Abstract
Oregano essential oil (OEO) is a promising natural preservative due to its strong antimicrobial properties. This study evaluated the effectiveness of low-salt brine enriched with OEO in inhibiting common foodborne pathogens in white brined cheese. The antimicrobial activity of OEO was tested against Escherichia coli and Staphylococcus aureus using standard microbiological techniques. The OEO-enriched brine showed strong bactericidal effects, with minimum bactericidal concentrations of 0.025% (v/v) against E. coli and 0.05% (v/v)—0.1% (v/v) against S. aureus. These effects were maintained over seven days and were not influenced by storage temperature. The findings support the use of OEO as an effective natural preservative for reducing salt content in white brined cheese without compromising microbiological safety.
Similar content being viewed by others
Introduction
Cheese is a widely consumed food product globally, obtained from curdled milk through whey removal and curd maturation in the presence of specific microbiota. White brined cheeses mature in brine containing salt (6–10% sodium chloride), where the high salt content acts as a selective factor for the microbiota that develops within it1. During the cheese production process, particularly during maturation, salt plays a crucial role in modulating the physicochemical and biochemical properties of the product, as well as in shaping its sensory characteristics2.
Excessive sodium chloride consumption has become a global health concern. According to the World Health Organization (WHO), most people consume sodium chloride at levels three times higher than the recommended intake3. Consequently, various strategies have been implemented to reduce salt concentrations in multiple food categories, including cheese. Reducing the salt concentration in cheese brine while maintaining its preservative properties is a technological approach that could offer significant health benefits to consumers. One such approach involves lowering the salt concentration in brine while supplementing it with additional components that work synergistically with sodium chloride to enhance or even surpass its preservative effects. According to Soltani4, the extent to which the salt concentration in brine can be reduced without compromising the physicochemical or organoleptic properties of the cheese ranges between 1% and 2.5%. However, at these low sodium chloride levels, additional agents are required to maintain the brine’s preservative stability. In this regard, the use of natural preservatives in cheese, particularly plant extracts that can complement the antimicrobial effects of salt, is widely discussed5,6.
Despite its probiotic benefits, cheese also serves as a suitable medium for pathogenic microorganisms. The most frequently reported pathogens include Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, and Salmonella spp.
Certain E. coli serotypes cause foodborne illnesses associated with severe pathological manifestations. Generally, the presence of these bacteria in food products indicates hygienic contamination, as they originate from the intestinal flora of animals and humans. The detection of glucuronidase activity is widely used for microbiological analysis of E. coli in food samples. The enzyme β-D-glucuronidase is produced by 94–96% of E. coli strains7. Another significant foodborne pathogen, particularly in cheese, is Staphylococcus aureus. This microorganism is primarily found in cheeses produced under poor hygienic conditions and can produce enterotoxins that pose a health risk to consumers. Microbiological analysis of staphylococci in food products focuses on detecting coagulase-positive staphylococci, as they are associated with significant health hazards.
Controlling microbial contamination in food products during both production and shelf life involves incorporating various categories of preservatives. In mature brined cheeses, high salt concentrations in the brine typically serve as the sole preservative, allowing the product to be stored for up to several months. Natural preservatives, particularly plant extracts and essential oils, are widely discussed as potential alternatives or supplements to salt in cheese preservation. Spices and their essential oils contain bioactive compounds that provide health benefits while preventing microbial contamination in food products. The antimicrobial properties of spices have been well-documented over the past few decades8. Essential oils and their active compounds have also been extensively studied for their effects against pathogenic microorganisms. Extracts and essential oils from aromatic and medicinal plants such as dill, oregano, rosemary, cumin, black pepper, sage, thyme, and parsley have demonstrated satisfactory in vitro antimicrobial activity against pathogens associated with cheese contamination9,10,11, highlighting their potential as preservatives. Studies consistently indicate that spices exhibit broad-spectrum activity against Gram-negative and Gram-positive bacteria, molds, and yeasts. The mechanism of action of plant extracts and essential oils is not yet fully elucidated. However, studies suggest that compounds from various chemical groups present in these natural products can target multiple sites within microbial cells. They may permeabilize or disrupt the cytoplasmic membrane, allowing the passage of non-specific compounds or causing the release of cytoplasmic contents. Additionally, they can inhibit ATPase, an enzyme responsible for cellular energy generation, ultimately leading to cell death12.
Oregano essential oil (OEO) is one of the natural compounds frequently associated with cheese formulations6. It has been used in traditional medicine since ancient times due to its well-known antioxidant and antimicrobial properties13. The primary antimicrobial component in OEO is the phenolic monoterpene carvacrol. The broad-spectrum activity of carvacrol extends to food spoilage microorganisms, pathogenic fungi, yeasts, and bacteria, including drug-resistant and biofilm-forming microorganisms14,15. The well-documented properties of OEO make it an attractive natural preservative for various food products, including cheese. In this context, enriching brine with OEO for white brined cheese could potentially allow for a reduction in its salt content while maintaining its preservative stability.
The present study aimed to investigate the properties of low-salt brine enriched with OEO as a reliable antimicrobial preservative for white brined cheese. The antimicrobial effects were evaluated against sanitary indicator bacteria such as E. coli and coagulase-positive staphylococci.
Materials and methods
Oregano essential oil
The OEO used in this study was commercially purchased and is 100% pure with certified organic ingredients. OEO used in this study was obtained from the brand doTERRA (USA). The product used had a batch number (L)233543Y and an expiration date of 12/2028. Manufacturer: doTERRA International, LLC, Pleasant Grove, Utah, USA.
Gas chromatography-mass spectrometry
For this analysis, a 7890A gas chromatograph coupled with a flame ionization detector (FID) and a 5975C mass spectrometric detector (Agilent Technologies, USA) was employed, and separation was carried out using a Stabilwax column (Restek, USA) with dimensions of 30 m in length, 0.25 mm in internal diameter, and a film thickness of 0.25 µm. The temperature program commenced at 65 °C, with a ramp to 170 °C at a rate of 1.5 °C/min, resulting in a total analysis time of 70 min. Both the injector and detector temperatures were set to 250 °C for both the injector and the FID. The carrier gases used were hydrogen and helium, each at a flow rate of 0.8 mL/min. The mass spectrometric detector operated within a scan range of m/z 40–450. A 1.0 µL sample was injected in split mode (100:1 split ratio). Compound identification was achieved by comparing retention times and Kovats retention indices (RI) against reference standards, supplemented by mass spectral data comparison utilizing the NIST’08 (National Institute of Standards and Technology, USA) and Adams Library databases16,17.
Cheese samples
For the purposes of this study, we used farm-produced white brined cheese (Yovchevi, Bulgaria), obtained directly from a local artisanal producer in the town of Rakovski, Southern Bulgaria. According to the producer, the cheese was prepared following a traditional Bulgarian recipe, using the following ingredients: cow’s milk, starter culture, rennet, and salt. The starter culture used is typical for the region and consistent with traditional Bulgarian brined cheese production. It includes thermophilic lactic acid bacteria, most notably Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus, which are known to dominate in the early stages of fermentation. The cheese was produced using pasteurized cow’s milk and ripened in brine with a salt concentration ranging between 6 and 10% NaCl prior to its experimental use.
The cheese and brine were stored in vacuum packaging, and prior to the commencement of this study, their organoleptic and physicochemical characteristics, as well as microbial safety, were analyzed (Table 2) as part of Project No. 22011, funded by the Science Fund of the Medical University—Varna, Bulgaria. The physicochemical, organoleptic parameters, and microbial safety of the cheese samples were determined using standard analytical methods18,19,20,21,22,23,24,25.
Microbial strains
The study utilized reference microbial strains of Staphylococcus aureus ATCC 29213 and Escherichia coli ATCC 25922, branded as Mecconti, MicroSwab (Italy). Both strains were initially cultured for the purposes of the study using Blood agar (HiMedia, India) for bacterial inoculation and incubation under aerobic conditions at 37 °C for 24 h.
Methodology for antimicrobial activity assessment
The study investigated the antimicrobial activity of brine with reduced salt content (2% NaCl) enriched with various concentrations of OEO against S. aureus ATCC 29213 and E. coli ATCC 25922. Initially, cheese brine was prepared using sterile distilled water with a salt concentration of 20 g/L. Salt was dissolved in hot water (95 °C/30 min) and homogenized for 15 min at room temperature. Then, solutions were refrigerated at 4 °C, before cheese immersion.
Four separate sets of eight samples each were prepared in sterile beakers, each containing 20 mL of brine and one of the following OEO concentrations: 0.012% (v/v), 0.025% (v/v), 0.05% (v/v), 0.1% (v/v), 0.5% (v/v), 1% (v/v), 2.5% (v/v), and 5% (v/v). To all samples, sliced pieces of white brined cheese was added, ensuring complete immersion in the brine. The cheese pieces were cut into cubes with dimensions of 2.0 ± 0.2 cm per side (approximately 5 g each), to ensure consistent surface area and facilitate reproducibility. Following the preparation of the brines and cheeses, two sets of samples were inoculated with 0.1 mL of a approximately 1.5 × 10⁸ CFU/mL, corresponding to the 0.5 McFarland standardized microbial culture of S. aureus ATCC 29213, while the other two sets were inoculated with the same amount of standardized bacterial suspension of E. coli ATCC 25922. A negative control was included, containing 20 mL of brine and 5 g of cheese, as well as positive controls for each of the tested strains, each containing 20 mL of brine, 5 g of cheese, and 0.1 mL of a standardized bacterial culture. All samples in the study were prepared in triplicate.
Two of the sample sets, one inoculated with E. coli and the other with S. aureus, were stored under temperature conditions ensuring optimal microbial growth at 37 °C. Additionally, the remaining two identical sets were stored at 4 °C. This allowed for a comparative evaluation of microbial behavior under different storage conditions. During the storage period, microbiological control of the brines and the cheeses within them was performed at three time points—3rd, 24th, and 168th hour (7 days) (Fig. 1).
Detection and enumeration of coagulase-positive staphylococci
For microbiological assessment, we followed the instructions outlined in ISO 6888:202224. At the control points, 1 mL of brine from each sample was mixed with 9 mL of Maximum Recovery Diluent (HiMedia, India), resulting in a 10⁻1 dilution. Serial dilutions up to 10⁻5 were prepared using the same technique. Similarly, 1 g of cheese from each sample was used to prepare dilutions up to 10⁻5. From each serial dilution, 0.1 mL samples were plated onto Baird-Parker agar (HiMedia, India) using a spread plate technique. All Petri dishes were incubated at 37 °C for 48 h, with intermediate results recorded at 24 h.
After the incubation period, the minimum bactericidal concentrations (MBCs) were determined for each set of samples. For all samples showing microbial growth, the typical colonies were counted, and CFU/mL (log CFU/mL) values were calculated for the brines, while CFU/g (log CFU/g) values were determined for the cheeses. Additionally, from each positive Baird-Parker agar sample, at least three typical colonies (black colonies with or without a clear zone or an opalescent ring around them) were tested for the presence of plasma-coagulase enzymes using a rabbit plasma test. Each colony was initially cultured in Brain Heart Infusion Broth (HiMedia, India) for 24 h at 37 °C. Subsequently, 0.1 mL of the suspension was transferred to 0.5 mL of rabbit plasma (Bul Bio, Bulgaria) and incubated at 37 °C. The rabbit plasma was monitored for the presence of coagulum/thrombus at the 2nd, 4th, and 6th hours of incubation. Samples that exhibited coagulation were considered positive for coagulase activity, while those that remained liquid were considered negative.
Detection and enumeration of β-glucuronidase-positive E. coli
All procedures were performed in accordance with ISO 16,649:2019 for the detection and enumeration of β-glucuronidase-positive E. coli25. At the microbiological control points, 1 mL of brine and 1 g of cheese from each sample were analyzed, and serial dilutions from 10⁻1 to 10⁻5 were prepared in Maximum Recovery Diluent. Next, 1 mL of each dilution was poured into sterile 90 mm Petri dishes (90 mm in diameter), and the sample was overlaid with a thin layer of TBX agar (HiMedia, India) cooled to 45 °C. All Petri dishes were incubated at 44 °C for 24 h.
After the storage period, minimum bactericidal concentrations (MBCs) were determined for each set of samples. For all samples with microbial growth, the typical colonies (blue to blue-green in color) were counted, and CFU/mL (log CFU/mL) values were calculated for the brines, while CFU/g (log CFU/g) values were determined for the cheeses. From each Petri dish, at least three colonies were subjected to biochemical profiling using the KIA test (Bul Bio, Bulgaria) and an indole test (Bul Bio, Bulgaria). Microbial colonies that were indole-positive, lactose- and glucose-positive (often with gas production), and negative for hydrogen sulfide production were confirmed as Escherichia coli.
Statistical analysis
The statistical analysis was conducted using ANOVA to assess the differences in the growth of S. aureus and E. coli at different OEO concentrations in both cheese and brine samples. ANOVA was performed to compare the average CFU values among the control group and the various OEO concentrations. The results were considered statistically significant if the p-value was less than 0.05. Post-hoc comparisons were carried out using Tukey’s test to determine which specific groups differed from each other. Tukey’s test was used to compare the CFU values between the control group and each OEO concentration, as well as between different OEO concentrations, with the goal of identifying the specific differences in microorganism growth. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY), at a significance level of p < 0.05.
Results
The chemical composition of OEO was comprehensively analyzed, and the identified constituents are summarized in Table 1. A total of 22 compounds were detected, collectively representing 99.88% of the total composition. Carvacrol (81.20%) was identified as the predominant component, followed by β-linalool (3.76%), p-cymene (3.69%), and ɣ-terpinene (3.52%).
To investigate the preservative potential of brine with OEO, the cheese samples, which were part of the studies, were carefully selected. For the purposes of our previous science project, its parameters (microbial safety, physicochemical and organoleptic characteristics) were examined, and the results are presented in Table 2.
Microbiological analysis at the 3rd hour after sample preparation
The initial microbiological assessment of the prepared samples was conducted at the third hour after their preparation. No presence of E. coli and coagulase-positive staphylococci was detected in the negative control, neither in the cheese nor in the brine. This confirms the absence of these bacterial species in the products before intentional microbial contamination. In the positive controls stored under both temperature conditions, the presence of tested bacterial strains was confirmed, with lower microbial counts observed in the samples stored at 4 °C. The brine with 2% sodium chloride did not demonstrate antimicrobial activity against E. coli and S. aureus.
In the samples containing OEO, antimicrobial activity was recorded with MBC = 0.1% (v/v) against S. aureus and a stronger bactericidal effect against E. coli with MBC = 0.025% (v/v). At this control, we found that different storage temperatures did not affect the MBC values of the brine with OEO against S. aureus and E. coli growth (Table 3).
Microbiological analysis at the 24th hour after sample preparation
At the second control point, the results once again confirmed microbial growth in the positive controls stored at 37 °C and 4 °C, with a slight increase in the growth of both tested strains.
In the samples with added OEO and stored at 37 °C, no change was observed in the MBC value against S. aureus. In contrast, in the cheese and brine sets stored at 4 °C, the MBC value was lower—0.05% (v/v). Regarding E. coli, the antimicrobial activity of the brines with EO for each set of samples remained unchanged from the previous control point, with an MBC of 0.025% (v/v) (Table 4).
Microbiological analysis at the 168th hour (7th day) after sample preparation
The assessment of the antimicrobial efficacy of the brine enriched with OEO seven days after the contamination of the samples allows for the evaluation of its potential in the long-term storage of brined cheeses. At both storage temperatures, the sets of samples demonstrated retention of the results from the previous measurement.
Similar to the other control points, microbial counts in the positive controls were significantly higher than those in the samples containing OEO. The brine with essential oil maintained its bactericidal effects against S. aureus, which was consistent with at the 24-h control point. Specifically, for the samples stored at 37 °C, the MBC was recorded at 0.1% (v/v), while for those stored at the lower temperature of 4 °C, the brine had a lower MBC of 0.05% (v/v).
Regarding E. coli, the MBC values remained unchanged over the seven-day period and were consistently recorded as 0.025% (v/v) throughout the entire observation period, irrespective of the sample type or storage temperature (Table 5).
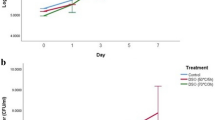
The obtained results on the dynamics of the antimicrobial efficacy of OEO-enriched brine against S. aureus and E. coli over a seven-day period and under two different storage temperature conditions are presented in Figs. 2 and 3.
All log CFU/g and log CFU/mL values are presented as mean values from three consecutive experiments. Data are presented as mean ± SD of three independent experiments. Statistical significance compared to the positive control was determined by one-way ANOVA with Tukey’s post hoc test (p < 0.05).
All log CFU/g and log CFU/mL values are presented as mean values from three consecutive experiments. Data are presented as mean ± SD of three independent experiments. Statistical significance compared to the positive control was determined by one-way ANOVA with Tukey’s post hoc test (p < 0.05).
The study assessed the impact of various concentrations OEO on the growth of S. aureus in cheese and brine samples. In the positive control group, S. aureus exhibited an average log colony-forming unit (CFU) count of 5.05 ± 0.67 in cheese and 4.94 ± 1.01 in brine. One-way ANOVA revealed a statistically significant difference among the groups (F = 72.3, p < 0.001). Tukey’s post hoc test indicated that bacterial growth was significantly higher in the control group compared to all OEO-treated groups, with no detectable growth observed at concentrations ≥ 0.1% in either cheese or brine (p < 0.05).
At 0.012% OEO, the bacterial count reached 2.91 ± 1.03 log CFU in cheese and 3.84 ± 1.56 log CFU in brine—significantly higher than at ≥ 0.1% (p < 0.05).
At 0.025% OEO, CFU counts were 2.43 ± 1.21 in cheese and 3.16 ± 0.98 in brine, which were also significantly higher than those at ≥ 0.1% (p < 0.05), but significantly lower than at 0.012% (p < 0.05).
For Escherichia coli, the positive control group showed average counts of 2.49 ± 0.87 log CFU in cheese and 3.36 ± 0.91 log CFU in brine. ANOVA analysis (F = 89.6, p < 0.001) confirmed significant differences among groups. Tukey’s post hoc test showed that the control group had significantly higher bacterial loads than the 0.012% OEO group (1.56 ± 1.01 in cheese, 1.91 ± 0.78 in brine) and the ≥ 0.025% groups, which showed complete inhibition (p < 0.05).
Moreover, the 0.012% OEO concentration yielded significantly higher CFU values than 0.025% (p < 0.05), where no bacterial growth was detected. Similarly, concentrations ≥ 0.05% also demonstrated complete inhibition, with statistically superior antimicrobial activity compared to 0.025% (p < 0.05).
Biochemical identification and plasma coagulase enzyme test
To confirm the presence of staphylococci, all Petri dishes showing microbial growth—characterized by black colonies, with or without an opalescent halo (Fig. 4)—were subjected to the plasma coagulase test. All samples tested positive for plasma coagulase enzyme production, confirming the identity of S. aureus.
Typical colonies of coagulase-positive staphylococci on Baird-Parker agar after inoculation of Staphylococcus aureus ATCC 29213 in cheese and brine. Images (a) and (b) show brine containing OEO. The results illustrate the ineffective inhibitory potential of OEO at a concentration of 0.12% (v/v). (a) Inoculation assessed after 24 h of cultivation, with no visible opalescent halo around the black colonies. (b) Colonies observed after 48 h of cultivation, showing a well-defined and visible opalescent halo surrounding the microbial colonies. (c) Microbial growth after 24 h of cultivation in the positive control sample, which does not contain OEO. All inoculations in (a), (b), and (c) were plated from a 10⁻1 dilution.
For microbiological control of E. coli, biochemical tests were performed on all samples exhibiting growth using Kligler Iron Agar (KIA) and the indole test. A minimum of three colonies with characteristic blue-green pigmentation were selected from each Petri dish for analysis (Fig. 5). The results confirmed the typical biochemical characteristics of E. coli—positive glucose and lactose fermentation, gas production, and indole positivity.
Discussion
The OEO used in this study was characterized by 22 constituents (Table 1), with carvacrol (81.20%) as the predominant compound, followed by β-linalool (3.76%), p-cymene (3.69%), and γ-terpinene (3.52%). This high carvacrol content aligns with previous studies26,27, which report levels ranging from 76.64% to 85.70%, depending on geographical origin, chemotype, extraction method, and environmental conditions. Pharmacologically, carvacrol is the primary bioactive component responsible for the antimicrobial activity of OEO. Its mechanism of action includes disruption of bacterial homeostasis through intracellular ATP depletion and increased cytoplasmic membrane permeability, leading to cellular dysfunction and death. This underpins the potential application of OEO as a natural antimicrobial agent, including against antibiotic-resistant bacteria14,15,28. Its bactericidal effects within the cell are primarily expressed through the blocking of microbial enzymes and interference with systems essential for cell survival29. Additionally, according to Walczak30, carvacrol inhibits biofilm formation by up to 86–100% for S. aureus and to a similar extent for some Gram-negative species. Another active component in OEO is thymol, which is also known for its antimicrobial properties31,32. Although, according to Honório33, the inhibitory effects of EO and carvacrol applied alone manifest at the same concentrations, emphasizing the role of carvacrol in the preservative potential of OEO.
This study presents data on the antimicrobial activity of OEO in brine with reduced sodium chloride content and demonstrates its potential for controlling pathogenic microbiota in white brined cheese. Focus was placed on the use of OEO as an active component for post-preservation of brined cheeses. Enriching the brine with OEO could allow for a reduction in sodium chloride content without compromising the microbiological safety of the product.
Control samples without OEO and with reduced salinity (from 6–10% to 2% NaCl) showed that this sodium chloride concentration allowed the growth of both studied pathogens. As expected, S. aureus demonstrated significant tolerance to the saline environment34, while E. coli was partially inhibited but not completely suppressed. According to Nogueira35, E. coli is inhibited at a minimum of 5% salinity, whereas S. aureus remains viable even at 15% NaCl.
The findings of this study confirm the antimicrobial potential of OEO in brined cheese preservation. The data indicate that OEO effectively inhibits the growth of S. aureus and E. coli, with a stronger bactericidal effect against E. coli. The results also demonstrate that the antimicrobial efficacy of OEO is influenced by storage temperature, with lower MBC values observed at 4 °C. This suggests that refrigeration enhances the preservative action of OEO, potentially extending the shelf life of brined cheeses. The sustained antimicrobial activity over a seven-day period underscores the potential of OEO-enriched brine as a natural preservative in dairy products.
A substantial number of publications have examined the antimicrobial efficacy of OEO in the context of its use as a preservative for cheese. Cortez36 discussed OEO and its potential preservative properties in Minas cheese against E. coli and S. aureus. The results they reported showed significantly higher MBC values, with the MBC of OEO being 0.25% (v/v) against E. coli and 1% (v/v) against S. aureus. The lower bactericidal concentrations of OEO obtained in our study are likely potentiated by the 2% saline solution included in the experiment.
Other contemporary studies have demonstrated the preservative effectiveness of OEO and/or carvacrol in various cheese-processing technologies. For instance, Kuorwel37 reported that starch-based films containing carvacrol effectively inhibited S. aureus in cheese. Similarly, another study38 found that OEO at just 0.02% (v/v) completely eliminated E. coli, S. aureus, and fungal strains during cheese ripening, demonstrating both antibacterial and antifungal properties by day six. Several authors emphasize that when evaluating the potential of OEO as food preservatives, its effects are not identical across different technological methods of incorporation and types of food. Moreover, it has been demonstrated that higher concentrations of OEO are typically required to achieve bactericidal effects in food systems compared to synthetic media2,11,39.
The influence of OEO on the physicochemical and organoleptic characteristics of various types of cheese is well established and thoroughly documented. In their study, Asensio39, added OEO to cottage cheese and, over 30 days of thermal storage, monitored chemical indicators of lipid oxidation, overall oxidation, and changes in fatty acid and organic acid profiles. The results showed that adding OEO to the cheese reduces the deterioration of quality parameters during storage, thereby extending the shelf life of the product. Additionally, Ritota7 reported that both fresh and dried oregano leaves, as well as OEO, contribute to improving the sensory characteristics of cheese. Moreover, when incorporated into the product, OEO not only exhibits preservative properties but also extend its shelf life.
Considering all aspects of the application of OEO in brine and cheese, the effect of OEO on the development and survival of lactic acid bacteria is of particular significance. The natural cheese microbiota and starter cultures are responsible for fermentation and imparting the desired sensory characteristics to the product, so maintaining their species composition, microbial count, and the appropriate low pH for their development is of utmost importance. There is substantial scientific evidence that several essential oils with antimicrobial activity against pathogens do not affect the population of lactic acid bacteria in the same concentration9,40, and the pH of the product does not change significantly either41. Marcial42, in assessing the influence of essential oils on cheese, reported that the essential oil had no effect on the growth or acidifying activity of lactic acid bacteria in the dairy product and even had a positive effect by improving the microbiological quality of the products during ripening. Other studies have also confirmed that the addition of essential oils does not significantly affect the pH or moisture content of cheese samples38.
Despite these promising results, certain limitations of our study must be acknowledged. To comprehensively evaluate the preservative potential of oregano essential oil in dairy systems, further investigations are needed to explore its efficacy against other major foodborne pathogens, such as Listeria monocytogenes and Salmonella spp. In addition, future research should focus on incorporating the essential oil directly into the cheesemaking process. This is particularly important, as the effective concentration demonstrated in brine may not be sufficient in the more complex cheese matrix, where interactions with fat and protein components could potentially diminish its antimicrobial activity.
In conclusion, the pilot data presented here, together with evidence from the literature, confirm the significant potential of OEO as a natural preservative for brined cheeses. Applying a consumable, pathogen-free product into a brine solution containing 0.1% (v/v) OEO appears to be an effective strategy for maintaining the microbiological safety and quality of the cheese. Moreover, OEO contributes positively to the flavor profile, does not adversely affect the organoleptic or physicochemical characteristics of the product, and facilitates the reduction of salt content in the brine—an increasingly important aspect from a public health and nutritional perspective.
Conclusion
The present study demonstrated that white brined cheese stored in brine enriched with OEO at concentrations between 0.05% (v/v) and 0.1% (v/v) effectively inhibited the growth of Staphylococcus aureus ATCC 29213 in both the brine and the cheese matrix (p < 0.05). The extent of the bactericidal effect varied depending on the storage temperature of the samples.
For Escherichia coli ATCC 25922, a minimum effective concentration of 0.025% (v/v) OEO was sufficient to inhibit bacterial growth in both cheese and brine, irrespective of storage temperature conditions (p < 0.05).
These findings indicate that OEO is a promising natural preservative for white brined cheese, active against the most commonly encountered foodborne pathogens—β-glucuronidase-producing Escherichia coli and coagulase-positive staphylococci.
Data availability
Data is provided within the manuscript.
References
The International Dairy Federation. Cheese and varieties part II: Cheese styles. https://www.fil-idf.org/wp-content/uploads/2021/02/Cheese-and-varieties-Part-2_-Cheese-styles-.pdf. Accessed Feb 2021 (2021).
Tidona, F., Zago, M., Carminati, D. & Giraffa, G. The reduction of salt in different cheese categories: Recent advances and future challenges. Front. Nutr. https://doi.org/10.3389/fnut.2022.859694 (2022).
Araújo, C. I. A. et al. How much can sodium chloride be substituted for potassium chloride without affecting the sensory acceptance of cracker-type biscuits?. Food Res. Int. https://doi.org/10.1016/j.foodres.2021.110798 (2021).
Soltani, M., Guzeler, N. & Hayaloglu, A. A. The influence of salt concentration on the chemical, ripening and sensory characteristics of Iranian white cheese manufactured by UF-Treated milk. J. Dairy Res. https://doi.org/10.1017/S0022029915000278 (2015).
Dupas, C. et al. Plants: A natural solution to enhance raw milk cheese preservation?. Food Res. Int. https://doi.org/10.1016/j.foodres.2019.108883 (2020).
Ritota, M. & Manzi, P. Natural preservatives from plant in cheese making. Animals (Basel) https://doi.org/10.3390/ani10040749 (2020).
Gouvea, F. S., Rosenthal, A. & Ferreira, E. H. R. Plant extract and essential oils added as antimicrobials to cheeses: A review. Ciênc Rural https://doi.org/10.1590/0103-8478cr20160908 (2017).
Liang, J., Huang, X. & Ma, G. Antimicrobial activities and mechanisms of extract and components of herbs in East Asia. RSC Adv. https://doi.org/10.1039/d2ra02389j (2022).
Moro, A., Librán, C. M., Berruga, M. I., Carmona, M. & Zalacain, A. Dairy matrix effect on the transference of rosemary (Rosmarinus officinalis) essential oil compounds during cheese making. J. Sci. Food Agric. https://doi.org/10.1002/jsfa.6853 (2015).
Caleja, C., Barros, L. & Antonio, A. L. Foeniculum vulgare Mill. as natural conservation enhancer and health promoter by incorporation in cottage cheese. J. Funct. Foods https://doi.org/10.1016/j.jff.2014.12.016 (2015).
Hassanien, M. F., Mahgoub, S. A. & El-Zahar, K. M. Soft cheese supplemented with black cumin oil: Impact on food borne pathogens and quality during storage. Saudi J. Biol. Sci. https://doi.org/10.1016/j.sjbs.2013.10.005 (2014).
Gill, A. O. & Holley, R. A. Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int. J. Food Microbiol. https://doi.org/10.1016/j.ijfoodmicro.2006.04.046 (2006).
Oniga, I. et al. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules https://doi.org/10.3390/molecules23082077 (2018).
Nostro, A. & Papalia, T. Antimicrobial activity of carvacrol: Current progress and future prospectives. Recent Pat Antiinfect. Drug Discov. https://doi.org/10.2174/157489112799829684 (2012).
Imran, M. et al. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. https://doi.org/10.1002/fsn3.2994 (2022).
Milovanović, I. L. et al. Evaluation of a GC-MS method for the analysis of oregano essential oil composition. Food Process. Qual. Saf. 36(3–4), 75–79 (2009).
Ahamad, J., Ali, M. & Naquvi, K. J. Analysis of essential oil of Origanum vulgare Linn. by GC and GC-MS. JGTPS. 9(3), 5786–5791 (2018).
ISO 6091: IDF 86:2010Dried milk—Determination of titratable acidity (Reference method) https://www.iso.org/standard/55777.html (2010).
Cheese and processed cheese—determination of the total solids content (Reference method) (ISO 5534) https://bds-bg.org/bg/project/show/bds:proj:50074. Accessed 29 Dec 2005 (2004).
Cheese and processed cheese products—determination of chloride content—potentiometric titration method. Fourth Edition 2006-10-01. https://cdn.standards.iteh.ai/samples/43922/41446e4ae977424a9f4b6453101325d3/ISO-5943-2006.pdf (2006).
Cheese—determination of fat content—Van Gulik method. https://bds-bg.org/bg/project/show/iso:proj:8750. Accessed 01 Jul 1975.
Microbiology of the food chain—Horizontal method for the detection, enumeration and serotyping of Salmonella—Part 1: Detection of Salmonella spp. - Amendment 1 Broader range of incubation temperatures, amendment to the status of Annex D, and correction of the composition of MSRV and SC (ISO 6579-1:2017/Amd 1:2020). https://bds-bg.org/en/project/show/bds:proj:108026. Accessed 16 Mar 2023 (2017).
Microbiology of the food chain—Horizontal method for the detection and enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection method (ISO 11290-1:2017). https://bds-bg.org/en/project/show/bds:proj:95459. Accessed 14 Nov 2018 (2017).
BDS ISO 6888-1:2022. Microbiology of the food chain - Horizontal method for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and other species)—Part 1: Method using Baird-Parker agar medium (ISO 6888-1:2021) https://bds-bg.org/en/project/show/bds:proj:108028. Accessed 17 Feb 2022 (2022).
BDS ISO 16649-2:2014. Microbiology of the food chain—horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli—Part 2: Colony-count technique at 44 °C using 5-bromo-4-chloro-3-indolyl beta-D-glucuronide. https://bds-bg.org/bg/project/show/bds:proj:86693. Accessed 21 Feb 2025 (2014).
Aelenei, P. et al. Essential oils and their components as modulators of antibiotic activity against Gram-negative bacteria. Med. (Basel) https://doi.org/10.3390/medicines3030019 (2016).
Penteado, A. L., Eschionato, R. A., de Souza, D. R. C. & Queiroz, S. D. N. Avaliação in vitro de atividade antimicrobiana de óleos essenciais contra Salmonella typhimurium e Staphylococcus aureus. http://www.alice.cnptia.embrapa.br/handle/doc/1137345. Accessed 21 Feb 2025 (2021).
Magi, G., Marini, E. & Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant group A Streptococci. Front Microbiol https://doi.org/10.3389/fmicb.2015.00165 (2015).
Burt, S. Essential oils: Their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022 (2004).
Walczak, M., Michalska-Sionkowska, M., Olkiewicz, D., Tarnawska, P. & Warżyńska, O. Potential of carvacrol and thymol in reducing biofilm formation on technical surfaces. Molecules https://doi.org/10.3390/molecules26092723 (2021).
Marchese, A. et al. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. https://doi.org/10.1016/j.foodchem.2016.04.111 (2016).
Vassiliou, E., Awoleye, O., Davis, A. & Mishra, S. Anti-inflammatory and antimicrobial properties of thyme oil and its main constituents. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24086936 (2023).
Honório, V. G. et al. Inhibition of Staphylococcus aureus cocktail using the synergies of oregano and rosemary essential oils or carvacrol and 1,8-cineole. Front Microbiol. https://doi.org/10.3389/fmicb.2015.01223 (2015).
de Santana, E. H. W., Beloti, V., Aragon-Alegro, L. C. & Mendonça, M. B. O. C. Staphilococci in food. Arq. Inst. Biol. https://doi.org/10.1590/1808-1657v77p5452010 (2015).
Nogueira, J. O. E. et al. Mechanism of action of various terpenes and phenylpropanoids against Escherichia coli and Staphylococcus aureus. FEMS Microbiol. Lett. https://doi.org/10.1093/femsle/fnab052 (2021).
Cortez, M., Gomes, A. & Carvalho, E. Antimicrobial action of Oregano (Origanum vulgare) and Rose-Mary (Rosmarinus officinalis) essential oils on Escherichia coli and Staphylococcus aureus inoculated in “Minas Frescal” cheese. Preprints (2023). https://doi.org/10.20944/preprints202306.0062.v1
Kuorwel, K. K., Cran, M. J., Sonneveld, K., Miltz, J. & Bigger, S. W. Antimicrobial activity of natural agents coated on starch-based films against Staphylococcus aureus. J. Food Sci. https://doi.org/10.1111/j.1750-3841.2011.02344.x (2011).
de Campos, A. C. L. P. et al. Antimicrobial effect of Origanum vulgare (L.) essential oil as an alternative for conventional additives in the Minas cheese manufacture. LWT https://doi.org/10.1016/j.lwt.2021.113063 (2022).
Asensio, C. M., Grosso, N. R. & Juliani, R. H. Quality preservation of organic cottage cheese using oregano essential oils. LWT https://doi.org/10.1016/j.lwt.2014.10.054 (2015).
Gammariello, D., Di Giulio, S., Conte, A. & Del Nobile, M. A. Effects of natural compounds on microbial safety and sensory quality of Fior di Latte cheese, a typical Italian cheese. J. Dairy Sci. https://doi.org/10.3168/jds.2008-1146 (2008).
de Carvalho, R. J. et al. Comparative inhibitory effects of Thymus vulgaris L. essential oil against Staphylococcus aureus, Listeria monocytogenes and mesophilic starter co-culture in cheese-mimicking models. Food Microbiol. https://doi.org/10.1016/j.fm.2015.07.003 (2015).
Marcial, G. E. et al. Influence of oregano essential oil on traditional Argentinean cheese elaboration: Effect on lactic starter cultures. Rev. Argent Microbiol. https://doi.org/10.1016/j.ram.2016.04.006 (2016).
Acknowledgements
This study was financially supported by European Union–Next Generation EU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, Project № BG-RRP-2.004-0009-C02.
Funding
This work was supported by the institutional Science Fund (Project No. 22011) of the Medical University of Varna.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by N. E., S. S., N. I., G. T., D. N., S. N., I. I. and E. G. The first draft of the manuscript was written by N. E. and S. S. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approcal
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ermenlieva, N., Stamova, S., Ivanova, N. et al. Pilot study on the potential of low-salt brine with oregano essential oil for post-preservation of white brined cheese. Sci Rep 15, 28151 (2025). https://doi.org/10.1038/s41598-025-13384-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13384-7