Abstract
Childhood maltreatment can disrupt brain development, leading to vulnerabilities in white matter (WM) microstructure, compromised brain integrity, and various psychiatric disorders. Among different forms of maltreatment, neglect is the most common; however, limited data exist on its specific impact on the brain. This study utilized diffusion tensor imaging to examine WM microstructure differences between neglected children without other types of maltreatment (Neglect, n = 21) and typically developing controls (TD, n = 106). Additionally, the study explored the relationship between WM microstructure alterations and psychosocial problems observed in neglected children. Neglected children exhibited larger axial diffusion (AD) in regions such as the right corticospinal tract (R.CST), right superior longitudinal fasciculus (R.SLF), and left cingulum (L.CG) compared to typically developing children. Increased AD in the R.CST, L.CG, and R.SLF was associated with conduct problems. These findings suggest that alterations in WM microstructure contributed to behavioral symptoms in neglected children.
Similar content being viewed by others
Introduction
Child neglect, defined as the failure to provide essential care during early development1 has been linked to impaired social and emotional skills2 and long-term adverse outcomes, including increased vulnerability to mental illness3 heightened suicide risk4 and other physical ailments5. Among all forms of maltreatment, child neglect remains the most prevalent, representing 76.0% of substantiated child abuse cases globally6. This form of maltreatment involves the persistent failure to meet a child’s basic needs—including adequate nutrition, clothing, shelter, supervision, and protection—placing the child’s health and development at serious risk. Despite its profound and lasting impact, neglect often receives less attention compared to more overt forms of maltreatment. Understanding the neurodevelopmental consequences of neglect requires a closer examination of its unique influence on brain structure and functional connectivity, utilizing advanced neuroimaging techniques.
Previous studies have explored the effects of neglect on brain structure and functional connectivity7,8,9. Teicher, et al.9 examined magnetic resonance imaging (MRI) data from 28 children with a history of abuse or neglect and 115 healthy controls, reporting reduced corpus callosum volume among maltreated children. Kawata, et al.7 extended these findings by examining the anterior cingulate cortex in 23 neglected children and 140 healthy controls, revealing larger gray matter volume in this region along with disruptions in the salience network’s functional connectivity, which exacerbated depressive symptoms. These studies highlight the critical role of structural abnormalities and functional connectivity disruptions in understanding neglect’s effects on the brain. However, existing research has significant gaps. Although structural and functional imaging studies have provided valuable insights, relatively few studies have utilized diffusion tensor imaging (DTI), a technique capable of detecting subtle microstructural abnormalities in white matter (WM)10.
DTI provides a unique and complementary perspective on WM microstructure compared to voxel-based morphometry (VBM) and functional connectivity analyses. Although VBM focuses on gray matter volume and density, providing structural insights, it lacks the sensitivity to detect subtle WM alterations essential for understanding neural communication11. Similarly, functional connectivity measures the temporal correlation of brain activity across regions, revealing functional integration and network dynamics without addressing the structural pathways that facilitate these connections. DTI excels in characterizing WM integrity by quantifying diffusion properties such as fractional anisotropy (FA) and axial diffusivity (AD), which serve as direct markers of axonal density, myelination, and microstructural organization11. This capability makes DTI particularly valuable for investigating how disruptions in WM pathways contribute to atypical connectivity and functional impairments. Studies employing DTI have identified microstructural changes in maltreated populations, including reduced FA in the thalamic radiation and cingulum, with these reductions being linked to neglect and associated anxiety symptoms12,13. These findings suggest that WM microstructural pathways play a crucial role in mediating functional connectivity alterations in maltreated children. Child neglect, particularly during critical developmental periods, significantly affects WM integrity14. Children who experience neglect have been associated with reduced FA in specific WM fiber tracts, suggesting impaired connectivity between brain regions responsible for emotional regulation, attention, and cognitive control14. The mechanisms underlying these alterations are thought to involve the chronic stress and deprivation characteristic of neglect, which disrupt neurodevelopmental processes such as axonal growth, synaptogenesis, and myelination15. These microstructural changes in WM are believed to contribute to the cognitive, emotional, and social impairments observed in neglected children, including difficulties in executive functioning, emotional regulation, and social interaction.
Identifying and understanding these WM alterations are critical for developing targeted interventions to mitigate the long-term impacts of neglect on brain development. Despite advancements in research, significant statistical and methodological challenges remain. Previous studies have failed to isolate the unique effects of neglect8 often grouping it with other maltreatment types, which obscures its specific impact. In this study, we proposed that the neural mechanisms underlying the psychosocial characteristics of neglected children were directly linked to the integrity of the microstructure of WM, which is associated with the pathways of connectivity between various brain regions. To test this hypothesis, we examined whether children who had primarily undergone social intervention due to neglect, supported by official maltreatment records, displayed distinct WM microstructural features and psychosocial traits compared to the general population. A comprehensive whole-brain analysis was conducted to detect WM microstructural atypicality using the tract-based spatial statistics (TBSS) analysis.
Results
Clinical demographics psychological assessment outcomes
This brain MRI study employed DTI to compare WM fiber tracts in the brains of neglected children without other types of maltreatment (Neglect, n = 21) with typically developing children (TD, n = 106). The study also explored how these differences in WM fiber tracts relate to specific psychosocial problems observed in neglected children. Table 1 summarizes the clinical demographics and psychological assessment outcomes for the participants in the dataset. Significant group differences were detected in the full-scale intelligence quotient (FSIQ), which was incorporated as a covariate in the group comparisons for brain imaging analyses beyond sex, age, and MRI type. As estimated, individuals in the Neglect group exhibited significantly greater difficulties in psychosocial behavior and cognitive function.
Tract-based Spatial statistics
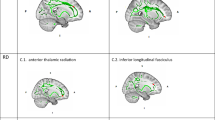
A TBSS analysis, with family-wise error (FWE) correction at P < 0.5 for cluster-level significance, was performed to examine microstructural differences in WM between the two groups. Significant findings included larger AD in the right cortical spinal tract (R.CST; Montreal Neurological Institute [MNI] coordinates: x = 28, y = − 15, z = 28; cluster size = 584 voxels; FWE corrected P = 0.015), the right superior longitudinal fasciculus (R.SLF; MNI coordinates: x = 32, y = − 23, z = 39; cluster size = 24 voxels; FWE corrected P = 0.045), and left cingulum (L.CG; MNI coordinates: x = − 17, y = 26, z = 27; cluster size = 67 voxels; FWE corrected P = 0.037) in the Neglect group compared to the TD group (Fig. 1). No other areas of increased or decreased FA, medial diffusivity (MD), or radial diffusivity (RD) were found with a corrected cluster probability value that approached significance. The AD values from the identified clusters in the R.CST, R.SLF, and L.CG were extracted and used for correlation analyses.
Structural differences in the white matter (WM) between neglected and typically developing (TD) groups. Larger WM fiber tracts were observed in the Neglect group compared to the TD group. Slices of the mean FA skeleton (green), overlaid with red clusters, depict significantly higher AD values in the Neglect group than in the TD group (Neglect > TD; FWE corrected P < 0.05, red-yellow). FWE, family-wise error; AD, axial diffusivity; FA, fractional anisotropy; L.CG, left cingulum; R.CST, right cortical spinal tract; R.SLF, right superior longitudinal fasciculus
AD values and psychological States correlation
The strength and difficulties questionnaire (SDQ) in conduct problems (CP) showed significant correlations with the AD values in the R.CST (r = 0.20, 95% confidence interval [Cl]: 0.05−0.34, false discovery rate [FDR] corrected P = 0.034; Power (1 − β) = 0.98), R.SLF (r = 0.19, 95% Cl: 0.04−0.32, FDR corrected P = 0.034; Power (1 − β) = 0.92), and L.CG (r = 0.24, 95% Cl: 0.10−0.37, FDR corrected P = 0.018; Power (1 − β) = 0.87) (Fig. 2). No other correlations were significant.
Correlations plots between CP and L.CG, CP and R.CST, and CP and R.SLF. Closed circles represent the Neglect group, and open circles represent the typically developing (TD) group. The shaded beige area indicates 95% confidence intervals for the regression line (dark yellow). L.CG, left cingulum; R.CST, right cortical spinal tract; R.SLF, right superior longitudinal fasciculus; CP, conduct problems.
Discussion
This study explored potential differences in WM tracts between children who experienced neglect and TD children. The results of the case-control analysis showed significantly larger AD values in the R.CST, R.SLF, and L.CG in neglected children compared to TD children. The larger values in the R.CST, R.SLF, and L.CG were associated with neglected experiences and current behavioral problems observed in children in this study.
Abnormalities in the WM tract maturation are associated with psychiatric and developmental disorders12,16,17,18. AD, which measures the magnitude of diffusion parallel to the fiber tracts, is a key indicator of WM microstructural integrity. Studies have shown that AD and RD typically decrease with age in most brain regions, likely reflecting increased axonal density or caliber19. Conversely, larger AD values in specific disease processes or developmental stages may indicate pathological changes caused by neuronal alterations19. Two studies have examined the impact of neglect on WM microstructure9,14 highlighting alterations in specific regions associated with cognitive and behavioral functions. Hanson, et al.14 utilized DTI to assess WM integrity in the FA of the prefrontal cortex in children raised in institutionalized settings who experienced early neglect. Their findings revealed more diffuse WM organization in the prefrontal cortex, correlating with neurocognitive deficits. Similarly, Teicher, et al.9 found that children who experienced neglect had significant reductions in the corpus callosum area, which is indicative of compromised interhemispheric communication, potentially contributing to cognitive and emotional difficulties. The variability in the MRI analysis techniques and participant backgrounds, including differences in maltreatment type, duration, and onset of neglect, significantly contributes to the inconsistencies observed across studies. No prior research has explored the microstructural characteristics of WM fibers in neglected children using diffusion-weighted imaging (DWI). Although previous studies have emphasized the importance of WM tract integrity in children exposed to neglect, the interpretation of WM microstructural changes in children who have experienced neglect exclusively remains unclear. Our study is the first to identify increased AD values in the R.CST, R.SLF, and L.CG in this context.
This study emphasized the role of larger R.CST and L.CG tract features in children who experienced neglect. The findings align with previous research that identifies atypical brain WM tracts associated with common symptoms in maltreated children, such as post-traumatic stress disorder (PTSD) and WM microstructural abnormalities linked to maltreatment20,21. The CST, primarily originating from the frontoparietal cortices, including the primary motor cortex, secondary motor area, and somatosensory cortex22 plays a critical role in voluntary motor control. Previous studies have also examined the CST in relation to inattention and impulsivity in children with attention-deficit/hyperactivity disorder (ADHD)20. Conversely, WM microstructural abnormalities in the CG, the WM core of the cingulate gyrus23,24 have been implicated in behavioral issues such as emotional dysregulation and aggression. The CG connects the limbic system to dorsal cortical brain systems, including the corticolimbic emotional system, the fronto-parietal attentional system, and the motor-control system23,25. It plays a vital role in emotion regulation, cognitive processing, autonomic nervous system responses, and motor control through its interaction with the CST25. The observed alterations in WM in child neglect align with findings from a recent meta-analysis, which reported consistent structural abnormalities across fronto-limbic and fronto-parietal pathways in individuals with a history of childhood maltreatment26. This study further underscores the importance of the CG in the context of child neglect, suggesting that WM microstructural abnormalities in the CG may contribute to the neuropathological mechanisms associated with neglect. The positive correlations observed between larger R.CST and CP, as well as between L.CG and CP, may reflect an acquired tendency in neglected children to exhibit heightened behavioral challenges. The increased AD values observed in the CST and CG in this study provide neurobiological evidence supporting these manifestations.
Additionally, we found another atypicality in the WM tracts of the Neglect group, specifically in the larger R.SLF. The SLF is a WM tract in the brain that has been implicated in ADHD27. The SLF is a major WM tract that connects the frontal and parietal regions of the brain, playing a crucial role in cognitive functions such as attention, language, and executive functions28. Alterations in the SLF have been associated with various behavioral and cognitive issues in children. In children with ADHD, atypical microstructural organization within the SLF has been associated with reduced fine motor competence29. Specifically, atypical fiber density in the R.SLF correlates with poorer performance on fine motor tasks. Furthermore, the SLF and CG are major WM tracts integral to various cognitive and emotional functions30. A study examining structural brain connectivity in children with behavioral problems found that atypical WM integrity, particularly in the SLF and CG, is associated with childhood conduct problems31. The present study observed positive correlations between CP and the R.SLF and between CP and the L.CG in neglected children, suggesting the potential acquisition of neurobiological features characteristic of ADHD. The observed WM alterations align with DTI-based structural connectome studies, indicating that childhood maltreatment compromises the integrity of large-scale structural networks, potentially affecting both cognitive and emotional functioning32. Although children exposed to maltreatment often do not meet diagnostic criteria for neurodevelopmental disorders, trauma-related experiences and difficulties in forming secure attachments may lead to symptoms that resemble such conditions33.
Although these WM tracts have distinct origins, they interact within the brain’s intricate neural network. The CST, originating in the motor cortex, may be influenced by cognitive and emotional processes mediated through its connections with the SLF and CG.
This study had several limitations. First, identifying the distinct effects of multiple factors within this cross-sectional study posed significant difficulties due to the intricate and multifaceted nature of neurodevelopmental processes. As neglect frequently occurs alongside poverty or prematurity, low birth weight, and malnutrition, distinguishing their individual contributions to brain development remains complex34. In this study, several steps were taken to minimize the potential confounding influence of these early-life biological risks. We conducted a retrospective review of gestational age and birth weight data (where available) and found no systematic group-level differences between the neglected and TD children. We also applied a stricter inclusion criterion by excluding children with FSIQ scores below 70, which may indirectly account for the effects of severe perinatal complications and malnutrition35,36,37. Additionally, to enhance the interpretability of the results and reduce variability related to cognitive impairment, we used a different participant sample from our previous study7. Moreover, we improved the analytical rigor by adjusting for age and sex as covariates in all correlation analyses to account for developmental influences. FDR correction was applied to all statistical comparisons involving brain regions and psychological states; thereby, reducing the risk of Type I errors. These methodological enhancements substantially strengthen the validity and generalizability of the present findings. Nonetheless, some residual limitations remain. For instance, early developmental factors could not be entirely ruled out, and future multimodal and longitudinal studies should assess these risks more directly. Furthermore, the findings may have limited generalizability due to the possible influence of social interventions aimed at assisting neglected children. Second, although significant group differences in behavioral measures such as CP and hyperactivity-inattention (HI) were observed, these were reported in Table 1 for descriptive purposes. No multiple comparison corrections were applied to these demographic and clinical characteristics statistics, in line with common practices in reporting demographic characteristics7,12. However, CP was subsequently analyzed using hypothesis-driven models with full adjustment for covariates (age, sex, MRI type, and FSIQ), and FDR correction was applied to all statistical tests involving brain regions and psychological states. Third, although multiple steps were taken to reduce the risk of Type I error—most notably through FDR correction of correlation analyses—this study did not correct for the number of psychological scales analyzed, which may have introduced residual inflation of false positives. Additionally, correlations were performed on the entire sample (rather than stratified by group), which may have introduced confounding due to group membership. Fourth, sex differences were not examined despite prior evidence suggesting gender-specific effects of neglect; future research should investigate this possibility. Finally, the MRI data were acquired using two different scanner types and protocols. Although MRI type was included as a covariate, the data were not harmonized across acquisition parameters, potentially affecting the consistency of the results. These limitations underscore the need for meticulous study design, careful participant selection, and rigorous data analysis in future investigations on the effects of neglect on brain development.
In conclusion, this study highlighted significant differences in WM tracts between children who experienced neglect and TD children, particularly in the R.CST, R.SLF, and L.CG. These findings provide valuable clinical insights for designing targeted interventions and therapies that address the specific developmental challenges faced by neglected children. Understanding the interplay between these WM tracts enhances our knowledge of the neurobiological impacts of neglect and lays a foundation for developing evidence-based strategies to support affected children in achieving healthier developmental trajectories.
Methods
Ethics statement
The study protocol was approved by the Ethics Committee of the University of Fukui, Japan (assurance no. 20220034, 20210004, 20130157, 20138012 [UMIN000013215], and 20110104). Written informed consent was obtained for all data used beyond the original study purposes, and secondary data analysis was approved by the Ethics Committee of the University of Fukui, Japan (assurance no. 20220039). This study was conducted in accordance with the Declaration of Helsinki38 and the Ethical Guidelines for Clinical Studies of the Ministry of Health, Labor, and Welfare of Japan. All parents or directors of a child welfare facility provided written informed consent. Further details can be found in the study previously published by Kawata, et al.7.
Participants
This study utilized a brain DWI MRI archival dataset of children exposed to maltreatment and TD children collected under several original study protocols (Supplementary Table S1). Twenty-one cases of neglect (ICD-10-CM Code T74.02) were included in the study (mean age = 13.5 years, standard deviation [SD] = 2.6). These cases did not involve any prior exposure to other forms of maltreatment, such as physical, emotional, or sexual abuse, as previously reported by Kawata, et al.7. The maltreated children in this study had experienced continuous or significant maltreatment to the extent that a serious decision was made by the Child Protection Service or equivalent, which involved legal intervention and potentially drastic changes to the child’s life (e.g., removal from biological parents and placement in residential childcare facilities). One hundred and six TD children without a history of maltreatment were extracted from the dataset and matched for age and sex (mean age = 12.5 years, SD = 2.6). These children had no prior diagnoses of autism spectrum disorder (ASD) or ADHD39. Recruitment of the TD children was described previously40,41. Participants with an FSIQ below 70 were excluded from the analysis.
Psychological assessment
The FSIQ was assessed using the Wechsler Intelligence Scale for Children (WISC) Fourth Edition42 or the Wechsler Adult Intelligence Scale (WAIS) Third Edition43 under the supervision of pediatric clinicians (AT). Caregivers provided ratings on the SDQ to assess various psychopathological domains, including emotional symptoms (ES), CP, HI, peer problems (PP), prosocial behavior (PB), and total behavioral difficulties44. Depressive symptoms were measured using the depression self-rating scale for children (DSRS-C)45. Further details can be found in the study previously published by Kawata, et al.7.
Image preprocessing and statistical analyses
This study integrated diverse datasets from original studies, with details on the resonance image acquisition protocols presented in Supplementary Table S1. The DTI data, initially stored in the Digital Imaging and Communication in Medicine (DICOM) image format, were converted into the Neuroimaging Informatics Technology Initiative (NIFTI) image format using MRIcron (version 1.0.10101102, https://www.nitrc.org/projects/mricron). No participants exhibited focal structural abnormalities in DWI scans. Preprocessing of the DTI data was conducted using MRtrix (version 3.0.4, https://mrtrix.org) and the TBSS pipeline at the Oxford Centre for Functional MRI of the Brain Software Library (FSL, version 6.0.7.6, https://fsl.fmrib.ox.ac.uk/). Corrections for the susceptibility-induced off-resonance field, eddy current, and movement-induced distortions were implemented using the eddy tool. Bias field correction was performed using the Advanced Normalization Tools (ANTs, version 2.4.2) bias correction algorithm. Masks were generated from bias-field-corrected images. The FSL toolbox DTIFIT fitted the preprocessed images to derive four parameters of the diffusion tensor model: FA, MD, AD, and RD. MD, representing the average diffusivity of molecular motion, was calculated. AD, indicating diffusivity parallel to the axonal fibers, corresponded to the first eigenvalue (L1). RD, reflecting diffusivity perpendicular to the axonal fibers, was computed as the average of the second (L2) and third (L3) eigenvalues.
We used TBSS, a voxel-wise approach, to investigate whole-brain fiber tracts, prioritizing sensitivity, objectivity, and interpretability in DTI analyses, as described previously. The TBSS tool in FSL facilitated the voxel-wise analysis of preprocessed FA, MD, AD, and RD values. With the nonlinear registration tool of FNIRT (FMRIB’s Nonlinear Registration Tool), the FA, MD, AD, and RD images of each participant were registered to the FMRIB58_FA_1 mm template in the MNI space. A mean FA image was computed with an FA threshold value of 0.2, and this image was thinned to create a mean FA skeleton. Each participant’s aligned FA map was projected onto the skeleton using the maximum FA value detected around the tract. Finally, the AD, RD, and MD skeletons were created by applying the projection parameters derived from the FA skeletonization process. By focusing only on voxels closest to the white matter fiber skeleton, TBSS minimized statistical errors associated with imperfect registration. Additionally, the non-parametric test approach eliminated the need for image smoothing; thus, reducing partial volume effects.
To compare FA, MD, AD, and RD values between the two groups while controlling for potential confounding variables, we included age, sex, MRI type, and FSIQ as covariates of no interest in the standard general linear model (GLM, version 8.6.13) design matrix in FSL. Voxel-wise statistical analyses of FA, MD, AD, and RD data were performed using FSL’s randomize tool (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/randomise/index.html). This tool employed a non-parametric permutation-based inference approach, incorporating the threshold-free cluster enhancement (TFCE) option. Analyses involved 5,000 permutations, with FWE correction applied at a significance threshold of P < 0.05 for each measure. The anatomical locations of significant clusters were identified using the JHU white-matter tractography atlas integrated into the FSL46.
Correlation between regional brain atypicality and psychological assessment outcomes
Demographic and clinical characteristics were compared using chi-squared (χ2 tests and independent sample t-tests. Pearson correlation analyses were conducted to investigate correlations between each AD value (dependent variables) identified in the group comparisons and psychological assessment scores (independent variables), including total SDQ scores, the five SDQ subscales, and DSRS-C outcomes. These analyses incorporated adjustments for relevant covariates (age, sex, FSIQ, and MRI type) consistent with the multiple linear regression models. All statistical analyses were performed using R software (version 2024.09.0, R Core Development Team, Toulouse, France, https://cran.rstudio.com/). Statistical significance was set at FDR corrected P < 0.05.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the risk of subject identification, subjects whose anonymity should be carefully protected. However, data and code may be provided to interested researchers upon reasonable request to the corresponding author, after clearance from the Research Ethics Committee.
References
Daley SF, G. D., Bethencourt Mirabal A, et al. Child abuse and Neglect. StatPearls (2025).
Haslam, Z. & Taylor, E. P. The relationship between child neglect and adolescent interpersonal functioning: A systematic review. Child. Abuse & Negl. 125, 105510. https://doi.org/10.1016/j.chiabu.2022.105510 (2022).
Adlington, K., Easter, A., Galloway, H. & Howard, L. M. Mental health is neglected in maternal ‘near miss’ research. BMJ 376, e069486. https://doi.org/10.1136/bmj-2021-069486 (2022).
Tang, S., Ports, K. A., Stone, D. M. & Lin, H. C. The mediating role of internalizing and externalizing symptoms in the association between child neglect and suicide attempt in adulthood. Int. J. Inj Contr Saf. Promot. 29, 112–122. https://doi.org/10.1080/17457300.2021.2007406 (2022).
Grummitt, L. R. et al. Associations of childhood emotional and physical neglect with mental health and substance use in young adults. Aust N Z. J. Psychiatry. 56, 365–375. https://doi.org/10.1177/00048674211025691 (2022).
Child welfare information gateway. Child Maltreatment. U.S. Department of Health and humaan Services, Adiministration for Children and Families, Children’s Bureau. (2023).
Kawata, N. Y. et al. Brain structures and functional connectivity in neglected children with no other types of maltreatment. NeuroImage 292, 120589. https://doi.org/10.1016/j.neuroimage.2024.120589 (2024).
Rakesh, D., Allen, N. B. & Whittle, S. Longitudinal changes in within-salience network functional connectivity mediate the relationship between childhood abuse and neglect, and mental health during adolescence. Psychol. Med. 53, 1552–1564. https://doi.org/10.1017/s0033291721003135 (2023).
Teicher, M. H. et al. Childhood neglect is associated with reduced corpus callosum area. Biol. Psychiatry. 56, 80–85. https://doi.org/10.1016/j.biopsych.2004.03.016 (2004).
Yoshida, S., Oishi, K., Faria, A. V. & Mori, S. Diffusion tensor imaging of normal brain development. Pediatr. Radiol. 43, 15–27. https://doi.org/10.1007/s00247-012-2496-x (2013).
Dhollander, T. et al. Fixel-based analysis of aiffusion MRI: methods, applications, challenges and opportunities. NeuroImage 241, 118417, https://doi.org/10.1016/j.neuroimage.2021.118417 (2021).
Makita, K. et al. White matter changes in children and adolescents with reactive attachment disorder: A diffusion tensor imaging study. Psychiatry Res. Neuroimaging. 303, 111129. https://doi.org/10.1016/j.pscychresns.2020.111129 (2020).
Tendolkar, I., Mårtensson, J., Kühn, S., Klumpers, F. & Fernández, G. Physical neglect during childhood alters white matter connectivity in healthy young males. Hum. Brain Mapp. 39, 1283–1290. https://doi.org/10.1002/hbm.23916 (2018).
Hanson, J. L. et al. Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child. Dev. 84, 1566–1578. https://doi.org/10.1111/cdev.12069 (2013).
Antontseva, E., Bondar, N., Reshetnikov, V. & Merkulova, T. The effects of chronic stress on brain myelination in humans and in various rodent models. Neuroscience 441, 226–238. https://doi.org/10.1016/j.neuroscience.2020.06.013 (2020).
Jung, M. et al. Thalamic volume is related to increased anterior thalamic radiations in children with reactive attachment disorder. Cereb. Cortex. 30, 4238–4245. https://doi.org/10.1093/cercor/bhaa051 (2020).
Vanes, L. D. et al. White matter tract Myelin maturation and its association with general psychopathology in adolescence and early adulthood. Hum. Brain Mapp. 41, 827–839. https://doi.org/10.1002/hbm.24842 (2020).
Weber, C. F. et al. Age-dependent white matter microstructural disintegrity in autism spectrum disorder. Front. NeuroSci. 16 https://doi.org/10.3389/fnins.2022.957018 (2022).
Kumar, R., Nguyen, H. D., Macey, P. M., Woo, M. A. & Harper, R. M. Regional brain axial and radial diffusivity changes during development. J. Neurosci. Res. 90, 346–355. https://doi.org/10.1002/jnr.22757 (2012).
Bu, X. et al. Quantitative tractography reveals changes in the corticospinal tract in drug-naïve children with attention-deficit/hyperactivity disorder. J. Psychiatry Neurosci. 45, 134–141. https://doi.org/10.1503/jpn.190024 (2020).
Cassiers, L. L. M. et al. Structural and functional brain abnormalities associated with exposure to different childhood trauma subtypes: A systematic review of neuroimaging findings. Front. Psychiatry. 9 https://doi.org/10.3389/fpsyt.2018.00329 (2018).
Moreno-Lopez, Y., Bichara, C., Delbecq, G., Isope, P. & Cordero-Erausquin, M. The corticospinal tract primarily modulates sensory inputs in the mouse lumbar cord. Elife 10 https://doi.org/10.7554/eLife.65304 (2021).
Vachha, B. A., Massoud, T. F. & Huang, S. Y. Anatomy of the cerebral cortex, lobes, and cerebellum. Neuroimaging Clin. N. Am. 32, 463–473. https://doi.org/10.1016/j.nic.2022.04.008 (2022).
Liu, Y. et al. The microstructural abnormalities of cingulum was related to patients with mild cognitive impairment: a diffusion kurtosis imaging study. Neurol. Sci. 44, 171–180. https://doi.org/10.1007/s10072-022-06408-x (2023).
Hung, Y. et al. Cingulum-Callosal white-matter microstructure associated with emotional dysregulation in children: A diffusion tensor imaging study. Neuroimage Clin. 27, 102266. https://doi.org/10.1016/j.nicl.2020.102266 (2020).
Lim, L., Howells, H., Radua, J. & Rubia, K. Aberrant structural connectivity in childhood maltreatment: A meta-analysis. Neurosci. Biobehavioral Reviews. 116, 406–414. https://doi.org/10.1016/j.neubiorev.2020.07.004 (2020).
Chiang, H. L. et al. Atypical development in white matter microstructures in ADHD: A longitudinal diffusion imaging study. Asian J. Psychiatry. 79, 103358. https://doi.org/10.1016/j.ajp.2022.103358 (2023).
Janelle, F., Iorio-Morin, C., D’Amour, S. & Fortin, D. Superior longitudinal fasciculus: A review of the anatomical descriptions with functional correlates. Front. Neurol. 13, 794618. https://doi.org/10.3389/fneur.2022.794618 (2022).
Hyde, C., Sciberras, E., Efron, D., Fuelscher, I. & Silk, T. Reduced fine motor competence in children with ADHD is associated with atypical microstructural organization within the superior longitudinal fasciculus. Brain Imaging Behav. 15, 727–737. https://doi.org/10.1007/s11682-020-00280-z (2021).
Catani, M. & de Thiebaut, M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44, 1105–1132. https://doi.org/10.1016/j.cortex.2008.05.004 (2008).
Burley, D. T., Genc, S. & Silk, T. J. Childhood conduct problems are associated with reduced white matter fibre density and morphology. J. Affect. Disord. 281, 638–645. https://doi.org/10.1016/j.jad.2020.11.098 (2021).
Puetz, V. B. et al. Altered brain network integrity after childhood maltreatment: A structural connectomic DTI-study. Hum. Brain Mapp. 38, 855–868. https://doi.org/10.1002/hbm.23423 (2017).
Cruz, D., Lichten, M., Berg, K. & George, P. Developmental trauma: conceptual framework, associated risks and comorbidities, and evaluation and treatment. Front. Psychiatry. 13, 800687. https://doi.org/10.3389/fpsyt.2022.800687 (2022).
Hock, R. S. et al. Relationship between infant malnutrition and childhood maltreatment in a Barbados lifespan cohort. Vulnerable Child. Youth Stud. 12, 304–313. https://doi.org/10.1080/17450128.2017.1371817 (2017).
Galler, J. R. et al. Neurodevelopmental effects of childhood malnutrition: A neuroimaging perspective. NeuroImage 231, 117828. https://doi.org/10.1016/j.neuroimage.2021.117828 (2021).
Waber, D. P. et al. Impaired IQ and academic skills in adults who experienced moderate to severe infantile malnutrition: a 40-year study. Nutr. Neurosci. 17, 58–64. https://doi.org/10.1179/1476830513y.0000000061 (2014).
Upadhyay, S. K., Agarwal, D. K. & Agarwal, K. N. Influence of malnutrition on intellectual development. Indian J. Med. Res. 90, 430–441 (1989).
World Medical, A. Declaration of Helsinki. Law Med. Health Care. 19, 264–165 (1991).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™ (American Psychiatric Publishing, Inc., 2013).
Mizuno, Y. et al. Structural brain abnormalities in children and adolescents with comorbid autism spectrum disorder and attention-deficit/hyperactivity disorder. Transl Psychiatry. 9, 332. https://doi.org/10.1038/s41398-019-0679-z (2019).
Fujisawa, T. X. et al. Association of epigenetic eifferences Ecreened in a few cases of monozygotic twins discordant for attention-deficit hyperactivity disorder with brain structures. Front. Neurosci. 15, 799761. https://doi.org/10.3389/fnins.2021.799761 (2021).
Wechsler, D. & Kodama, H. Wechsler Intelligence Scale for Children, Fourth Ed 1. (The Psychological Corporation, 2003).
Wechsler, D. Wechsler Adult Intelligence Scale, third edition. Psychological Corporation, San Antonio, TX. (1997).
Stone, L. L., Otten, R., Engels, R. C., Vermulst, A. A. & Janssens, J. M. Psychometric properties of the parent and teacher versions of the strengths and difficulties questionnaire for 4- to 12-year-olds: a review. Clin. Child. Fam Psychol. Rev. 13, 254–274. https://doi.org/10.1007/s10567-010-0071-2 (2010).
Murata, T. Examination of Birleson depression scale for school children in Japan. Jpn J. Latest Psychiatry. 1, 131–138 (1996).
Mori, S. et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40, 570–582. https://doi.org/10.1016/j.neuroimage.2007.12.035 (2008).
Acknowledgements
This work was supported by JSPS KAKENHI Early-Career Scientists [grant number 22K13677 to NYSK], JSPS KAKENHI Fostering Joint International Research [grant number 22KK0218 to NYSK], Grant-in-Aid for Challenging Exploratory Research (Houga) [grant number 21K18499 to AT], AMED [grant number JP20gk0110052 to AT], JSPS KAKENHI Scientific Research (A) [grant number 19H00617 to AT], Grant-in-Aid for“Creating a Safe and Secure Living Environment in the Changing Public and Private Spheres” from the Japan Science and Technology Corporation (JST)/Research Institute of Science and Technology for Society (RISTEX to AT), Research Grant from Japan-United States Brain Research Cooperative Program (AT), the a research grant from the Strategic Budget to Realize University Missions (FY 2022 to AT), JSPS KAKENHI Scientific Research (B) [grant number 23K25644 to TXF], JSPS KAKENHI Scientific Research (C) [grant number 21K02352 to KM and AT], and JSPS KAKENHI Advanced Bioimaging Support (ABiS) [Grant Number JP22H04926]. We thank the Research Center for Child Mental Development of the University of Fukui staff for their clerical support and Dr. Shinichiro Takiguchi, Dr. Sakae Mizushima, Dr. Yoshifumi Mizuno, and Dr. Daisuke Saito for collecting the data.
Author information
Authors and Affiliations
Contributions
NYSK contributed to the conceptualization, methodology, formal analysis, visualization, writing – original draft, writing – review, and editing. KM contributed to the MRI data analysis. AY was responsible for data organization. TXF, KM, AY, HO, and AT contributed to the collected the data, and the revision of the manuscript. AT and TXF contributed to the conception, methodology, and revised the manuscript critically for intellectual content. All authors approved of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kawata, N.Y., Fujisawa, T.X., Makita, K. et al. White matter microstructure abnormalities in children experiencing neglect without other forms of maltreatment. Sci Rep 15, 27282 (2025). https://doi.org/10.1038/s41598-025-13363-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13363-y