Abstract
To investigate the mechanism and potential targets of Yiqi Ziyin (YQZY) for treating immune thrombocytopenia (ITP). Prednisone and YQZY were orally administered to ITP mice. Post-treatment, blood samples were taken to evaluate the blood picture. The spleen was obtained to observe histomorphological changes and calculate the spleen index. The active components and predicted related targets of YQZY were obtained based on the TCMSP database. The ITP-related targets were screened by GeneCards and DisGeNET databases. Then, functional enrichment analyses were conducted to examine the potential regulatory pathways of YQZY in ITP, which was validated by western blot assay. Additionally, three different networks were constructed to identify the predominant targets and ingredients of YQZY in the ITP treatment, followed by molecular docking analysis. YQZY significantly upregulated platelet counts and improved the other blood index abnormalities caused by anti-platelet serum injection. Totally 60 active ingredients were acquired, and 85 common targets of active ingredients and ITP. The PI3K-Akt pathway was the consistent pathway of functional enrichment analyses. Western blot assay showed that the protein levels in the prednisone + YQZY group had a tendency to the normal group. Finally, CASP3 and TNF were identified as the core targets that had strong binding to the active ingredients. YQZY might be a potential alternative approach in treating ITP through the PI3K-Akt pathway.
Similar content being viewed by others
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disease characterized by enhanced platelet destruction and impaired platelet production, leading to a low platelet level, rendering a bleeding risk1. Its incidence is approximately 2–5/100,000 annually in the general population, which is higher in females than in males according to the guidelines issued by the American Society of Hematology2. The pathogenesis of ITP is not completely clear, with a complicated process. A major ITP trigger is the action of autoantibodies to immunoglobulin G on platelets, resulting in the formation of antigen-antibody complexes that are involved in phagocytosis and destruction by macrophages in the spleen via the Fc receptor3. However, anti-platelet antibodies can be detected in 50%‐70% of patients, so a negative result does not rule out a diagnosis4. ITP patients are at increased risk of bruising, bleeding on the skin, and rare severe bleeding, including intracranial hemorrhage5. Besides, the physical and psychological symptoms seriously affected the ITP patients’ quality of life6. Pharmaceutical medicines such as prednisone served as the standard therapy to increase platelet counts and minimize bleeding events for ITP patients7. Nevertheless, sole use of these drugs might be accompanied by harmful side effects since ITP is a chronic disease that requires long-term treatment8.
Traditional Chinese medicine (TCM) as a complementary and auxiliary strategy has been successfully applied for treating immune-related diseases, including ITP9. ITP has been named “purpura disease”, which was differentiated as “spleen deficiency” in TCM. Yiqi Ziyin (YQZY) is a Chinese formula developed according to the TCM syndrome analysis of ITP. Qi represents the energy or essential source that is disrupted in disease. Although there is no similar scientific concept in the West, in the issue of Blood, Ni et al. report the use of the hypomethylating agent decitabine to rebalance energy metabolism through the LKB1/AMPK pathway, which enhances immune platelet counts10. The YQZY includes herbs such as Astragalus membranaceus (Fisch.) Bunge (Huangqi in Chinese, HQ), Rubia cordifolia L. (Qiancao in Chinese, QC), Pseudostellaria heterophylla (Miq.) Pax ex Pax et Hoffm. (Taizishen in Chinese, TZS), Lithospermum erythrorhizon Siebold & Zucc. (Zicao in Chinese, ZC), and Phragmites australis (Cav.) (Ganlugen in Chinese, GLG). A previous study has shown that platelet counts were upregulated and symptoms of ITP patients were improved upon the administration of Yiqi Tongyang Decoction, which might be related to regulating T lymphocyte levels11. In addition, Shengxuexiaoban is a widely used capsule in ITP treatment, which increased the level of CD4 + Treg cells in the peripheral blood of ITP patients12. In clinical practice, YQZY has been shown to alleviate bleeding symptoms with sustained therapeutic effects, while also reducing the adverse reactions associated with first- and second-line treatments for ITP, and it has become one of the emerging focal points in the research of novel clinical therapeutic strategies13. However, the underlying mechanism of YQZY in treating ITP is still unclear.
In this study, the therapeutic effect of prednisone and YQZY on ITP was examined by establishing the ITP mouse model. Then, a network pharmacology approach was adopted to investigate the mechanism of action and molecular targets of YQZY for the ITP treatment. We first identified the active ingredients of YQZY and their related targets, followed by obtaining the ITP-associated targets. The functional enrichment analyses were performed to explore the possible mechanisms, which were validated by the western blot assay. Finally, the molecular docking analysis was performed to verify the interaction between active ingredients and hub targets.
Materials and methods
Animals
BALB/c mice (aged 8 weeks; weighted ~ 18–22 g) and guinea pigs (weighted 340 ± 10 g) were purchased from Zhejiang Academy of Medical Sciences (Zhejiang, China). The mice were kept in an individually ventilated cage (IVC) system at constant temperature (26 ℃) and humidity. All experiment procedures involving animals complied with the ARRIVE guidelines and were approved by the Institutional Animal Care and Use Committee, ZJCLA (Approval No. ZJCLA-IACUC-20010554). All experiments were performed in accordance with relevant guidelines and regulations. Our study was conducted following the Declaration of Helsinki.
ITP mice model establishment and treatment
Whole blood was collected from the BALB/c mice into an EDTA/Na2 test tube, which was then centrifuged at 800 rpm-min–1 for 10 min. After that, the platelets were obtained to form water-in-oil form as an antigen, which was injected subcutaneously into the plantar and dorsal areas of guinea pigs, at least 4 points each time, 150 µL each point. In the 6th week, the non-anticoagulant whole blood taken from the guinea pig heart was centrifuged at 3000 rpm-min− 1 for 20 min to obtain anti-platelet serum (GP-APS). The APS was removed from the refrigerator and placed in a water bath. An equal volume of 5% red blood cell suspension from BALB/c mice was added to GP-APS. Saline was diluted with GP-APS at a concentration of 1:4.
The mice were randomly divided into the normal healthy control group (n = 6), the prednisone group (n = 8), and the prednisone + YQZY combination group (n = 8). Healthy mice without any treatment served as the normal group. The prednisone group and the combination group were injected with GP-APS (100 µL each) on days 1, 3, 5, 7, 9, 11, and 13 to induce chronic and persistent thrombocytopenia, and the prednisone (2 mg/mL) and YQZY decoction (1.325 g/kg, showing best effects in preliminary experiments) was administrated from day 8 and lasted for 10 days.
The general condition of the mice
The activity, mental status, skin conditions, and weight were recorded.
Hematological analysis
The anticoagulant whole blood was collected with an EDTA anticoagulant tube, and the optimal concentration ratio of EDTA to blood was 1.5 mg/ml, and then was subjected to the Mindary automatic blood cell analyzer (BC-2800vet) for detailed hematological analysis.
Hematoxylin-eosin (HE) staining
After the experiment, isoflurane was used for Euthanasia, and the spleen of the mice was taken and weighed. First, the paraffin section was put into xylene, anhydrous ethanol, and 75% alcohol before washing with tap water. For HE staining, the hematoxylin was stained for 3–5 min, washed with tap water, differentiated with differentiation solution, washed with tap water, counterstained with blue, and rinsed with running water. Then, the sections were dehydrated with 85% and 95% gradient alcohol for 5 min each, and were stained in an eosin staining solution for 5 min. Thereafter, the slices were placed for 5 min each in absolute ethanol I, absolute ethanol II, absolute ethanol III, dimethyl I, and xylene II in turn to be transparent, and the slices were sealed with neutral gum. Microscopic examination, image collection, and analysis were performed.
Active ingredients in YQZY
Drugs are usually taken orally in clinical treatment. Drug likeness (DL) and oral bioavailability (OB) are considered the major parameters affecting drug absorption in the gastrointestinal mucosa14. We retrieved effective components of YQZY in the Traditional Chinese Medicine System Pharmacology (TCMSP) (https://tcmsp-e.com/tcmsp.php) by searching “Huangqin”, “Taizishen”, “Zicao”, “Qiancao”, and “Ganlugen”, with DL ≥ 0.18 and OB ≥ 30% as the threshold15. The molecular structure, structural information, and Canonical SMILES of the active ingredients were acquired from PubChem (https://pubchem.ncbi.nlm.nih.gov/).
Targets of the active ingredients
The targets of the active ingredients in YQZY were predicted through the Swiss Target Prediction database (http://www.swisstargetprediction.ch/index.php) using their structure data, with the sapiens was “homo sapiens”. All the targets were standardized in UniProt (https://www.uniprot.org/).
Targets related to ITP
The ITP-related genes were obtained from GeneCards (https://www.genecards.org/) and DisGeNET (https://www.disgenet.org/home/) databases. Using “immune thrombocytopenia” as the keyword, ITP-related targets obtained from these databases were 545 and 338, respectively, which were then standardized in UniProt.
Functional enrichment analysis
Gene ontology (GO) annotation describes the characteristics of the genes from cellular component (CC), molecular function (MF), as well as biological process (BP). Kyoto Encyclopedia of Genes and Genomes (KEGG)16,17,18 is a comprehensive database integrating genomic, chemical, and system functions (www.kegg.jp/kegg/kegg1.html). GO and KEGG analyses were conducted using the clusterProfiler in the R package.
Network construction and visualization
The common targets of YQZY and ITP were obtained through Venn analysis (http://bioinformatics.psb.ugent.be/webtools/Venn/). The protein-protein interaction (PPI) of the common targets was predicted in the String database (https://string-db.org/) by inputting “homo sapiens” in the Organism column. A PPI network of common targets, an active ingredients-common targets network, and a disease-herbs-active ingredients-hub genes network were constructed and visualized through Cytoscape (version 3.8.0), which is a tool for analysis and visualization of biological networks. In accordance with the network topology, betweenness centrality (BC) and degree centrality (DC) are the vital parameters to assess node criticality in network. DC reflects the number of direct interactions a node has, indicating its potential influence within the network, while BC measures the extent to which a node lies on the shortest paths between other nodes, representing its role in information flow and network control. Nodes with higher quantitative values typically exhibit stronger regulatory or functional importance in biological systems19. In our analysis, BC > 10 or DC ≥ 40 were adopted as the threshold to identify hub genes within the PPI network.
Molecular docking analysis
Using the RCSB PDB database (http://www.rcsb.org/), we downloaded the 3D structure file of the hub targets, and the chemical structure file of active ingredients was obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/). Then, both the structure files were prepared by AutoDock Tools 1.5.6 software before the molecular docking process, including removing water molecules and adding hydrogen atoms, which were subsequently saved as PDBQT format files for later use. The binding energy (affinity) for molecular docking was taken as the final result and visualized by PyMOL software. Meanwhile, the binding energy ≤ − 5.0 kcal/mol was used as a criterion to evaluate the binding performance of the ligands and receptors20.
Western blot analysis
Totally 50 µg in each sample was boiled for 3 min in SDS-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and subjected to SDS-PAGE using 10% running gels and transferred onto PVDF membranes. The membranes were blocked with 5% BSA for 2 h at room temperature and then incubated overnight at 4 °C with primary antibodies. The primary antibodies included in this study were anti-BAX (ET1603-34, HUABIO), anti-BCL-2 (ET1702-53, HUABIO), anti-p-PI3K (4228 S, HUABIO), anti-PI3K (ET1608-70, HUABIO), anti-p-AKT (ET1607-73, HUABIO), anti-AKT (ET1609-51, HUABIO), anti-p-ERK (4370 S, CST), anti-ERK (ET1601-29, HUABIO), anti-IKBKB (ET1611-23, HUABIO), and anti-β-actin (20536-1-AP, Proteintech). An HRP-conjugated antibody was used as the secondary antibody. Bands were visualized using the enhanced chemiluminescence method.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 9.0. The ANOVA was used to analyze the differences among at least three groups, followed by Tukey’s HSD post-hoc test for pairwise comparisons. P < 0.05 was considered statistically significant.
Results
Effect of prednisone and YQZY on the general condition of the mice
Before modeling, mice in each group had smooth hair and were active and flexible. After 8 days of modeling, the mice in the control group had a good mental state and a normal diet. In the prednisone group, the mice were in a poor state, with listlessness and hair loss around the lips, some of which lost weight, and two died. In the combination group, mice exhibited slow movement and drowsiness, as well as scattered and erect hair, and a reduction in hair around the lips.
Effect of prednisone and YQZY on spleen index
To explore the influence of drugs on spleen index, prednisone and YQZY were administered to the ITP mice for 10 consecutive days. At the end of the experiment, the mice were sacrificed, and the spleens were removed and weighed. The spleen index (spleen coefficient = spleen weight/body weight) was calculated (Table S1). The spleen was then fixed with formaldehyde, embedded in paraffin, sectioned, and stained with HE to observe the morphological changes. Compared with the normal control group, the spleen of the model group was enlarged, and the spleen index was significantly increased. Whereas, no remarkable between-group differences were observed in terms of body weight (Fig. 1A and C). The normal spleen tissue had clear tissue structure with clear capsule, red pulp, and white pulp (Fig. 1D). Compared with the prednisone group, the structure and morphology of white and red pulp in the combination group began to improve, and the lymphocyte infiltration and megakaryocyte abnormality were significantly improved (Fig. 1E and F).
Effect of prednisone and Yiqi Ziyin on the spleen. (A) The spleen of the mice in three groups. (B) The weight of mice in the three groups. (C) The spleen index in the three groups. The histomorphological observations of spleen in (D) normal group, (E) PDS group, and (F) PDS + YQZY group. PDS, prednisone; YQZY, Yiqi Ziyin.
Effect of prednisone and YQZY on the blood picture
To evaluate the impact of drugs on blood picture, platelets, white blood cells (WBC), hemoglobin (HB), and red blood cells (RBC) were measured. Compared with the control group, the number of platelets in the model treatment group was significantly reduced, suggesting that the ITP model was successfully established. On day 3 after treatment, there were no significant differences in WBC, HB, and RBC among the three groups (Fig. 2A). On day 10 after treatment, the number of platelets increased, which was significantly higher in the combination group compared to the prednisone group (P < 0.05). Besides, the combination group significantly improved the peripheral blood WBC, HB, and RBC disorders caused by GP-APS injection, making these indices tend to be normal. Notably, the combination group showed a more pronounced effect than the prednisone group (Fig. 2B).
Common targets of active ingredients and ITP
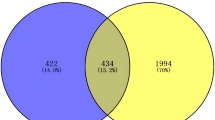
The mouse experiments have demonstrated that YQZY plays an essential role in treating IPT. Therefore, we conducted a systematic pharmacological study to reveal the therapeutic mechanism of YQZY for ITP21. First, we retrieved 60 active ingredients in the YQZY from the TCMSP database according to the selection criteria, of which 20 were from HQ, 8 from TZS, 12 from ZC, 19 from QC, and 1 from GLG (Table S2). By screening the Swiss Target Prediction database, 744 targets associated with the active ingredients in the YQZY were obtained (Table S3). Through GeneCards and DisGeNET databases, we acquired 689 ITP-related targets (Table S4). Following this, the Venn analysis revealed 85 common targets between ITP and active ingredients of YQZY (Figure S1).
Functional enrichment analysis of ITP-related genes
To investigate the underlying mechanism of ITP, the GO and KEGG analyses of ITP-related genes were conducted. In terms of BP, these genes were mainly involved in defense response, and response to biotic stimulus (Fig. 3A). The major CCs were cell surface, intrinsic component of plasma membrane, and side of membrane (Fig. 3B). As for MFs, they mainly participated in signaling receptor binding, identical protein binding, and receptor regulator activity (Fig. 3C). The primary KEGG pathways included PI3K-Akt pathway, Th17 cell differentiation, Th1 and Th2 cell differentiation, and apoptosis (Fig. 3D).
Functional enrichment analysis of common genes
To further examine the mechanism of YQZY in treating ITP, enrichment analysis was performed on the 85 common targets. As shown in Fig. 4A, the major BPs were cell proliferation, apoptosis, and regulation of response to stress. The main CCs were cell surface, chromatin, and side of membrane (Fig. 4B). The primary MFs were enzyme binding, identical protein binding, and signaling receptor binding (Fig. 4C). As for KEGG terms, they were involved in PI3K-Akt pathway, c-type lectin receptor signaling pathway, influenza A, TNF signaling pathway, and HIF-1 signaling pathway (Fig. 4D). Combined with the previous KEGG results of the ITP-related genes, the PI3K-Akt pathway shared the same regulatory pathway.
Hub genes identification
To identify the hub targets of YQZY in ITP treatment, the 85 common targets were imported into the String database to construct a PPI network, and the results were visualized in the Cytoscape software. The network has 85 nodes and 795 edges. By setting DC ≥ 40 as the threshold, we finally obtained 9 hub genes, including TNF, ALB, STAT3, TP53, MMP9, IL2, CASP3, HIF1A, and PTPRC (Fig. 5).
Active ingredients-common targets network construction
To obtain the vital active ingredients in YQZY, the active ingredients-common targets were constructed. The 85 common targets were related to 31 active ingredients (Table S5). The interaction network with 116 nodes and 85 edges was exhibited in Fig. 6. Based on the BC value > 10, MOL000438 ((3R)-3-(2-hydroxy-3,4-dimethoxyphenyl)chroman-7-ol), MOL000398 (isoflavanone), MOL000449 (Stigmasterol), MOL000380 ((6aR,11aR)-9,10-dimethoxy-6a,11a-dihydro-6 H-benzofurano[3,2-c]chromen-3-ol), MOL000417 (Calycosin), MOL002464 (1-Monolinolein), and MOL006139 (1,3-dimethoxy-2-carboxyanthraquinone) were defined as vital active ingredients.
Disease-herbs-active ingredients-hub genes network construction
Subsequently, the disease-herbs-active ingredients-hub genes network was constructed according to the effective components directly associated with the hub targets (Fig. 7). The network contains 39 nodes (1 disease, 9 hub genes, 25 active ingredients, and 4 herbs) and 73 edges. Among the 25 active ingredients, MOL000380, MOL000417, MOL000438, MOL000398, and MOL006139 are also the vital ingredients (with BC value > 10) generated by the active ingredients-common targets network, which were considered as pivotal ingredients for YQZY in the treatment of ITP.
Active ingredients and hub targets interaction
The 5 essential active ingredients and 9 hub targets were used as ligands and receptors, respectively. Table 1 represents the molecular docking results. The stability of ligands and receptors is determined by the binding energy, which is more stable with the lower binding energy. Thus, the ligands with the lowest binding energy to the receptor were screened out by the criteria (Fig. 8). Notably, (6aR,11aR)-9,10-dimethoxy-6a,11a-dihydro-6 H-benzofurano[3,2-c]chromen-3-ol demonstrated the strongest binding affinity to CASP3, with a binding energy of -7.66 kcal/mol, indicating a highly stable interaction (Fig. 8A). The binding energy of (3R)-3-(2-hydroxy-3,4-dimethoxyphenyl)chroman-7-ol to CASP3 (Fig. 8B) and Calycosin to TNF (Fig. 8C) was − 5.61 and − 5.04 kcal/mol, respectively. The above findings revealed a satisfactory binding performance of the active ingredients to protein targets.
Validation of yqzy’s effect on PI3K-Akt pathway
In the above analysis, we found that the therapeutic effects of YQZY on ITP may involve the PI3K-Akt signaling pathway. To further validate this association, we examined the impact of YQZY on the expression levels of proteins within the PI3K-Akt pathway. As shown in Fig. 9, compared to the prednisone group, the combination treatment group exhibited higher levels of BAX, p-Akt, Akt, p-ERK, and ERK, with some protein levels approaching those observed in the normal group. No significant differences were observed in other proteins among the three groups. These findings further suggest that YQZY may exert its therapeutic effects on ITP by modulating the PI3K-Akt signaling pathway.
Discussion
Herein, we established the ITP mice model and explored the impact of prednisone and YQZY on the mice with regard to the general condition, organ index, and blood picture. After exhibiting the potential robust value of YQZY in treating ITP, we used a network pharmacology approach to investigate the underlying mechanism of YQZY in the ITP treatment and identified the hub targets as well as pivotal active ingredients, which were validated by the molecular docking analysis.
ITP is an autoimmune hemorrhagic disease caused by the failure of the spleen to manage blood with reduced platelet counts and increased bleeding tendency, including petechiae in the skin or epistaxis22. In addition, ITP patients may also present with fatigue, dry cough, or shortness of breath23. Zhang et al. previously demonstrated that ITP mice exhibited decreased physical performance and peripheral platelet counts, but increased spleen index24. Similarly, we found that the physical and mental status of the ITP mice was poor, and the spleen index was upregulated. The mice in the PDS + YQZY group presented better performance than those in the PDS group, but no significant difference in spleen index was observed between the two groups. The combination group significantly improved the peripheral blood WBC, HB, and RBC disorders caused by GP-APS injection, making these indices tend to be normal, revealing the significance of YQZY in the ITP treatment.
The combination of two or more compatible TCM usually improves clinical efficacy through synergies. ITP is characterized by bleeding and blood deficiency; thus, hemostasis and blood enrichment are required for the treatment of ITP. We used five herbs in the YXZY decoction, including HQ, QC, TZS, ZC, and GLG, to supplement Qi and nourish Yin. As one of the promising and widely-used TCM to invigorate Qi, HQ regulates the immune system and elevates energy levels25. QC contributes to stopping bleeding, eliminating stasis, and cooling blood26. ZC possesses biological activities such as anti-tumor, anti-bacterial, and anti-inflammatory effects27. TZS contributes to replenishing Qi, benefiting blood, and invigorating the spleen, which has been a commonly used medicine in treating spleen asthenia, fatigue, and cough28. GLG clears away heat and fire, produces fluid to quench thirst, and eliminates irritability29. Therefore, the YQZY decoction as an auxiliary method to treat ITP might contribute to supplementing Qi, controlling bleeding, and alleviating fatigue through the synergistic effects of these herbs. Through data mining, 60 active ingredients were retrieved in the YQZY. The targets associated with active ingredients and ITP were 744 and 689, respectively.
Following this, the functional enrichment analysis results showed that ITP-related genes were involved in the PI3K-Akt pathway, Th17 cell differentiation, Th1 and Th2 cell differentiation, and apoptosis. The common targets participated in the PI3K-Akt pathway, HIF-1 signaling pathway, and TNF signaling pathway. T lymphocytes, such as Th17, Tregs, and Th1/Th2 cells, were closely associated with the progression of autoimmune diseases, including ITP30,31. Th1 cells mediate cellular immunity and cytotoxicity, mainly featured with IFN-gamma, TNF-alpha, and IL-2 secretion. Th2 cells stimulate B cell proliferation and antibody production by secreting cytokines such as IL-4 and IL-1031. Moreover, HIFs dysregulation increases reactive oxygen species that are dramatically downregulated under the conditions of fatigue32,33. Notably, the PI3K-Akt pathway was the common pathway that regulated the ITP-related targets and common targets. The study revealed that the PI3K-Akt pathway was essential in balancing the innate immune response34. Platelet autophagy regulated by this pathway might inhibit platelet apoptosis and improve platelet viability, prolonging the life span of platelets from patients with ITP35. These indicate that YQZY might treat ITP to increase platelet counts and improve diverse symptoms such as fatigue and bleeding through the PI3K-Akt pathway. A previous study has demonstrated that inhibiting the PI3K-Akt pathway can suppress the overactivation and proliferation of CD4 + T cells in ITP, thereby restoring their cellular function36. Additionally, components of the YQZY, such as HQ, TZS, and ZC, have been shown to regulate CD4 + T cell differentiation37,38,39,40. We hypothesize that YQZY may improve immune balance in ITP patients by modulating CD4 + T cell differentiation through the PI3K-Akt signaling pathway.
Network construction and molecular docking indicated that CASP3, MMP9, TNF, and IL2 might be the core target genes, especially CASP3 and TNF. The active ingredients had strong binding to the CASP3 and TNF, with binding energy less than − 5.0 kcal/mol. As an execution protease, CASP3 is a primary apoptotic regulator responsible for platelet apoptosis in ITP41,42. TNF regulates various BPs such as coagulation, lipid metabolism, cell apoptosis, differentiation, and proliferation43. It’s noteworthy that TNF plays a crucial role in the regulation of the PI3K-Akt pathway44. According to Xin et al., the traditional Chinese medicine formula, Baixianfeng decoction, can inhibit the overexpression of the apoptosis-related protein caspase-3 by suppressing the TNF/PI3K/Akt pathway45. Importantly, the integration of network pharmacology and experimental approaches has proven to be an effective strategy for elucidating the mechanisms of multi-component herbal formulae, as demonstrated in previous studies46,47. In our study, we demonstrated through in vitro experiments that YQZY treatment affects the expression levels of apoptosis-related proteins and PI3K-Akt signaling-related proteins. This finding further suggests that YQZY may exert its therapeutic effects on ITP by modulating the PI3K-Akt pathway; however, additional in vivo and in vitro studies are needed to confirm this mechanism.
For strengths, this study innovatively combined network pharmacology, and molecular docking with in vivo and in vitro experiments to comprehensively explore the therapeutic effect of YQZY on ITP and its underlying mechanism. Several essential active ingredients and hub targets have been identified, but were not validated by experiments, which should be future research to provide novel insights into clinical drug development. However, this study also has certain limitations. The identification of active compounds and targets relied mainly on the TCMSP database, which may not cover newly discovered components, potentially leading to omissions. Future studies should consider integrating multiple databases to enhance coverage. Additionally, our experimental validation focused on the PI3K-Akt signaling pathway, while the effects of YQZY on other predicted pathways, such as HIF-1 and TNF signaling, require further investigation. Moreover, the predictive nature of network pharmacology may not fully account for pharmacokinetics and in vivo complexity, which could influence the interpretation of results.
In conclusion, YQZY might enhance the platelet count and improve ITP through modulating the PI3K-Akt pathway. The systemic pharmacology results might provide a direction for screening indicators of the follow-up clinical efficacy of YQZY and provide a scientific basis for YQZY in the ITP treatment.
Data availability
The dataset used and/or analyzed during the current study is available from the corresponding author (Li-qiang Wu) on reasonable request.
References
Lambert, M. P. & Gernsheimer, T. B. Clinical updates in adult immune thrombocytopenia. Blood 129, 2829–2835. https://doi.org/10.1182/blood-2017-03-754119 (2017).
Neunert, C. et al. American society of hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 3, 3829–3866. https://doi.org/10.1182/bloodadvances.2019000966 (2019).
Zufferey, A., Kapur, R. & Semple, J. W. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J. Clin. Med. 6, 856. https://doi.org/10.3390/jcm6020016 (2017).
Warner, M. N., Moore, J. C., Warkentin, T. E., Santos, A. V. & Kelton, J. G. A prospective study of protein-specific assays used to investigate idiopathic thrombocytopenic purpura. Br. J. Haematol. 104, 442–447. https://doi.org/10.1046/j.1365-2141.1999.01218.x (1999).
Lin, J. et al. Cost of Bleeding-related episodes in adult patients with primary immune thrombocytopenia: a Population-based retrospective cohort study of administrative claims data for commercial payers in the united States. Clin. Ther. 39, 603–609e601. https://doi.org/10.1016/j.clinthera.2017.01.023 (2017).
Lopez, M. F. et al. [Cost-per-responder analysis comparing Romiplostim to rituximab in the treatment of adult primary immune thrombocytopenia in spain]. Med. Clin. (Barc). 144, 389–396. https://doi.org/10.1016/j.medcli.2013.11.035 (2015).
Kuhne, T. Treatment of pediatric primary immune thrombocytopenia with thrombopoietin receptor agonists. Semin Hematol. 52, 25–30. https://doi.org/10.1053/j.seminhematol.2014.10.004 (2015).
Jiang, Y. et al. Elucidation of the mechanisms and molecular targets of Yiqi shexue formula for treatment of primary immune thrombocytopenia based on network Pharmacology. Front. Pharmacol. 10, 1136. https://doi.org/10.3389/fphar.2019.01136 (2019).
Liu, W. B., Li, S., Yu, X. L., Dai, T. Y. & Gao, R. L. Research progress on Chinese medicine Immunomodulatory intervention for chronic primary immune thrombocytopenia: targeting cellular immunity. Chin. J. Integr. Med. 25, 483–489. https://doi.org/10.1007/s11655-019-3031-9 (2019).
Ni, X. et al. Low-dose decitabine modulates myeloid-derived suppressor cell fitness via LKB1 in immune thrombocytopenia. Blood 140, 2818–2834. https://doi.org/10.1182/blood.2022016029 (2022).
Yang, X. P., Ma, R., Yang, X. H., Zhu, H. L. & Xu, Y. G. Effect of Yiqi Tongyang Decoction () on blood T cell subsets in patients with chronic immune thrombocytopenia. Chin. J. Integr. Med. 23, 709–713. https://doi.org/10.1007/s11655-016-2278-7 (2017).
He, Y. Z., Lu, R. F., Zhu, C. & Hua, J. Y. Qian five rhinoceros Gindeng (QFRG) protects against development of immune thrombocytopenia via miR-181a Inhibition of TLR-4 expression. Int. J. Clin. Exp. Med. 8, 6986–6993 (2015).
Zhou, Y. H. et al. Multi-central clinical research into treating 80 cases of chronic thrombocytopenia with qi-supplementing and yin-nourishing therapy and Western medicine. J. Tradit Chin. Med. 31, 277–281. https://doi.org/10.1016/s0254-6272(12)60004-1 (2011).
Li, X., Tang, H., Tang, Q. & Chen, W. Decoding the mechanism of Huanglian Jiedu Decoction in treating pneumonia based on network Pharmacology and molecular Docking. Front. Cell. Dev. Biol. 9, 638366. https://doi.org/10.3389/fcell.2021.638366 (2021).
Feng, W., Ao, H., Yue, S. & Peng, C. Systems Pharmacology reveals the unique mechanism features of Shenzhu capsule for treatment of ulcerative colitis in comparison with synthetic drugs. Sci. Rep. 8, 16160. https://doi.org/10.1038/s41598-018-34509-1 (2018).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592. https://doi.org/10.1093/nar/gkac963 (2023).
Xu, M., Shi, J., Min, Z., Zhu, H. & Sun, W. A network Pharmacology approach to uncover the molecular mechanisms of herbal formula Kang-Bai-Ling for treatment of vitiligo. Evid. Based Complement. Alternat Med. 2019, 3053458. https://doi.org/10.1155/2019/3053458 (2019).
Li, C. et al. A systems Pharmacology approach for identifying the multiple mechanisms of action for the Rougui-Fuzi herb pair in the treatment of cardiocerebral vascular diseases. Evid. Based Complement. Alternat Med. 2020, 5196302. https://doi.org/10.1155/2020/5196302 (2020).
Li, S. Network Pharmacology evaluation method Guidance - Draft. World J. Traditional Chin. Med. 7, 146–154. https://doi.org/10.4103/wjtcm.wjtcm_11_21 (2021).
Wang, M. J. et al. Mechanism and molecular targets of Ejiao Siwu Decoction for treating primary immune thrombocytopenia based on High-Performance liquid chromatograph, network pharmacology, molecular Docking and cytokines validation. Front. Med. (Lausanne). 9, 891230. https://doi.org/10.3389/fmed.2022.891230 (2022).
Miadlikowska, E. et al. Serum biomarkers of lung fibrosis in interstitial pneumonia with autoimmune Features-What do we already know?? J. Clin. Med. 11, 856. https://doi.org/10.3390/jcm11010079 (2021).
Zhang, Y. et al. Jianpi Yiqi shexue ameliorates immune thrombocytopenia related fatigue by regulating mitochondrial function. Ann. Palliat. Med. 10, 156–168. https://doi.org/10.21037/apm-20-2153 (2021).
Chen, Z. et al. Astragali radix (Huangqi): a promising edible Immunomodulatory herbal medicine. J. Ethnopharmacol. 258, 112895. https://doi.org/10.1016/j.jep.2020.112895 (2020).
Shan, M. et al. A review of the botany, phytochemistry, Pharmacology and toxicology of rubiae radix et rhizoma. Molecules 21, 856. https://doi.org/10.3390/molecules21121747 (2016).
Kang, T. K. et al. Roots of lithospermum erythrorhizon promotes retinal cell survival in optic nerve crush-induced retinal degeneration. Exp. Eye Res. 203, 108419. https://doi.org/10.1016/j.exer.2020.108419 (2021).
Hu, D. J., Shakerian, F., Zhao, J. & Li, S. P. Chemistry, Pharmacology and analysis of Pseudostellaria heterophylla: a mini-review. Chin. Med. 14, 21. https://doi.org/10.1186/s13020-019-0243-z (2019).
Ren, Y. et al. Traditional uses, phytochemistry, Pharmacology and toxicology of rhizoma phragmitis: A narrative review. Chin. J. Integr. Med. 28, 1127–1136. https://doi.org/10.1007/s11655-022-3572-1 (2022).
McKenzie, C. G., Guo, L., Freedman, J. & Semple, J. W. Cellular immune dysfunction in immune thrombocytopenia (ITP). Br. J. Haematol. 163, 10–23. https://doi.org/10.1111/bjh.12480 (2013).
Ji, X., Zhang, L., Peng, J. & Hou, M. T cell immune abnormalities in immune thrombocytopenia. J. Hematol. Oncol. 7, 72. https://doi.org/10.1186/s13045-014-0072-6 (2014).
Prabhakar, N. R., Peng, Y. J. & Nanduri, J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Invest. 130, 5042–5051. https://doi.org/10.1172/JCI137560 (2020).
Cheng, A. J. et al. Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J. Physiol. 594, 5149–5160. https://doi.org/10.1113/JP270650 (2016).
Rohani, M. G. et al. PAR1- and PAR2-induced innate immune markers are negatively regulated by PI3K/Akt signaling pathway in oral keratinocytes. BMC Immunol. 11, 53. https://doi.org/10.1186/1471-2172-11-53 (2010).
Wang, C. Y. et al. Enhancing autophagy protects platelets in immune thrombocytopenia patients. Ann. Transl Med. 7, 134. https://doi.org/10.21037/atm.2019.03.04 (2019).
Xu, P. et al. Atorvastatin restores imbalance of cluster of differentiation 4 (CD4)(+) T cells in immune thrombocytopenia in vivo and in vitro. Br. J. Haematol. 201, 530–541. https://doi.org/10.1111/bjh.17938 (2023).
Kang, H., Ahn, K. S., Cho, C. & Bae, H. S. Immunomodulatory effect of astragali radix extract on murine TH1/TH2 cell lineage development. Biol. Pharm. Bull. 27, 1946–1950. https://doi.org/10.1248/bpb.27.1946 (2004).
Song, Q., Kobayashi, T., Xiu, L. M., Hong, T. & Cyong, J. C. Effects of astragali root and hedysari root on the murine B and T cell differentiation. J. Ethnopharmacol. 73, 111–119. https://doi.org/10.1016/s0378-8741(00)00273-7 (2000).
Choi, Y. Y. et al. Immunomodulatory effects of Pseudostellaria heterophylla (Miquel) pax on regulation of Th1/Th2 levels in mice with atopic dermatitis. Mol. Med. Rep. 15, 649–656. https://doi.org/10.3892/mmr.2016.6093 (2017).
Oh, J. S., Lee, S. J. & Choung, S. Y. Lithospermum erythrorhizon alleviates atopic Dermatitis-like skin lesions by restoring immune balance and skin barrier function in 2.4-Dinitrochlorobenzene-Induced nc/nga mice. Nutrients 13, 563. https://doi.org/10.3390/nu13093209 (2021).
Jeelani, R. et al. Hypochlorous acid reversibly inhibits caspase-3: a potential regulator of apoptosis. Free Radic Res. 54, 43–56. https://doi.org/10.1080/10715762.2019.1694675 (2020).
Houwerzijl, E. J. et al. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with idiopathic thrombocytopenic purpura. Blood 103, 500–506. https://doi.org/10.1182/blood-2003-01-0275 (2004).
Nie, H. et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat. Med. 19, 322–328. https://doi.org/10.1038/nm.3085 (2013).
Gao, Y. et al. Systemic Pharmacological verification of Guizhi fuling Decoction in treating endometriosis-associated pain. J. Ethnopharmacol. 297, 115540. https://doi.org/10.1016/j.jep.2022.115540 (2022).
Wei, X. et al. Systemic Pharmacological verification of Baixianfeng Decoction regulating TNF-PI3K-Akt-NF-kappaB pathway in treating rheumatoid arthritis. Bioorg. Chem. 119, 105519. https://doi.org/10.1016/j.bioorg.2021.105519 (2022).
Li, S., Lu, A. P., Wang, Y. Y. & Li, Y. D. Suppressive effects of a Chinese herbal medicine qing-luo-yin extract on the angiogenesis of collagen-induced arthritis in rats. Am. J. Chin. Med. 31, 713–720. https://doi.org/10.1142/S0192415X03001430 (2003).
Zhang, S. et al. Deciphering the Pharmacological mechanisms of Guizhi-Fuling capsule on primary dysmenorrhea through network Pharmacology. Front. Pharmacol. 12, 613104. https://doi.org/10.3389/fphar.2021.613104 (2021).
Funding
This work was supported by the following grants: Science and Technology Programme for Traditional Chinese Medicine in Zhejiang Province (No. 2024ZL407).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: Jun Wang and Li-qiang Wu.(II) Collection and assembly of data: Jun Wang and Bo Wang.(III) Data analysis and interpretation: Jun Wang, Yun Zhang, Li-qiang Wu.(IV) Manuscript writing: All authors.(V) Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Institutional Animal Care and Use Committee, ZJCLA with the approval number: ZJCLA-IACUC-20010554, dated November 17, 2023. Our study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, J., Wang, B., Zhang, Y. et al. Integrating network pharmacology and experimental validation to explore the mechanism of Yiqi Ziyin in treating immune thrombocytopenia. Sci Rep 15, 28640 (2025). https://doi.org/10.1038/s41598-025-14105-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14105-w