Abstract
Muscle weakness is a significant concern in older women, as it increases the risk of falls and fractures. To develop practical tools for early detection, it is important to understand how hand grip strength relates to gait parameters in non-laboratory settings. This study examined the relationship between hand grip strength and gait characteristics in older women, using a foot-mounted sensor during natural walking. Publicly available data from 55 women aged > 70 years were analyzed. Each woman wore an inertial measurement unit attached to their shoe and completed a 30-min walking task. Hand grip strength was used as a general indicator of muscle strength. The mean and coefficient of variation (CV) were calculated for 18 gait parameters, including spatiotemporal parameters and foot pitch angles. Hand grip strength showed significant correlations with several mean gait parameters, including the percentage of the stance phase (r = −0.57) and the timing of the minimum foot pitch angle (r = −0.59). While hand grip strength was significantly correlated with the CV of several gait parameters, the strongest r value was −0.34. These findings suggest that mean gait parameters may better reflect hand grip strength than their CV.
Similar content being viewed by others
Introduction
Muscle weakness in older adults is a significant concern because it impairs balance1,2,3 and substantially increases the risk of falls4,5,6. These falls often lead to fractures such as distal radius7,8,, lumbar compression8, and femoral neck fractures7,8,9,10,11 that can greatly reduce the ability to perform daily activities12,13,14. Muscle weakness tends to develop earlier in women than in men, likely due to menopause15. Additionally, women face higher rates of osteoporosis16, sarcopenia17,18, and fracture risk16 compared to men. For these reasons, early assessment of muscle strength is critical for timely intervention and effective fall prevention, particularly in older women.
Several devices are currently available to assess muscle strength, including the Biodex system19 and hand-held dynamometers20,21. However, the Biodex system is expensive and impractical for regular or personal use. While hand-held dynamometers are more affordable, they require individuals to exert maximum voluntary effort, which may be difficult for some older women to sustain consistently. These challenges highlight the need for alternative ways to assess muscle strength that leverage data gathered during everyday activities.
Inertial measurement units (IMUs) are inexpensive, lightweight, compact, and easy to use in everyday life. Collecting gait data while wearing shoes equipped with IMUs offers a significant practical advantage22,23. Although previous studies have reported associations between muscle strength and gait parameters24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39, no studies have examined these relationships using foot-mounted sensors in non-laboratory settings. Moreover, gait characteristics are known to differ between laboratory and non-laboratory settings40,41, making it important to explore these associations in non-laboratory settings. Understanding these connections could help assess muscle strength through gait analysis during normal daily walking.
This study aimed to investigate the relationship between hand grip strength and gait parameters in older women using a foot-mounted sensor in non-laboratory settings. Hand grip strength was used as an indicator of muscle strength in this study, as it is strongly correlated with lower limb strength42 and is widely used in diagnosing sarcopenia43.
Methods
Database and data collection
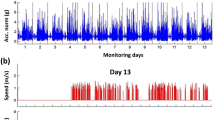
We used the GSTRIDE database, previously described in a published study44 and publicly available in Zenodo45. A brief overview of the dataset is provided here for reference44. The study included 163 adults aged > 70 years (45 men, 118 women). The original study assessed fall history over the last year, along with a range of anatomical, functional, and cognitive variables, including body mass, height, body mass index (BMI), and the Global Deterioration Scale (GDS). Additional assessments included the 4-meter walk test (duration and gait speed); frailty indicators such as hand grip strength (measured using an analog hydraulic hand dynamometer [JAMAR Dynamometer, Talexco, Spain] and a digital dynamometer [MAP 80K1S Dynamometer, KERN & SOHN, Germany])44; the Short Physical Performance Battery; the Timed Up and Go test; the Short Falls Efficacy Scale–International; and gait data collected via IMUs. For gait data collection, an IMU was attached to the top of the participant’s foot (left or right) using an elastic strap. The coordinate system was defined as follows: x axis (medial/lateral), y axis (anterior/posterior), and z axis (superior/inferior). The participants first stood still for 30 s, then began walking in a non-laboratory setting (i.e., indoors or outdoors). Acceleration and angular velocity were recorded throughout a 30-min walking session. Two types of IMUs were used: LSM6DSRX (STMicroelectronics, CH) and Physilog 6 S (GaitUp, CH)44. Although both types were included in the dataset, a previous study46 found that differences between the devices had minimal impact on gait parameter estimates, with mean relative errors ranging from 2.26 to 5.04%. The study44 was approved by the Ethics Committee for Research with Medicines of Hospital Universitario La Paz (Ref. HULP: PI-4486) and conducted within the framework of the Analysis of the Gait Pattern through the Design of an Electronic Prototype and a Monitoring App project (G-STRIDE, Ref. M2451). All methods were performed in accordance with the relevant guidelines and regulations. All participants or their relatives provided written informed consent before participation.
Data analysis
The sample size was determined using R version 4.3.0 (R Development Core Team). Correlation analysis was performed to investigate the relationship between hand grip strength and gait parameters. An a priori power analysis was conducted using the following parameters: an effect size (Cohen’s r) of 0.37, a significance level (α) of 0.05, and statistical power (1–β) of 0.80. The effect size was based on a previous study47, which reported a correlation coefficient of − 0.37 between gait velocity and the Standardized Frailty Criteria. We used the pwr.r.test function from the pwr package to calculate the required sample size (see Supplementary material for details). The sample size required for this study was determined to be 54.
Of the 163 participants, gait data from 55 were included in the analysis based on the criteria outlined below (Figure 1; Table 1). The inclusion criterion was female sex. A previous study48 reported that women have lower muscle strength than men, and other studies16,49 have also found a higher prevalence of osteoporosis and increased risk of fractures in women compared to men. Therefore, the present study focused on women. The exclusion criteria were as follows: (1) GDS score > 1 and (2) BMI > 30 kg/m². Cognitive function50,51,52 and BMI53 may influence gait parameters. Therefore, these exclusion criteria were applied to minimize potential confounding factors.
Calibrated IMU data were provided in the TXT format45. We developed custom scripts to calculate all the Gait Parameters using MATLAB version R2024b (MathWorks) (see Gait Parameters section for further details). First, the IMU data (acceleration and angular velocity) were imported and filtered using a fourth-order Butterworth filter with a zero-phase lag and a cutoff frequency of 10 Hz54. Given its high reliability in detecting gait events, the zero-crossing method55 was used to identify heel contact and toe-off based on angular velocity along the x-axis (Figure 2). Foot flat was defined as the midpoint between heel contact and toe-off56 (Figure 2).
The IMU orientation was estimated using a Kalman filter algorithm. Gravitational acceleration was calculated from 15 s of standing data and used to remove the gravitational component along the z-axis of the global coordinate system (GCS). The IMU velocity was obtained by integrating the acceleration data between two consecutive ipsilateral foot-flat events. A zero-velocity update was applied to correct velocity estimates57. The IMU trajectory was then computed by integrating the corrected velocity. It was assumed that the IMU’s height (Z = 0) remained constant between the foot flat and the subsequent ipsilateral foot flat events. Based on this assumption, drift along the z-axis was corrected using the same method described previously57. Finally, the trajectory was rotated so that the Y-axis aligned with the vector connecting the IMU positions at the two foot-flat events.
Several outliers were identified in the gait parameters of the dataset. To enhance the robustness of our analysis, we removed these outliers based on predefined criteria. Specifically, we calculated the mean and standard deviation (SD) of stride time for each participant across multiple trials. Gait trials were excluded if their stride times fell outside the range of mean ± 2 SD. Additionally, trials were excluded if the maximum foot pitch angle exceeded 50 degrees, or the minimum foot pitch angle was below −80 degrees. Fifty gait cycles without outliers were included for analysis for each participant.
Gait parameters
Muscle weakness may influence both the mean and variability of gait parameters22,23,31,58. Therefore, this study focused on mean values and coefficient of variation (CV). Figure 3 shows details of spatial gait parameters and foot pitch angles. Gait speed was calculated as the ratio of stride length to stride time (toe-off to the next toe-off). Stride length normalized to body height, was defined as the distance between the foot flat and the subsequent ipsilateral foot flat position. Cadence was calculated using stride time (heel contact to the next heel contact), which was defined as the interval between two consecutive heel contacts on the same side. Stance time was defined as the duration from heel contact to toe-off, whereas swing time was defined as the duration from toe-off to the next heel contact. The percentage of the stance phase was calculated by dividing stance time by stride time (heel contact to the next heel contact). Since stance and swing phase percentages are linearly dependent, only the percentage of the stance phase was analyzed. Foot pitch angle was computed in the sagittal plane using the Z-axis of the GCS and the local coordinate system of the foot. Pitch angles at heel contact and toe-off, as well as the minimum and maximum pitch angles during the stride, were extracted. The range of foot pitch angles was calculated as the difference between the minimum and maximum angles. The minimum and maximum foot pitch angle times were defined as the durations from heel contact to their respective occurrence within the stride. These times were then normalized to stride time (heel contact to the next heel contact) and expressed as percentages of stride duration (i.e., [minimum or maximum foot pitch angle time/stride time] × 100). The walk ratio was calculated as step length (half of the stride length) divided by cadence. Step speed was defined as the average foot velocity during the swing phase.
Spatial gait parameters and foot pitch angles. In the sagittal plane, when viewed from the right side of the foot, counterclockwise foot rotation (dorsiflexion) is represented by positive values, and clockwise rotation (plantarflexion) by negative values. A foot orientation parallel to the ground corresponds to 0 degrees. The red, green, and blue circles indicate heel contact, foot flat, and toe-off, respectively.
Muscle strength
Handgrip strength is widely used in the diagnosis of sarcopenia43, as it reflects overall muscle strength. In this study, it was used as an index of muscle strength. The database provided the maximum hand grip strength value for each participant⁴⁴ (rather than a mean value). Handgrip strength was measured using both an analog hydraulic hand dynamometer (JAMAR Dynamometer, Talexco, Spain) and a digital dynamometer (MAP 80K1S Dynamometer, KERN & SOHN, Germany)44.
Statistical analysis
The normality of each variable was assessed using the Shapiro-Wilk test. Based on the results, either Pearson or Spearman correlation coefficients were used to assess the relationships between variables. Statistical significance was set at p < 0.05. Based on a previous study59, effect sizes (Cohen’s r) were calculated and defined as negligible (Cohen’s r < 0.1), small (0.1 ≤ Cohen’s r < 0.3), medium (0.3 ≤ Cohen’s r < 0.5), and large (Cohen’s r ≥ 0.5). All statistical analyses were performed using R.
Results
Table 2 shows the relationship between mean gait parameters and hand grip strength. Significant positive correlations were observed between gait speed, stride length, cadence, foot pitch angle at heel contact, maximum foot pitch angle, range of foot pitch angle, and foot speed during the swing phase. In contrast, significant negative correlations were observed for stride time, stance time, percentage of stance phase, minimum foot pitch angle, minimum foot pitch angle time, maximum foot pitch angle time, and timing of the minimum foot pitch angle.
Table 3 shows the relationships between the CV of gait parameters and hand grip strength. Significant negative correlations were found between gait speed, cadence, stride time, stance time, swing time, percentage of stance phase, minimum foot pitch angle, range of foot pitch angle, minimum foot pitch angle time, maximum foot pitch angle time, timing of minimum foot pitch angle, walk ratio, and foot speed during the swing phase.
Discussion
This study examined the relationship between hand grip strength and gait parameters in older women using a foot-mounted sensor in non-laboratory settings. The key findings revealed significant correlations between hand grip strength and several mean gait parameters, including gait speed (r = 0.49 [medium]), percentage of the stance phase (r = −0.57 [large]), and timing of the minimum foot pitch angle (r = −0.59 [large]), as shown in Table 2. Our results suggest that a walking pattern associated with lower hand grip strength is characterized by reduced gait speed, resulting from shorter stride length and lower cadence, as well as an increased percentage of the stance phase. Although previous studies have reported associations between muscle strength and gait parameters24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39, only a few have used shoe-mounted IMUs to investigate these relationships29,31. Furthermore, all existing studies conducted their walking tests in controlled laboratory environments. Given that gait parameters can differ between laboratory and non-laboratory settings40,41, it is important to examine these associations in non-laboratory settings. To the best of our knowledge, this is the first study to investigate the relationship between hand grip strength and gait parameters in older women using foot-mounted sensors in non-laboratory settings. These findings may help support the development of practical methods for assessing muscle strength using gait data collected during natural walking in non-laboratory settings.
The present study demonstrated a positive association between hand grip strength and mean gait speed with a medium effect size (r = 0.49). This finding is consistent with a previous simulation study60 reporting that muscle weakness leads to reduced gait speed, and with an experimental study25 reporting a similar association. Although, in our study, gait speed measured using foot-mounted sensors in non-laboratory settings was estimated through numerical integration of acceleration data, our results suggest that gait speed can serve as a useful indicator for assessing hand grip strength in older women.
In the present study, reduced hand grip strength was negatively associated with stance time (r = −0.48 [medium]), the percentage of the stance phase (r = −0.57 [large]), and the timing of the minimum foot pitch angle (r = −0.59 [large]). A previous study31 assessed gait parameters using a gait analysis system that incorporated shoe-type data loggers with embedded IMUs on both outsoles during a straight 19-meter overground walk. The researchers examined the relationships between gait parameters and hand grip strength and reported a negative association between hand grip strength and stance phase duration during preferred-speed walking (r = −0.233). Therefore, previous findings regarding stance phase duration are consistent with our results. However, the previous study31 did not evaluate the percentage of the stance phase or the timing of the minimum foot pitch angle. Since these two variables demonstrated stronger associations with hand grip strength than stance time in our analysis, we suggest that they may serve as more sensitive indicators for assessing muscle strength in older adults.
Previous studies have indicated that increased variability in gait parameters correlates with higher fall risks61,62,63,64 and muscle weakness31. Therefore, we investigated the relationship between hand grip strength and the mean gait parameters and their CVs. Significant associations were found between hand grip strength and several CVs of gait parameters, including gait speed (r = −0.32 [medium]) and swing time (r = −0.34 [medium]), as detailed in Table 3. While hand grip strength showed significant correlation with the CVs of several gait parameters, the strongest correlation observed was r = −0.34 (swing time). The effect size for CVs and mean gait parameters ranged from 0.27 to 0.34 (small to medium) and 0.28 to 0.59 (small to large), respectively. A previous study65 has indicated that gait variability is also influenced by cognitive function. However, the present study only included older women without cognitive decline (i.e., GDS = 1). Notably, if older women with cognitive decline were analyzed, different results may be obtained. Since effect sizes (e.g., small, medium, and large) reflect the strength of the relationship between hand grip strength and gait parameters measured using a foot-mounted sensor, our findings suggest that the CVs of gait parameters might be insignificant. In contrast, the mean values of gait parameters may be more important than their CVs for older women without cognitive decline when assessing hand grip strength.
We also conducted additional analyses that retained outliers, recognizing that these data points may offer valuable insights in a community-based sample. However, the correlation coefficients did not change significantly (Supplementary Tables S1 and S2). Specifically, hand grip strength remained significantly associated with gait speed (r = 0.49 [medium] in Table 2 vs. r = 0.48 [medium] in Supplementary Table S1), percentage of the stance phase (r = −0.57 [large] vs. r = −0.51 [large]), and timing of the minimum foot pitch angle (r = −0.59 [large] vs. r = −0.48 [medium]). Although the impact of outlier removal was minimal, excluding outliers may still be beneficial for improving accuracy and interpretability.
A systematic review66 noted that most studies using IMUs during walking have placed the sensors on the lower back, due to its proximity to the body’s center of mass. One such study67 reported that gait variability measured from a lower-back IMU was associated with muscle strength. However, wearing a device in that location can interfere with daily activities, pressing against chairs when seated, striking the floor when lying down, or making it difficult to dress and undress. These practical limitations may reduce compliance in non-laboratory settings. In contrast, shoe-mounted IMUs offer a more user-friendly alternative, as they require no behavioral changes, participants simply wear their shoes as usual. A previous study using the same dataset⁴⁷ found that this placement was comfortable and did not affect walking behavior. Additionally, a systematic review55 reported that foot-based algorithms outperform trunk-based ones in detecting gait events. Thus, foot-mounted IMUs enable accurate gait analysis while offering superior practicality for daily use.
The Asian Working Group for Sarcopenia (2019) recommends using hand grip strength as a standard measure of muscle strength[43. Furthermore, previous research⁴² has shown strong correlations between grip strength and lower limb strength, including hip, knee, and ankle muscle groups. Based on this evidence, we used hand grip strength as a proxy for overall muscle strength in this study. However, since grip strength does not directly reflect lower limb strength, results might differ if other measures (e.g., knee extension strength) were assessed. Still, our findings provide meaningful insight into the potential for assessing muscle strength from gait data collected during natural walking in non-laboratory settings.
This study has some limitations. First, gait data were obtained from 30-min walking sessions conducted in non-laboratory settings, which may not fully reflect typical daily walking patterns. Therefore, further investigation is required to confirm the generalizability of the findings to daily walking. Second, the study included older women only so the results may not be generalizable to middle-aged men and women or older men. Further research is needed to explore the relationship between hand grip strength and gait parameters measured using a foot-mounted IMU in these populations.
Conclusion
This study investigated the relationships between hand grip strength and gait parameters in older women using a foot-mounted sensor in non-laboratory settings. These findings demonstrated significant associations between hand grip strength and both the mean values and CVs of the gait parameters. These results offer valuable insights into the potential for assessing muscle strength in older adults using gait data collected during natural walking in non-laboratory environments.
Data availability
The datasets analyzed during the current study are available at https://zenodo.org/records/8003441.
References
Song, Q. et al. Relationship of proprioception, cutaneous sensitivity, and muscle strength with the balance control among older adults. J. Sport Health Sci. 10, 585–593 (2021).
Shahtahmassebi, B., Hebert, J. J., Hecimovich, M. D. & Fairchild, T. J. Associations between trunk muscle morphology, strength and function in older adults. Sci. Rep. 7, 10907 (2017).
Tavakkoli Oskouei, S. et al. Is ankle plantar flexor strength associated with balance and walking speed in healthy people? A systematic review and meta-analysis. Phys. Ther. 101, pzab018 (2021).
Horlings, C. G. C., van Engelen, B. G. M., Allum, J. H. J. & Bloem, B. R. A weak balance: the contribution of muscle weakness to postural instability and falls. Nat. Clin. Pract. Neurol. 4, 504–515 (2008).
Moreland, J. D., Richardson, J. A., Goldsmith, C. H. & Clase, C. M. Muscle weakness and falls in older adults: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 52, 1121–1129 (2004).
Whipple, R. H., Wolfson, L. I. & Amerman, P. M. The relationship of knee and ankle weakness to falls in nursing home residents: an isokinetic study. J. Am. Geriatr. Soc. 35, 13–20 (1987).
Nevitt, M. C. & Cummings, S. R. Type of fall and risk of hip and wrist fractures: the study of osteoporotic fractures. The study of osteoporotic fractures research group. J. Am. Geriatr. Soc. 41, 1226–1234 (1993).
Yu, W. Y., Hwang, H. F. & Lin, M. R. Variations in situational risk factors for fractures of the distal forearm, hip, and vertebrae in older women. BMC Geriatr. 21, 214 (2021).
Greenspan, S. L. et al. Fall direction, bone mineral density, and function: risk factors for hip fracture in frail nursing home elderly. Am. J. Med. 104, 539–545 (1998).
Greenspan, S. L., Myers, E. R., Maitland, L. A., Resnick, N. M. & Hayes, W. C. Fall severity and bone mineral density as risk factors for hip fracture in ambulatory elderly. JAMA 271, 128–133 (1994).
Yang, Y. et al. The effect of fall biomechanics on risk for hip fracture in older adults: A cohort study of video-captured falls in long-term care. J. Bone Min. Res. 35, 1914–1922 (2020).
Alarcón, T., González-Montalvo, J. I., Gotor, P., Madero, R. & Otero, A. Activities of daily living after hip fracture: profile and rate of recovery during 2 years of follow-up. Osteoporos. Int. 22, 1609–1613 (2011).
Iwata, S. et al. Osteoporosis, spinal degenerative disorders, and their association with low back pain, activities of daily living, and physical performance in a general population. Sci. Rep. 14, 15860 (2024).
Vergara, I. et al. Wrist fractures and their impact in daily living functionality on elderly people: A prospective cohort study. BMC Geriatr. 16, 11 (2016).
Phillips, S. K., Rook, K. M., Siddle, N. C., Bruce, S. A. & Woledge, R. C. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin. Sci. (Lond). 84, 95–98 (1993).
Cawthon, P. M. Gender differences in osteoporosis and fractures. Clin. Orthop. Relat. Res. 469, 1900–1905 (2011).
Meng, S. et al. The prevalence of sarcopenia and risk factors in the older adult in china: a systematic review and meta-analysis. Front. Public. Health. 12, 1415398 (2024).
Hwang, J. & Park, S. Gender-specific risk factors and prevalence for sarcopenia among community-dwelling young-old adults. Int. J. Environ. Res. Public. Health. 19, 7232 (2022).
Si, X., Liu, Y., Feng, X. & Shao, Y. A study on the effects of different isokinetic testing modes of knee flexion-extension muscle strength ratios on lower extremity stiffness during jumping. Sci. Rep. 15, 9402 (2025).
do Amaral, C. M. S. S. B. et al. Low handgrip strength is associated with worse functional outcomes in long COVID. Sci. Rep. 14, 2049 (2024).
Saygin, D. et al. Hand-held dynamometry for assessment of muscle strength in patients with inflammatory myopathies. Rheumatology 60, 2146–2156 (2021).
Yamamoto, A. et al. Using in-shoe inertial measurement unit sensors to understand daily-life gait characteristics in patients with distal radius fractures during 6 months of recovery: cross-sectional study. JMIR Mhealth Uhealth. 12, e55178 (2024).
Yamamoto, A. et al. Foot characteristics of the daily-life gait in postmenopausal females with distal radius fractures: a cross-sectional study. BMC Musculoskelet. Disord. 24, 706 (2023).
Abdul Jabbar, K. et al. Fast gait Spatiotemporal parameters in adults and association with muscle strength - The Yishun study. Gait Posture. 85, 217–223 (2021).
Buchner, D. M., Larson, E. B., Wagner, E. H. & Koepsell, T. D. Lateur, B. J. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing. 25, 386–391 (1996). de.
Guadagnin, E. C., Priario, L. A. A., Carpes, F. P. & Vaz, M. A. Correlation between lower limb isometric strength and muscle structure with normal and challenged gait performance in older adults. Gait Posture. 73, 101–107 (2019).
Harris-Love, M. O., Benson, K., Leasure, E., Adams, B. & McIntosh, V. The influence of upper and lower extremity strength on performance-based sarcopenia assessment tests. J. Funct. Morphol. Kinesiol. 3, 53 (2018).
Hayashida, I., Tanimoto, Y., Takahashi, Y., Kusabiraki, T. & Tamaki, J. Correlation between muscle strength and muscle mass, and their association with walking speed, in community-dwelling elderly Japanese individuals. PLOS ONE. 9, e111810 (2014).
Huang, C. et al. Healthcare application of in-shoe motion sensor for older adults: frailty assessment using foot motion during gait. Sens. (Basel). 23, 5446 (2023).
Kanayama, A. et al. Examination of the impact of strength and velocity of the knee and ankle on gait speed in community-dwelling older adults. Healthc. (Basel). 10, 2093 (2022).
Kim, B., Youm, C., Park, H., Lee, M. & Choi, H. Association of muscle mass, muscle strength, and muscle function with gait ability assessed using inertial measurement unit sensors in older women. Int. J. Environ. Res. Public. Health. 19, 9901 (2022).
Mateu Serra-Prat, E. P. Muscle strength, sarcopenia and frailty associations with balance and gait parameters: A cross-sectional study. Eur. J. Geriatr. Gerontol. 1, 61–66 (2019).
Millor, N. et al. High density muscle size and muscle power are associated with both gait and sit-to-stand kinematic parameters in frail nonagenarians. J. Biomech. 105, 109766 (2020).
Misu, S. et al. Association between toe flexor strength and Spatiotemporal gait parameters in community-dwelling older people. J. Neuroeng. Rehabil. 11, 143 (2014).
Mori, K. et al. Gait characteristics of dynapenia, sarcopenia, and presarcopenia in community-dwelling Japanese older women: A cross-sectional study. Healthc. (Basel). 10, 1905 (2022).
Soltani, A. et al. Real-world gait speed estimation, frailty and handgrip strength: a cohort-based study. Sci. Rep. 11, 18966 (2021).
Stotz, A., Hamacher, D. & Zech, A. Relationship between muscle strength and gait parameters in healthy older women and men. Int. J. Environ. Res. Public. Health. 20, 5362 (2023).
Yamagiwa, D. et al. Examination of gait characteristics related to sarcopenia in community-dwelling older adults: A study focusing on plantar pressure. J. Cachexia Sarcopenia Muscle. 16, e13634 (2024).
Peoples, B. M. et al. Functional lower extremity strength influences stepping strategy in community-dwelling older adults during single and dual-task walking. Sci. Rep. 14, 13379 (2024).
Czech, M. D. et al. Age and environment-related differences in gait in healthy adults using wearables. NPJ Digit. Med. 3, 127 (2020).
Schmitt, A. C. et al. Walking indoors, outdoors, and on a treadmill: gait differences in healthy young and older adults. Gait Posture. 90, 468–474 (2021).
Strandkvist, V. et al. Hand grip strength is strongly associated with lower limb strength but only weakly with postural control in community-dwelling older adults. Arch. Gerontol. Geriatr. 94, 104345 (2021).
Chen, L. K. et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 21, 300–307e2 (2020).
García-de-Villa, S. et al. A database with frailty, functional and inertial gait metrics for the research of fall causes in older adults. Sci. Data. 10, 566 (2023).
García-Villamil Neira, G. et al. GSTRIDE: A database of frailty and functional assessments with inertial gait data from elderly fallers and non-fallers populations. Zenodo https://doi.org/10.5281/zenodo.8003441 (2022).
García-Villamil, G., Ruiz, L., Jiménez, A. R., Seco, F. & Rodríguez-Sánchez, M. C. Influence of IMU’s measurement noise on the accuracy of stride-length estimation for gait analysis. IPIN 2021 WiP Proceedings 1–16 (2021).
Álvarez, M. N. et al. Assessing falls in the elderly population using G-STRIDE foot-mounted inertial sensor. Sci. Rep. 13, 9208 (2023).
Nuzzo, J. L. Narrative review of sex differences in muscle strength, endurance, activation, size, fiber type, and strength training participation rates, preferences, motivations, injuries, and neuromuscular adaptations. J. Strength. Cond Res. 37, 494–536 (2023).
Alswat, K. A. Gender disparities in osteoporosis. J. Clin. Med. Res. 9, 382–387 (2017).
Hao, W. et al. Association of gait with global cognitive function and cognitive domains detected by MoCA-J among community-dwelling older adults: a cross-sectional study. BMC Geriatr. 21, 523 (2021).
Luo, J., Su, L., Ndeke, J. M., Wang, F. & Hendryx, M. Gait speed, handgrip strength, and cognitive impairment among older women - A multistate analysis. Exp. Gerontol. 169, 111947 (2022).
Seo, K. et al. Association between daily gait speed patterns and cognitive impairment in community-dwelling older adults. Sci. Rep. 13, 2783 (2023).
Chehab, E. F., Andriacchi, T. P. & Favre, J. Speed, age, sex, and body mass index provide a rigorous basis for comparing the kinematic and kinetic profiles of the lower extremity during walking. J. Biomech. 58, 11–20 (2017).
Salminen, M., Perttunen, J., Avela, J. & Vehkaoja, A. A novel method for accurate division of the gait cycle into seven phases using Shank angular velocity. Gait Posture. 111, 1–7 (2024).
Pacini Panebianco, G., Bisi, M. C., Stagni, R. & Fantozzi, S. Analysis of the performance of 17 algorithms from a systematic review: influence of sensor position, analysed variable and computational approach in gait timing Estimation from IMU measurements. Gait Posture. 66, 76–82 (2018).
Fukushi, K. et al. On-line algorithms of stride-parameter Estimation for in-shoe motion-sensor system. IEEE Sens. J. 22, 9636–9648 (2022).
Laidig, D. et al. Calibration-free gait assessment by foot-worn inertial sensors. Front. Digit. Health. 3, 736418 (2021).
Yamamoto, A. et al. Gait characteristics in patients with distal radius fracture using an in-shoe inertial measurement system at various gait speeds. Gait Posture. 107, 317–323 (2024).
Cohen, J. A power primer. Psychol. Bull. 112, 155–159 (1992).
Kudo, S., Fujimoto, M. & Nagano, A. Effects of aging-related muscle degeneration on dynamic stability during walking: a musculoskeletal computer simulation study. Front. Bioeng. Biotechnol. 12, 1524751 (2025).
Callisaya, M. L. et al. Gait, gait variability and the risk of multiple incident falls in older people: a population-based study. Age Ageing. 40, 481–487 (2011).
Hausdorff, J. M., Rios, D. A. & Edelberg, H. K. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch. Phys. Med. Rehabil. 82, 1050–1056 (2001).
Verghese, J., Holtzer, R., Lipton, R. B. & Wang, C. Quantitative gait markers and incident fall risk in older adults. J. Gerontol. Biol. Sci. Med. Sci. 64A, 896–901 (2009).
García-de-Villa, S. et al. Validation of an IMU-based gait analysis method for assessment of fall risk against traditional methods. IEEE J. Biomedical Health Inf. 29, 107–117 (2025).
Martin, K. L. et al. Cognitive function, gait, and gait variability in older people: a population-based study. J. Gerontol. Biol. Sci. Med. Sci. 68, 726–732 (2013).
Mobbs, R. J. et al. Gait metrics analysis utilizing single-point inertial measurement units: A systematic review. Mhealth 8, 9 (2022).
Bogen, B., Moe-Nilssen, R., Aaslund, M. K. & Ranhoff, A. H. Muscle strength as a predictor of gait variability after two years in community-living older adults. J. Frailty Aging. 9, 23–29 (2020).
Author information
Authors and Affiliations
Contributions
T.I. contributed to all aspects of the study, including conceptualization of the research design, data analysis, and manuscript preparation. T.T. conceptualized the research design and critically revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Inai, T., Takabayashi, T. Relationships between hand grip strength and gait parameters measured using a foot-mounted sensor in non-laboratory settings in older women. Sci Rep 15, 29375 (2025). https://doi.org/10.1038/s41598-025-14442-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14442-w