant breast cancers. Biomaterials 57, 93–106. https://doi.org/10.1016/j.biomaterials.2015.04.006 (2015).
Li, Y. et al. Gram-scale synthesis of highly biocompatible and intravenous injectable hafnium oxide nanocrystal with enhanced radiotherapy efficacy for cancer theranostic. Biomaterials 226, 119538. https://doi.org/10.1016/j.biomaterials.2019.119538 (2020).
Hua, S. et al. Multistage-responsive clustered nanosystem to improve tumor accumulation and penetration for photothermal/enhanced radiation synergistic therapy. Biomaterials 268, 120590. https://doi.org/10.1016/j.biomaterials.2020.120590 (2021).
Funding
This research was funded by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission, Grant number 2021RC071.
Author information
Authors and Affiliations
Contributions
H.Z.: Draft writing. Y.Q.: Draft writing. S.H.: Data collection. Z.N.: Corresponding author, Funding support. R.H.: Data collection. L.C.: Data collection. X.Z.: Resources. J.H.: Corresponding author, Project management. H.H.: Corresponding author, Supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, H., Qi, Y., Hua, S. et al. Constructing pH-sensitive silica-sulfide hafnium dot nanotherapeutics for synergistically enhanced chemoradiotherapy. Sci Rep 15, 28298 (2025). https://doi.org/10.1038/s41598-025-13072-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13072-6
Keywords
This article is cited by
-
Dual-Targeting and pH-Responsive Graphene Oxide Nano-Platform for Synergistic Photothermal/Chemotherapy of NSCLC
Journal of Inorganic and Organometallic Polymers and Materials (2026)