Abstract
Fearful body expressions convey critical information that is rapidly and preferentially processed, facilitating swift motor reactions to potential dangers. Consistent evidence has shown that even the subliminal presentation of fear-related expressions can impact visual processing and autonomic responses, increasing sensory vigilance for monitoring potential threats. However, it remains unclear whether the presentation of non-visible emotional bodies modulates corticospinal excitability (CSE) in the observer. To investigate this, we asked 22 healthy participants to perform a sex discrimination task involving neutral target body postures, preceded by the brief subliminal presentation of fearful, happy, or neutral body postures. CSE was tested using Transcranial Magnetic Stimulation (TMS) at early time points (70, 90, and 110 ms) after target stimulus onset. Results showed a significant CSE reduction in the dominant hemisphere for subliminal fearful primes compared to happy and neutral primes. This CSE suppression was independent of the time of stimulation, participants’ subjective or objective awareness, metacognitive sensitivity, or personality traits. Our findings highlight an early automatic activation of the motor system in response to subliminal fearful stimuli, supporting the view that fearful expressions, even when not consciously perceived, activate basic survival mechanisms for monitoring and preparing fast motor responses to potential threats.
Similar content being viewed by others
Introduction
Consistent evidence has shown that negative stimuli signaling potential danger can be processed outside conscious awareness, thereby facilitating rapid threat detection and bolstering protective behaviors1,2,3. Among these, fearful expressions stand out as particularly salient social cues, conveying the presence of a possible threat in the surroundings, without specifying its source3,4. Because of this ambiguity, fearful expressions are thought to serve as a general alarm signal, prompting heightened vigilance and orienting attention toward potential danger in the surroundings. Interestingly, unattended or unseen fearful expressions from faces or bodies, either due to experimental manipulation or permanent brain damage, modulate spontaneous expressive reactions, physiological changes, or subsequent judgments to consciously reported stimuli5,6,7,8,9,10,11,12,13,14. For example, Zhan and de Gelder15 demonstrated that subliminal perception of fearful body postures, using continuous flash suppression, enhanced conscious discrimination of visible angry expressions. This finding supports the notion that fearful stimuli capture attention and facilitate processing other negative emotions, indicating a complex neural prioritization for unconscious fear perception in rapid threat detection.
Collectively, neuroimaging studies in both healthy and brain-damaged patients point to a subcortical network for non-conscious thereat detection centered on the amygdala. Concerning the timing, magnetoencephalography (MEG) studies have identified a dissociation in amygdala responses to threat signals. Early amygdala responses (~ 60 ms post-stimulus onset) occur automatically, independent of conscious attention, while later responses (~ 280 ms) are modulated by voluntary attention16. More conclusively, human intracranial electrophysiology found rapid responses (~ 70 ms) in the lateral amygdala, specifically to fearful facial expressions, which occur much earlier than similar fear responses in the visual cortex, typically emerging in the temporal regions around 170 ms post-stimulus17.
Beyond increasing sensory vigilance at pre-attentive or non-conscious processing stages, fearful expressions consistently influence the motor system by engaging preparatory responses such as freezing or orienting immobility18,19,20,21,22,23. These states, which involve a transient suppression of overt movements, are widely recognized as active defensive strategies that enhance perceptual sensitivity and facilitate rapid transitions to fight-or-flight responses when necessary24,25,26,27.
Our previous studies have demonstrated that conscious perception of negative scenes28, facial expressions20,29, or emotional bodily postures rapidly affects motor excitability18,19,20,21,22,23,30. In particular, observing fearful body expressions has been found to consistently suppress motor excitability18,23,31. On the other hand, Quettier et al.32 used a modified Stop Signal Task with subliminal emotional primes and showed that undetected fear stimuli enhanced participants’ ability to inhibit motor responses, implying an indirect modulation of motor excitability. However, only one study directly investigated whether subliminally presented emotional stimuli can modulate corticospinal excitability (CSE), a reliable measure of the motor system’s functional state that reflects shifts in the balance between excitatory and inhibitory activity in response to experimental manipulations33. In two different experiments, the authors presented fearful, angry, neutral bodies or objects, with TMS pulses delivered over the left primary motor cortex (M1) at different time points post-stimulus onset (200, 400, 500, 700 ms), but found no conclusive evidence of non-conscious fearful stimuli altering CSE. Therefore, it remains unclear whether subliminally presented fearful stimuli can modulate CSE, particularly at early time windows, as shown for consciously perceived stimuli.
To address these gaps, we investigated whether CSE could be implicitly modulated by rapid (16 ms) masked presentations of emotional bodily expressions while participants performed a sex categorization task on neutral body postures. We probed CSE by stimulating both the left and right M1 at 70, 90, and 110 ms post-stimulus – time points corresponding to the latency of the P1, the earliest cortical ERP component that is consistently modulated by fearful expressions34,35,36. Previous studies by our group have demonstrated a reduction in CSE within the 70–90 ms23, 100–125 ms21 and 150 ms22 intervals, as well as decreased intracortical facilitation (ICF) at 100–125 ms18, thus indicating that consciously perceived fearful stimuli reduce motor excitability in this early post-stimulus time window18,21,23. In line with the previous findings, we considered the selected time points (70, 90, 110 ms) as the ideal latencies to investigate whether CSE can be also affected by subliminally presented fearful stimuli. Finally, building on our previous TMS evidence18,21,23, no time-specific effects are expected, as earlier studies showed inhibitory activity across overlapping time intervals rather than at distinct latencies, suggesting a temporally broad modulation of motor excitability within the early post-stimulus window (70–110 ms).
Given the evolutionary need to respond quickly to impending signals of fear, often manifesting in freezing, we hypothesized that subliminal fearful bodily postures would reduce CSE relative to happy and neutral postures, even when task-unrelated. We also assessed prime visibility in a separate session (awareness check) to test whether individual differences in stimulus awareness might impact CSE. Consistent with our predictions, fearful bodily expressions reduced CSE in the left dominant hemisphere across all tested TMS time points, regardless of participants’ awareness of the prime stimuli. These results suggest that fearful bodily expressions are a privileged signal influencing motor readiness both rapidly and independently of conscious perception.
Methods
Participants
Twenty-two healthy young adults were involved in the study (11 female, mean age ± standard deviation: 24.45 years ± 2.42). The sample size was established using G*Power software37 by conducting a power analysis that aimed for a high power (1 e b) of 0.95, while keeping the significance threshold (a) at 0.05. Based on the MEP modulations patterns observed in our prior work18,19,23,38, we expected a MEP decrease in response to Prime Body Expressions during the task, with a large effect size (f = 0.4). This sample size was also consistent with the typical sample used across ten experimental groups in the aforementioned studies, each typically consisting of 15 participants. The experiments were carried out at the Center for studies and research in Cognitive Neuroscience, Department of Psychology, University of Bologna. All participants were right-handed, unaware of the study’s objectives, and reported no history of neurological or psychiatric conditions, visual impairments, medicine intake, or contraindications for TMS39. The University of Bologna’s Bioethics Committee approved the study, which was conducted in compliance with legal requirements and international guidelines (Declaration of Helsinki, 2013). Prior to the experiment, all participants gave their informed consent and received a brief overview of the experimental procedures. No discomfort or adverse effects related to TMS were reported or observed.
Visual stimuli
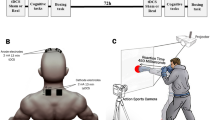
Different images were displayed on a 15.4-inch screen positioned approximately 60 cm from the participants. A total of 48 pictures depicting four different actors (two males and two females) in both emotional and neutral poses (Fig. 1.a) were chosen from a validated database19,21,38. These images were equally categorized into 16 instances of fearful postures, 16 of happy postures, and 16 of neutral body movements (Fig. 1). The emotional expressions were elicited through emotionally engaging instructions aimed at generating spontaneous and naturalistic reactions, rather than posed gestures. Images were then selected based on pilot studies assessing emotional intensity and recognition accuracy, ensuring the inclusion of stimuli with high emotional clarity and minimal ambiguity. For details see38.
Transcranial magnetic stimulation and electromyography recording
The experiment began with setting up the electrode montage, identifying the optimal scalp position, and determining the resting motor threshold (rMT). To assess CSE, we elicited MEPs by stimulating the hand M1 representation in the left and right hemispheres. Specifically, we targeted the motor representation of the first dorsal interosseous (FDI), using a Magstim 200 transcranial magnetic stimulator (Magstim, Whiteland, Dyfed, UK) equipped with a 70-mm figure-of-eight focal coil. Electromyography (EMG) signals from both the right and left FDI were recorded with a Biopac MP-35 device (Biopac, U.S.A.), band-pass filtered from 30 to 500 Hz, sampled at 5000 Hz, digitized, and stored for offline analysis. Pairs of silver-chloride surface electrodes were positioned in a belly-tendon configuration over the FDI of the two hands, with ground electrodes placed on the wrist. The coil was held tangentially to the scalp, with its handle oriented backward and laterally at a 45° angle from the midline to generate a posterior-anterior current flow across the central sulcus. The optimal coil position was defined as the M1 site where stimulation consistently produced the largest MEP in the contralateral FDI. The rMT was defined as the minimum stimulus intensity needed to evoke MEPs of at least 50 µV in 5 out of 10 consecutive pulses40. TMS was applied at 120% of the rMT across two sessions, each involving a different behavioral task (see below). Within each session, two blocks were performed, with stimulation applied to the left M1 in one block and to the right M1 in the other. Mean stimulation intensity (mean % of maximal stimulator output ± SD) was statistically comparable between the right M1 block (57.68 ± 8.09) and left M1 block (57.77 ± 8.14; t42 = 0.04, p = 0.97). We consistently monitored for any EMG muscle contractions, and their absence was visually confirmed during the entire experiment.
Procedure and experimental design
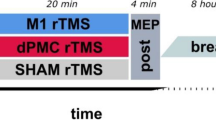
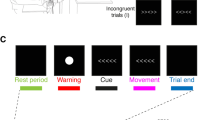
The experiments were programmed using Matlab software, which controlled the presentation of the images and the triggering of TMS pulses. In the first TMS session (main experiment), we assessed CSE during the presentation of visual stimuli. MEPs induced by stimulation of the left and right M1 were recorded in two separate experimental blocks of 144 trials each (Fig. 1.b), with M1 stimulation applied to the left M1 in one block and to the right M1 in the other. The order of the two blocks was counterbalanced across participants. Participants performed a sex discrimination task: a neutral body picture was presented as the target stimulus, and participants had to decide whether the depicted model was male or female. Target stimuli were preceded by the brief presentation of prime stimuli, following this procedure: each trial began with a gray screen displayed for 1 s, followed by a random-dot mask lasting 16 ms. This mask was generated by scrambling the corresponding sample stimulus using custom-made image segmentation software. Subsequently, the prime stimulus —depicting a happy, fearful, or neutral body posture— was presented at the center of the random-dot mask for 16 ms. This was immediately followed by another mask lasting 33 ms and then the target stimulus, which always depicted a neutral body posture. For each prime-target pair, the same model was depicted in both the prime and target stimuli to ensure consistency in body sex and actor identity.
Participants were instructed to perform the sex discrimination task on the target stimulus by providing a verbal response, which was recorded by an experimenter pressing a key on the computer. To avoid altering CSE due to verbal response41,42, participants were told to respond a few seconds after the TMS pulse, during the presentation of the question screen43. After their response, a white screen was presented for 6 s. In this way, we ensured a low TMS frequency, in order to prevent CSE changes caused by TMS itself44 throughout the experiment. The target stimuli were presented for 80, 100, or 120 ms and TMS pulses were delivered at 70, 90, and 110 ms from stimulus onset. Stimulus duration was randomly distributed across each block.
Following the first TMS session (main experiment), we performed two awareness checks. The first awareness check (subjective) was carried out without TMS and aimed to assess participants’ subjective awareness of the prime body posture. Participants were asked a series of questions in the following order: (1) Did you see anything besides the target body? (2) Did you see anything right before the target body? (3) There was actually a flicker before each target body: what did you see? (4) Did you see a body? (5) What expression did you see? Verbal reports indicating awareness of a body and/or expression were considered evidence that the prime presentations were not strictly subliminal32,45. Next, participants were debriefed about the use of subliminal primes.
The second awareness check (objective) was designed to assess participants’ objective awareness of the prime body stimuli while receiving TMS pulses over M1. The procedure mirrored the timing and structure of the main experiment: TMS pulses were delivered over the two M1 to simulate the same potential neural interference of TMS present during CSE measurement. However, no MEPs were recorded, as participants were asked to discriminate the prime stimulus by providing a manual response (forced choices: happy, fearful, neutral). Participants provided their answers to the emotion discrimination task by pressing a computer key a few seconds after the TMS pulse. On each trial, participants also judged their confidence on a 4-point Likert scale. Information about participants’ awareness and confidence was collected in two separate blocks of 48 trials, one for each hemisphere.
Subjective measures: personality questionnaires
In our previous study18, we observed that CSE reduction when observing fearful body postures was associated with interindividual differences in behavioral inhibition system (BIS) scale, while behavioral activation system (BAS) scale or State and Trait-Anxiety Inventory (STAI-Y2) showed no consistent predictive value. Therefore, to assess whether similar inter-individual differences may also predict CSE modulation for unconscious fearful body expressions presentation, after the two TMS sessions, we asked participants to fill out the Italian versions of the BIS/BAS46,47, the form Y2 of the STAI48. The BIS/BAS scales are based on the idea that two primary motivational systems influence behavior. The BIS is responsible for regulating responses to potential threats, promoting caution, fear, and anxiety when faced with danger. In contrast, the BAS drives approach behaviors, motivating individuals to pursue rewards and positive outcomes, and to avoid punishment. The BIS scale, which includes 7 items, measures an individual’s sensitivity to threats, while the BAS scale, consisting of 13 items, assesses the tendency to seek out rewards and pleasurable experiences. On the other hand, the STAI-Y2 is a general anxiety questionnaire that measures how frequently a person experiences anxiety. Unlike the BIS/BAS scales, which focus on responses to specific threats and rewards, the STAI-Y2 provides a broader assessment of overall anxiety levels, measuring the general tendency toward anxiety rather than specific triggers.
Data analysis
Neurophysiological and behavioral data were processed off-line. The mean MEP amplitude in each condition was measured peak-to-peak (in mV). MEPs associated with incorrect answers were discarded from the analysis (11%), in line with high task accuracy (89% ± 31%). Since background EMG is known to affect motor excitability49MEPs with preceding background EMG deviating from the mean by more than 2 S.D. were removed from further analysis (~ 3%).
MEP amplitudes were first analyzed using a four-way ANOVA with Hemisphere (2 levels: Right and Left), Time (3 levels: 70, 90 and 110 ms), actor Sex (2 levels: Female, Male), and Prime Body Expression (3 levels: Happy, Fearful and Neutral) as within-subjects factors. Post-hoc comparisons were carried out by means of the Newman-Keuls test. Effect size indices for main effects and interactions were computed using partial eta2, whereas Cohen’s d was computed for post hoc comparisons.
The ANOVAs showed a CSE reduction for the prime showing fearful body postures relative to happy and neutral prime body postures in the left hemisphere, and this effect was independent of the factor Time. To explore the relations between this motor suppression and key personality traits18correlation and regression analyses were performed. An index representing the early motor modulation [the mean CSE effect for fearful postures in the left hemisphere (average across time and actor sex) minus the mean CSE of all the other conditions, was entered as the dependent variable in a stepwise regression model, whereas questionnaire scores were entered as predictors (criteria probability of F-to-enter: ≤ 0.05; F-to-remove: ≥0.1).
Moreover, to account for participants’ objective awareness of subliminal primes, mean scores of accuracy collected during the two control experiments (i.e., TMS over right or left M1) were entered in a two-way ANOVA with Hemisphere (2 levels: Right and Left) x Prime Body Expression (3 levels: Happy, Fearful and Neutral) as within-subjects factors. This analysis tested whether the MEP modulation observed in the main experiment (see Results) could be attributed to specific interferential effects of TMS on the discrimination of prime stimuli.
Since the mean accuracy data exceeded the chance level (33%), we conducted additional analyses to determine whether subjective awareness influenced CSE modulation. Participants were classified as either ‘aware’ or ‘unaware’ of the prime based on individual performance assessed using two-sided binomial tests. Those with accuracy significantly above chance (p < 0.05) were categorized as ‘aware’, while the rest were classified as ‘unaware’. Then, to investigate whether the reduction of CSE was influenced by interindividual differences in awareness, a mixed factors 3-way ANOVA was performed on MEP amplitudes with Awareness (2 levels: Aware, Unaware) as the between-subjects factor and Hemisphere and Prime Body Expression as within-subjects factors. The same ANOVA was calculated on a subsample of MEP selecting the first 48 stimuli presentation (i.e., 16 body postures for each body expression) for each hemisphere to have a comparable number of measurements in the main experiment (i.e., during the sex discrimination task on the target stimulus) compared to the control experiment (i.e., during the emotion discrimination task on the prime stimulus).
To assess participants’ metacognitive sensitivity, we compared meta-d’ to d’ scores, with the target signal being fear, and noise conditions represented by other prime body expressions (e.g., neutral or happy). Meta-d’ was calculated specifically for trials where participants reported high confidence, defined as the top 50% of confidence scores (those rated ≥ 3 on a 1 to 4 scale). This approach allowed us to focus on trials where participants felt subjectively certain of their responses, thereby providing a clearer measure of metacognitive sensitivity in relation to actual detection ability. A paired samples t-test was conducted to examine potential differences between meta-d’ and d’ scores, revealing the degree to which participants’ confidence corresponded to their detection accuracy. Additionally, we analyzed criteria for both meta-d’ and d’ scores to assess response bias across conditions, helping to determine whether participants maintained consistent decision thresholds regardless of confidence level.
To further explore the relationship between metacognitive sensitivity and personality traits, correlation analyses were performed between meta-d’ and scores from the BIS, BAS, and STAI-Y2. This was done to evaluate whether individual differences in these traits might predict variations in metacognitive sensitivity. Finally, to assess whether participants’ level of metacognitive sensitivity influenced CSE modulation, we used meta-d’ as a criterion to split participants into two groups: those with high metacognitive sensitivity (meta-d’ > 1) and those with low metacognitive sensitivity (meta-d’ ≤ 1). This allowed us to examine whether variations in metacognitive sensitivity were associated with differential effects on CSE. A mixed-factor ANOVA was conducted across metacognitive sensitivity levels, Hemispheres, and different Prime Body Expressions on MEP amplitude.
For all ANOVAs, effect size indices for main effects and interactions were computed using partial eta2 (ηp2, whereas Cohen’s d was computed for post hoc comparisons.
Results
Neurophysiological data
The Hemisphere × Time × Sex × Prime Body Expression ANOVA on MEP amplitudes showed a significant Hemisphere × Prime Body Expression interaction (F2,42 = 7.88; p = 0.001; ηp2 = 0.27; Fig. 2). Post-hoc analyses showed that lower MEPs were elicited in the left hemisphere when participants observed fearful postures (mean amplitude ± S.D. = 1.21 ± 0.11 mV) rather than happy (1.30 ± 0.12 mV, p = 0.01; d = 0.76) and neutral expressions (1.29 ± 0.12 mV, p = 0.02; d = 0.69), which in turn did not significantly differ from one another (p = 0.73; d = 0.08). Moreover, MEP amplitudes recorded for fearful postures in the left hemisphere were lower than fearful (1.32 ± 0.21 mV, p = 0.002, d = 0.68), happy (1.28 ± 0.20 mV, p = 0.04; d = 0.42), and neutral postures (1.27 ± 0.19 mV, p = 0.04; d = 0.37) recorded in the right hemisphere. No significant modulations were found in the right hemisphere (all p ≥ 0.31). In sum, the analysis indicated that body expressions modulated MEPs only in the left hemisphere session, with lower amplitudes for fearful relative to happy and neutral bodies, but not in the right hemisphere. No other main effects or interactions were significant (all F ≤ 2.42, p ≥ 0.10; all ηp2 ≤ 0.0007). In particular, the Hemisphere × Prime Body interaction was not qualified by higher-order interactions with either Time or Sex (0.43 ≤ F ≤ 2.42, 0.78 ≥ p ≥ 0.10), suggesting that the left-M1 CSE reduction for fearful postures did not vary as function of stimulation latencies and sex of the actors.
CSE modulations during the sex discrimination task. MEP amplitude (mV) was recorded from FDI muscle. Data show the Hemisphere × Body Posture interaction (average of the three Times and Sex). Asterisks (*) denote significant post-hoc comparisons (p < 0.05). Error bars indicate s.e.m. Different scales were used for mean (on the left) and individual data (on the right).
Relation between changes in motor excitability and personality traits
The reduction in CSE for observed fearful body expressions in the left M1 was found across all time intervals. A series of correlations and a multiple regression analysis were carried out to test whether this neurophysiological effect was related to individual differences in affective personality traits. A MEP contrast computed based on the results of the ANOVA (i.e., [the mean CSE modulation for fearful postures in the left hemisphere (average across time and sex) minus the mean CSE of all the other conditions was entered into the analysis as a dependent variable, and participants’ scores from the BIS, BAS, and STAI-Y2 scales were entered as predictors. The regression model and simple correlations between the magnitude of CSE suppression and BIS, BAS, and STAI-Y2 scores were not significant (− 0.02 ≤ r ≤ 0.41, all p ≥ 0.09). Thus, the suppression of the CSE in response to unconscious fearful body postures was not significantly associated with personality variables in our sample, unlike what has been observed with consciously perceived stimuli18.
Subjective awareness check
Out of 22 participants, four explicitly reported the presence of the body postures as primes. To ensure that the results were not influenced by this factor, we repeated the same Hemisphere × Time × Sex × Prime Body Expression ANOVA after excluding these four participants. The results confirmed the significant Hemisphere × Prime Body Expression interaction (F2,34 = 7.43, p = 0.002, ηp2 = 0.30). This suggests that the fear-specific modulations are detectable even when the prime stimuli are not consciously perceived, indicating that subjective prime visibility is not crucial for eliciting CSE suppression.
Objective awareness check
The Hemisphere × Prime Body Expression ANOVA conducted on the accuracy scores collected during the emotion discrimination task showed no significant main effects of Hemisphere (F1,21 = 0.88; p = 0.36; ηp2 = 0.04) or Prime Body Expression (F2,42 = 0.43; p = 0.65; ηp2 = 0.02), and no significant Hemisphere × Prime Body Expression interaction (F2,42 = 0.07; p = 0.93; ηp2 = 0.003), indicating that neither active stimulation of the two hemispheres nor the presentation of different body postures significantly impacted perceptual awareness in our sample (see Table 1).
The binomial test was conducted to categorize participants as ‘aware’ and ‘unaware’. Half of the subjects were able to discriminate above chance the presented body postures (mean accuracy scores for 11 participants out of 22 were higher than 33% (p ≤ 0.05), whereas the remaining participants were unable to discriminate postures above chance level. Then, the Awareness × Hemisphere × Prime Body Expression on MEP amplitude mixed factors ANOVA revealed no main effect of Awareness (F1,20 = 0.16, p = 0.69; ηp2 = 0.0001), and no significant interactions involving Awareness (all F ≤ 0.97; all p ≥ 0.38). We can therefore conclude that the effect we found in the CSE (i.e., reduction in the CSE for fearful relative to happy and neutral body postures in the left hemisphere) did not significantly differ across aware and unaware participants.
The same Awareness × Hemisphere × Prime Body Posture mixed factors ANOVA was repeated on a subsample of MEP (the first 48 presented stimuli, see method session) and results showed no main effect of the factor Awareness (F1,20 = 0.28; p = 0.60; ηp2 = 0.01) and no significant interactions (all F ≤ 0.46; all p ≥ 0.63). These results additionally support the idea that prime visibility did not consistently influence CSE suppression in our experiment (Fig. 3).
Awareness effect on CSE modulations during the sex discrimination task. MEP amplitude (mV) was recorded from FDI muscle. Participants were classified as aware or unaware according to their accuracy scores in the emotion discrimination task and the binomial test. Data show the Awareness × Hemisphere × Prime Body Expression interaction. Error bars indicate s.e.m. Different scales were used for mean (on the left) and individual data (on the right).
Confidence analysis
A paired samples t-test revealed a significant difference between d’ and meta-d’, indicating that participants’ metacognitive sensitivity was higher (meta-d’ mean ± S.D. = 0.97 ± 0.91) than their objective detection ability (d’ mean ± S.D. = 0.49 ± 0.56; t19 = 3.07, p = 0.006, d = 0.64). This suggests a moderate effect size, with participants showing greater alignment between confidence and accuracy on high-confidence trials. Additionally, there was no significant difference in criterion values between meta criterion (mean ± S.D. = 0.39 ± 0.36) and criterion (mean ± S.D. = 0.45 ± 0.23), indicating that participants did not significantly change their response bias across conditions, regardless of confidence level (t19 = -0.68, p = 0.50, d = 0.18). These findings suggest that participants could accurately gauge their performance on trials where they felt more confident, reflected in a higher meta-d’ compared to d’. However, their criterion for emotion discrimination remained consistent across both high-confidence and general trials, demonstrating no significant shift in response bias. Simple correlations between the magnitude of meta-d’ and BIS, BAS, and STAI-Y2 scores were not significant (-0.27 ≤ r ≤ 0.12, all p ≥ 0.24). Then, the Meta-d’ × Hemisphere × Prime Body Expression on MEP amplitude mixed factors ANOVA revealed no main effect of the factor Meta-d’ (F1,18 = 2.35, p = 0.14; ηp2 = 0.11) and no significant interactions involving Meta-d’ were found (all F < 0.75; all p > 0.12; Fig. 4). Thus, we observed no influence of participants’ metacognitive sensitivity in the effect we found in the CSE (i.e., reduction in the CSE for fearful relative to happy and neutral body postures in the left hemisphere).
Comparison of CSE modulations during the sex discrimination task based on meta-d’ scores. Participants were classified into two groups: Meta-d’ > 1 and Meta-d’ < 1. Corticospinal excitability (CSE) was assessed by recording motor-evoked potential (MEP) amplitude (mV) from the FDI muscle. The data illustrate the interaction between meta-d’ scores and Hemisphere on MEP amplitude. Error bars represent the standard error of the mean (s.e.m.).
Discussion
The human brain rapidly and efficiently processes significant environmental information, even when it lies outside our attention or awareness. Previous EEG studies have shown that early visual components (e.g., P1, N170, and P200) are influenced by the subliminal presentation of fearful stimuli50,51,52,53,54,55,56,57. However, whether the motor system is similarly affected at the early stages of processing remains unclear. To address this issue, we tested CSE in the left (dominant) and right M1 of healthy participants performing a sex discrimination task on neutral body postures. Subliminal task-irrelevant body postures (negative, positive, or neutral) were flashed before the target neutral stimuli, and CSE was probed at early latencies (70, 90, and 110 ms) post-stimulus onset18,19,21,23. Additionally, participants’ personality traits, subjective and objective awareness of stimulus detection, and metacognitive sensitivity (i.e., the ability to gauge one’s performance accurately) were considered potential modulators58,59.
We found that subliminally presented fearful body postures reduced CSE in the left, dominant hemisphere (i.e., lower MEP in the right hand). This supports our hypothesis that task-irrelevant subliminal fearful stimuli modulate the motor system at early processing stages, likely preceding conscious visual awareness. As expected, this CSE suppression was independent of stimulation timing, consistent with prior findings of CSE reduction in response to supraliminal fearful postures at similar early time windows18,19,21,22.
Importantly, the modulation was lateralized to the left M1, highlighting the engagement of dominant-hemisphere circuits involved in action planning, inhibitory control, and attentional processes (e.g60,61. This lateralization is particularly relevant considering that all participants in our study were right-handed, the left hemisphere is typically dominant for motor control in such individuals62,63. Previous studies have suggested that left M1 shows greater CSE modulation during tasks requiring rapid motor responses, suggesting a functional asymmetry that facilitates fast action execution64,65, including increased readiness to emotional stimuli66.
Left-lateralized modulation may therefore indicate that early motor inhibition processes are preferentially initiated in the dominant hemisphere, serving as an initial gating mechanism. Engaging early inhibitory control in the dominant hemisphere may allow for fast and efficient modulation of motor output, given its leading role in voluntary movement and action planning in right-handed individuals. However, we do not interpret this effect as hand-specific motor inhibition. Rather, we suggest that the left M1 may serve as a functional entry point for a broader motor suppression cascade, rapidly recruiting bilateral networks to support a transient, global freezing-like response—consistent with previous findings of widespread bilateral inhibition at slightly later latencies (100–125 ms22,31. In real-life conditions, such transient early inhibition may serve an adaptive function: a brief suppression of motor output may act as a preparatory step that prevents premature or maladaptive actions, enhances vigilance, and allows for rapid contextual assessment24,25,26,27. This pause would then facilitate the timely recruitment of broader, bilateral motor programs required for an effective full-body defensive response.
Interestingly, in our recent work, CSE suppression in the left M1 was observed when participants actively monitored emotional features of supraliminal body postures during an emotion recognition task; in contrast, the same stimuli elicited CSE facilitation when participants performed a gender recognition task, despite involving the same emotional body postures21. These earlier findings suggest that motor inhibition during conscious perception of emotional body postures is modulated by task demands and emerges specifically when attention is directed toward emotional features. In contrast, here, fearful stimuli were presented subliminally and were task-irrelevant, yet still elicited a reduction in CSE. This provides compelling evidence that threat-related motor suppression can occur automatically, without conscious awareness or explicit attentional engagement. Such automatic inhibition likely reflects an early, preattentive defensive mechanism—such as freezing—that prepares the organism for rapid and adaptive responses to potential danger24,25,26,27.
Our study provides the first evidence of the impact of subliminal body expressions on the observer’s CSE. This contrasts with Engelen et al.33, who reported null findings when using continuous flash suppression (CFS) paradigms and later stimulation intervals (200–700 ms post-stimulus). The discrepancy may stem from differences in timing and paradigms, as our study targeted early time windows (70–100 ms), while later intervals capture more general action-related effects22,38. Furthermore, the gradual contrast increase inherent to CFS might attenuate early perceptual processing, thereby limiting the stimulus’s impact on MEP amplitude.
Our findings suggest that CSE suppression was independent of participants’ subjective or objective awareness of prime stimuli, as well as metacognitive sensitivity. Excluding participants reporting subjective awareness did not alter the observed CSE suppression. Additionally, metacognitive sensitivity—the confidence-accuracy relationship—was unrelated to CSE modulation or personality traits, reinforcing the role of unconscious mechanisms in motor system responses to subliminal fearful cues.
Unlike previous studies linking personality traits, particularly BIS-related traits, to heightened sensitivity to fear-related stimuli under conscious conditions18,22, we found no such association under subliminal conditions in our study. Although this should be interpreted with caution—given that a lack of statistical significance does not imply the absence of an effect, especially with a relatively small sample—the dissociation between the present and prior18,22 findings may suggest that the motor system’s response to subliminal fear operates independently of individual personality differences. With these caveats, our results appear to support the “low-road” pathway hypothesis67, which emphasizes rapid subcortical processing via structures such as the pulvinar, caudate nucleus, and amygdala12,68,69. This pathway enables preparatory motor responses, such as freezing or avoidance, bypassing slower cortical processing. Subcortical structures also project downstream to influence spinal cord responses70,71. In contrast, consciously perceived fear engages the “high road,” integrating personal experiences and contextual factors, and is more influenced by traits like BIS72,73.
This study highlights the motor system’s rapid, automatic response to subliminal threats, likely mediated by an evolutionary conserved mechanism for survival. The observed independence from personality traits and subjective/objective awareness further underscores its automatic nature. Further research is needed to elucidate the neurophysiological underpinnings and functional or clinical implications of these motor responses. Additionally, exploring the temporal dynamics and hemispheric specialization of subliminal threat processing could refine our understanding of these mechanisms. For example, the structural and functional asymmetry of the left M1, including its greater volume of descending motor fibers, may explain its role in facilitating swift motor responses to emotional stimuli74,75.
In conclusion, this work provides compelling evidence that the motor system can rapidly and selectively respond to subliminal fearful cues. These findings emphasize the importance of temporal precision and subcortical mechanisms in understanding the interplay between emotion and motor control, offering novel insights into the rapid and unconscious preparation of defensive actions in response to perceived threats.
Data availability
The datasets generated during this study are available at Open Science Framework Repository (https://osf.io/vsu72/?view_only=32eb5091bf9f485eaf035cf42105b8c2).
References
Morris, J. S. et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 121, 47–57 (1998).
Morris, J. S., Öhman, A. & Dolan, R. J. Conscious and unconscious emotional learning in the human amygdala. Nature 393, 467–470 (1998).
Whalen, P. J. et al. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J. Neurosci. 18, 411–418 (1998).
Davis, M. & Whalen, P. J. The amygdala: vigilance and emotion. Mol. Psychiatry. 6, 13–34 (2001).
Bertini, C., Cecere, R. & Làdavas, E. I am blind, but I ‘ see’ fear. Cortex 49, 985–993 (2013).
Cecere, R., Bertini, C., Maier, M. E. & Làdavas, E. Unseen fearful faces influence face encoding: evidence from erps in hemianopic patients. J. Cogn. Neurosci. 26, 2564–2577 (2014).
De Gelder, B., Vroomen, J., Pourtois, G. & Weiskrantz, L. Non-conscious recognition of affect in the absence of striate cortex. Neuroreport 10, 3759–3763 (1999).
Làdavas, E. & Bertini, C. Right hemisphere dominance for unconscious emotionally salient stimuli. Brain Sci. 11 (11), 823 (2021).
Phelps, E. A. & LeDoux, J. E. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 48, 175–187 (2005).
Stienen, B. M. C. & de Gelder, B. Fear modulates visual awareness similarly for facial and bodily expressions. Front. Hum. Neurosci. 5, 132 (2011).
Stienen, B. M. C. & de Gelder, B. Fear detection and visual awareness in perceiving bodily expressions. Emotion 11, 1182–1189 (2011).
Tamietto, M. et al. Unseen facial and bodily expressions trigger fast emotional reactions. Proc. Natl. Acad. Sci. 106, 17661–17666 (2009).
Yang, J., Xu, X., Du, X., Shi, C. & Fang, F. Effects of unconscious processing on implicit memory for fearful faces. PLoS One. 6, e14641 (2011).
Yang, Y. H. & Yeh, S. L. Unconscious processing of facial expression as revealed by affective priming under continuous flash suppression. Psychon Bull. Rev. 25, 2215–2223 (2018).
Zhan, M. & de Gelder, B. Unconscious fearful body perception enhances discrimination of conscious anger expressions under continuous flash suppression. Neuropsychologia 128, 325–331 (2019).
Luo, Q., Holroyd, T., Jones, M., Hendler, T. & Blair, J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage 34, 839–847 (2007).
Méndez-Bértolo, C. et al. A fast pathway for fear in human amygdala. Nat. Neurosci. 19, 1041–1049 (2016).
Borgomaneri, S., Vitale, F. & Avenanti, A. Behavioral Inhibition system sensitivity enhances motor cortex suppression when watching fearful body expressions. Brain Struct. Funct. 222, 3267–3282 (2017).
Borgomaneri, S., Vitale, F. & Avenanti, A. Early motor reactivity to observed human body postures is affected by body expression, not gender. Neuropsychologia 146, 107541 (2020).
Borgomaneri, S., Vitale, F., Battaglia, S. & Avenanti, A. Early right motor cortex response to happy and fearful facial expressions: A TMS motor-evoked potential study. Brain Sci. 11, 1203 (2021).
Borgomaneri, S., Vitale, F., Battaglia, S., de Vega, M. & Avenanti, A. Task-related modulation of motor response to emotional bodies: A TMS motor-evoked potential study. Cortex 171, 235–246 (2024).
Borgomaneri, S., Gazzola, V. & Avenanti, A. Transcranial magnetic stimulation reveals two functionally distinct stages of motor cortex involvement during perception of emotional body Language. Brain Struct. Funct. 220, 2765–2781 (2015).
Borgomaneri, S., Vitale, F. & Avenanti, A. Early changes in corticospinal excitability when seeing fearful body expressions. Sci. Rep. 5, 14122 (2015).
Fanselow, M. S. Neural organization of the defensive behavior system responsible for fear. Psychon Bull. Rev. 1, 429–438 (1994).
Hagenaars, M. A., Oitzl, M. & Roelofs, K. Updating freeze: aligning animal and human research. Neurosci. Biobehav Rev. 47, 165–176 (2014).
Frijda, N. H. Impulsive action and motivation. Biol. Psychol. 84, 570–579 (2010).
Lang, P. J. & Bradley, M. M. Emotion and the motivational brain. Biol. Psychol. 84, 437–450 (2010).
Borgomaneri, S., Gazzola, V. & Avenanti, A. Temporal dynamics of motor cortex excitability during perception of natural emotional scenes. Soc. Cogn. Affect. Neurosci. 9, 1451–1457 (2014).
Vicario, C. M. et al. Pictures of disgusting foods and disgusted facial expressions suppress the tongue motor cortex. Soc. Cogn. Affect. Neurosci. 12, 352–362 (2017).
Botta, A., Lagravinese, G., Bove, M., Pelosin, E., Bonassi, G., Avenanti, A., & Avanzino, L. Sensorimotor inhibition during emotional processing. Sci. Rep. 12, 6998 (2022).
Borgomaneri, S., Vitale, F., Gazzola, V. & Avenanti, A. Seeing fearful body Language rapidly freezes the observer’s motor cortex. Cortex 65, 232–245 (2015).
Quettier, T. et al. Individual differences in intracortical Inhibition predict action control when facing emotional stimuli. Front. Psychol. 15, 1391723 (2024).
Engelen, T., Zhan, M., Sack, A. T. & de Gelder, B. The influence of conscious and unconscious body threat expressions on motor evoked potentials studied with continuous flash suppression. Front. Neurosci. 12, 480 (2018).
Williams, L. M., Palmer, D., Liddell, B. J., Song, L. & Gordon, E. The ‘when’ and ‘where’ of perceiving signals of threat versus non-threat. Neuroimage 31, 458–467 (2006).
Vuilleumier, P. & Pourtois, G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia 45, 174–194 (2007).
van Heijnsbergen, C. C. R. J., Meeren, H. K. M., Grèzes, J. & de Gelder, B. Rapid detection of fear in body expressions, an ERP study. Brain Res. 1186, 233–241 (2007).
Faul, F., Erdfelder, E., Lang, A. G. G. & Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 39, 175–191 (2007).
Borgomaneri, S., Gazzola, V. & Avenanti, A. Motor mapping of implied actions during perception of emotional body Language. Brain Stimul. 5, 70–76 (2012).
Rossi, S. et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin. Neurophysiol. 132, 269–306 (2021).
Rossini, P. M. et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 126, 1071–1107 (2015).
Tokimura, H., Tokimura, Y., Oliviero, A., Asakura, T. & Rothwell, J. C. Speech-induced changes in corticospinal excitability. Ann. Neurol. 40, 628–634 (1996).
Meister, I. G. et al. Motor cortex hand area and speech: implications for the development of Language. Neuropsychologia 41, 401–406 (2003).
Tidoni, E., Borgomaneri, S., di Pellegrino, G. & Avenanti, A. Action simulation plays a critical role in deceptive action recognition. J. Neurosci. 33, 611–623 (2013).
Chen, R. et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48, 1398–1403 (1997).
Sweeny, T. D., Grabowecky, M., Suzuki, S. & Paller, K. A. Long-lasting effects of subliminal affective priming from facial expressions. Conscious. Cogn. 18, 929–938 (2009).
Carver, C. S. & White, T. L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J. Pers. Soc. Psychol. 67, 319–333 (1994).
Leone, L., Perugini, M., Bagozzi, R. P., Pierro, A. & Mannetti, L. Construct validity and generalizability of the Carver - White behavioural Inhibition system / Behavioural activation system scales. Eur. J. Pers. 15, 373–390 (2001).
Spielberger, C. D. The State-Trait Anxiety Inventory (STAI) (Consulting Psychologist, 1983).
Devanne, H., Lavoie, B. A. & Capaday, C. Input-output properties and gain changes in the human corticospinal pathway. Exp. Brain Res. 114, 329–338 (1997).
Liddell, B. J., Williams, L. M., Rathjen, J., Shevrin, H. & Gordon, E. A Temporal dissociation of subliminal versus supraliminal fear perception: an event-related potential study. J. Cogn. Neurosci. 16, 479–486 (2004).
Williams, M. A., Moss, S. A. & Bradshaw, J. L. A unique look at face processing: the impact of masked faces on the processing of facial features. Cognition 91, 155–172 (2004).
Kiss, M. & Eimer, M. ERPs reveal subliminal processing of fearful faces. Psychophysiology 45, 318–326 (2008).
Pegna, A. J., Landis, T. & Khateb, A. Electrophysiological evidence for early non-conscious processing of fearful facial expressions. Int. J. Psychophysiol. 70, 127–136 (2008).
Pegna, A. J., Darque, A., Berrut, C. & Khateb, A. Early ERP modulation for task-irrelevant subliminal faces. Front. Psychol. 2, 1–10 (2011).
Lu, Y., Zhang, W. N. N., Hu, W. & Luo, Y. J. J. Understanding the subliminal affective priming effect of facial stimuli: an ERP study. Neurosci. Lett. 502, 182–185 (2011).
Lee, S. A., Kim, C. Y., Shim, M. & Lee, S. H. Gender differences in neural responses to perceptually invisible fearful face—an ERP study. Front. Behav. Neurosci. 11, 1–12 (2017).
Tanaka, M. et al. Gender differences in subliminal affective face priming: A high-density ERP study. Brain Behav. 11, e02060 (2021).
Maniscalco, B. & Lau, H. A signal detection theoretic approach for estimating metacognitive sensitivity from confidence ratings. Conscious. Cogn. 21, 422–430 (2012).
Trajkovic, J., Gregorio, F., Di, Avenanti, A., Thut, G. & Romei, V. Two oscillatory correlates of attention control in the Alpha-Band with distinct consequences on perceptual gain and metacognition. J. Neurosci. 43, 3548–3556 (2023).
Aron, A. R., Robbins, T. W. & Poldrack, R. A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 8, 170–177 (2004).
Serrien, D. J., Ivry, R. B. & Swinnen, S. P. Dynamics of hemispheric specialization and integration in the context of motor control. Nat. Rev. Neurosci. 7, 160–166 (2006).
van den Berg, F. E., Swinnen, S. P. & Wenderoth, N. Excitability of the motor cortex ipsilateral to the moving body side depends on Spatio-Temporal task complexity and hemispheric specialization. PLoS One. 6, e17742 (2011).
Caligiuri, M. P. et al. A functional magnetic resonance imaging study of cortical asymmetry in bipolar disorder. Bipolar Disord. 6, 183–196 (2004).
Klein, P. A., Duque, J., Labruna, L. & Ivry, R. B. Comparison of the two cerebral hemispheres in inhibitory processes operative during movement Preparation. Neuroimage 125, 220–232 (2016).
Vines, B. W., Nair, D. & Schlaug, G. Modulating activity in the motor cortex affects performance for the two hands differently depending upon which hemisphere is stimulated. Eur. J. Neurosci. 28, 1667–1673 (2008).
Komeilipoor, N., Pizzolato, F., Daffertshofer, A. & Cesari, P. Excitability of motor cortices as a function of emotional sounds. PLoS One. 8, e63060 (2013).
LeDoux, J. Emotional networks and motor control: a fearful view. Prog Brain Res. 107, 437–446 (1996).
van de Riet, W. A. C., Grèzes, J. & de Gelder, B. Specific and common brain regions involved in the perception of faces and bodies and the representation of their emotional expressions. Soc. Neurosci. 4, 101–120 (2009).
Tamietto, M. & de Gelder, B. Neural bases of the non-conscious perception of emotional signals. Nat. Rev. Neurosci. 11, 697–709 (2010).
Tamietto, M., Pullens, P., de Gelder, B., Weiskrantz, L. & Goebel, R. Subcortical connections to human amygdala and changes following destruction of the visual cortex. Curr. Biol. 22, 1449–1455 (2012).
Grèzes, J., Valabrègue, R., Gholipour, B. & Chevallier, C. A direct amygdala-motor pathway for emotional displays to influence action: A diffusion tensor imaging study. Hum. Brain Mapp. 35, 5974–5983 (2014).
Amodio, D. M., Master, S. L., Yee, C. M. & Taylor, S. E. Neurocognitive components of the behavioral Inhibition and activation systems: implications for theories of self-regulation. Psychophysiology 45, 11–19 (2008).
Gray, J. A. & McNaughton, N. The neuropsychology of anxiety: Reprise. In Nebraska Symposium on Motivation, 1995: Perspectives on Anxiety, Panic, and Fear (ed. Hope D. A) 61–134 (University of Nebraska Press, 1996).
Frenkel-Toledo, S. et al. Lesion location impact on functional recovery of the hemiparetic upper limb. PLoS One. 14, e0219738 (2019).
Stefanou, M. I., Galevska, D., Zrenner, C., Ziemann, U. & Nieminen, J. O. Interhemispheric symmetry of µ-rhythm phase-dependency of corticospinal excitability. Sci. Rep. 10, 1–9 (2020).
Acknowledgements
S.Bo was supported by Next Generation EU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP) PRIN 2022 (grant no. 2022XKZBFC—CUP J53D23008340001): the influence of emotions on action control: brain network plasticity and potential trans-diagnostic applications (D DN. 104 02.02.2022) and Bial Foundation, Portugal (033/22). The views and opinions expressed are solely those of the authors and do not necessarily reflect those of the European Union, nor can the European Union be held responsible for them. S.Ba and AA were supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), and project MNESYS (PE0000006)—a multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022) and Bial Foundation, Portugal (235/22). MT is supported by ERC Proof of Concept “PRISM” (grant id. 101158). AA was also supported grants from the Bial Foundation, Portugal (304/2022), Universidad Católica Del Maule, Chile [CDPDS2022]; and Fondazione del Monte di Bologna e Ravenna, Italy [1402 bis/2021]. The authors wish to thank Gianluigi Serio and Giulia Donati for their help in data collection.
Author information
Authors and Affiliations
Contributions
A.A. and S.Bo. developed the study concept and contributed to the study design; S.Bo. performed testing and data collection; T.Q. performed the data analysis; S.Bo., M.A., T.Q. wrote the manuscript; all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borgomaneri, S., Quettier, T., Ambrosecchia, M. et al. Early changes in corticospinal excitability for subliminally presented fearful body postures. Sci Rep 15, 29088 (2025). https://doi.org/10.1038/s41598-025-13185-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13185-y