Abstract
Trichotillomania (TTM), an understudied psychiatric disorder, was investigated using Sapap3 knockout (KO) mice to elucidate nucleus accumbens (NAc) circuit dysfunction and oxytocin’s therapeutic potential. Under aversive conditions, KO mice exhibited TTM-like behavioral phenotypes compared to wild-type (WT) controls: elevated anxiety-like behavior (reduced total distance 33328.45 ± 6703.97 mm vs. WT 47787.22 ± 12221.33 mm; decreased standing episodes 34.20 ± 19.41 vs. WT 58.10 ± 15.55; increased immobility duration 175.05 ± 54.46 s vs. WT 90.23 ± 70.22 s, all p < 0.05), excessive grooming duration (467.43 ± 94.98 s vs. WT 391.62 ± 86.44 s, p < 0.05), and impaired social interaction characterized by elevated aggression (70 ± 10% victory rate in tube-dominance test vs. WT, p < 0.05). Calcium imaging revealed NAc neuronal hypoactivity (peak ΔF/F0: 2.44 ± 1.67% vs. WT 6.92 ± 2.08%, p < 0.05). Molecular analyses revealed: (1) Dopaminergic signaling alterations (increased dopamine: 26.95 ± 2.04 pg/mL vs. WT 22.43 ± 1.85 pg/mL, p < 0.05; D1 receptor up-regulation 1.29-fold and D2 down-regulation 0.89-fold). (2) Synaptic plasticity disruptions (CREB overexpression: 1.71-fold, p < 0.01; SHANK upregulation: 1.18-fold, p < 0.05). (3) SAPAP3-SHANK3 interaction deficits confirmed by immunofluorescence (compensatory SHANK3 up-regulation 1.46-fold). Oxytocin effects were paradoxical: acute administration exacerbated total grooming duration (550.45 ± 33.65 s vs. KO baseline 467.43 ± 94.98 s, p < 0.05) but reduced grooming bouts (50.80 ± 28.20 vs. 95.30 ± 31.92, p < 0.01) and attenuated aggression (victory rate against WT decreased to 65 ± 5%). Sex-stratified analysis revealed enhanced grooming severity in female KO mice (the grooming duration 526.59 ± 25.69 s vs. male KO 408.26 ± 104.33 s, p < 0.05). These findings highlight NAc circuit dysfunction and complex oxytocin effects in TTM, suggesting therapeutic targets while emphasizing the need for sex-stratified.
Similar content being viewed by others
Introduction
Trichotillomania (TTM) is a complex mental health condition characterized by the recurrent urge to pull out hair, leading to noticeable hair loss and dysfunction1. According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) of the American Psychiatric Association, TTM is classified under “Obsessive-Compulsive and related disorders (OCRDs)“1. A study involving a large sample of U.S. adults showed that TTM is relatively common (1.7%), causes severe distress (average 5.1/7), and often co-occurs with other mental health disorders (in 79% of cases)1. Earlier studies suggest that while TTM prevalence is similar in both sexes, its symptoms and impacts vary by sex2. Effective treatments for TTM include psychological therapies, such as cognitive and behavioral therapies. However, pharmacological treatments show variable efficacy and side effects, and there is limited research on their effectiveness3. Therefore, in-depth research into the classification, neurobiological mechanisms, prevalence characteristics, and treatment methods of TTM is of significant scientific and societal importance for providing more precise and effective treatment options, alleviating patients’ suffering, and improving their quality of life. Previous studies have enhanced our understanding of TTM, revealing its neurobiological complexity and overlap with OCRDs4. Notably, SAP90/PSD95 associated protein 3 (SAPAP3) has been implicated in TTM5.
SAPAP3 is a critical synaptic-associated protein predominantly expressed in the basal ganglia, such as the caudate nucleus. It plays a pivotal role in regulating synaptic structure and function through interactions with PSD-95 (Postsynaptic density-95) and SHANK protein families6. Research by van den Boom et al. suggests a link between Sapap3 deficiency and compulsive checking behaviors as well as impaired cognitive flexibility7. Furthermore, studies in Sapap3 knockout mice have demonstrated excessive grooming behaviors akin to those observed in Obsessive-Compulsive Disorder (OCD) patients, accompanied by facial injuries and increased anxiety8. Boardman et al. reported a significant association between rs11583978 in Sapap3 and TTM (n = 45, P = 0.024), supporting the hypothesis that Sapap3is a genetic risk factor for TTM and may share genetic vulnerabilities with OCD9.
A landmark study by Welch et al. revealed that Sapap3 knockout mice exhibit OCD-like behaviors, providing crucial insights into the role of Sapap3 in neurobiology and behavioral regulation10. These mice displayed corticostriatal synaptic defects, affecting neural signal transmission efficiency and leading to behavioral changes such as excessive grooming, facial self-injury, and increased anxiety, which mirror the clinical manifestations of OCD patients. These findings underscore SAPAP3’s importance in maintaining normal synaptic function and behavior, establishing Sapap3 knockout mice as a valuable model for studying OCRD pathophysiology5. It is worth noting that these mice exhibited excessive grooming behaviors, which can lead to characteristic skin damage, reproducing the compulsive plucking and skin damage rituals observed in TTM patients. Aripiprazole is a first-line therapeutic drug for tic disorders and TTM. In Sapap3 KO mice, systemic administration of aripiprazole can effectively inhibit tic and compulsive grooming behaviors (while retaining the normal grooming sequence), strongly verifying the effectiveness of Sapap3 KO mice as a model for studying the neurobiological mechanism of TTM11. The pathogenesis of OCD is multifaceted, involving genetics, environment, and neurobiology12. Among these, dopaminergic system imbalance is considered a key factor13. Dopamine receptors, crucial for neurotransmitter signal transduction, have been implicated in the onset and progression of mental illnesses, making them a significant target for research14. Dopamine (DA) exerts its physiological effects by binding to D1 and D2 receptors, and interacting with Glutamic acid (Glu)15. Among them, D1 receptors are mainly involved in the regulation of cognitive, emotional, and motor functions, while D2 receptors are mainly involved in reward, motivation, and emotional regulation15. Studies have shown that the expression levels of dopamine receptors D1 and D2 are altered in OCD patients and animal models, suggesting that they may play a role in the pathogenesis of OCD16,17,18. Hence, investigating the role of the dopamine signaling pathway in TTM pathogenesis is of paramount scientific significance. Studies have reported that the spatial working memory of TTM groups is impaired19. Additionally, evidence suggests that disorder of reward processing at the nucleus accumbens (NAc) level may play a key role in the pathophysiology of TTM20. It is noteworthy that medium spiny neurons (MSNs) in the NAc constitute the fundamental functional units of this brain region21. MSNs are characterized by distinct membrane surface receptors, primarily D1-like (for direct pathway) and D2-like (for indirect pathway) receptors. These two pathways exert opposing effects on motor regulation and jointly regulate learning and decision-making processes in the brain22. The above-mentioned research suggests that the dopamine system may play a core role in the pathogenesis of TTM, especially mediated by the MSNs in NAc.
When delving into the regulatory mechanism of the dopaminergic system on TTM, the synergistic effects of other neurotransmitters and hormones cannot be ignored. Oxytocin, a neuropeptide crucial in the endocrine and central nervous systems of mammals, binds to the oxytocin receptor (OXTR), which is widely distributed in the NAc of the brain23. It influences not only childbirth and lactation but also social behavior, emotional regulation, and cognitive functions24. Studies have shown that the DNA methylation levels of the two target sequences of exon III of the oxytocin receptor gene (OXTR) in the peripheral blood of patients with OCD are higher than those in the healthy control group, and are related to the severity of OCD25. Future studies need to verify the direct effect of oxytocin on the hair-pulling behavior of Sapap3 KO mice, and thereby explore its efficacy on TTM.
Based on the association between Sapap3 gene deletion and TTM pathological behavior initially revealed by existing studies, we propose the following core hypotheses: Defects in the Sapap3 gene disrupt the synaptic scaffold function of SAPAP3 and SHANK protein in NAc, leading to impaired glutamate delivery and imbalance of the dopamine receptor signaling pathway, and ultimately inducing grooming behavior driven by the reward system. To further verify this hypothesis and reveal its underlying mechanism, we designed and implemented a comprehensive set of research methods: we conducted a series of behavioral tests to fully assess the impact of Sapap3 deficiency on the behavior of mice. These tests include our further simulation of stress-induced pathological grooming through the light aversion experiment, which is consistent with the stress-triggering mechanism in TTM patients1. In addition, a tube-dominance test to monitor changes in social behavior under stress. To gain a deeper understanding of the effects of Sapap3 defects at the neural level, we employed calcium imaging techniques to monitor changes in the activity of NAc neurons in Sapap3 KO mice during specific behavioral processes. Calcium imaging is a powerful tool for visualizing neuronal activity with high spatial and temporal resolution. By measuring changes in intracellular calcium concentrations, calcium imaging allows researchers to monitor the activation of neurons in real-time26. In our study, calcium imaging techniques were used to investigate the neural correlates of Sapap3 defects and their impact on grooming behaviors. This approach provided insights into the neural mechanisms underlying Sapap3-mediated behavioral changes and helped to elucidate the role of SAPAP3 in synaptic transmission and neurobiology. Additionally, to verify possible interactions between SAPAP3 and SHANK proteins, we used double-label immunofluorescence to observe the co-localization of these two proteins at the neuronal level. This step helps reveal the possible neurobiological consequences of SAPAP3 obstructed interactions with SHANK proteins. Finally, to explore potential therapeutic strategies, we evaluated the efficacy of oxytocin in treating TTM-like behaviors. This step offers preliminary evidence for therapeutic intervention and clues for future treatment development in TTM and related neuropsychiatric disorders. By combining these various research methods, we hope to gain a comprehensive understanding of the role of Sapap3 in TTM pathogenesis and to identify novel therapeutic targets for the treatment of this disorder.
Materials and methods
Statement
All experimental procedures were conducted in strict accordance with relevant ethical guidelines, animal welfare standards, and laboratory regulations. Specifically, the study was approved by the Ethics Committee of Ningxia Medical University General Hospital (KYLL-2024-0340), and all animal protocols adhered to the ARRIVE guidelines (https://arriveguidelines.org) and AVMA Euthanasia Guidelines (https://www.avma.org). Animals were sourced from institutions certified under international welfare standards, and all surgical interventions and euthanasia were performed under anesthesia (isoflurane inhalation) or cervical dislocation to minimize suffering. Experimental materials and reagents were used as per manufacturer instructions and laboratory protocols, equipment operation followed standardized procedures, and data analysis employed validated statistical methods with rigorous quality control to ensure result reliability.
Experimental animals
SPF-grade C57BL/6J mice aged 4 months (21–27 g) were used. Sapap3 knockout (KO) mice were purchased from Shanghai Southern Model Organisms Center Co., Ltd. and housed in the Experimental Animal Center of Ningxia Medical University under standard conditions: 22 ± 2 °C, 60% humidity, and a 12 h light/dark cycle. Mice had free access to food and water and were grouped in cages of eight.
The study subjects consisted of littermate-matched Sapap3 KO mice and their corresponding wild-type (WT) littermate controls, all derived from the same breeding cohorts. The mice were divided into three independent cohorts, which were respectively used for: (1) For 10 Sapap3 KO mice (5 males, 5 females) and 10 WT control mice (5 males, 5 females), open field experiments were conducted first, followed by the aversive stimulus task. Quantitative Real-time PCR (qRT-PCR) and Enzyme-Linked Immunosorbent Assay (ELISA) were performed on tissue samples collected 2 h after completing these behavioral experiments; (2) 7 Sapap3 KO male mice and 3 WT male controls were taken for fiber-optic calcium recording; (3) 20 Sapap3 KO mice (10 males, 10 females) and 20 WT controls (10 males, 10 females) were selected for tube-dominance test and oxytocin treatment intervention tests. All behavioral tests were conducted during the same circadian rhythm period (13:00–19:00). Before each experiment began, mice were allowed to freely explore the experimental site for 5 min to promote environmental adaptation. After the behavioral test was completed, we wiped the device with 75% alcohol, dried it thoroughly with a hair dryer, and then started the next test to prevent the odors among different mice from affecting their behavior.
Behavioral experiments
The behavioral experiment took Sapap3 KO and WT mice as the subjects. Firstly, the open-field test and the aversive stimulus task were used to quantitatively analyze the repetitive grooming behavior and anxiety level. Stress social test was used to evaluate the effect of the knockout on the behavioral phenotype.
(1) TTM-Like Behavioral Analysis.
-
a.
(a) Open-field test Mice were placed in the center of the open-field apparatus (40 cm×40 cm×30 cm) under a dark condition (no light) and allowed to freely explore for 10 min, while being recorded by a camera above. Offline analysis was performed using tracking software to assess behavioral parameters. The repetitive grooming behavior and anxiety level were recorded for 10 min and scored using the VisuTrack animal behavior Analysis System (Shanghai Xinruan), it can synchronously record behavioral parameters under light or dark conditions, and the data is automatically collected and stored by the supporting software. Time spent in the center was taken as a sign of less anxiety-like behavior, while staying close to the walls pointed to higher anxiety-like levels. In addition, total distance (mm), immobility duration (s), and number of standing episodes (the mouse supports its weight with its hind limbs and maintains a stable upright posture) were measured.
-
b.
(b) Aversive stimulus task Mice were placed in a modified open-field apparatus, and grooming behavior under aversive conditions was observed and compared between the two groups of mice. We placed a 15-watt light above the open -field apparatus in a dark room (about 10 cm away), restricting mice’s space and intensifying their aversion to light to study the effect of Sapap3gene knockout on stress-related behaviors in mice. The evaluation indicators were the same as Open-field Test.
(2) Tube-dominance test In order to further evaluate the social behaviors of WT and Sapap3 KO mice under stress, we conducted a social hierarchy test. The apparatus utilized for this purpose was a transparent polyvinyl chloride (PV) tube with a diameter of 3 cm and a length of 45 cm. In the social interaction test, mice of different genotypes (WT/KO) were randomly were randomly same-sex paired. The social behavior of the mice was continuously recorded until one of the mice exited the tube. The mouse that exited first was considered to be the subordinate one in the pair.
Detection of molecular markers in the D1 and D2 signaling pathways
To gain a deeper understanding of the specific roles of the D1 and D2 signaling pathways in the pathogenesis of TTM, molecular markers in these pathways will be detected in brain tissue using molecular biology techniques such as qRT-PCR and ELISA. We euthanized the mice by cervical dislocation and promptly isolated the NAc. Each sample comprised six mice (three males and three females), and we carried out every technical procedure for each sample in triplicate.
(1) qRT-PCR RNA Extraction: Total RNA was extracted from the NAc using a kit from Simgen Company. The extraction process strictly adhered to the manufacturer’s instructions. The mRNA levels of DRD1, DRD2, cAMP, PKC, CREB, and SHANK will be detected using qRT-PCR. Reverse Transcription: Reverse transcription reactions were performed in accordance with the manufacturer’s protocol to synthesize complementary DNA (cDNA) from the extracted RNA.qRT-PCR: The SYBR Premix Ex Taq II (Perfect Real Time) reagent kit from Simgen Company was used for PCR amplification. The qRT-PCR reaction protocol consisted of an pre-denaturation at 95 °C for 30 s, followed by 40 cycles of PCR reaction (95 °C for 5 s, 60 °C for 30 s), and a melting curve analysis (1 min at each 0.5 °C increment from 57 °C to 90 °C, with fluorescence collection at each step). The reaction volume was 20 µL. Relative gene expression changes were calculated using the Ct (2 −ΔΔCT) method, and all gene expression levels were normalized to the expression levels of GAPDH levels. The primers will be synthesized by Huada Genomics Co., Ltd (Table 1).
(2) ELISA detection Sample Preparation: Brain tissue was ground on ice in PBS (0.01 M, pH 7.4) at a ratio of 0.1 g tissue to 1 mL PBS until homogenized. The homogenate was then transferred to an EP tube and stored at -20 °C overnight. After two freeze-thaw cycles to disrupt cell membranes, the sample was centrifuged at 3000 RPM /min for 5 min at 4 °C to collect the supernatant. ELISA Procedure: The detection steps were performed following the instructions of the commercial ELISA kit from Ruifan Biotechnology Company. A standard curve was constructed by plotting the known concentrations of the standards against their corresponding OD values. The concentrations of Glu and DA in the test samples were calculated using this formula.
(3) Immunofluorescence After the mice were sacrificed for neck dislocation, a flat needle was quickly inserted from the apex of the heart into the left ventricle to the aorta and fixed. A cut was made in the right atrial appendage, and 50–60 mL of pre-cooled normal saline was pumped in. Clear effusion was observed. Instead, 30mL of 4% paraformaldehyde was pumped in. The perfusion was completed when the muscles of the mice became stiff. The mouse’s head was decapitated and craniotomy was performed to obtain the brain. It was fixed in 4% paraformaldehyde overnight at 4℃. Paraffin sections were prepared by gradient ethanol dehydration, xylene transparency, and paraffin wax permeation and encapsulation. After dewaxing the sections to water, the antigens were successively repaired under high pressure, the endogenous enzymes were inactivated with 3% hydrogen peroxide, and the sections were blocked with 5% BSA. The SAPAP3 primary antibody (Rabbit Anti-SAPAP3 antibody, BIOSS, bs-11316R, 1:200) was incubated overnight and the horseradish enzyme secondary antibody was incubated for 50 min successively, and then CY3-TSA(goat anti-rabbit CY3-Tyramide) developed color. The antibodies were removed by thermal repair. After the target was permeated, the sections were blocked for the second time. Incubate the SHANK3 primary antibody (Rabbit Anti-SHANK3 antibody, BIOSS, bs-12143R, 1:200) overnight and the 488 fluorescent secondary antibody (goat anti-rabbit IgG/488) for 45 min. After staining the nuclei with DAPI (ready-to-use DAPI staining solution, KeyGEN BioTECH), seal the slides for microscopic examination(BX53, OLYMPUS). The results showed CY3 (red), 488 (green), and DAPI (blue) as SAPAP3, SHANK3, and the nuclei. Due to breeding reasons and insufficient data on female mice, only the data from male mice are presented here, with 3 male mice in each group.
Fiber-optic calcium recording in the NAc
Using an advanced fiber-optic recording system (Rayward Fiber Optic Recorder, Shenzhen Rayward Co., Ltd.), our goal is to closely monitor the changes in neuronal calcium signals in the NAc of Sapap3 KO mice under aversive conditions compared with WT mice. In this comprehensive neuroscience study, animals were initially anesthetized with 1–2% isoflurane. Subsequently, precise unilateral injections of PT-0148rAAV-hSyn-GCaMP6m-WPRE-hGH polyA (Wuhan Shumi Brain Science and Technology Co., Ltd.) were administered into the NAc at the following coordinates: AP = ± 1.6 mm; ML = ± 1.2 mm; DV = -4.3 mm. Following the injection, an optical fiber ceramic sleeve with short optical fibers was implanted at the injection site and secured within the brain. Animals were then given a two-week recovery period to allow for viral expression. Utilizing a fiber-optic imaging system, a 470 nm laser was used to excite the green fluorescent calcium signal. Grooming behavior was captured using a dedicated video analysis system, with the time stamps synchronized with the calcium signal recordings. Due to considerations in breeding and the challenges of calcium imaging technology that may reduce the success rate of the experiment, this article only provides data on male mice.
Oxytocin treatment
Drug injection details: oxytocin (Shanghai Jianglai Biotechnology Co., LTD) was administered at a dose of 10 mg/kg, diluted in normal saline, with a total intraperitoneal injection volume of 2 mL/kg body weight. The behavioral experiment was conducted one hour after an injection of oxytocin. This is to study the immediate behavioral response triggered by one-time oxytocin treatment.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). All behavioral and physiological data were expressed as mean ± SD. To examine the influence of sex (male/female) and genotype (Sapap3 KO/WT) on grooming behavior, a two-way ANOVA was performed to assess main effects and their interaction. Significant interactions (p < 0.05) were followed by Šidák’s post hoc tests to compare group means. All statistical results report the F-value, degrees of freedom and exact p-value. The comparison between the two groups of data was conducted using the t-test. All statistical analyses were conducted and plotted using GraphPad Prism 9.0.
Results
Differences between Sapap3 knockout mice and WT mice in comparative behavioral experiments
(1) Differences in grooming behavior.
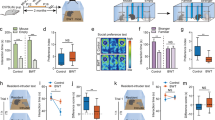
The results showed that environmental factors had a significant main effect on the indicators of grooming behavior (p < 0.05). Specifically, the grooming bouts (F1,36= 17.32, p < 0.05) and grooming durations (F1,36= 24.02, p < 0.05) were significantly different under different environmental conditions (Fig. 1A). In contrast, the main effect of genotype on the indicators of grooming behavior was not significant (p > 0.05). However, in terms of the grooming durations, there was a significant interaction between environmental factors and genotype (F1,36= 4.950, p < 0.05). Under the aversive condition, the KO mice exhibited significantly increased grooming duration (467.43 ± 94.98 s vs. WT 391.62 ± 86.44 s, p < 0.05). There was no interaction between sex and genotype (F₁,₈= 0.1948, p > 0.05). Specifically, female KO mice showed prolonged grooming compared to male KO mice (526.59 ± 25.69 s vs. 408.26 ± 104.33 s, p < 0.05; Fig. 1B–C).
The comprehensive graph title integrates the behavioral differences between Sapap3 KO mice and WT mice under different conditions, including grooming behavior, and anxiety-like behavior. Specifically, Part A highlights the grooming time of WT and KO mice under different light conditions (p < 0.05). Among them, AD represents environmental factors (A under light aversion stimulation and D under dark conditions). Parts B and C reveal the sex differences in grooming behavior. Among them, F represents the female and M represents the male. Part D and Part E describes the anxiety levels of mice. Part D shows the differences in exploration behavior and assesses the total distance in the opening test. Among them, the WT mice under dark conditions were WTD, the Sapap3 KO mice under dark conditions were KOD, the WT mice under light aversion stimulation were WTA, and the Sapap3 KO mice under light aversion stimulation were KOA. Part E shows the number of standing episodes and the immobility duration. * indicates p < 0.05, ** indicates p < 0.01, and *** indicates p < 0.0001.
(2) Anxiety-like behaviors.
As shown in Fig. 1D and E, there was a significant interaction between genotypes and environmental factors (F1,36=9.866, p < 0.05), jointly affected the total distance of mice. However, when analyzing the main effects of environmental factors and genotypes separately, neither of them showed significance (p > 0.05). The post hoc tests result showed that under light-aversion stimulation conditions, compared with the WT group, the total distance of KO mice was significantly reduced (33328.45 ± 6703.97 mm vs. WT 47787.22 ± 12221.33 mm, p < 0.05), the number of standing episodes was also significantly decreased (34.20 ± 19.41 vs. WT 58.10 ± 15.55, p < 0.05), while the immobility duration was significantly increased (175.05 ± 54.46 s vs. WT 90.23 ± 70.22 s, p < 0.05). Further comparison of the motor performance of KO mice under different environmental conditions revealed that when they were in a light-averting stimulus environment, compared with the dark environment, the total distance was significantly reduced (KOA 33328.45 ± 6703.97 mm vs. KOD 46334.43 ± 11525.74 mm, p < 0.05), and the immobility duration was significantly increased (KOA 175.05 ± 54.46 s vs. KOD 25.18 ± 13.15 s, p < 0.05).
(3) Tube-dominance test.
As shown in Table 2, there was a significant interaction between genotypes and environmental factors (F1,36= 8.471, p < 0.05), jointly affected the aggressive behaviors of mice. The propensity of the mice to exhibit aggressive behaviors in the tube-dominance test was found to be dependent upon genotype (F1,36= 8.471, p < 0.05). After many rounds of testing, in the final KO versus WT mice duel, KO mice showed a significant advantage with 70 ± 10% victory rate vs. WT, p < 0.05. Overall, KO mice initiated more aggressive responses than WT mice during tube testing. It’s basically the act of forcing a cage mate out of the tube.
Detection of molecular indicators in D1 and D2 signaling pathways
The results of related gene expression showed that in NAc, the relative expression of DRD2 was down-regulated (0.89-fold), while the expression of other genes was up-regulated, and the relative expression of CREB and SHANK were significantly different (D1 receptor 1.29-fold; CREB 1.71-fold, p < 0.01; SHANK 1.18-fold, p < 0.05; Fig. 2A).
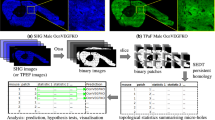
Detection of D1 and D2 signaling pathways and co-localization of SHANK3 and SAPAP3 in mouse NAc (A) Relative expression detection of genes related to dopamine receptor signaling pathways in mouse NAc. (B) The content of neurotransmitters in mouse NAc. (C) Immunofluorescence showed the co-localization of SHANK3 (green) and SAPAP3 (red) in the NAc of mouse brains. The fluorescence signals of SAPAP3 and SHANK3 significantly overlap in the same area (indicated by white arrows, and the overlapping signals are indicated by yellow), suggesting their close spatial relationship and potential interaction within the NAc synaptic structure. (D) Immunofluorescence intensity results of SAPAP3 and SHANK3 genes in NAc. * indicates p < 0.05, and ** represents p < 0.01.
Based on the ELISA results (Fig. 2B), we observed no difference in Glu concentrations in the NAc between Sapap3 KO mice and WT mice. The DA concentration in the NAc of Sapap3 KO mice was significantly higher than that in WT mice (KO 26.95 ± 2.04 pg/mL vs. WT 22.43 ± 1.85 pg/mL, p < 0.05).
Immunofluorescence results showed that SAPAP3 and SHANK3 fluorescence signals overlapped significantly in the same region, indicating a colocalization relationship in NAc (Fig. 2C). The expression of SAPAP3 in NAc of KO mice (i.e. immunofluorescence intensity, Fig. 2D) was significantly lower than that of WT mice (0.14-fold, p < 0.05), indicating that Sapap3 KO mice were successfully modeling. Meanwhile, the SHANK3 in KO mice was higher than that in WT (1.46-fold, p > 0.05).
Results of calcium fiber photometry in the NAc
As shown in Fig. 3A, GCaMP6m was stably expressed in the NAc brain region. Neuronal calcium imaging reveals that peak frequency and peak mean are crucial parameters reflecting neuronal activity characteristics, which are associated with grooming behavior in mice. In KO mice, a lower peak value (ΔF/F0: 2.44 ± 1.67% vs. WT 6.92 ± 2.08%, p < 0.05) and peak frequency (ΔF/F0: 0.12 ± 0.02 vs. WT 0.13 ± 0.01, p > 0.05) were observed during grooming behavior compared to WT mice (Fig. 3B and C).
Results of Calcium Fiber Photometry in the NAc. (A) Schematic of virus injection and representative images of AAV-hSyn-GCaMp6m injection in NAc (the brain area circled in red is NAc, Scale bar: 1000 μm). (B) Calcium release trend diagram of mice grooming behavior. (C) The Peak Frequency and Peak value (ΔF/F) of calcium ion release during grooming behavior. * indicated p < 0.05.
Examining the therapeutic efficacy of Oxytocin treatment
An oxytocin treatment regimen was administered in Sapap3 KO mice to evaluate its effect on grooming behavior. Post-treatment observations showed that compared with Sapap3 KO mice, oxytocin treatment did significantly reduce the grooming bout in KO mice (50.80 ± 28.20 vs. KO baseline 95.30 ± 31.92, p < 0.01), while the grooming duration also significantly increased (550.45 ± 33.65 s vs. KO baseline 467.43 ± 94.98 s, p < 0.05; Fig. 4). Furthermore, oxytoc in alleviated the aggressive behavior of KO mice (victory rate against WT decreased to 65 ± 5%, p < 0.05; Table 3).
Discussion
The Sapap3 gene, which has been identified as significantly associated with human TTM, has received extensive attention in recent years. In our study, Sapap3 KO mice showed significantly increased anxiety and higher grooming behavior, especially significantly longer grooming duration. This finding is consistent with the findings of Lamothe et al.11. However, compared to previously reported studies, our study provides more detailed data on behavioral responses to light-averse stimuli, including reduced total distance, reduced standing episodes, and increased immobility duration. These results further highlight the importance of the Sapap3 gene in regulating anxiety states in response to aversive stimuli and reveal abnormal behavior in Sapap3 KO mice exposed to environmental stimuli.
This study shows that light stimulation, as an environmental stressor, significantly alters the grooming behavior pattern of mice. It is speculated that by triggering changes in neurological activities, the grooming behavior changes with the environment. Although the main effect of genotype is not significant, there is an interaction with environmental factors in the grooming duration, indicating that genotype may regulate the stress sensitivity of mice and affect the role of the environment in grooming behavior. Both sex and genotype have significant main effects on the grooming duration. Moreover, the grooming duration of female mice in the KO group was significantly longer than that of male mice. This sex-specific response may reflect the influence of hormones on OCRDs27. It is worth noting that Sapap3 gene knockout mice model can capture the sex-related susceptibility factors, which may provide clues to understand whether there are sex differences in stereotyped behavior in patients with TTM (human TTM shows a clear female-dominant feature, with a ratio of 10:1)28,29,30. Future research should further verify whether the hormonal cycle affects the phenotypic manifestations related to SAPAP3.
Our investigation into the social behavior and cognitive ability of Sapap3 KO mice revealed an increase in aggression, which is consistent with behavioral patterns often observed in patients with OCRDs31. Furthermore, Sapap3 KO mice showed significant cognitive decline characterized by lack of motivation, lethargy, and depression-like behavioral symptoms, which resonate with OCD comorbidities reported by Overbeek et al.32. By linking Sapap3 deficiency to changes in aggression, dominance, and cognitive impairment, our study underscores the need for further exploration of the neural mechanisms driving these behavioral changes, which may have positive implications for understanding and treating disorders like TTM that share similar behavioral characteristics.
When comparing calcium signaling in the NAc during grooming behavior between Sapap3 KO and WT mice, significant differences were observed. Consistent with prior research33,34 our study revealed that during grooming, the average peak of calcium concentration in Sapap3 KO mice was significantly reduced, and the peak frequency of neuronal activity slightly decreased. Despite this reduced activity intensity, Sapap3 KO mice spent more time grooming, suggesting that neurons need a longer time to complete the task. Conversely, the grooming bout was lower, which might indicate that they do not need to do so frequently to gain a sense of comfort. This shift in grooming behavior may stem from decreased neuronal activity efficiency and intensity due to Sapap3 knockout, leading to prolonged grooming durations but lower frequencies. In contrast, WT mice exhibited efficient and balanced neuronal activity, enabling quicker grooming task completion and more frequent grooming sessions to maintain comfort.
In the Sapap3 KO mice model, we also observed a series of neurobiological changes closely related to abnormal behaviors such as grooming, anxiety, and social disorders. Specifically, although Glu, the primary excitatory neurotransmitter in the central nervous system, did not significantly change in the TTM mice model, indicating that Sapap3 may not directly regulate Glu release or reuptake processes35. DA, a neurotransmitter that plays a crucial role in reward mechanisms, motor control, and various emotional functions36 increased in levels after Sapap3 knockout. This change may explain some of the behavioral alterations observed in the TTM mice model.
Furthermore, in the TTM mice model, we found that D1 receptor expression was up-regulated, while D2 receptor expression was down-regulated, suggesting that the increase in dopamine levels in the NAc activated MSNs in the direct pathway, thereby affecting the behavioral manifestations of mice. Additionally, literature also indicates that increased CREB expression in the NAc increases the likelihood of aversive behaviors37. This conclusion aligns with the increased grooming behavior observed in the TTM mice model under aversive stimuli and the significant increase in relative CREB expression in the NAc. In addition, we detected for the first time the interaction between SAPAP3 and SHANK proteins in neurons, offering new perspectives on Sapap3’s synaptic role. SHANK proteins, critical for postsynaptic density organization6showed altered expression in KO mice, potentially representing compensatory responses to maintain synaptic integrity. While colocalization and interaction between SAPAP3 and SHANK were confirmed, the functional consequences of SHANK up-regulation in compensating for Sapap3 deficiency warrant deeper investigation.
Based on the above experimental results and literature evidence, we draw the following conclusions: (1) Neuronal activity and hair grooming behavior: Sapap3 KO mice exhibit long-duration, low-frequency abnormal hair grooming behavior, which is related to the decreased intensity and efficiency of neuronal activity, suggesting the neural activity basis of TTM hair grooming disorder. (2) Sapap3 and neurotransmitter levels: Sapap3 indirectly affects DA levels rather than Glu, indicating that it may participate in hair grooming behavior by regulating DA release, reuptake, or metabolism. (3) NAc neural regulatory pathway: Sapap3 deficiency disrupts the balance of neuronal activity in the direct/indirect pathways of NAc, possibly related to multiple behavioral defects such as hair grooming behavior, anxiety-like manifestations, and social function abnormalities. (4) Sapap3 and CREB association: The increased expression of CREB in the NAc region is correlated with aversive behavior and exacerbated hair grooming, suggesting that Sapap3 deficiency may regulate the excitability of MSNs through CREB. This finding is consistent with the research on the role of CREB in OCD-related circuits38. However, it must be emphasized that the mechanism of action in this study remains a hypothetical conclusion, and the functional significance of CREB (such as the recovery effect of overexpression/knockdown) needs to be clarified through subsequent genetic manipulation experiments. (5) Limitations of SHANK compensation: SHANK up-regulation suggests a potential compensatory mechanism, but it is still unclear whether this response can alleviate the effect of Sapap3 deficiency. It needs to be verified through intervention experiments to confirm its functional importance. (6) Neural circuit hypothesis of aggressive behavior: The increase in aggressive behavior in KO mice may be directly related to the dopamine dysregulation in the NAc. According to reports, by specifically inducing ΔFosB in the D1-MSNs of the NAc, aggressive behavior can be enhanced39 so it is speculated that Sapap3 deficiency causes dopamine signal disorder in the NAc, that is, the excessive activation of D1 receptors ultimately leads to the aggravation of aggressive behavior. Subsequently, this hypothesis will be verified at the NAc neural circuit level through techniques such as optogenetics.
When comparing the grooming behavior of Sapap3 KO mice and those treated with oxytocin, we observed significant differences: Oxytocin treatment further prolonged the grooming duration of the KO mice40 although oxytocin reduced the number of grooming bouts, the contradictory increase in total duration challenged the traditional perception that oxytocin alleviates stereotyped behaviors41. The tube dominance experiment confirmed that oxytocin can improve the aggressive social behavior of KO mice (consistent with the literature on oxytocin alleviating aggressive behavior24. However, it is important to emphasize that these findings are limited to a single acute administration (dose: 10 mg/kg). Considering the complex dose-dependent nature of oxytocin in OCD models42 future studies must use dose-response curves and long-term administration paradigms to rule out potential regulatory effects and avoid over generalizing the current results to other scenarios.
Conclusions
TTM, an OCRD in DSM-5, remains enigmatic despite sharing neurobiology with other mental disorders. Our research offers insights into Sapap3’s role in TTM. Highlights include: (1) Clarify the key role of NAc behind TTM-like behaviors. (2) It is emphasized that the treatment of TTM with oxytocin requires meticulous consideration. (3) Determine potential drug targets through dopamine receptor system analysis. (4) Establishing SAPAP3-SHANK interactions as critical components of TTM pathophysiology. (5) Raising important questions about sex-dependent disease manifestations.
Although these findings may provide valuable research directions for the treatment of TTM, they must be interpreted in the context of current experimental limitations. The main limitations are: (1) The molecular and imaging analyses only used male cohorts, which prevented the elucidation of the regulatory mechanism of sex hormones on the SAPAP3-SHANK pathway. This limitation significantly hindered the translation of the research results to the clinical population of TTM with a higher incidence in females (a ratio of 10:1). (2) The sample sizes for core behavioral tests were small (males or females: n = 5/group), although they were in line with the conventional sample size range for rodent behavioral studies43 but statistical power (1.3) only detects > 62% effect sizes, which might weaken the ability to interpret behavioral phenotypes. (3) We actively excluded the conditioned place preference (CPP) experiment because the experimental design of this experiment had a fundamental interpretational dilemma (such as using human food odors instead of the familiar rewards for mice). Given that the CPP data neither reliably reflects the reward mechanism nor can it avoid introducing misleading conclusions, it has been excluded.
Data availability
We guarantee the authenticity of the data, but do not disclose the data, if necessary, you can email fjq7887215@163.com to obtain the data.
References
Grant, J. E. et al. Prevalence, gender correlates, and co-morbidity of trichotillomania. Psychiatry Res. 288, 112948. https://doi.org/10.1016/j.psychres.2020.112948 (2020).
Grant, J. E. et al. Sex differences in trichotillomania. Annals Clin. Psychiatry: Official J. Am. Acad. Clin. Psychiatrists Vol. 28 (2), 118–124 (2016).
Christensen, Rachel, E. et al. Recent advances in trichotillomania: a narrative review. Acta dermatovenerologica Alpina, Pannonica, et Adriatica vol. 32,4 : 151–157. (2023).
Grünblatt, E. Genetics of OCD and Related Disorders; Searching for Shared Factors. Current topics in behavioral neurosciences vol. 49 : 1–16. (2021). https://doi.org/10.1007/7854_2020_194
Lamothe, H. et al. Jan. The Sapap3–/– mouse reconsidered as a comorbid model expressing a spectrum of pathological repetitive behaviours. Translational psychiatry vol. 13,1 26. 30 (2023). https://doi.org/10.1038/s41398-023-02323-7
Cunha, C. et al. Dysregulation of DNA methylation during development as a potential mechanism contributing to obsessive compulsive disorders and autism. Neurology Neurobiol. 3(1), 2–4 (2020).
van den Boom, Bastijn, J. G. et al. Behavioral flexibility in a mouse model for obsessive-compulsive disorder: Impaired Pavlovian reversal learning in SAPAP3 mutants. Genes, brain, and behavior vol. 18,4 : e12557. (2019). https://doi.org/10.1111/gbb.12557
Corbit, Victoria, L. et al. Strengthened inputs from secondary motor cortex to striatum in a mouse model of compulsive behavior. J. Neuroscience: Official J. Soc. Neurosci. Vol. 39 (15), 2965–2975. https://doi.org/10.1523/JNEUROSCI.1728-18.2018 (2019).
Boardman, L. et al. Investigating SAPAP3 variants in the etiology of obsessive-compulsive disorder and trichotillomania in the South African white population. Comprehensive Psychiatry 52,2 (2011): 181–187. https://doi.org/10.1016/j.comppsych.2010.05.007
Welch, J. M. et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nat. Vol. 448, 894–900. https://doi.org/10.1038/nature06104 (2007).
Wilson, C. et al. Experience-dependent grooming microstructure alterations and Gastrointestinal dysfunction in the SAPAP3 knockout mouse model of compulsive behaviour. J. Affect. Disord. 363, 520–531. https://doi.org/10.1016/j.jad.2024.07.143 (2024).
Narayanaswamy, J. C. et al. Neuro-hemodynamic endophenotypes of emotional interference in OCD: fMRI study using emotion counting Stroop task. Asian J. Psychiatry. 39, 35–41. https://doi.org/10.1016/j.ajp.2018.11.015 (2019).
Hsieh, H. J. et al. L-3,4-Dihydroxy-6-[F-18]fluorophenylalanine positron emission tomography demonstrating dopaminergic system abnormality in the brains of obsessive-compulsive disorder patients. Psychiatry Clin. Neurosciences 68,4 (2014): 292–298. https://doi.org/10.1111/pcn.12139
Perreault, Melissa, L. et al. Heteromeric dopamine receptor signaling complexes: emerging neurobiology and disease relevance. Neuropsychopharmacology: Official Publication Am. Coll. Neuropsychopharmacology 39,1 (2014): 156–168. https://doi.org/10.1038/npp.2013.148
Bertran-Gonzalez, J. et al. Restoring the youthful state of striatal plasticity in aged mice re-enables cognitive control of action. Curr. Biology: CB Vol. 33, 1997–2007e5. https://doi.org/10.1016/j.cub.2023.04.020 (2023).
Mitra, S. et al. Postpartum Lactation-Mediated behavioral outcomes and drug responses in a spontaneous mouse model of Obsessive-Compulsive disorder. ACS Chem. Neurosci. Vol. 8 (12), 2683–2697. https://doi.org/10.1021/acschemneuro.7b00231 (2017).
Liu, Y. et al. Brain functional specialization in obsessive-compulsive disorder associated with neurotransmitter profiles. J. Affect. Disord. 329, 477–482. https://doi.org/10.1016/j.jad.2023.02.146 (2023).
Ducasse, D. et al. D2 and D3 dopamine receptor affinity predicts effectiveness of antipsychotic drugs in obsessive-compulsive disorders: a metaregression analysis. Psychopharmacology vol. 231,18 (2014): 3765-70. https://doi.org/10.1007/s00213-014-3516-3
Chamberlain, S. R. et al. A neuropsychological comparison of obsessive-compulsive disorder and trichotillomania. Neuropsychologia vol. 45,4 : 654 – 62. (2007). https://doi.org/10.1016/j.neuropsychologia.2006.07.016
White, M. P. et al. Disordered reward processing and functional connectivity in trichotillomania: a pilot study. J. Psychiatric Res. Vol. 47, 1264–1272. https://doi.org/10.1016/j.jpsychires.2013.05.014 (2013).
Ghosal, S. et al. Mitofusin-2 in nucleus accumbens D2-MSNs regulates social dominance and neuronal function. Cell. Rep. Vol. 42, 112776. https://doi.org/10.1016/j.celrep.2023.112776 (2023).
Kravitz, Alexxai, V. et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nat. Vol. 466, 622–626. https://doi.org/10.1038/nature09159 (2010).
Keebaugh, A. C., Larry, J. & Young Increasing Oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm. Behav. Vol. 60 (5), 498–504. https://doi.org/10.1016/j.yhbeh.2011.07.018 (2011).
Zhou, H. et al. Neuropeptides affecting social behavior in mammals: Oxytocin. Peptides vol. 177 : 171223. (2024). https://doi.org/10.1016/j.peptides.2024.171223
Cappi, C. et al. Nov. Epigenetic evidence for involvement of the oxytocin receptor gene in obsessive-compulsive disorder. BMC neuroscience vol. 17,1 79. 30 (2016). https://doi.org/10.1186/s12868-016-0313-4
Inayat, S. et al. Weak-hyperactive hippocampal CA1 neurons in the prodromal stage of alzheimer’s disease in hybrid AppNL–G–F/NL–G–F × Thy1-GCaMP6s+/– mice suggest disrupted plasticity. Neurobiol. Aging. 130, 154–171. https://doi.org/10.1016/j.neurobiolaging.2023.06.002 (2023).
Lochner, C. et al. Gender in obsessive-compulsive disorder: clinical and genetic findings. Eur. Neuropsychopharmacology: J. Eur. Coll. Neuropsychopharmacol. Vol. 14 (2), 105–113. https://doi.org/10.1016/S0924-977X(03)00063-4 (2004).
Grant, J. E., Samuel, R., Chamberlain & Trichotillomania Am. J. Psychiatry Vol 173,9 : 868–874. doi:https://doi.org/10.1176/appi.ajp.2016.15111432 (2016).
Christenson, G. A. et al. Adult men and women with trichotillomania. A comparison of male and female characteristics. Psychosom. Vol. 35 (2), 142–149. https://doi.org/10.1016/s0033-3182(94)71788-6 (1994).
Lochner, C. et al. Chronic hair-pulling: phenomenology-based subtypes. J. Anxiety Disorders Vol. 24 (2), 196–202. https://doi.org/10.1016/j.janxdis.2009.10.008 (2010).
Gehlenborg, J. et al. Implicit aggressive self-concept in patients with obsessive-compulsive disorder: results from an approach-avoidance task. J. Behav. Ther. Exp. Psychiatry. 83, 101927. https://doi.org/10.1016/j.jbtep.2023.101927 (2024).
Overbeek, T. et al. Comorbidity of obsessive-compulsive disorder and depression: prevalence, symptom severity, and treatment effect. J. Clin. Psychiatry Vol. 63, 1106–1112. https://doi.org/10.4088/jcp.v63n1204 (2002).
Carlson, Emily, J. et al. Neurocognitive test performance in relation to symptom severity and age of onset of trichotillomania. J. Obsessive-Compulsive Relat. Disorders. 42.000, 6 (2024).
Ramírez-Armenta, Kathia, I. et al. Optogenetic Inhibition of indirect pathway neurons in the dorsomedial striatum reduces excessive grooming in Sapap3-knockout mice. Neuropsychopharmacology: Official Publication Am. Coll. Neuropsychopharmacol. Vol. 47 (2), 477–487. https://doi.org/10.1038/s41386-021-01161-9 (2022).
Lian, Y. N. et al. The role of glutamate and its receptors in central nervous system in stress-induced hyperalgesia. Int. J. Neurosci. Vol. 128 (3), 283–290. https://doi.org/10.1080/00207454.2017.1387112 (2018).
Manning, E. E. et al. May. Disruption of prepulse inhibition is associated with compulsive behavior severity and nucleus accumbens dopamine receptor changes in Sapap3 knockout mice. Scientific reports vol. 11,1 9442. 3 (2021). https://doi.org/10.1038/s41598-021-88769-5
Zhai, Q. L. et al. Perampanel ameliorates nitroglycerin-induced migraine through Inhibition of the cAMP/PKA/CREB signaling pathway in the trigeminal ganglion in rats. Korean J. Pain Vol. 36 (3), 335–346. https://doi.org/10.3344/kjp.23039 (2023).
Rohbani, K. et al. Parental morphine exposure affects repetitive grooming actions and marble burying behavior in the offspring: potential relevance for obsessive-compulsive like behavior. Eur. J. Pharmacol. 865, 172757. https://doi.org/10.1016/j.ejphar.2019.172757 (2019).
Aleyasin, H. et al. Cell-Type-Specific role of ∆fosb in nucleus accumbens in modulating intermale aggression. J. Neuroscience: Official J. Soc. Neurosci. Vol. 38, 5913–5924. https://doi.org/10.1523/JNEUROSCI.0296-18.2018 (2018).
Drago, F. et al. Oxytocin potently enhances novelty-induced grooming behavior in the rat. Brain Res. Vol. 368 (2), 287–295. https://doi.org/10.1016/0006-8993(86)90573-1 (1986).
Dai, Y. C. et al. Oct. Neonatal Oxytocin Treatment Ameliorates Autistic-Like Behaviors and Oxytocin Deficiency in Valproic Acid-Induced Rat Model of Autism. Frontiers in cellular neuroscience vol. 12 355. 9 (2018). https://doi.org/10.3389/fncel.2018.00355
Leckman, J. F. et al. The role of central oxytocin in obsessive compulsive disorder and related normal behavior. Psychoneuroendocrinology vol. 19,8 : 723 – 49. (1994). https://doi.org/10.1016/0306-4530(94)90021-3
Maravet Baig, K. et al. Mice deficient in AKAP13 (BRX) develop compulsive-like behavior and increased body weight. Brain Res. Bull. 140, 72–79. https://doi.org/10.1016/j.brainresbull.2018.04.005 (2018).
Funding
The study was funded by Supported by the National Natural Science Foundation of China (Grant No. 82460277) and Ningxia Medical University’s scientific research project (Grant No. XT2024031).
Author information
Authors and Affiliations
Contributions
Conceptualization: Jianqun Fang. Formal analysis: Yuan Wang, Jingjing Yu, Yundong Chen. Visualization: Yuan Wang, Jingjing Yu, Rui Ma, Yundong Chen. Writing—original draft: Yuan Wang, Jingjing Yu, Rui Ma and Shihao Lu. Writing—Review & Editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Yu, J., Ma, R. et al. Exploring the nucleus accumbens circuit and oxytocin therapy in a Sapap3 knockout mouse model of trichotillomania. Sci Rep 15, 28492 (2025). https://doi.org/10.1038/s41598-025-14076-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14076-y