Abstract
It has been confirmed that tamoxifen (TAM) use is associated with thromboembolic risks; however, it remains uncertain which specific thromboembolic events warrant prioritized surveillance beyond those already listed in the prescribing information. This study was conducted to assess the relationship between TAM use and reported thromboembolic events using the latest FDA Adverse Event Reporting System (FAERS) data. Tamoxifen-related AEs reported in FAERS between January 2004 and December 2024 were extracted, screened for thromboembolic cases, and subjected to disproportionality analysis to identify significant signals. A clinical prioritization scoring system was then applied to rank signal relevance, and multivariate regression analyses were performed to identify factors associated with thromboembolic outcomes. Among 385 TAM-associated thromboembolic events, 28 significant signals were detected. Newly detected signals comprised unilateral paralysis, retinal artery occlusion, and atrial thrombosis. Male sex, older age, and elevated body mass were identified as risk indicators for thromboembolism. These findings may inform enhanced clinical surveillance and TAM risk stratification; however, further validation is warranted.
Similar content being viewed by others
Introduction
Since the 1970s, tamoxifen, a selective estrogen receptor modulator (SERM), has been employed as a cornerstone of adjuvant therapy for hormone receptor–positive breast cancer. It has been demonstrated by large-scale meta-analyses with 15-year follow-up that five-year tamoxifen therapy reduces recurrence risk by 39% and breast cancer mortality by 30%, and that extending therapy to ten years further enhances survival outcomes1,2. The mechanism of action is characterized by estrogen antagonism in breast tissue, while partial estrogen agonist activity is concurrently observed in other systems (e.g., cardiovascular and skeletal)3. This tissue-selective profile may be intimately linked to the observed thrombotic risk4.
According to clinical data, using tamoxifen raises the risk of venous thromboembolism (VTE) by two to three times5and the risk increases with treatment duration4,6,7. According to the NSABP P-1 trial, patients on tamoxifen had a higher risk of stroke, pulmonary embolism, and deep vein thrombosis, and these events were more prevalent in women 50 years of age or older8. Despite a clear clinical consensus regarding tamoxifen-associated thrombotic risk in female patients, the risk in male breast cancer (MBC) patients—who represent approximately 1% of all cases—has remained understudied9. It should be noted that current MBC treatment guidelines are derived from limited retrospective studies and extrapolated from female breast cancer (FBC) management, and that most ongoing breast cancer clinical trials continue to exclude male participants10,11. Given inherent physiological differences between sexes, direct application of FBC management strategies to MBC warrants caution. Furthermore, although thromboembolic events (TEs) are designated as a black box warning on tamoxifen labeling12the specific thrombus types and their clinical monitoring priorities remain undefined.
The FDA Adverse Event Reporting System (FAERS) is recognized as a global pharmacovigilance database13. In this study, a comprehensive pharmacovigilance analysis was conducted using FAERS data to investigate TAM-associated thromboembolic risk, encompassing sex-related differences and rare subtypes, with the aim of enhancing patient safety in real-world clinical practice.
Materials and methods
Data sources and processing
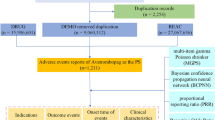
To ensure complete case inclusion, all seven structured FAERS data files were downloaded, including patient demographics (DEMO), drug exposure (DRUG), adverse events (REAC), outcomes (OUTC), report sources (RPSR), therapy dates (THER), and medical indications (INDI). All tamoxifen-associated reports from the first quarter of 2004 (Q1 2004) to the fourth quarter of 2024 (Q4 2024) were retrieved from the FAERS database through the official portal (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html).
In accordance with FDA guidelines for pharmacovigilance studies, a hierarchical deduplication strategy was implemented. Primary case identification numbers were used as the primary identifier, with duplicate entries being removed based on the highest report number and the most recent FDA receipt date. A search was conducted to identify cases related to TAM, with the following keywords employed as search criteria: including “TAMOXIFEN”, “SOLTAMOX”, and “NOLVADEX”. For the purpose of subsequent analysis, reports designating tamoxifen as the “Primary Suspect (PS)” were retained.
Data mining
Baseline characteristics—including sex distribution, age categories, reporting region, and reporter type—were described using descriptive statistics. Thromboembolic events were identified via a Standardized MedDRA Query (narrow scope: Embolic and Thrombotic Events [SMQ 20000081]) and mapped to Preferred Terms (PTs; e.g., pulmonary embolism, deep vein thrombosis) and System Organ Classes (SOCs; e.g., eye disorders, blood and lymphatic system disorders). Non–drug-related PTs (e.g., malignancy-associated thrombosis, product quality issues) and reports with date inconsistencies (e.g., event dates preceding treatment initiation) were excluded. A 2 × 2 contingency table (Supplementary Table S1) was employed for disproportionality analysis, and signals were detected using four algorithms: Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Empirical Bayes Geometric Mean (EBGM). Positive signals were defined as those meeting predefined significance thresholds across all four algorithms simultaneously (see Supplementary Table S2 for criteria). All data processing was performed in R (v4.3.1), with duplicate reports removed prior to statistical analysis, and the study flow diagram is presented in Fig. 1.
Clinical prioritization of disproportionality signals
The clinical priority of TEs was assessed using a semi-quantitative scoring scale based on the European Medicines Agency lists of Designated Medical Events (DME) and Important Medical Events (IME)14. The system was stratified and evaluated on the following five dimensions: (1) Number of target events, (2) Lower limit of ROR, (3) Mortality proportion, (4) IMEs or DMEs and (5) Biological plausibility. Among them, IME is defined as a serious AE with standardized diagnostic criteria, and DME specifically refers to rare drug-related serious AEs that require immediate initiation of regulatory review. The scoring criteria for each dimension are detailed in Supplementary Table S3. Each dimension was categorized into three levels (0–2 points) according to preset thresholds, and only the highest score was taken when multiple criteria were met within the same dimension. A total score of 0–4 points is categorized as weak priority, 5–7 points as moderate priority, and 8–10 points as strong priority.
Subgroup analysis and risk factor assessment
A subgroup analysis stratified by gender was conducted to systematically compare the distribution of tamoxifen-related thromboembolic events between sexes. Multivariable logistic regression was then performed to evaluate associations between selected risk factors (age, sex, and body weight) and the risk of thromboembolism, yielding adjusted odds ratios (ORs) with 95% confidence intervals. Due to substantial missingness and inconsistent coding in the FAERS database, important covariates such as comorbidities and concomitant medications could not be reliably included in the model. Therefore, only variables with high reporting completeness—namely, age, sex, and body weight—were retained to ensure analytical robustness. Two-sided tests were used for all analyses. To control for type I error due to multiple comparisons across 28 Preferred Terms, a Bonferroni-adjusted significance threshold of p < 0.001 was applied, despite the exploratory nature of signal detection.
Results
Descriptive analysis
This pharmacovigilance study utilized data from the US FDA Adverse Event Reporting System (FAERS) database (2004–2024). The raw dataset initially contained 22,249,476 demographic records (DEMO), from which 3,621,809 duplicate entries were removed using FDA-recommended deduplication protocols, resulting in 18,627,667 unique cases. Through linkage analysis with DRUG (n = 67,864,973) and REAC (n = 54,514,632), 4,465 adverse event (AE) reports were identified in which tamoxifen (TAM) was designated as the PS medication. Among these, 385 cases were specifically attributed to thromboembolic events directly associated with TAM therapy.
The characteristics of patients who suffered from TAM-related AEs are summarized in Table 1. A female predominance (78.7%) was observed among the 385 thromboembolic AE cases, slightly lower than the 84.0% female representation in all TAM-associated AEs (n = 4,465). The 50–64.9 age cohort accounted for 50.4% of thromboembolic events, exceeding the 44.9% prevalence in general TAM-related AEs. Notably, 31.7% of thromboembolism patients had body weights between 50 and 100 kg, compared to 21.9% in the broader AE cohort. Geographically, the United States contributed 37.7% of thromboembolism reports, with France (19.0%) and the UK (15.3%) comprising secondary clusters. Hospitalization rates for thromboembolic events (40.8%) substantially exceeded those for all AEs (17.6%). The mortality rate in the TE group (7%) was significantly higher than the overall rate (4.5%).
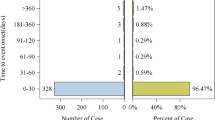
After the FDA updated the black box warning for thrombotic risk in the drug labeling of TAM in 200512, the number of TAM-related thromboembolic adverse reactions reported peaked in 2007 (Fig. 2), accounting for 13.8% of all adverse reactions reported in that year. Although the total number of TAM adverse reaction reports has been increasing year after year since then, the proportion of thromboembolic events has stabilized at about 5% after a period of increase. These data suggest that pharmacovigilance monitoring of TAM-induced TE should be strengthened in clinical practice.
Disproportionality analysis
According to the SMQ narrow classification system, TAM-related thromboembolic events were classified into three categories: Arterial Thromboembolism (SMQ 20000082), Venous Thromboembolism (SMQ 20000084), and Unspecified Thromboembolism (SMQ 20000083). The study incorporated a total of 437 adverse event (AE) reports, of which 28 exhibited positive signals (meeting the four disproportionality analyses). The specific distributional characteristics are detailed in Table 2.
The Sankey diagram (Fig. 3A) demonstrated a substantial discrepancy in the distribution of the 28 PTs between SMQ classification and SOC. The category of venous thromboembolism exhibited the highest proportion (70.9%, 310/437) (Supplementary Table S4), encompassing 19 PTs (Fig. 3B), with Pulmonary Embolism (n = 123, ROR = 5.59) and Deep Vein Thrombosis (n = 79, ROR = 5.17) as the predominant event types. Notably, Budd-Chiari syndrome (n = 5, ROR = 88.50), cerebral venous thrombosis (n = 24, ROR = 58.1), and ophthalmic vein thrombosis (n = 3, ROR = 55.84) showed higher signal intensity.
Arterial thromboembolic events accounted for 11.0% (48/437) of events involving 5 PTs, dominated by ischemic stroke (n = 20, ROR = 4.88) and peripheral artery thrombosis (n = 12, ROR = 36.87), with coronary artery embolism (n = 3, ROR = 44.05) presenting a significant risk signal. Unclassified thromboembolic events accounted for 18.1% (79/437) of cases and were dominated by thrombosis (n = 63, ROR = 3.35), with antiphospholipid syndrome (n = 6, ROR = 16.93) presenting a particularly prominent risk signal.
At the SOC level (Fig. 3C), the majority of PT subtypes were concentrated in the categories of Vascular disorders, Nervous system disorders, and Eye disorders. Conversely, respiratory, thoracic, and mediastinal disorders, comprising only two PTs, accounted for 30.4% (133/437) of cases. This is attributable to the high prevalence of pulmonary embolism in this cohort. Supplementary Table S5 presents a detailed exposition of the specific distribution characteristics of the SOC.
Disproportionality analysis of TAM. (A) Sankey diagram illustrating the hierarchical flow from Standardized MedDRA Queries (SMQs) to Preferred Terms (PTs) and subsequently to System Organ Classes (SOCs). Each band width reflects the number of cases assigned to a given category. (B) The proportion of TAM-related thromboembolism AE reports classified by SMQ. (C) The proportion of TAM-related thromboembolism AE reports classified by SOC.
Clinical prioritization of the disproportionality signals
As shown in Supplementary Table S6, among the 28 positive signals related to TEs, a total of 22 PTs (78.57%) was classified as IMEs. The priority stratification indicated that 16 AEs (57.1%) had a moderate clinical priority (scores 5–7), while 12 AEs (42.9%) had a weak priority (scores 0–4). Among the moderate-priority AEs, Pulmonary Embolism (n = 123, ROR025 = 4.68, score = 6), Deep Vein Thrombosis (n = 79, ROR025 = 4.14, score = 6), Thrombosis (n = 62, ROR025 = 2.61, score = 6), Coronary Artery Embolism (n = 3, ROR025 = 14.12, score = 6) and Cerebral Venous Thrombosis (n = 24, ROR025 = 38.82, score = 6) ranked in the top five. The AE type with the highest number of deaths was Pulmonary Embolism (n = 10), followed by Deep Venous Thrombosis (n = 8). Notably, the highest mortality rate (100%) was observed in Coronary Artery Embolism. New thromboembolic signals included Monoplegia (ROR025 = 3.97), Retinal Artery Occlusion (ROR025 = 4.85), and Atrial Thrombosis (ROR025 = 1.97). Their clinical significance was underscored by quantitative indicators (ROR values, number of deaths, scores) and structured classification.
Subgroup analysis and risk factor assessment
Table 3 delineates the gender-stratified ROR (with 95% confidence intervals) for TEs, categorized into arterial (ATE), venous (VTE), and unspecified thromboembolism. Males exhibited significantly higher ROR than females in most TE subtypes. For example, in VTE subtypes, males demonstrated higher risks for pulmonary embolism (ROR = 15.98 vs. 5.09 in females) and deep vein thrombosis (ROR = 12.05 vs. 4.65). In particular, in ATE subtypes, coronary artery embolism showed a striking male predominance (ROR = 851.62). In addition, certain thromboembolic subtypes lacked sex-specific ROR estimates, particularly in males. This was not due to biological exclusivity, but rather to low reporting frequencies or zero-event counts in the FAERS database, which precluded stable estimation. These missing data points likely reflect underreporting or the rarity of these events, rather than the absence of risk.
To gain a deeper insight into potential influencing factors, a multivariate regression analysis was conducted based on reports of thromboembolism associated with TAM. The significant predictors of risk of thromboembolic events are shown in Table 4. In a multivariate regression analysis, male sex, advanced age and high weight were significantly associated with the risk of thromboembolic events. Male gender was associated with a 2.97-fold increase in risk compared to female gender (p < 0.001). Each higher age category was associated with an exponential increase in risk, with the > 85 years age group showing an 11.92-fold increase in risk (p < 0.001). Weight > 80 kg was independently associated with increased risk of thromboembolic events (OR = 2.71, 95% CI: 1.70–4.30, p < 0.001), whereas the 60–80 kg group showed no significant association (OR = 1.03, 95% CI: 0.66–1.60, p = 0.901). The intercept term indicated low baseline risk in the reference group (OR = 0.02, 95% CI 0.01–0.06).
Discussion
It has been demonstrated in early clinical studies18 that tamoxifen use is associated with increased mortality from stroke and pulmonary embolism. In the present study, demographics, temporal trends, and clinical heterogeneity of TAM-associated TE were systematically characterized by analyzing 385 thromboembolic events among 4,465 TAM-related adverse reports. Key findings included: (1) increased thrombotic susceptibility among men, older adults, and individuals with elevated body mass; (2) novel thromboembolic signals, including monoplegia, retinal artery occlusion, and atrial thrombosis; and (3) identification of high-risk thrombotic subtypes prioritized for clinical monitoring.
Women accounted for 78.7% of thromboembolic cases, a finding consistent with data from registry studies: the incidence of thromboembolism was higher in patients with FBC than in MBC15 after treatment with TAM, but the adjusted risk was elevated in men by a factor of 2.97, especially in arterial events (e.g., coronary artery embolism: male ROR = 851.62). This paradoxical phenomenon may be related to the lower basal estrogen levels in men and the exacerbation of the thrombogenic pathway by the partial estrogen agonism of tamoxifen16. The thrombogenic potential of tamoxifen is likely multifactorial. While it functions as an estrogen receptor antagonist in breast tissue, tamoxifen also exhibits partial estrogen agonist activity in hepatic and vascular tissues17,18. Studies have demonstrated that tamoxifen upregulates the synthesis of procoagulant factors (e.g., fibrinogen, prothrombin) and downregulates natural anticoagulants such as protein S19,20. In vascular endothelial cells, it has been shown to enhance the expression of adhesion molecules and promote platelet aggregation, further contributing to a prothrombotic state21. These effects may be particularly pronounced in male patients, whose low baseline estrogen levels could amplify the thrombogenic consequences of tamoxifen’s estrogenic activity.
The observed discrepancy between our findings and previous reports of TAM’s cardiovascular benefits may be explained by differences in the types of vascular outcomes assessed. Most earlier studies focused on chronic atherosclerotic endpoints (e.g., myocardial infarction)22,23whereas our analysis identified acute embolic events (e.g., coronary artery embolism, cerebral venous thrombosis). Furthermore, real-world users, including off-label male recipients, may differ substantially from postmenopausal women enrolled in clinical trials, further contributing to divergent risk profiles. These findings underscore the importance of stratified risk evaluation and warrant further mechanistic investigations. In addition MBC has a worse prognosis and lower overall survival compared to FBC, even after adjusting for age, race and treatment24,25suggesting the need for gender-specific risk management programs.
The age-related risk increased exponentially, with patients > 85 years of age showing an 11.92-fold increase in thromboembolic risk compared with those < 45 years (95% CI: 3.28–43.35). This trend is consistent with previous studies26 in which people aged 50-64.9 years accounted for 50.4% of thrombotic events. A threshold effect was observed for body weight, with patients weighing > 80 kg exhibiting a 2.71-fold increase in thromboembolism risk (95% CI: 1.70–4.30). However, 58.96% of thromboembolism cases lacked weight data, which is a limitation that should be addressed in future studies.
Previous studies16,27 have shown that tamoxifen may increase the risk of venous thrombosis but has no significant effect or even a protective effect on arterial thrombosis (including coronary events), as it reduces coronary plaque in vivo, improves lipid levels, reduces C-reactive protein, and modulates nitric oxide production. In breast cancer treatment, the risk of major adverse cardiovascular events (MACE, including coronary artery disease, myocardial infarction, etc.) was significantly reduced in TAM users compared with aromatase inhibitors (AIs)20suggesting a potential cardiovascular benefit. However, we identified 28 thromboembolic signals by disproportionality analysis, with 11% being arterial thrombotic events (including coronary embolism), although dominated by pulmonary embolism (ROR = 5.59) and deep vein thrombosis (ROR = 5.17). The following discrepancy between this finding and previous studies may be due to the fact that most of the literature attributes arterial thromboembolism to traditional cardiovascular risk factors (e.g., myocardial infarction, arrhythmia) and does not comprehensively and systematically analyze the contribution of TAM to all types of arterial embolism. Moreover, when analyzed by gender stratification, men had a higher incidence of arterial thromboembolic events than women, and the mortality rate of arterial events was extremely high - coronary artery embolism had a 100% mortality rate (3/3). This is in contrast to the findings of previous studies, which tended to discuss overall thrombotic risk and did not discuss it by age groups, and further analysis of the factors influencing the cardiovascular events of tamoxifen is needed to clarify specific risk indicators.
Although thromboembolic risks associated with tamoxifen are already highlighted in its prescribing information, including a boxed warning, our study provides several important refinements to the existing safety knowledge. First, by employing a structured classification and prioritization framework (e.g., IME and DME categories), we offer a more nuanced understanding of thromboembolic signal strength, aiding in clinical triage. Second, the use of FAERS pharmacovigilance data captures long-term, real-world exposure patterns and patient subgroups not typically represented in controlled trials. Third, we detected several thromboembolic signals that are currently absent from drug labeling, indicating potential emerging risks. Together, these enhancements help bridge the gap between regulatory awareness and clinical risk mitigation.
In this study, disproportionality signals were further analyzed using a scoring scale to prioritize safety concerns and minimize unwarranted alerts. Clinical prioritization analysis of TAM-associated thromboembolic adverse events (AEs) revealed that most events were classified as important medical events (IMEs), highlighting their potential impact on patient health. Among all AEs, 57.1% were assigned intermediate priority, with pulmonary embolism (n = 123, score = 6), deep vein thrombosis (n = 79, score = 6), thrombosis (n = 62, score = 6), coronary artery embolism (n = 3, score = 6), and cerebral vein thrombosis (n = 24, score = 6) comprising the top five. Enhanced clinical monitoring is therefore recommended. Notably, three of the 28 AEs were identified as novel and unexpected safety signals not included in the current drug labeling. These signals comprised monoplegia (ROR = 7.94), retinal artery occlusion (ROR = 10.80), and atrial thrombosis (ROR = 6.12). This underscores the need for detailed analysis of existing patient records and strengthened post-marketing surveillance to enable real-time monitoring. The specific impact of TAM on these AEs and their underlying mechanisms remains insufficiently investigated and warrant further clinical exploration. Given the pervasive risk of TAM-associated thromboembolism, particularly with newly documented signals, clinicians should recognize these concerns as critical safety alerts.
In light of these findings, it is important to translate signal detection into concrete clinical strategies to mitigate thromboembolic risk. Based on the stratified risk profiles identified in this study, we recommend that male patients prescribed tamoxifen, particularly those over 65 years of age or with body weight exceeding 80 kg, undergo enhanced surveillance. This may include baseline and periodic Doppler ultrasound examinations for lower extremity deep vein thrombosis, symptom-based monitoring for signs such as dyspnea or unilateral limb swelling, and early cardiology or hematology consultation in patients with pre-existing cardiovascular or prothrombotic conditions28. In the event of new-onset symptoms suggestive of thromboembolism—such as chest pain, shortness of breath, focal neurological deficits, or limb edema—clinicians should promptly initiate diagnostic evaluation and consider temporary discontinuation of tamoxifen until the cause is clarified29. Furthermore, in male breast cancer patients, individualized risk–benefit assessments may support the substitution of aromatase inhibitors plus gonadotropin-releasing hormone analogues when the thrombotic risk is deemed excessive. These recommendations underscore the need for sex-specific, risk-adapted monitoring protocols and highlight a broader gap in existing clinical guidelines for tamoxifen safety management.
This study has several limitations inherent to the nature of spontaneous reporting systems. First, the FAERS database is a passive surveillance system that is subject to underreporting bias, particularly for less severe or subclinical events. As a result, the observed signals may overrepresent severe thromboembolic outcomes such as pulmonary embolism and coronary artery embolism, while underestimating the incidence of milder events. Second, a substantial proportion (58.96%) of thromboembolic cases lacked complete body weight data, which may have introduced bias into the risk estimation for overweight or obese individuals. Although sensitivity analyses were attempted to account for this, the absence of this key covariate limits the precision of our multivariable modeling. Third, despite the application of rigorous deduplication algorithms and standardized disproportionality methods, the FAERS database lacks denominator data (i.e., total number of exposed patients), which precludes absolute risk estimation. Finally, causality cannot be definitively established from signal detection results, and the findings should be interpreted as hypothesis-generating rather than confirmatory.
Conclusion
The present pharmacovigilance study confirms that tamoxifen significantly increases the risk of arteriovenous thrombosis and that there are stratified differences in risk by sex, age, and weight. The identification of male-specific vulnerability, age-indexed growth patterns of risk, and high-signal thrombotic subtypes (e.g., cerebral venous thrombosis, ROR = 58.10) reshapes surveillance priorities for the TAM-treated population. Although the inherent limitations of spontaneous reporting systems affect the precision of risk quantification, the results of this study call for improved clinical surveillance protocols, including gender-specific screening, geriatric dose optimization, and targeted imaging assessment of high-risk events.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files). The FAERS datasets analyzed during the current study are available on FDA websites (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html).
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus Tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: A patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 23, 382–392 (2022).
Burstein, H. J., Lacchetti, C. & Griggs, J. J. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J. Oncol. Pract. 15, 106–107 (2019).
Jordan, V. C. The science of selective Estrogen receptor modulators: concept to clinical practice. Clin. Cancer Res. : Off J. Am. Assoc. Cancer Res. 12, 5010–5013 (2006).
Decensi, A. et al. Effect of Tamoxifen on venous thromboembolic events in a breast cancer prevention trial. Circulation 111, 650–656 (2005).
Deitcher, S. R. & Gomes, M. P. V. The risk of venous thromboembolic disease associated with adjuvant hormone therapy for breast carcinoma: a systematic review. Cancer 101, 439–449 (2004).
Cuzick, J. et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet (lond Engl). 381, 1827–1834 (2013).
Adomaityte, J., Farooq, M. & Qayyum, R. Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis. Thromb. Haemost. 99, 338–342 (2008).
Fisher, B. et al. Tamoxifen for prevention of breast cancer: report of the National surgical adjuvant breast and bowel project P-1 study. J. Natl. Cancer Inst. 90, 1371–1388 (1998).
Oke, O., Niu, J., Chavez-MacGregor, M., Zhao, H. & Giordano, S. H. Adjuvant Tamoxifen adherence in men with early-stage breast cancer. Cancer 128, 59–64 (2022).
Leon-Ferre, R. A. et al. A contemporary review of male breast cancer: current evidence and unanswered questions. Cancer Metastasis Rev. 37, 599–614 (2018).
Losurdo, A. et al. Controversies in clinicopathological characteristics and treatment strategies of male breast cancer: a review of the literature. Crit. Rev. Oncol. Hematol. 113, 283–291 (2017).
Patel, P. & Jacobs, T. F. Tamoxifen. in Statpearls (StatPearls Publishing, Treasure Island (FL), (2025).
Sakaeda, T., Tamon, A., Kadoyama, K. & Okuno, Y. Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10, 796–803 (2013).
Wang, J. et al. Exploring novel adverse events of Nefecon. Kidney Int. Rep. 9, 2705–2717 (2024).
Eggemann, H. et al. Tamoxifen treatment for male breast cancer and risk of thromboembolism: prospective cohort analysis. Br. J. Cancer. 120, 301–305 (2019).
Braithwaite, R. S. et al. Meta-analysis of vascular and neoplastic events associated with Tamoxifen. J. Gen. Intern. Med. 18, 937–947 (2003).
Pérez-Cremades, D., Cheng, H. S. & Feinberg, M. W. Revisiting hormonal control of vascular injury and repair. Circ. Res. 127, 1488–1490 (2020).
Liang, X. et al. DLGAP1-AS2 promotes Estrogen receptor signalling and confers Tamoxifen resistance in breast cancer. Mol. Biol. Rep. 49, 3939–3947 (2022).
Tran, H. C. M. et al. The procoagulant signature of cancer cells drives fibrin network formation in tumor microenvironment and impacts its quality. Implications in cancer cell migration and the resistance to anticancer agents. Thromb. Res. 238, 172–183 (2024).
Okekawa, A. et al. Platelet-derived growth factor signaling in pericytes promotes hypothalamic inflammation and obesity. Mol. Med. (camb Mass.). 30, 21 (2024).
Tamargo, I. A. et al. HEG1 protects against atherosclerosis by regulating stable flow-induced KLF2/4 expression in endothelial cells. Circulation 149, 1183–1201 (2024).
Lai, S. W., Lin, C. L. & Liao, K. F. Association between Tamoxifen use and acute myocardial infarction in women with breast cancer. Med. (Baltim). 98, e13925 (2019).
Sun, J. C., Sun, Z. F., He, C. J., Zhai, C. L. & Qian, G. Association between aromatase inhibitors and myocardial infarction morbidity in women with breast cancer: a meta-analysis of observational studies. Cancer Control: J. Moffitt Cancer Cent. 29, 10732748221132512 (2022).
Weir, J., Zhao, Y. D., Herman, T. & Algan, Ö. Clinicopathologic features and radiation therapy utilization in patients with male breast cancer: a National cancer database study. Breast Cancer: Basic. Clin. Res. 12, 1178223418770687 (2018).
Wang, F. et al. Overall mortality after diagnosis of breast cancer in men vs women. JAMA Oncol. 5, 1589–1596 (2019).
Huang, A. et al. Long-term trends in the incidence of male breast cancer and nomogram for predicting survival in male breast cancer patients: a population-based epidemiologic study. Sci. Rep. 15, 2027 (2025).
Matthews, A. A. et al. Endocrine therapy use and cardiovascular risk in postmenopausal breast cancer survivors. Heart (br Card Soc). 107, 1327–1335 (2021).
Key, N. S. et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO guideline update. J. Clin. Oncol. : Off J. Am. Soc. Clin. Oncol. 41, 3063–3071 (2023).
Falanga, A. et al. Venous thromboembolism in cancer patients: ESMO clinical practice guideline. Ann. Oncol. : Off J. Eur. Soc. Med. Oncol. 34, 452–467 (2023).
Acknowledgements
Thanks to the FDA Adverse Event Reporting System (FAERS) for sharing data and code.
Author information
Authors and Affiliations
Contributions
Jiajia Jia and Chunyu Tian: Conceptualization, Methodology, Data curation, Formal analysis, Writing-review & editing. Wenchao Han and Qi Ma: Data curation, Validation, Supervision, Writing-review & editing. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Because this study was an observational study using global open database (FAERS) with anonymized information, not involving treatment intervention or collection of human samples, informed consent was exempted.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jia, J., Tian, C., Han, W. et al. Real-world assessment of thromboembolic risk associated with tamoxifen. Sci Rep 15, 27820 (2025). https://doi.org/10.1038/s41598-025-13585-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13585-0