Abstract
Cholecystectomy has been reported to be associated with an increased risk of metabolic dysfunction-associated steatotic liver disease (MASLD), but the extent and modifying factors of this association remain unclear. We analyzed 661,122 individuals enrolled from January 1, 2009 to December 31, 2019 in the Korean National Health Insurance Service-National Sample Cohort. Among them, 4,664 patients who underwent cholecystectomy were matched by age, sex, and other factors. MASLD was defined using a fatty liver index > 60. The cholecystectomy group had a 1.48-fold higher risk of MASLD compared to those without cholecystectomy. This risk was modified by the presence of underlying cardiometabolic risk factors. Compared to patients with fewer than three cardiometabolic risk factors and no cholecystectomy, the risk of MASLD increased 4.45-fold in patients with cholecystectomy and ≥ 3 cardiometabolic risk factors. In contrast, those with fewer than three risk factors showed only a 1.22-fold increase. Multivariate analysis including interaction terms showed an adjusted hazard ratio of 5.26 (95% CI, 2.35–11.78) for patients with cholecystectomy and ≥ 3 cardiometabolic risk factors. Cholecystectomy increases the risk of MASLD, particularly in individuals with multiple cardiometabolic risk factors. Comprehensive screening and aggressive management of these factors are essential before and after cholecystectomy.
Similar content being viewed by others
Introduction
Cholecystectomy is a surgical procedure indicated for the treatment of various gallbladder diseases and is one of the most commonly performed surgeries worldwide1,2. The main indication for cholecystectomy is gallstone disease, and with overall favorable clinical outcomes, it is considered a relatively safe surgery with low morbidity and mortality3. In this context, the number of cholecystectomy cases in the Republic of Korea has been markedly increased, with the age-standardized rate per 100,000 based on the 2010 population, increasing sharply from 67.7 in 2003 to 211.4 in 20174. However, although cholecystectomy has been widely performed owing to assumed minimal to no adverse effects on health, cholecystectomy has been recently associated with an increased risk of metabolic consequences, such as dyslipidemia and hyperglycemia5,6,7,8,9. Moreover, cholecystectomy may increase the risk of developing nonalcoholic fatty liver disease (NAFLD)10,11,12,13. NAFLD is the most common chronic liver disease worldwide, characterized by hepatic lipid accumulation associated with insulin resistance14. It is considered the hepatic manifestation of metabolic syndrome and is associated with an increased risk of liver-related outcomes, cardiovascular events, chronic kidney disease, and malignancies, leading to a high socioeconomic burden15. Therefore, the impact of cholecystectomy on the development of NAFLD should be rigorously evaluated. However, most previous studies reporting on the association between cholecystectomy and NAFLD have been conducted with a cross-sectional design, and no large-scale longitudinal study on this topic has been conducted to date. Additionally, a recent consensus by an international panel of experts has recommended renaming NAFLD to metabolic dysfunction-associated steatotic liver disease (MASLD), a designation defined for patients with hepatic steatosis who have one or more of five cardiometabolic risk factors (CMRF), emphasizing the critical role of cardiometabolic profile in the pathogenesis of MASLD16. Although the term NAFLD has been replaced with MASLD, no studies have yet investigated the association between MASLD and cholecystectomy. Therefore, we conducted a large-scale nationwide cohort study to demonstrate the association between cholecystectomy and MASLD development using the Korean National Health Insurance Service-National Sample Cohort 2.0 data17,18. In addition, no study has identified high-risk groups with an increased risk of developing MASLD after cholecystectomy. Since cardiometabolic risk factors are considered to be associated with MASLD development, we also aimed to investigate whether the risk of new-onset MASLD following cholecystectomy varies depending on the number of individual cardiometabolic risk factors.
Materials and methods
Data source
We analyzed a population-based cohort data set from the Korean National Health Insurance Service-National Sample Cohort 2.0 (NHIS-NSC 2.0), which includes approximately 2% of the total Korean population in 200617,18. The Korean government incorporated retrospective and prospective follow-up data from 2002 to 2019 and stratified the total population into 2,142 strata based on age, sex, region, eligibility status, and income level. From each stratum, the government randomly selected 2.1% (n = 1,021,208) to be included in the NHIS-NSC 2.0 cohort. As the Korean NHIS covers about 97% of the total Koreans, this cohort is representative of the entire Korean population18. This study was approved by the Institutional Review Board of the Chungnam National University Hospital (IRB number: 2024-04-031), and permission was granted to use the NHIS health check-up data (NHIS-2024-2-146). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Study population
The cohort of patients who underwent cholecystectomy was constructed using the NHIS-NCS 2.0 database. We included individuals from the NHIS-NSC who were over 18 years as of 2009 and had not died before December 31, 2009. Individuals who undergone cholecystectomy were identified using the procedure codes Q7380 and Q7410 from the health insurance procedure classification. Patients with the fatty liver index (FLI) ≥ 60 at any time prior to the date of cholecystectomy were excluded from the study cohort19. The NHIS database enables longitudinal tracking of diagnoses and procedures, allowing us to distinguish between pre-existing and incident SLD. Participants were followed using repeated health screening data within the NHIS database, which includes serial laboratory results and anthropometric measurements.
Definition of MASLD
MASLD was defined as the presence of SLD and at least one of the five cardiometabolic risk factors, with no other discernible cause. The presence of SLD was defined as the FLI, a validated prediction models for fatty liver disease, ≥ 60.19 In this study, steatotic liver disease was defined as a case where the FLI was 60 or higher at least once during the follow-up period. The FLI is calculated using the following formula.
Fatty Liver Index = ey/(1 + ey) × 100, where y = 0.953 × ln(triglycerides, mg/dL) + 0.139 × BMI, kg/m2 + 0.718 × ln(GGT, U/L) + 0.053 × waist circumference, cm – 15.745)
Cardiometabolic risk factors were defined as follows. (1) Body mass index ≥ 23 kg/m2 or a high waist circumference (≥ 90 cm for men and ≥ 85 cm for women), (2) fasting serum glucose ≥ 100 mg/dL, type 2 diabetes, or a prescription record of antidiabetic medications, (3) blood pressure ≥ 130/85 mmHg or a prescription record of antihypertensive medications, (4) triglycerides ≥ 150 mg/dL or a prescription record of lipid-lowering medications, and (5) a low high-density lipoprotein cholesterol (≤ 40 mg/dL for men and ≤ 50 mg/dL for women)16. Additionally, since MASLD is associated with minimal or no alcohol consumption, we set the alcohol intake threshold at less than 210 g per week for men and less than 140 g per week for women to differentiate MASLD from metabolic dysfunction and alcohol-associated steatotic liver disease16. We also excluded cases of MASLD occurring within the first year after cholecystectomy, as early MASLD events may reflect pre-existing liver disease rather than new-onset disease following cholecystectomy.
Definition of variables
Family income status was determined based on the decile distribution of household insurance premiums and categorized into low (1st–3rd deciles), middle (4th–7th deciles), and high (8th–10th deciles) income groups. Cigarette smoking status was classified into three groups: never smoker, former smoker, and current smoker. Alcohol intake was grouped into three levels: 0 g per week, less than 140 g per week, and 140 g or more per week. Exercise intensity was measured in metabolic equivalents (METs) and categorized into three levels: less than 500 METs, 500–1,000 METs, and 1,000 METs or more. Height and weight were measured by trained personnel following a standardized protocol. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m²). Hypertension, diabetes mellitus, and dyslipidemia was defined based on self-reported diagnoses using diagnosis-specific questionnaires or by the presence of relevant billing codes (HTN: I10, I11, I12, I13, and I15; Diabetes mellitus: E10, E11, E12, E13, and E14; Dyslipidemia: E78, E780, E781, E782, E783, E784, and E785).
Statistical analysis
We expressed participant characteristics as means with standard deviation or as numbers and percentages. Student’s t-test or chi-square test was used to estimate statistical differences between two groups. The cumulative incidence of new-onset MASLD was calculated by product limit (Kaplan-Meier) method of survival probability and compared across the groups using the Kaplan-Meier method and the log-rank test. Incidence rate (IR) of MASLD was presented as the incidence per 1000 person-years. Multivariate analysis was performed with a Cox proportional hazards analysis to assess the associations of cholecystectomy with the development of MASLD. The proportional hazards assumption was evaluated graphically by log–log plots, and there was no significant departure from proportionality in hazards over time. Hazard ratio (HR) and 95% confidence interval (CI) were calculated. Model 1 was an unadjusted model, and Model 2 was adjusted for sex, age, status of income, activity group, BMI, triglyceride, HDL-cholesterol, total cholesterol, fasting blood sugar, aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase. Model 3 included variable from model 2 plus interaction terms (Cholecystectomy & CMRF, groups*Sex; Cholecystectomy & CMRF, groups*Age; Cholecystectomy & CMRF, groups*BMI) which the significant interactions were detected. Variables selection was done by clinical knowledge or by the stepwise method, which is both forward selection and backward elimination. P-values < 0.05 were considered statistically significant. All analyses were performed with SAS Enterprise Guide version 8.3 (SAS Institute, Cary, NC, USA) and RStudio Server version 2023.03.1 Build 446 (2009–2023 Posit Software, PBC, Boston, MA, USA; https://posit.co/download/rstudio-server/), and R software (version 4.3.0; R Foundation for Statistical Computing, Vienna, Austria).
Propensity score matching
To mitigate selection bias from potential confounders between the cholecystectomy group and the non-cholecystectomy group, propensity score matching (PSM) analysis was conducted. This analysis included matching variables included age, age group, sex, cardiometabolic risk factors, income status, cigarette smoking, drinking alcohol amount (grams per week), alcohol drinking group, activity group, walking activity frequency (per week), body mass index (kg/m²), systolic and diastolic blood pressure (mmHg), total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol, fasting plasma glucose, BMI group, and comorbidities (hypertension, diabetes mellitus, dyslipidemia).The PSM analysis was performed using the matchit() function from the MatchIt (version 4.5.0; Ho, Imai, King, and Stuart) with nearest neighbor method with a 1:3 ratio, without replacement, with no caliper. Balance diagnostics to determine the quality of the matching was evaluated in Supplementary Fig. 1.
Results
Flow of analysis
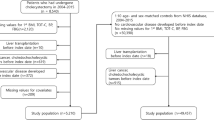
From January 1, 2009 to December 31, 2019, a total of 661,122 patients were enrolled in the study. Among these patients, 18,816 patients aged < 18 years and 227 patients who died before December 31, 2009 were excluded. Therefore, the remaining 642,079 patients comprising 10,194 patients who underwent cholecystectomy and 631,885 who did not were included in the study. Among the 10,194 patients who underwent cholecystectomy, 5,178 patients were excluded owing to a fatty liver index (FLI) of > 60. After further excluding cases with missing matching variables (n = 352), 4,664 cholecystectomy matched cases and their 13,992 non-cholecystectomy matched controls were analyzed in this study (Fig. 1). The mean follow-up period was 5.35 years, generating 99,767 person-years.
Baseline characteristics before and after PSM
Table 1 presents the baseline characteristics of the participants who underwent and did not undergo cholecystectomy before and after PSM. After matching, the cholecystectomy group (n = 4,664) and the matched non-cholecystectomy group (n = 13,992) were well balanced across all covariates, including age, sex, cardiometabolic risk factors, socioeconomic status, lifestyle factors, and laboratory values. No significant differences were observed between the two groups for any matching variable after propensity score matching, suggesting that the PSM effectively minimized potential selection bias (p > 0.05) (Table 1). Moreover, the distribution of propensity scores before and after matching is illustrated in supplementary Fig. 1. Prior to matching, there were notable differences in the distribution of propensity scores between the treated and control groups, with the raw control group showing a higher concentration at lower propensity scores compared to the raw treated group. After propensity score matching, the distributions for the matched treated and matched control groups were well balanced, with substantial overlap across the range of propensity scores. This indicates that the matching procedure was effective in achieving comparability between the groups, thereby minimizing confounding and improving the validity of subsequent analyses. Moreover, before propensity score matching, patients who underwent cholecystectomy exhibited a significantly higher mean number of CMRF compared to non-cholecystectomy patients, as evidenced by greater mean values and standard deviations (e.g., 3.13 ± 1.23 vs. 2.53 ± 1.32, p < 0.001). This baseline imbalance confirmed that cholecystectomy patients had a substantially greater cardiometabolic risk burden prior to surgical intervention. After propensity score matching, the CMRF burden was effectively balanced between groups. The mean and standard deviation of CMRF counts showed no statistically significant difference between matched cholecystectomy and non-cholecystectomy groups (e.g., 3.13 ± 1.23 vs. 3.11 ± 1.25, p = 0.213), demonstrating that the matching procedure successfully eliminated baseline differences in metabolic risk profiles. This balancing validates subsequent comparisons of MASLD outcomes between the cohorts (Supplementary Table 1). Furthermore, both before and after propensity score matching, there were significant differences in the mean (SD) values of the number of CMRF across the four groups stratified by cholecystectomy status and CMRF burden (cholecystectomy with CMRF ≥ 3, cholecystectomy with CMRF < 3, non-cholecystectomy with CMRF ≥ 3, and non-cholecystectomy with CMRF < 3). For example, before matching, the mean (SD) number of CMRF was 3.80 (0.71) in the cholecystectomy with CMRF ≥ 3 group and 1.55 (0.58) in the cholecystectomy with CMRF < 3 group, while after matching, the values were 3.81 (0.71) and 1.55 (0.58), respectively, with similar patterns in the non-cholecystectomy groups. However, when comparing within each CMRF stratum (i.e., between cholecystectomy and non-cholecystectomy groups with the same CMRF burden), before propensity score matching, there were statistically significant differences in the mean (SD) number of CMRF between cholecystectomy and non-cholecystectomy groups both in those with CMRF ≥ 3 and in those with CMRF < 3. After propensity score matching, a significant difference persisted between cholecystectomy and non-cholecystectomy groups in the CMRF < 3 stratum, whereas the difference was no longer significant in the CMRF ≥ 3 stratum. These results indicate that, prior to matching, baseline metabolic profiles differed between surgical and non-surgical groups across both CMRF strata, but after matching, only the low CMRF burden group continued to show residual metabolic differences, while the high CMRF burden group did not (Supplementary Table 2).
MASLD risk in cholecystectomy
After matching, the cholecystectomy group (n = 4,664) and the non-cholecystectomy group (n = 13,992) were followed for a total of 16,133.9 and 83,633.2 person-years, respectively. The overall incidence rate of MASLD was 29.87 per 1,000 person-years in the cholecystectomy group, compared to 20.37 per 1,000 person-years in the non-cholecystectomy group. The crude hazard ratio for MASLD in the cholecystectomy group versus the non-cholecystectomy group was 1.48 (95% CI: 1.34–1.64), indicating a significantly higher risk of MASLD among individuals who underwent cholecystectomy. When stratified by CMRF burden, the incidence rate of MASLD was highest in the subgroup with both cholecystectomy and three or more CMRFs, with an incidence rate of 27.77 per 1,000 person-years. In contrast, the incidence rate in the group with cholecystectomy and fewer than three CMRFs was 9.06 per 1,000 person-years. For the non-cholecystectomy group, the incidence rates were 25.70 per 1,000 person-years for those with three or more CMRFs and 8.78 per 1,000 person-years for those with fewer than three CMRFs. The hazard ratios reflected these trends: compared to the reference group (non-cholecystectomy, CMRF < 3), the HR for MASLD was 4.45 (95% CI: 3.79–5.22) in the cholecystectomy group with CMRF ≥ 3, 1.22 (95% CI: 0.90–1.64) in the cholecystectomy group with CMRF < 3, and 2.94 (95% CI: 2.56–3.37) in the non-cholecystectomy group with CMRF ≥ 3. In summary, Table 2 demonstrates that the risk of developing MASLD is substantially higher in individuals with a history of cholecystectomy, particularly when accompanied by a high burden of cardiometabolic risk factors. The incidence rates and hazard ratios indicate a clear additive effect of cholecystectomy and multiple CMRFs on MASLD development.
Subgroup analysis for MASLD risk according to cholecystectomy and cardiometabolic risk factors
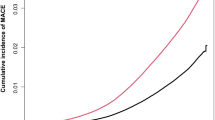
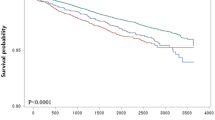
Cumulative incidence of MASLD as estimated by the Kaplan-Meier method, stratified by cholecystectomy status and CMRF burden (≥ 3 or < 3). 5-year cumulative incidence risk of MASLD was 17.10 (95% CI 5.41–18.75) for the cholecystectomy group with over three cardiometabolic risk factors, 6.29 (95% CI 4.44–8.10) for the cholecystectomy group fewer than three cardiometabolic risk factors, 12.48 (95% CI 11.78–13.17) for the non-cholecystectomy group with over three cardiometabolic risk factors, and 3.87 (95% CI 3.25–4.49) for the non-cholecystectomy group with fewer than three cardiometabolic risk factors. At 96 months, the cumulative incidence of MASLD was highest in the cholecystectomy group with CMRF ≥ 3 (23.48%, 95% CI: 21.03–25.85), followed by the non-cholecystectomy group with CMRF ≥ 3 (19.05%, 95% CI: 18.12–19.98), the non-cholecystectomy group with CMRF < 3 (7.48%, 95% CI: 6.47–8.48), and the cholecystectomy group with CMRF < 3 (6.92%, 95% CI: 4.87–8.93). Notably, there were no new-onset MASLD cases between 72 and 96 months in the cholecystectomy (+), CMRF < 3 group, and between 84 and 96 months in the cholecystectomy (+), CMRF ≥ 3 group, indicating a plateau in cumulative incidence during late follow-up (Table 3). Moreover, he cumulative hazard of new-onset MASLD according to cholecystectomy status in the propensity score-matched population. The cumulative hazard was consistently higher in the cholecystectomy group compared to the non-cholecystectomy group throughout the follow-up period (log-rank p < 0.0001). The number at risk at each time point is shown, confirming robust follow-up across both groups (Fig. 2).
Further stratification of the cumulative hazard of MASLD by both cholecystectomy status and CMRF burden were shown in Fig. 3. The cumulative hazard was highest in the cholecystectomy group with CMRF ≥ 3, followed by the non-cholecystectomy group with CMRF ≥ 3, the cholecystectomy group with CMRF < 3, and the non-cholecystectomy group with CMRF < 3. Multiple comparison testing confirmed that the cumulative hazard in the cholecystectomy (+), CMRF ≥ 3 group was significantly higher than in all other groups (log-rank p < 0.0001 for all relevant comparisons). The number at risk for each group at each time point is also provided, supporting the validity of the survival analysis (Fig. 3).
In Table 4, the adjusted hazard ratios (aHRs) for MASLD according to cholecystectomy status and CMRF burden. In the fully adjusted model (Model 3), which incorporated interaction terms for cholecystectomy, CMRF, sex, age, and BMI, the risk of MASLD was highest in the cholecystectomy group with CMRF ≥ 3 (aHR: 5.26, 95% CI: 2.35–11.78, p < 0.001), followed by the non-cholecystectomy group with CMRF ≥ 3 (aHR: 1.65, 95% CI: 0.82–3.31, p = 0.158), and the cholecystectomy group with CMRF < 3 (aHR: 0.57, 95% CI: 0.11–2.35, p = 0.521), compared to the reference group (non-cholecystectomy, CMRF < 3). These findings remained consistent across all adjustment models, demonstrating that both cholecystectomy and a higher burden of cardiometabolic risk factors independently and additively increase the risk of MASLD.
In summary, both cholecystectomy and a higher burden of cardiometabolic risk factors were independently and additively associated with an increased risk of MASLD. The risk was most pronounced in individuals with both cholecystectomy and three or more CMRFs, as demonstrated by the highest cumulative incidence and hazard in this group over the entire follow-up period.
Cholecystectomy indications and hospital visit routes
The distribution of primary diagnoses among patients who underwent cholecystectomy during the study period. The most common indication for cholecystectomy was gallstone-related disease, including cholelithiasis and cholecystitis, accounting for the majority of cases. Other indications included gallbladder polyps, biliary dyskinesia, and less frequently, gallbladder neoplasms. This distribution reflects typical clinical practice, where benign gallbladder disease is the predominant reason for surgical intervention (Supplementary Table 3). For these patients, the routes of hospital presentation for the study cohort were presented (N = 5,013). Of these, 1,139 patients (22.72%) visited via the emergency room, while 3,874 (77.28%) presented via the outpatient clinic. The emergency room group was further subdivided into arrivals from other medical institutions (n = 93, 1.85%), by ambulance (n = 38, 0.76%), and by other means (n = 1,008, 20.12%). Similarly, the outpatient clinic group included arrivals from other medical institutions (n = 260, 5.18%), by ambulance (n = 9, 0.18%), and by other means (n = 3,605, 71.92%). These data provide insight into the healthcare utilization patterns and referral pathways within the study population (Supplementary Table 4).
Sensitivity analysis based on alcohol consumption in males and females
The results of sensitivity analyses evaluating the association between cholecystectomy, CMRF burden, and the risk of MASLD-stratified by sex and levels of alcohol consumption-are presented in supplementary Table 5. These analyses demonstrate that the increased risk of MASLD associated with cholecystectomy and higher CMRF burden was consistent in both males and females, regardless of alcohol intake. This finding supports the robustness of the main results and indicates that the observed associations are not confounded by differences in alcohol consumption patterns between sexes. Supplementary Fig. 2 graphically displays the subdistribution hazard ratios and 95% confidence intervals for MASLD according to cholecystectomy status (Yes/No), CMRF burden (< 3/≥3), sex (male/female), and strata of alcohol consumption (< 70 g, < 140 g, and < 210 g per week). In the figure, results for males are depicted as red circles and for females as blue triangles, with separate panels for each alcohol consumption category. The reference group in each comparison is the non-cholecystectomy, CMRF < 3 subgroup. Across all levels of alcohol consumption and in both sexes, the risk of MASLD is highest in individuals with both cholecystectomy and a high CMRF burden. Although confidence intervals are wider in subgroups with fewer events, the overall pattern remains consistent, reinforcing the additive effect of cholecystectomy and metabolic risk on MASLD development, independent of sex and alcohol intake.
Discussion
In this large-scale longitudinal cohort study involving 18,656 Korean adults, we demonstrated that cholecystectomy was associated with an increased risk of new-onset MASLD, particularly in individuals with three or more cardiometabolic risk factors. Individuals who underwent cholecystectomy had a 1.48-fold higher risk of developing MASLD than those who did not undergo cholecystectomy. The gallbladder stores and concentrates bile during fasting and releases it rhythmically through the fasting and postprandial phases, thereby playing a critical role in modulating the daily cycle of bile acids20. Bile acids, of which bile is the major constituent, not only facilitate lipid absorption but also act as essential signaling molecules, regulating gene expression associated with bile acid metabolism, as well as glucose, lipid, and energy metabolism, via the G protein-coupled bile acid receptor 1, farnesoid X nuclear receptor, and fibroblast growth factor 1920,21. Therefore, the loss of the gallbladder’s reservoir and concentrating functions following cholecystectomy may lead to metabolic disturbances, such as altered glucose and lipid homeostasis by altering the enterohepatic circulation of bile acids6. Clinical and experimental evidence has supported that cholecystectomy may contribute to the development of dysglycemia and dyslipidemia. Sonne at al. have reported that patients who underwent cholecystectomy exhibited a slight deterioration in postprandial glycemic control7. In addition, a cross-sectional and prospective study of 1,612 Chinese adults has indicated an association between cholecystectomy and a higher risk of both prevalent prediabetes and diabetes, as well as an increased risk of ≥ 10% in fasting plasma glucose levels (odds ratio [OR] 2.5; 95% CI 1.2–5.4) and ≥ 10% in hemoglobin A1c levels (OR 2.6; 95% CI 1.4–4.8)8. Meanwhile, according to a case–control study comparing patients undergoing cholecystectomy with controls without gallbladder disease or who did not undergo cholecystectomy, patients who underwent cholecystectomy exhibited higher plasma triglyceride, total cholesterol, and low-density lipoprotein cholesterol levels, but lower plasma high-density lipoprotein cholesterol levels than controls9.
Cholecystectomy may contribute to NAFLD development. Amigo et al. have reported that serum and hepatic triglycerides levels, as well as hepatic very low-density lipoprotein production, increased in cholecystectomized mice, suggesting that free fatty acid transport from adipose tissue to the liver is elevated in cholecystectomized mice10. Thus, cholecystectomy may promote hepatic lipid accumulation. Two cross-sectional studies conducted following this research have suggested that cholecystectomy may increase the risk of developing NAFLD. The first is a large retrospective cross-sectional study of adults in the United States, where Ruhl and Everhart et al. have reported that individuals who had undergone cholecystectomy have 2.4 times higher odds of developing NAFLD than those without gallstone disease11. The second is a cross-sectional study conducted on an Asian population, in which Kwak et al. have reported that individuals who underwent cholecystectomy have a 35% higher risk of NAFLD than those who had not undergone cholecystectomy12. Consistent with these two studies, a pilot study involving 26 patients who underwent cholecystectomy and 16 controls has reported increased hepatic fat content, assess by magnetic resonance imaging, after 24 months of follow-up in patients who underwent cholecystectomy, whereas no significant changes were observed in the control group13. Although other similar studies have suggested a possible relationship between cholecystectomy and NAFLD, they were all limited by a cross-sectional design or small sample size. Because gallstone disease, the primary indication for cholecystectomy, shares metabolic disturbances as predisposing factors with NAFLD, it is difficult in cross-sectional studies to determine whether metabolic disturbances resulting from cholecystectomy lead to NAFLD or whether pre-existing metabolic disturbances contribute to both gallstone disease and the development of NAFLD22,23,24,25,26,27. Consequently, the causal relationship between cholecystectomy and NAFLD remains unclear. Our study overcame the limitations of previous studies by using a nationally representative cohort database and employing propensity score matching to achieve baseline comparability in metabolic burden between the cholecystectomy and non-cholecystectomy groups. Moreover, we assessed MASLD development following cholecystectomy, a revised diagnostic category that replaces the former NAFLD classification, rather than NAFLD. To the best of our knowledge, our large-scale longitudinal study is the first to demonstrate the temporal and causal relationship between cholecystectomy and new-onset MASLD.
Additionally, the increased risk of MASLD following cholecystectomy is influenced by the number of cardiometabolic risk factors. With the recent change in terminology from NAFLD to MASLD, five cardiometabolic risk factors were included in the diagnostic criteria for MASLD16. These cardiometabolic risk factors were selected to identify patients in whom insulin resistance is the main cause of hepatic steatosis28. Several recent studies have reported that cardiometabolic risk factors were associated with an increased risk of NAFLD, liver-related outcomes, cardiovascular events, and all-cause mortality in a number-dependent manner29,30,31,32. In particular, a retrospective cohort study of 16,152 Chinese adults has indicated that individuals with more cardiometabolic risk factors have an higher risk of developing NAFLD29. In the present study, multivariable analysis revealed that individuals with three or more cardiometabolic risk factors who underwent cholecystectomy had a 5.26-fold higher risk of developing MASLD (HR, 5.26; 95% CI, 2.35–11.78; P < 0.001) than individuals with fewer than three cardiometabolic risk factors who did not undergo cholecystectomy. By contrast, individuals with fewer than three cardiometabolic risk factors who underwent cholecystectomy did not exhibit a statistically significant increase in MASLD risk compared to individuals with fewer than three cardiometabolic risk factors who did not undergo cholecystectomy. Thus, cholecystectomy may increase the risk of developing MASLD through the synergistic effect with cardiometabolic risk factors. Furthermore, risk stratification revealed that the markedly higher risk of developing MASLD after cholecystectomy in individuals with three or more cardiometabolic risk factors compared to those with fewer than three cardiometabolic risk factors underscores the importance of careful patient selection for cholecystectomy in these high-risk individuals. Moreover, if cholecystectomy is mandatory for individuals with three or more cardiometabolic risk factors, risk factors should be more aggressively managed to prevent MASLD development, and more frequent monitoring to detect its onset should be adopted in this group. In determining whether to perform cholecystectomy, clinicians should weigh the clinical balance between the potential development of MASLD associated with cholecystectomy and the complications of untreated gallbladder disease. For example, in patients with symptomatic gallstones accompanied by typical biliary pain, cholecystectomy should be performed to prevent recurrent biliary colic, acute cholecystitis, or gallstone pancreatitis. However, in cases where symptoms are atypical or mild, and the patient has three or more cardiometabolic risk factors, oral bile acid dissolution therapy may be considered as the preferred initial approach over cholecystectomy. In asymptomatic patients with gallstones and a high cardiometabolic burden who also present with potentially controversial risk factors for gallbladder cancer, such as gallstones ≥ 3 cm, porcelain gallbladder, or gallbladder polyps without other risk factors aside from gallstones, the appropriateness of cholecystectomy requires further investigation to evaluate its risk–benefit profile in this context33,34,35,36,37,38,39.
The present study has some limitations. First, we defined MASLD using the FLI score rather than liver biopsy, which is the gold standard for diagnosing SLD. However, the FLI has been established as an effective screening tool for detecting SLD19. It is calculated using routinely collected epidemiological data, including BMI, waist circumference, triglycerides, and gamma-glutamyl transferase, and has been extensively validated in Asian populations, including Koreans40,41,42,43,44,45. Performing repeated liver biopsies or even magnetic resonance imaging-proton density fat fraction (MRI-PDFF), a non-invasive alternative to liver biopsy, to evaluate the incidence of MASLD in large population-based cohorts is not feasible. Therefore, using well-established non-invasive tests, such as the FLI, to predict the incidence of MASLD is a practical approach. However, this indirect diagnostic method may result in misclassification, particularly in borderline or transient cases. The lack of alternative FLI thresholds or secondary definitions of steatosis thus represents a limitation of this study. Second, although we adjusted for multiple covariates, residual confounding effects due to unknown or unmeasured covariates may still exist. Therefore, it is difficult to completely rule out the possibility that underlying, unidentified metabolic abnormalities associated with the development of MASLD may have also contributed to gallstone formation and the subsequent need for cholecystectomy. Third, because the majority of our participants were South Korean, the findings may have limited generalizability to populations with different ethnic backgrounds. Finally, participants who underwent cholecystectomy may have been subject to more frequent clinical monitoring, potentially introducing surveillance bias. Future prospective cohort studies that apply standardized follow-up protocols to both cholecystectomy and non-cholecystectomy groups are warranted to minimize this bias and more accurately elucidate the temporal relationship between cholecystectomy and MASLD development.
In conclusion, our study demonstrated an increased risk of developing new-onset MASLD in individuals who underwent cholecystectomy. In particular, the risk of MASLD following cholecystectomy was particularly elevated in individuals with more cardiometabolic risk factors. Therefore, cardiometabolic risk factors of patients planning to undergo cholecystectomy should be preoperatively assessed. If a patient has three or more cardiometabolic risk factors, the risk of MASLD substantially increases postoperatively, necessitating a more cautious approach to surgical decision-making for these patients. Furthermore, after cholecystectomy, MASLD development should be closely monitored, and cardiometabolic risk factors should be effectively managed to reduce the risk of MASLD in this population.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Russo, M. W. et al. Digestive and liver diseases statistics, 2004. Gastroenterology 126, 1448–1453. https://doi.org/10.1053/j.gastro.2004.01.025 (2004).
Everhart, J. E. & Ruhl, C. E. Burden of digestive diseases in the united States part III: liver, biliary tract, and pancreas. Gastroenterology 136, 1134–1144. https://doi.org/10.1053/j.gastro.2009.02.038 (2009).
European Association for the Study of the Liver. Electronic address, E. E. E. EASL clinical practice guidelines on the prevention, diagnosis and treatment of gallstones. J. Hepatol. 65, 146–181. https://doi.org/10.1016/j.jhep.2016.03.005 (2016).
Jeon, C. H., Hong, J., Jung, J., Moon, J. Y. & Seo, H. S. Chronological trends in patients undergoing cholecystectomy in korea: a nationwide health insurance claims study. Ann. Surg. Treat. Res. 102, 205–213. https://doi.org/10.4174/astr.2022.102.4.205 (2022).
Chen, Y., Wu, S. & Tian, Y. Cholecystectomy as a risk factor of metabolic syndrome: from epidemiologic clues to biochemical mechanisms. Lab. Invest. 98, 7–14. https://doi.org/10.1038/labinvest.2017.95 (2018).
Di Ciaula, A., Garruti, G., Wang, D. Q. & Portincasa, P. Cholecystectomy and risk of metabolic syndrome. Eur. J. Intern. Med. 53, 3–11. https://doi.org/10.1016/j.ejim.2018.04.019 (2018).
Sonne, D. P. et al. Postprandial gut hormone responses and glucose metabolism in cholecystectomized patients. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G413–419. https://doi.org/10.1152/ajpgi.00435.2012 (2013).
Sang, M. et al. Cholecystectomy is associated with dysglycaemia: Cross-sectional and prospective analyses. Diabetes Obes. Metab. 24, 1656–1660. https://doi.org/10.1111/dom.14730 (2022).
Chavez-Tapia, N. C. et al. Association between cholecystectomy for gallstone disease and risk factors for cardiovascular disease. Ann. Hepatol. 11, 85–89 (2012).
Amigo, L. et al. Cholecystectomy increases hepatic triglyceride content and very-low-density lipoproteins production in mice. Liver Int. 31, 52–64. https://doi.org/10.1111/j.1478-3231.2010.02361.x (2011).
Ruhl, C. E. & Everhart, J. E. Relationship of non-alcoholic fatty liver disease with cholecystectomy in the US population. Am. J. Gastroenterol. 108, 952–958. https://doi.org/10.1038/ajg.2013.70 (2013).
Kwak, M. S. et al. Cholecystectomy is independently associated with nonalcoholic fatty liver disease in an Asian population. World J. Gastroenterol. 21, 6287–6295. https://doi.org/10.3748/wjg.v21.i20.6287 (2015).
Cortes, V., Quezada, N., Uribe, S., Arrese, M. & Nervi, F. Effect of cholecystectomy on hepatic fat accumulation and insulin resistance in non-obese Hispanic patients: a pilot study. Lipids Health Dis. 16, 129. https://doi.org/10.1186/s12944-017-0525-3 (2017).
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77, 1335–1347. https://doi.org/10.1097/HEP.0000000000000004 (2023).
Rinella, M. E. et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 77, 1797–1835. https://doi.org/10.1097/HEP.0000000000000323 (2023).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 78, 1966–1986. https://doi.org/10.1097/HEP.0000000000000520 (2023).
Cheol Seong, S. et al. Data resource profile: the National health information database of the National health insurance service in South Korea. Int. J. Epidemiol. 46, 799–800. https://doi.org/10.1093/ije/dyw253 (2017).
Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort profile: the National health insurance Service-National sample cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 46, e15. https://doi.org/10.1093/ije/dyv319 (2017).
Bedogni, G. et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6 https://doi.org/10.1186/1471-230X-6-33 (2006).
Di Ciaula, A. et al. Bile acid physiology. Ann. Hepatol. 16, s4–s14. https://doi.org/10.5604/01.3001.0010.5493 (2017).
Garruti, G., Wang, D. Q., Di Ciaula, A. & Portincasa, P. Cholecystectomy: a way forward and back to metabolic syndrome? Lab. Invest. 98, 4–6. https://doi.org/10.1038/labinvest.2017.129 (2018).
Misciagna, G., Guerra, V., Di Leo, A., Correale, M. & Trevisan, M. Insulin and gall stones: a population case control study in Southern Italy. Gut 47, 144–147. https://doi.org/10.1136/gut.47.1.144 (2000).
Shaffer, E. A. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr. Gastroenterol. Rep. 7, 132–140. https://doi.org/10.1007/s11894-005-0051-8 (2005).
Volzke, H. et al. Independent risk factors for gallstone formation in a region with high cholelithiasis prevalence. Digestion 71, 97–105. https://doi.org/10.1159/000084525 (2005).
Portincasa, P., Moschetta, A. & Palasciano, G. Cholesterol gallstone disease. Lancet 368, 230–239. https://doi.org/10.1016/S0140-6736(06)69044-2 (2006).
Stender, S., Frikke-Schmidt, R., Nordestgaard, B. G. & Tybjaerg-Hansen, A. The ABCG5/8 cholesterol transporter and myocardial infarction versus gallstone disease. J. Am. Coll. Cardiol. 63, 2121–2128. https://doi.org/10.1016/j.jacc.2013.12.055 (2014).
Jaruvongvanich, V., Sanguankeo, A. & Upala, S. Significant association between gallstone disease and nonalcoholic fatty liver disease: A systematic review and Meta-Analysis. Dig. Dis. Sci. 61, 2389–2396. https://doi.org/10.1007/s10620-016-4125-2 (2016).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 79, 1542–1556. https://doi.org/10.1016/j.jhep.2023.06.003 (2023).
He, L., Zheng, W., Liao, Y., Kong, W. & Zeng, T. Individuals with cardiometabolic risk factors are at higher risk for early-onset NAFLD. J. Hepatol. 81, e99–e101. https://doi.org/10.1016/j.jhep.2024.04.027 (2024).
Kanwal, F. et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology 71, 808–819. https://doi.org/10.1002/hep.31014 (2020).
Tamaki, N. et al. Cardiometabolic criteria as predictors and treatment targets of liver-related events and cardiovascular events in metabolic dysfunction-associated steatotic liver disease. Aliment. Pharmacol. Ther. 60, 1033–1041. https://doi.org/10.1111/apt.18205 (2024).
Li, M., Chen, W., Deng, Y. & Xie, W. Impacts of cardiometabolic risk factors and alcohol consumption on all-cause mortality among MASLD and its subgroups. Nutr. Metab. Cardiovasc. Dis. 34, 2085–2094. https://doi.org/10.1016/j.numecd.2024.05.018 (2024).
Lowenfels, A. B., Walker, A. M., Althaus, D. P., Townsend, G. & Domellof, L. Gallstone growth, size, and risk of gallbladder cancer: an interracial study. Int. J. Epidemiol. 18, 50–54. https://doi.org/10.1093/ije/18.1.50 (1989).
Stephen, A. E. & Berger, D. L. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery 129, 699–703. https://doi.org/10.1067/msy.2001.113888 (2001).
Park, J. K. et al. Management strategies for gallbladder polyps: is it possible to predict malignant gallbladder polyps? Gut Liver. 2, 88–94. https://doi.org/10.5009/gnl.2008.2.2.88 (2008).
Aldouri, A. Q. et al. The risk of gallbladder cancer from polyps in a large multiethnic series. Eur. J. Surg. Oncol. 35, 48–51. https://doi.org/10.1016/j.ejso.2008.01.036 (2009).
Schnelldorfer, T. Porcelain gallbladder: a benign process or concern for malignancy? J. Gastrointest. Surg. 17, 1161–1168. https://doi.org/10.1007/s11605-013-2170-0 (2013).
Elmasry, M. et al. The risk of malignancy in ultrasound detected gallbladder polyps: A systematic review. Int J. Surg 33 Pt A. 28–35. https://doi.org/10.1016/j.ijsu.2016.07.061 (2016).
Jain, K. & Garg, P. K. Could type and size of gallstones influence gallbladder carcinogenesis?? Ann. Surg. 263, e57. https://doi.org/10.1097/SLA.0000000000001083 (2016).
Huang, X. et al. Validation of the fatty liver index for nonalcoholic fatty liver disease in Middle-Aged and elderly Chinese. Med. (Baltim). 94, e1682. https://doi.org/10.1097/MD.0000000000001682 (2015).
Yang, B. L. et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large-scale cross-sectional study in Taiwan. PLoS One. 10, e0120443. https://doi.org/10.1371/journal.pone.0120443 (2015).
Hsu, C. L. et al. Role of fatty liver index and metabolic factors in the prediction of nonalcoholic fatty liver disease in a lean population receiving health checkup. Clin. Transl Gastroenterol. 10, 1–8. https://doi.org/10.14309/ctg.0000000000000042 (2019).
Han, A. L. & Lee, H. K. Comparison of the Diagnostic Performance of Steatosis Indices for Discrimination of CT-Diagnosed Metabolic Dysfunction-Associated Fatty Liver Disease. Metabolites 12, (2022). https://doi.org/10.3390/metabo12070664
Han, A. L. Validation of fatty liver index as a marker for metabolic dysfunction-associated fatty liver disease. Diabetol. Metab. Syndr. 14 https://doi.org/10.1186/s13098-022-00811-2 (2022).
Nomura, T. et al. Validation of fatty liver index as a predictor of hepatic steatosis in Asian populations: impact of alcohol consumption and sex. Hepatol. Res. 53, 968–977. https://doi.org/10.1111/hepr.13935 (2023).
Acknowledgements
This study is based on data sourced from the Korean National Health Insurance Service (research administration number, NHIS-2024-2-146), and the results of the study are not related to the National Health Insurance Service.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (RS-2023-00210903). This research was also supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2020-KH088690).
Author information
Authors and Affiliations
Contributions
Conceptualization: Hyuk Soo Eun, Soon-Ki Ahn. Data curation: Soon-Ki Ahn. Formal analysis: Hyuk Soo Eun, Soon-Ki Ahn, Hong Jae Jeon, Woo Sun Rou, Seok Hyun Kim, Byung Seok Lee. Funding acquisition: Hyuk Soo Eun. Methodology: Hyuk Soo Eun, Soon-Ki Ahn, Hong Jae Jeon. Project administration: Hyuk Soo Eun, Soon-Ki Ahn. Writing-original article: Hong Jae Jeon, Hyuk Soo Eun, Soon-Ki Ahn. Writing-review & editing: Woo Sun Rou, Seok Hyun Kim, Byung Seok Lee.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Review Board of the Chungnam National University Hospital (IRB number: 2024-04-031).
Informed consent
The requirement for written informed consent was waived due to the use of anonymized data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jeon, H.J., Eun, H.S., Rou, W.S. et al. Association between cholecystectomy and the risk of new-onset metabolic dysfunction-associated steatotic liver disease: a risk-stratified cohort study. Sci Rep 15, 28223 (2025). https://doi.org/10.1038/s41598-025-13556-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13556-5