Abstract
The TLR4/NLRP3 inflammasome pathway drives lung cancer cell proliferation, migration, and invasiveness. However, no in vivo treatments targeting this pathway in lung cancer exist. Resveratrol, an anti-inflammatory compound, inhibits the NLRP3 inflammasome but requires high doses due to its hydrophobicity and instability. This study aimed to enhance the therapeutic efficacy of resveratrol by encapsulating it in liposomes to effectively target the TLR4/NLRP3 inflammasome pathway in lung cancer. In this study, the efficacy of resveratrol liposome was tested in vitro through cell proliferation and migration assays and gene expression analysis. In vivo effects were evaluated in a lung cancer mouse model using histological and molecular techniques. Resveratrol liposome significantly inhibited lung cancer cell proliferation and migration, and induced apoptosis in orthotopic lung cancer models. Additionally, by suppressing TLR4/NLRP3 inflammasome-related genes, resveratrol liposomes diminished levels of IL-1β and IL-18 both locally at tumor sites and systemically, thus modulating the tumor immune microenvironment by decreasing MDSCs and increasing CD4+ and CD8+ T cells. In conclusion, resveratrol liposome alleviates lung cancer progression by targeting the TLR4/NLRP3 inflammasome pathway and enhancing anti-tumor immune responses, highlighting its potential as a promising therapeutic strategy for lung cancer treatment.
Similar content being viewed by others
Introduction
Lung cancer is the second most commonly diagnosed cancer and exhibits the highest mortality rates in both men and women1,2. Chronic inflammation, recognized as the “seventh hallmark of cancer”, is a critical factor in lung cancer development3. The inflammatory microenvironment, consisting of infiltrated immune cells, cytokines, chemokines, and growth factors, induces oxidative stress and creates an immunosuppressive milieu. This environment leads to the dysregulation of relevant pathways, which can activate oncogenes or silence tumor suppressor genes, thereby promoting tumor initiation and progression, as well as facilitating angiogenesis, metastasis, chemoresistance, and immune evasion4,5,6.
Chronic lung inflammation is closely linked to lung cancer progression, TLR4/NLRP3 signaling pathway plays a crucial role in this process within the inflammatory microenvironment, driving cellular proliferation and cell cycle dysregulation7,8,9. Inflammasomes, particularly the NLRP3 inflammasome, are pivotal mediators of inflammation associated with lung cancer7,10. Upon activation, the NLRP3 inflammasome, comprising NLRP3, the adaptor protein ASC, and pro-caspase-1, triggers the proteolytic cleavage of pro-caspase-1 into its active forms (caspase-1 p10 or p20), subsequently processing pro-IL-1β and pro-IL-18 into their mature, bioactive forms11,12. High levels of IL-1β and IL-18 expression have been observed in lung cancer cell lines and tissues compared with cancer-adjacent normal tissues13. The overexpression and release of IL-1β and IL-18 recruit immunosuppressive myeloid-derived suppressor cell (MDSC) subsets to infiltrate tumors, thereby promoting tumor growth14,15. TLR4, which recognizes pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), is upregulated in lung cancer16. Studies have indicated that TLR4 activation promotes malignant tumor growth and resistance to chemotherapy, whereas inhibition of TLR4 activation delays tumor growth and prolongs animal survival17,18,19.
Resveratrol, a polyphenol found in grape and mulberry skins, commonly used in red wine production, exhibits a broad range of biological activities, including anti-inflammatory, antioxidant, anticancer, anti-aging, antiplatelet aggregation, immunomodulatory, and chemopreventive effects20. These benefits are largely due to its antioxidant and anti-inflammatory properties. Resveratrol is known to downregulate TLR4, mitigating neuroinflammation following focal cerebral ischemia21. High doses of resveratrol activate AMPK, which negatively regulates the NLRP3 inflammasome22. Additionally, pretreatment with resveratrol effectively reduces NLRP3 expression and eliminates IL-1β secretion in human mesenchymal stem cells induced exposed to ionizing radiation23. However, the specific role of resveratrol in modulating the overactivated TLR4/NLRP3 pathway in lung cancer remains unclear. Moreover, the therapeutic potential of resveratrol hindered by suboptimal pharmacokinetic properties, including poor solubility, chemical instability, and rapid metabolism, which results in low bioavailability and restricted delivery to pathological sites24.
To overcome the limitations and investigate the in vivo pharmacological activity of resveratrol, particularly its modulatory effects on the TLR4/NLRP3 axis in lung cancer, liposomes is employed as nano-delivery systems for resveratrol due to its high lipophilicity and encapsulation efficiency. Liposomes are particularly suitable for delivering lipophilic compounds like resveratrol, as they can improve pharmacokinetics and tumor accumulation via the enhanced permeability and retention (EPR) effect25,26,27. In this study, we employed liposomal encapsulation to facilitate the in vivo delivery of resveratrol and investigate its potential modulatory effects on the TLR4/NLRP3 inflammasome signaling axis in lung cancer.
Materials and methods
Reagents and materials
Resveratrol, cholesterol, and lecithin were purchased from Maclin Co., Ltd. Lipopolysaccharide (LPS) and TUNEL assay kit were obtained from Beyotime Biotechnology. Adenosine triphosphate (ATP) was procured from Aladdin. The CCK-8 assay kit and streptomycin-penicillin dual antibodies were sourced from Meilun Biotech Co., Ltd. Dulbecco’s Modified Eagle Medium (DMEM) culture medium was purchased from Gibco, while fetal bovine serum (FBS) was obtained from Punuosai Biotech Co., Ltd. VivoGlo™ Luciferin was purchased from Promega Biotechnology Co., Ltd. Mouse IL-1β ELISA kit (F2040-A) and IL-18 ELISA kit (F2169-A) were procured from Fankew. RNAiso Plus kit was obtained from Takara. HiScript III RT SuperMix for qPCR (+ gDNA wiper) kit and ChamQ Universal SYBR qPCR Master Mix kit were procured from Vazyme Biotech Co., Ltd. APC-Cy7 Rat Anti-Mouse CD45 (30-F11), PE Hamster Anti-Mouse CD3e (145-2C11), APC Rat Anti-Mouse CD4 (RM4-5), PerCP-Cy5.5 Rat Anti-Mouse CD8a (53 − 6.7), PerCP-Cy5.5 Rat Anti-CD11b (M1/70), and PE-Cy7 Rat Anti-Mouse CD86 (GL1) were purchased from BD Pharmingen. NLRP3 antibody (ET1610-93), Caspase-1 antibody (ET1608-69), Caspase-1 p20 antibody (ER1905-47), and IRAK4 antibody (EM1709-86) were acquired from Hangzhou Huaan Biotechnology Co., Ltd.; TLR4 polyclonal antibody (A5258) and IL-18 antibody (A23076) were acquired from Abclonal; MyD88 polyclonal antibody (K001785P) was obtained from Solarbio; ASC antibody (#67824) and IL-1β (#12242) were purchased from Cell Signaling Technology; TAK1 (phospho S439) antibody (EPR2863), Donkey Anti-Rabbit IgG H&L (Alexa Fluor® 647) (ab150075), and Goat Anti-Mouse IgG H&L (Alexa Fluor® 488) (ab150113) were purchased from Abcam.
Animal
C57BL/6J male mice (4–6 weeks old) were obtained from the SPF (Beijing) Biotechnology Co., Ltd. Mice were housed in a pathogen-free animal barrier facility. The in vivo experiments were initiated with mice aged 6 to 8 weeks. All animal experiments and procedures were performed in compliance with ethical regulations and the approval of the Animal Care & Welfare Committee of Guangxi Medical University Institutional (No. 202310001). We confirm that all methods were performed in accordance with the relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines.
Cell culture
The Luciferase expression Lewis lung carcinoma (LLC-luc) cell line was purchased from Hefei Wanwu Biotechnology Co. Ltd. (Hefei, China). LLC-luc cells were grown in DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Cells were cultured in a 37 ℃, 5% CO2 incubator.
Preparation and characterization of Resveratrol liposome
Resveratrol liposome (Rsv-Lip) was prepared by the thin film hydration method. Briefly, lecithin (102 mg) and cholesterol (18 mg) were dispersed into 6 mL of chloroform according to the mass ratio of 85:15. Resveratrol (5 mg) was dissolved in a 6 mL 1:1 methanol/chloroform solvent mixture. Lipid stocks and resveratrol solution were mixed, followed by rotary evaporation for 15 min at 38 °C to yield a thin film. The lipid films were then hydrated with PBS (pH 7.4) at 55 °C for 10 min, and the resulting suspension was ultrasonically dispersed for 7 min to obtain uniform liposomes. Blank liposomes (Blank-Lip) were prepared using a similar process without the addition of resveratrol. The encapsulation efficiency of resveratrol liposomes was determined with by ultrafiltration centrifugation and ultraviolet spectrophotometry at absorption wavelength of 306 nm. The particle sizes and zeta potential of liposomes were measured by a Malvern Zetasizer Nano ZS (Malvern Instruments, UK).
Cell viability assay
Cell Counting Kit-8 (CCK‐8) assay was used to estimate the cytotoxicity of resveratrol and resveratrol liposome according to the manufacturer’s instructions. Briefly, LLC-luc cells were seeded in a 96‐well plate with 1 × 104 cells per well for 24 h. Resveratrol and resveratrol liposome were added at the same series of concentrations from 0.5 µM to 150 µM, and blank liposome were added with the same volume of resveratrol liposome for 24 h. For NLRP3 inflammasome activation, these cells were stimulated by 1 µg/mL LPS for 3 h. Subsequently, 5 mmol/L ATP was added with or without resveratrol and resveratrol liposome for the last half-hour. Then resveratrol resveratrol liposome or blank liposome were added to the cells for another 24 h with complete culture medium. 10 µL CCK‐8 solution was added to each well, which was subsequently incubated at 37 °C (5% CO2) for 1 h. A microplate reader was used to measure the optical density at 450 nm.
Cell migration assays
Cell migration was examined using a scratch assay. LLC-luc cells (1.5 × 105) were seeded in 12-well plates. Once the cells reached 90–100% confluence, forming a monolayer, a sterile 200 µL pipette tip was used to generate a scratch on the cell monolayer. The cells were then treated with 20 µM of resveratrol and resveratrol liposome for 4 h. Subsequently, the cells were induced by LPS at a concentration of 1 µg/mL for 3 h, followed by 5 mmol/L ATP for an additional 30 min. The wounds were photographed at baseline and 24 h later using a phase contrast microscope (Leica, Germany). The scratch healing area were analysis using Manual Wound_healing_size_tool28, and the migration rate were calculated by the following formula: migration rate = (initial area - residual area)/initial area ×100%.
Cellular uptake of Resveratrol liposomes
To evaluate the cellular uptake of resveratrol liposomes, DiI dye was using to label resveratrol liposomes. LLC cells (1 × 106) were cultured in 24 well plate at 37 °C for 24 h. Free DiI dye or DiI-label resveratrol liposomes was added to the cells for 4 h. Cellular uptake of DiI-label resveratrol liposomes was quantitatively analyzed by flow cytometry. Data were analysis using FlowJo V10.8.
Mouse orthotopic transplantation of lung cancer model
LLC-luc cells (2 × 105) suspended in DMEM medium were intrathoracically injected into C57BL/6 male mice. The mice were randomly assigned into five groups with five mice in each group. Resveratrol (3 mg/kg), resveratrol liposome (3 mg/kg), or blank liposome (100 µL) were administered through intraperitoneal injection on days 5, 8, and 11. Tumor growth was monitored by bioluminescent imaging. Luciferase-expressing tumor-bearing mice were injected intraperitoneally with 10 µL/g body weight of 15 mg/mL luciferin, and 12 min after injection, the mice were imaged with an IVIS Spectrum Imaging System (PerkinElmer). The bioluminescence intensity of the tumor was analysed using Living Image 4.5.4.
Biodistribution of Resveratrol liposomes post systemic deliver
To evaluate the biodistribution of resveratrol liposomes in vivo, DiR dye was using to label resveratrol liposomes according to prior biodistribution studies29,30. C57BL/6 mice bearing LLC Orthotopic tumors were intraperitoneally injected with 100 µL DiR-label resveratrol liposomes or free DiR (0.25 mg/mL) in 5 days post tumor implantation. After 24 h, and vital organs were harvested. The fluorescent signal within each organ was measured with In Vivo NIR-II Optical Imaging System (MARS-FAST).
Hematoxylin and Eosin (H&E) staining
H&E staining was performed as previously reported31. Tumor and the major organ (heart, liver, spleen and kidney) were initially fixed in a 4% paraformaldehyde solution for 24 h, subsequently embedded in paraffin and sectioned into 6 μm. The sections underwent deparaffinization and rehydration. For histological observation, tumor and tissue sections were stained with hematoxylin and eosin and examined under a microscope.
TUNEL staining
The deparaffinized and rehydrated tumor sections were permeabilized with proteinase K for 30 min, followed by washing with PBS. Subsequently, the TUNEL assay kit was applied as per the manufacturer’s protocol.
Transmission electron microscopy (TEM)
The tissue was pre-fixed with 3% glutaraldehyde, followed by further fixation with 1% osmium tetroxide. Dehydration was carried out stepwise using acetone. The sample was then embedded sequentially in dehydrating agent and Epon-812 embedding resin. Ultra-thin sections of 60–90 nm were transferred onto copper grids using an ultra-microtome. The sections were stained at room temperature with uranyl acetate for 10–15 min, followed by lead citrate staining for 1–2 min. Observations of the sections were conducted using a transmission electron microscope (JEOL, JEM-1400FLASH).
Immunohistochemistry and Immunofluorescence
The tissues were initially preserved in 10% neutral buffered formalin at 4 °C for 48 h and subsequently moved to 70% ethanol prior to undergoing paraffin embedding. Sections of 10 μm thickness were prepared from the paraffin blocks, followed by deparaffinization and rehydration. For antigen retrieval, citrate buffer pH 6 was used in a pressure cooker for 15 min. Sections were treated with 3% H2O2 in methanol, followed by blocking with 5% BSA in PBS, and then incubated with specific primary antibodies, followed by DAB chromogenic solution or Anti-Rabbit Alexa Fluor® 647 (Abcam, 1:800) and DAPI for 1.5 h at room temperature. Primary antibodies used were: NLRP3 (1:500, Hangzhou Huaan, ET1610-93), Caspase-1 p20 (1:500, Hangzhou Huaan, ER1905-47), ASC (1:1000, Cell Signaling Technology, #67824), TLR4 (1:500, Abclonal, A5258), MyD88 (1:200, Solarbio, K001785P); IL-1β (1:200, Cell Signaling Technology, #12242), IL-18 (1:1000, Abclonal, A23076).
ELISA assay
To determine the IL-1β and IL-18 concentrations in serum, an ELISA assay was performed. Mice were sacrificed, and serum was collected at the endpoint of the experiment. For IL-1β and IL-18 determination, all samples were processed and analyzed according to the manufacturer’s instructions. The results were obtained using a microplate spectrophotometer.
Western blot analysis
Western blot analysis was carried out using a standard protocol. Briefly, tumor tissue was collected at the endpoints for western blot analysis. Total protein was extracted and separated by 10% SDS-PAGE, and the separated proteins were transferred to PVDF membranes. Primary antibodies used included: NLRP3 antibody (Hangzhou Huaan, ET1610-93), Caspase-1 p20 antibody (Hangzhou Huaan, ER1905-47), IRAK4 antibody (Hangzhou Huaan, EM1709-86), TLR4 polyclonal antibody (Abclonal, A5258), MyD88 polyclonal antibody (Solarbio, K001785P), ASC antibody (Cell Signaling Technology, #67824), TAK1 (phospho S439) antibody (Abcam, EPR2863), and GADPH (Servicebio, GB15002). After washing, the membrane was incubated with HRP-coupled secondary antibody (goat anti-rabbit, Servicebio) at room temperature for 1 h. After removing the secondary antibodies and washing, protein bands on the membrane were visualized with a Tanon 4600 chemiluminescence imaging system (China).
RT-qPCR
RNA from tumor tissue or tumor cells was isolated using the RNAiso Plus kit (Takara). cDNA preparation and RT-PCR using SYBR-Green (Vazyme) were performed on a QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems). The relative mRNA expression levels for target genes were calculated using the 2−ΔΔCt method with GADPH as the internal standard control. The sequences of primers used for qPCR analysis are listed in the Table 1.
Flow cytometry
Single-cell dissociation from tumor tissue and subsequent flow cytometry analysis of tumor-infiltrating immune cells were performed. Tumors were incubated in dissociation buffer (0.5 mg/mL collagenase IV and 0.2 mg/mL DNase I) at 37 °C for 1 h to generate a single-cell suspension. After filtration through a 300-mesh sieve and washing with PBS, cells were collected by centrifugation. Red blood cells were lysed, and cells were collected by centrifugation and resuspended in flow cytometry buffer. Cell counting was performed, and 2 ~ 5 × 106 cells were stained. CD45 antibody was used to label total lymphocytes, CD3, CD4, and CD8 antibodies were used to label T cells, and CD11b and Gr-1 were used to label MDSC cells. Flow cytometry was then utilized to analyze the immune cells in tumor tissues. Data were analyzed using FlowJo software. A representative flow cytometry gating strategy is displayed in Supplementary Fig. S12.
Statistical analysis
Data were presented as mean ± SD. Statistical analyses were performed using GraphPad Prism 8.0 software. One-way ANOVA followed by Dunnett’s post hoc test was conducted for comparisons among multiple groups. A p-value < 0.05 was considered statistically significant. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
Resveratrol liposome reduces proliferation, growth, and migration of LLC tumor cells by inhibiting NLRP3 inflammasome activation
We developed a resveratrol liposome with a particle size of 115.20 ± 0.95 nm (PDI = 0.117) and a surface potential of −18.40 ± 1.06 mV, similar to a blank liposome (Fig. S1). The encapsulation efficiency of resveratrol liposome was 74.15%, and the concentration of resveratrol in the liposome solution was as high as 0.2966 mg/mL, which was 4.40-fold enhancement over the solubility of resveratrol reported in the literature (0.05 mg/mL in water)32. The cytotoxic effects of resveratrol liposome on LLC cells were evaluated using the CCK-8 assay after treatment with different concentrations of resveratrol, resveratrol liposome, and blank liposome for 24 h. The results revealed that resveratrol and resveratrol liposome exhibited similar cytotoxic effects on LLC cells, with IC50 values of 23.52 ± 5.25 µM and 19.20 ± 0.88 µM, respectively, after 24 h of incubation (Fig. 1A-B). Blank liposome did not exhibit any cytotoxic effects on LLC cells at any concentration, indicating that resveratrol is essential for the induction of tumor cell apoptosis by resveratrol liposome (Fig. S2). Moreover, activation of the NLRP3 inflammasome using LPS in combination with ATP promoted LLC cell proliferation (130.72 ± 8.78%) compared to non-activated cells. However, resveratrol and resveratrol liposome effectively killed inflammasome-activated LLC cells with IC50 values of 28.88 ± 8.38 µM and24.82 ± 5.68 µM, respectively, after 24 h of incubation (Fig. 1A-B).
The inflammatory response of lung cancer cells through the NLRP3 inflammasome pathway not only promotes cancer cell growth but also enhances cancer cell invasion and metastasis. Therefore, the effects of resveratrol and its liposome on the inhibition of lung cancer cell migration were investigated using a wound healing assay in LPS-ATP-induced LLC lung cancer cells. The study found that LPS-ATP-induced LLC lung cancer cells had a higher migration capability than non-induced LLC cells (Fig. 1C and Fig. S3). However, treatment with resveratrol and resveratrol liposome significantly inhibited cancer cell migration in LPS-ATP-induced LLC lung tumor cells (Fig. 1C and Fig. S3).
The inhibition of NLRP3 and pro-inflammatory cytokine gene expressions in LPS-ATP-induced LLC cells was assessed using RT-qPCR. The results demonstrated that treatment of LLC cells with LPS and ATP induced the gene expression of NLRP3, ASC, and inflammasome-related cytokines IL-1β and IL-18 (Fig. 1D). Conversely, treatment with resveratrol and resveratrol liposome significantly inhibited the expression of these genes in LLC cells stimulated with LPS and ATP (Fig. 1D).
In vitro antitumor effect of resveratrol liposomes. (A-B) LLC cells were treated with 0-150 µM of (A) resveratrol (Rsv) and (B) resveratrol liposome (Rsv-Lip) for 24 h with or without inflammasome activation (n = 4); (C) Migration image of LPS-ATP induced LLC cells after treatment with 25 µM of resveratrol and resveratrol liposome for 24 h; (D) mRNA relative expression of NLRP3 inflammasome pathway in LPS-ATP induced LLC cells (n = 3). Data are presented as mean ± standard deviation (***p < 0.001, ****p < 0.0001, ns indicates no statistically significant difference between samples).
Resveratrol liposome attenuates the growth rate of orthotopic lung cancer
To further investigate the antitumor effect of resveratrol liposome in vivo, luciferase-expressing LLC (LLC-Luc) cells were intrathoracically injected into C57BL/6J male mice. The establishment of an orthotopic model of lung cancer and drug administration procedure are described in Fig. 2A. Mice with established orthotopic lung cancer were subjected to bioluminescence imaging before drug administration (day 5 post-modeling) and after two administrations (day 10 post-modeling). As shown in Fig. 2B and Fig. S4, compared to the control group, the bioluminescence intensity was significant weaker (p < 0.001) in the resveratrol liposome groups, indicating a deceleration in tumor growth rate. In contrast, the tumor growth rate in the blank liposome treatment group, which did not receive the drug, showed no difference from the control group. At the experiment’s endpoint, images of mouse lungs and tumors, along with lung tumor weights, are presented in Fig. 2C and D. The average tumor weight of the resveratrol liposome treatment group was significantly lower than that in the control group (p < 0.05). Although there was a strong trend for a decrease in bioluminescence signal and tumor weight with resveratrol treatment, the difference was not statistically significant (Fig. S4 and Fig. 2D), and ANOVA analysis indicated no statistically significant difference between the blank liposome group and the control group. Evaluation of mouse survival also demonstrated that both resveratrol and resveratrol liposome extended the median survival of mice with orthotopic lung cancer from 15 days to 20.5 days and 22 days, respectively (Fig. 2E). The differences in the in vivo efficacy between resveratrol and resveratrol liposomes may be attributed to the biodistribution of resveratrol liposomes. As shown in the Fig. S5, the DiR fluorescence intensity of DiR-labeled resveratrol liposomes was significantly higher than that of the free DiR in lung and tumor. The uptake of resveratrol liposomes by LLC lung cancer cells is also stronger than that of the free dye (Fig. S6).
Furthermore, due to cancer cell compression in lung spaces causing respiratory distress in late-stage lung cancer mice, body weight decreased after the 10th day of modeling. The resveratrol liposome group exhibited better anti-tumor effects, slowing down the rate of weight loss in mice compared to the model control group (p < 0.05) (Fig. S7A). At the experiment’s end, organ tissues (heart, liver, kidneys, and spleen) were weighed to evaluate effects on the organs. Compared to the healthy control group, the model control group showed significant differences in heart weight, while no significant differences were observed in liver, kidney, and spleen weights among all treatment groups and the healthy control group (Fig. S7B). The biochemical blood indicators remained within normal ranges, indicating no significant liver and kidney function damage occurred following resveratrol liposome treatment (Fig. S8A). Moreover, as shown in Fig. S8B, histological analysis revealed no pathological changes in the main organs (heart, liver, spleen and kidney) of mice. These findings suggest that resveratrol liposome exhibits promising biological safety.
In vivo anti-tumor evaluation of resveratrol liposomes. (A) Schematic of the lung tumor model in which C57BL/6J mice were intrathoracically injected with LLC-luc cells (2 × 105 cells), and then treated with Blank Lip, Rsv, or Rsv-Lip inoculated i.p. on days 5, 8, and 11. (B) Representative images of tumor bioluminescence of tumor growth (n = 5 mice/group); (C) Photographs and (D) weight of tumors at the experimental endpoint (n = 5 mice/group); (E) Survival curves (n = 6 mice/group). Data are presented as mean ± standard deviation, n = 5 (*p < 0.05, ****p < 0.0001, ns indicates no statistically significant difference between samples).
Resveratrol liposome increases apoptosis of lung cancer
Pathological changes in tumor tissues were demonstrated with H&E staining, TUNEL staining, and TEM. As shown in Fig. 3A, LLC orthotopic lung cancer mice displayed dense tumor growth with severe invasion and compression of normal lung cells compared to healthy mice. Blank liposomes showed no notable difference from the model control group in H&E staining. Conversely, both resveratrol and resveratrol liposome significantly reduced tumor size, with the latter also showing lower cell nucleus density, indicating potential apoptosis induction. TUNEL staining revealed apoptotic cell areas of 0.14 ± 0.06%, 0.22 ± 0.13%, 2.73 ± 2.06%, and 8.06 ± 4.87% in control, blank liposome, resveratrol, and resveratrol liposome groups, respectively (Fig. 3B and Fig. S9). The resveratrol liposome group exhibited significantly higher apoptotic rates than the model control group, highlighting its role in directly promoting tumor cell apoptosis and inhibiting tumor growth.
We further observed structural changes in the subcellular organelles of the tissues after drug administration by transmission electron microscopy. As shown in Fig. 3C, in healthy mouse lung tissues, endothelial cell nuclei displayed uniform chromatin distribution and intact nuclear membranes. Mitochondria exhibited normal morphology with well-defined cristae and homogeneous stroma, alongside a normal endoplasmic reticulum. Cell membranes were continuous with clear tight junctions and a uniform basement membrane. In the model group, tumor cell nuclei were irregularly shaped with reduced heterochromatin located mainly beneath the nuclear membrane. Mitochondria showed clear cristae and a uniform matrix. The endoplasmic reticulum structure was normal, indicating strong growth capacity. The blank liposome treatment group exhibited microstructures similar to the model group. However, the resveratrol group showed signs of apoptosis: tumor cell nuclei were irregular with condensed chromatin and increased electron density. Mitochondria were swollen with fractured cristae and lysed stroma. Notably, resveratrol liposome induced more pronounced apoptosis: tumor cell nuclei showed irregular shapes with condensed chromatin and increased electron density. Nuclear membrane structure was blurred, and mitochondria exhibited extensive swelling, cristae fragmentation, and lysed stroma, indicative of significant apoptosis induction.
Resveratrol liposome downregulates the TLR4 pathway in orthotopic lung cancer
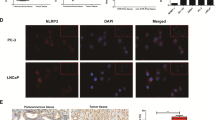
TLR4 and its adaptor protein MyD88 are highly expressed in various tumors and can activate the NLRP3/ASC/Caspase-1 inflammasome pathway, promoting the release of multiple factors from tumor cells and thereby facilitating tumor initiation, progression, and exacerbation33. Immunofluorescence images of TLR4 and MyD88 expression following treatment with resveratrol and its liposomes are presented in Fig. 4A. Treatment led to a notable reduction in red fluorescence intensity of both TLR4 and MyD88. Quantitative analysis revealed a 3.23-fold and 8.12-fold decrease in TLR4 fluorescence intensity after treatment with resveratrol and resveratrol liposomes, respectively (Fig. 4B), and an 8.67-fold and 10.79-fold decrease in MyD88 fluorescence intensity (Fig. 4C). These results reveal that the TLR4/MyD88 pathway downregulation activities of resveratrol liposome are stronger than those of resveratrol in vivo.
Upon binding with the adaptor protein MyD88, TLR4 recruits IRAK, initiating downstream signaling involving TRAF6, TAK1, and TAK1-binding proteins 1 and 2 (TAB 1 and 2), culminating in the expression of inflammatory factors. qPCR analysis of mRNA transcription levels of these downstream molecules in the TLR4/MyD88 pathway demonstrated that resveratrol and its liposomes effectively downregulated IRAK4, TRAF6, TAK1, TAB1, and TAB2 genes (Fig. 4D). This downregulation attenuates the signal transduction mediated by TLR4/MyD88, suggesting a mechanism by which resveratrol suppresses inflammatory responses.
Next, we performed Western blot analysis to evaluate protein expression after resveratrol and its liposome treatment in orthotopic lung cancer. The results demonstrate that resveratrol liposome effectively decreased the expression of TLR-4, MyD88, IRAK4, and p-TAK1 proteins in lung tumors, as illustrated in Fig. 4E-F and Fig. S10, suggesting that the reduction in TLR4 expression leads to a decrease in downstream signaling pathways.
Resveratrol liposome downregulates the TLR4 pathway in lung cancer. (A) Representative images of TLR4 and MyD88 immunofluorescence in tumor tissues (scale bar: 50 μm); (B-C) Fluorescence intensity of TLR4 and MyD88 in tumor tissues; (D) mRNA relative expression of the TLR4/MyD88 pathway in tumor tissues; (E-F) Protein expressions of TLR4, MyD88, IRAK4, and p-TAK1 in tumor tissues measured by Western blotting; original western blot scans are shown in Supplementary Fig. 10. Data are presented as mean ± standard deviation; (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns indicates no statistically significant difference between samples).
Resveratrol liposome downregulates the NLRP3/ASC/Caspase-1 pathway in orthotopic lung cancer
In addition to directly inducing the expression of inflammatory factors, TLR4 receptors on immune cells can activate NF-κB and upregulate mRNA transcription of NLRP3, which then binds with its adaptor protein ASC. The CARD domain of ASC interacts with the CARD of caspase-1, forming inflammasomes that generate active effector protein caspase-1. Caspase-1 cleaves GSDMD, triggering pyroptosis and initiating an inflammatory response34. The aforementioned experiments demonstrated that resveratrol liposome downregulated the TLR4/MyD88 pathway. Therefore, we further investigated their effects on the NLRP3/ASC/Caspase-1 inflammasome pathway.
qPCR analysis showed that resveratrol and its liposomes significantly downregulated the expression of genes associated with the inflammasome (Fig. 5A). Immunofluorescence staining of NLRP3 and caspase-1-P20 in tumor tissues from the resveratrol and its liposome groups revealed a notable decrease in red fluorescence intensity, indicating significant downregulation of the NLRP3/ASC/Caspase-1 inflammasome pathway (Fig. 5B). Western blot analysis also revealed that resveratrol liposome significantly reduced the expression of NLRP3, ASC, caspase-1, and caspase-1(P20) proteins in the lung tumor (Fig. 5C-D and and Fig. S11). Together, these results support that resveratrol liposome effectively reduces the mRNA and protein expression of the NLRP3 inflammasome pathway in lung cancer tissues.
Resveratrol liposome downregulated the NLRP3/ASC/Caspase-1 pathway. (A) qPCR analysis of mRNA relative expression levels of NLRP3, Prcard, caspase-1, and GSDMD in tumor tissues; (B) Representative images of immunofluorescence staining for TLR4 and MyD88 in tumor tissues (scale bar: 50 μm); (C-D) Protein expressions of NLRP3, ASC, Casp-1, Casp-1(P20) in tumor tissues measured by Western blotting; original western blot scans are shown in Supplementary Fig. 11. Data are presented as mean ± standard deviation (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns indicates no statistically significant difference between samples).
Resveratrol liposome downregulates Pro-inflammatory cytokines levels in lung cancer mice
Activation of the inflammasome pathway in lung cancer leads to the activation of inflammatory factors IL-1β and IL-18 by caspase-1, which is constitutively expressed in lung epithelial cell nuclei and plays a critical role in pulmonary inflammatory diseases. Following administration of resveratrol and resveratrol liposomes, immunohistochemical staining and qPCR analysis demonstrated a significant downregulation of IL-1β and IL-18 gene transcription and protein expression levels in lung tumor tissues (Fig. 6A-B). This indicates that resveratrol inhibits the TLR4 receptor-mediated inflammasome pathway, thereby reducing inflammation levels in lung tumors. ELISA examination of IL-1β and IL-18 concentrations in serum also showed a significant decrease compared to the model control group (Fig. 6C). Importantly, these levels did not significantly differ from those in the healthy control group, suggesting that treatment with resveratrol liposome can regulate inflammation levels to a healthy state.
Regulation of inflammation levels by resveratrol liposome in lung cancer mice. (A) mRNA relative expression levels of IL-1β and IL-18 in tumor tissues; (B) Serum levels of IL-1β and IL-18 measured by ELISA; Data are presented as mean ± standard deviation, n = 5 (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns indicates no statistically significant difference between samples).
Effects of Resveratrol liposome on the tumor immune microenvironment in lung cancer mice
Activation of the NLRP3/ASC/Caspase-1 inflammasome pathway induces chronic tumor inflammation, leading to increased infiltration of immunosuppressive MDSCs and a consequent immunosuppressive microenvironment that reduces the infiltration of tumor-killing CD4+ and CD8+ T cells35. Following administration of resveratrol and resveratrol liposomes, the proportion of MDSCs at tumor sites significantly decreased from 26.00 ± 1.01% in the control group to 17.93 ± 1.27% and 14.27 ± 2.90%, respectively (Fig. 7A-B). This represents decreases of 31.74% and 82.24%, with significant differences compared to the control group. Reduction in MDSC infiltration mitigates the immunosuppressive effect of the tumor microenvironment. Consequently, compared to the control group (CD3+CD4+ T cells 6.90 ± 2.36%, CD3+CD8+ T cells 14.13 ± 2.98%), treatment with resveratrol and its liposomes increased the infiltration of CD3+CD4+ T cells to 15.93 ± 2.91% and 20.23 ± 4.24%, respectively, and CD3+CD8+ T cells to 19.73 ± 0.90% and 21.97 ± 0.93%, respectively (Fig. 7C-D). This represents increases of 131% and 197% in CD3+CD4+ T cells, and 39% and 117% in CD3+CD8+ T cells, enhancing the anti-tumor immune response. Additionally, the expression levels of effector cytokines in tumor tissue were quantified by qPCR. As shown in Fig. 7E, resveratrol liposome significantly increased the expression of GzmA, GzmB, and IFN-γ, which are associated with increased T-cell infiltration. Altogether, resveratrol liposome decreases MDSC infiltration and enhances infiltration and function of tumor-killing T cells, thereby effectively reshaping the immunosuppressive tumor microenvironment in lung cancer.
Effects of resveratrol liposome on immune cells in the lung tumor microenvironment. (A) Representative flow cytometry plots of MDSCs (CD11b+Gr-1+), CD3+CD4+ T cells, and CD3+CD8+ T cells; (B-D) Proportions of MDSCs, CD3+CD4+ T cells, and CD3+CD8+ T cells in tumor sites detected by flow cytometry; (E) mRNA relative expression levels of GzmA, GzmB, and IFN-γ in tumor tissues. Data are presented as mean ± standard deviation, n = 3–5 (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns indicates no statistically significant difference between samples).
Discussion
Lung cancer is closely associated with chronic inflammation, characterized by infiltration of inflammatory cells and accumulation of pro-inflammatory factors including cytokines, chemokines, and prostaglandins that stimulate cell proliferation, angiogenesis, tissue remodeling, and metastasis5,36,37. Natural polyphenols, such as resveratrol, have shown potential in suppressing cancer-related inflammation by reducing ROS levels, mitochondrial damage, and mtDNA damage38,39. Previous studies have demonstrated resveratrol’s capacity to inhibit various types of cancer cells and its potential as a therapeutic strategy for non-small cell lung cancer through modulation of the Akt pathway40,41. However, the pharmacokinetic properties of resveratrol are suboptimal due to its poor solubility, chemical instability, and rapid metabolism, resulting in low bioavailability and limited in vivo anti-tumor efficacy42. In this work, we confirmed that resveratrol exhibited low anti-tumor efficiency lung cancer mice, even though it showed inhibitory capacity on the proliferation and migration of tumor cells in vitro. To overcome this limitation, we encapsulated resveratrol in liposomes in order to enhance its in vivo anti-tumor efficacy. Our results showed that resveratrol liposome markedly slowed tumor growth and significantly enhanced tumor cell apoptosis in lung cancer mice. The improved efficacy can be attributed to the increased solubility and bioavailability of resveratrol provided by the liposome encapsulation, as well as the ability of the nanoscale liposomes to concentrate the drug at the tumor site via the enhanced permeability and retention (EPR) effect.
The TLR4/NLRP3 signaling pathway is overactivated in the lung cancer-associated inflammatory microenvironment, driving cellular proliferation and cell cycle dysregulation8,9. The NLRP3 inflammasome, a key mediator of inflammatory responses, has been implicated in cancer initiation and progression. Studies have shown elevated expression levels of NLRP3, ASC, and caspase-1 in lung cancer cells and tissues compared to adjacent normal tissues13,42. Additionally, in vitro studies indicate that activation of the NLRP3 inflammasome exacerbates the proliferation and migration capabilities of A549 cells10,43,44. An analysis reveals that patients with lung adenocarcinomas with high NLRP3 expression have a poorer prognosis than those with low levels45. Moreover, patients with lower expression of NLRP3 and its pathway genes exhibit prolonged survival in lung squamous cell carcinoma, suggesting the NLRP3 inflammasome as a potential novel target for lung cancer therapy46. TLR4 is notably involved in the activation of NLRP3 inflammasome47. Upon activation by PAMPs and DAMPs, TLR4 primarily activates two signaling pathways: (1) the MyD88-dependent pathway, recruiting IRAK1, IRAK4, TRAF6, and sequentially activating TAK1; and (2) the TRIF-dependent pathway, recruiting TRAF6 and activating TAK1 to activate NF-κB, MAPK pathways and NLRP3 inflammasome48, induced the dysregulation of pro-inflammatory cytokines such as IL-1 and IL-18. However, there is a current deficiency in in vivo investigations focusing on TLR4/NLRP3 axis as a therapeutic target for lung cancer. Our studies identified that resveratrol liposome effectively down-regulates the expression of genes and proteins related to the TLR4 pathway and the NLRP3 pathway in orthotopic lung cancer tissues. Specifically, our results demonstrated that resveratrol liposome effectively decreased the expression of TLR4 and its downstream proteins MyD88, IRAK4, and p-TAK1 in lung cancer tissues. Furthermore, the downregulation of NLRP3, ASC, and caspase-1 by resveratrol liposomes led to a significant reduction in IL-1β and IL-18 secretion, both locally within the tumor and systemically. These finding suggest that inhibition of overactivated TLR4/NLRP3 signalling pathway could be an effective strategy for regulating lung cancer-associated inflammation and decelerating lung cancer progression.
Activation of TLR4/NLRP3 signalling pathway causes the expression, maturation and secretion of IL-1β and IL-18, which are key mediators of the inflammatory response49. IL-1β a critical pro-inflammatory cytokine whose genetic polymorphisms are closely associated with the development of various tumors, including pancreatic, breast, lung, and colon cancers50,51,52. Previous studies suggest IL-1β recruits MDSCs to the tumor microenvironment, thereby inhibiting anti-tumor immunity through activation of the NF-κB pathway, which can be abrogated by an anti-IL-1β blocking antibody15,53. Blocking IL-1β further increases the recruitment of CD8+ T cells and enhances the expression of cytotoxic CD8+ T cell markers Gzmb and IFN-γ, results that are consistent with our study15. IL-18 promotes angiogenesis via Src and JNK kinases and mediates immunosuppression in the tumor microenvironment by inducing the accumulation of MDSCs14,54,55. Upon reducing IL-1β and IL-18 secretion with resveratrol liposome, we observed a significant decrease in tumor MDSC cell infiltration, increased infiltration of CD4+ T cells and CD8+ T cells, and upregulation of GzmA, GzmB, and IFN-γ in tumors, all contributing to enhanced anti-tumor immune responses.
Nevertheless, there are some limitations to this study. In the biodistribution experiments, we observed that DiR-labeled Rsv-Lip showed greater accumulation in tissues compared to free DiR. However, this comparison was not made directly with free resveratrol, which introduces a certain degree of limitation in interpreting the results. Although we found that inhibition of the TLR4/NLRP3 pathway using resveratrol liposomes exhibited anti-lung cancer activity, demonstrating the potential of targeting this pathway for the treatment of lung cancer in vivo, the ability of resveratrol liposomes to inhibit the progression of lung cancer by themselves is limited. Future research should consider combining resveratrol liposomes with other therapies to achieve better therapeutic effects.
Conclusion
This study underscores the great promise for future application of resveratrol liposome targeting the TLR4/NLRP3 inflammasome pathway for lung cancer treatment in vivo. Our results conclusively demonstrate that resveratrol liposome effectively inhibits tumor growth in LLC orthotopic lung cancer models, significantly extending survival with negligible adverse effects. By inducing apoptosis in tumor cells and robustly suppressing the tumor TLR4 pathway, resveratrol liposome significantly reduces NLRP3/ASC/Caspase-1 inflammasome activation, leading to diminished levels of IL-1β and IL-18 both locally at tumor sites and systemically. Treatment reduces tumor infiltration by MDSCs and enhances infiltration of CD3+CD4+ and CD3+CD8+ T cells, thus reshaping the immunosuppressive tumor microenvironment and exerting potent anti-tumor effects.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- ASC:
-
Apoptosis-associated speck-like protein containing caspase recruitment domain
- ATP:
-
Adenosine triphosphate
- Blank-Lip:
-
Blank liposomes
- CARD:
-
Chimeric proteins or interaction domains
- CCK-8:
-
Cell Counting Kit‐8
- DAMPs:
-
Damage-associated molecular patterns
- ELISA:
-
Enzyme linked immunosorbent assay
- EPR:
-
Permeability and retention
- GSDMD:
-
Gasdermin-D
- GzmA:
-
Granzyme A
- GzmB:
-
Granzyme B
- IFN-γ:
-
Interferon-γ
- IL-18:
-
Interleukin-18
- IL-1β:
-
Interleukin-1β
- IRAK4:
-
Interleukin 1 receptor associated kinase 4
- LLC:
-
Lewis lung carcinoma
- LPS:
-
Lipopolysaccharide
- MDSCs:
-
Myeloid-derived suppressor cells
- MyD88:
-
Myeloid differentiation primary response protein 88
- NLRP3:
-
Nod-like receptor protein containing pyrin 3
- PAMPs:
-
Pathogen-associated molecular patterns
- PDI:
-
Polymer dispersity index
- Rsv:
-
Resveratrol
- Rsv-Lip:
-
Resveratrol liposome
- RT-qPCR:
-
Reverse transcription polymerase chain reaction
- TAB1:
-
TGF-beta activated kinase 1 binding protein 1
- TAB2:
-
TGF-beta activated kinase 1 binding protein 2
- TAK1:
-
TGF-beta activated kinase 1
- TEM:
-
Transmission electron microscopy
- TLR4:
-
Toll like receptor 4
- TRAF6:
-
TNF receptor associated factor 6
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling
References
Kratzer, T. B. et al. Lung cancer statistics, 2023. Cancer 130, 1330–1348 (2024).
Siegel, R. L., Giaquinto, A. N., Jemal, A. & Cancer statistics CA. Cancer J Clin 74: 12–49 (2024). (2024).
Huang, C. F. et al. NLRP3 inflammasome activation promotes inflammation-induced carcinogenesis in head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. 36, 116 (2017).
Bakhoum, S. F. & Cantley, L. C. The multifaceted role of chromosomal instability in cancer and its microenvironment. Cell 174, 1347–1360 (2018).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Zhang, Y. et al. Polarization of tumor-associated macrophages by TLR7/8 conjugated radiosensitive peptide hydrogel for overcoming tumor radioresistance. Bioact Mater. 16, 359–371 (2022).
Christenson, S. & Hersh, C. P. Found in translation: Multi-omics assessment of the chronic obstructive pulmonary Disease-Lung cancer interaction. Am. J. Respir Crit. Care Med. 200, 276–277 (2019).
Liang, M. et al. Cancer-derived Exosomal TRIM59 regulates macrophage NLRP3 inflammasome activation to promote lung cancer progression. J. Exp. Clin. Cancer Res. 39, 176 (2020).
Zhan, Z. et al. Autophagy facilitates TLR4- and TLR3-triggered migration and invasion of lung cancer cells through the promotion of TRAF6 ubiquitination. Autophagy 10, 257–268 (2014).
Wang, Y. et al. Activation of NLRP3 inflammasome enhances the proliferation and migration of A549 lung cancer cells. Oncol. Rep. 35, 2053–2064 (2016).
Choi, A. J. & Ryter, S. W. Inflammasomes: molecular regulation and implications for metabolic and cognitive diseases. Mol. Cells. 37, 441–448 (2014).
He, Y., Hara, H. & Nunez, G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 41, 1012–1021 (2016).
Kong, H. et al. Differential expression of inflammasomes in lung cancer cell lines and tissues. Tumour Biol. 36, 7501–7513 (2015).
Lim, H. X., Kim, T. S. & Poh, C. L. Understanding the differentiation, expansion, recruitment and suppressive activities of Myeloid-Derived suppressor cells in cancers. Int. J. Mol. Sci. 21, 3599 (2020).
Wang, D. et al. IL-1beta is an Androgen-Responsive target in macrophages for immunotherapy of prostate cancer. Adv. Sci. (Weinh). 10, e2206889 (2023).
Hoden, B. et al. Understanding the role of Toll-like receptors in lung cancer immunity and immunotherapy. Front. Immunol. 13, 1033483 (2022).
Domenis, R. et al. Toll-like Receptor-4 activation boosts the immunosuppressive properties of tumor Cells-derived exosomes. Sci. Rep. 9, 8457 (2019).
Kelly, M. G. et al. TLR-4 signaling promotes tumor growth and Paclitaxel chemoresistance in ovarian cancer. Cancer Res. 66, 3859–3868 (2006).
Pastille, E. et al. Inhibition of TLR4 signaling impedes tumor growth in Colitis-Associated colon cancer. Front. Immunol. 12, 669747 (2021).
Tozser, J. & Benko, S. Natural Compounds as Regulators of NLRP3 Inflammasome-Mediated IL-1beta Production. Mediators Inflamm : 5460302 (2016). (2016).
Lei, J. R. et al. Resveratrol downregulates the TLR4 signaling pathway to reduce brain damage in a rat model of focal cerebral ischemia. Exp. Ther. Med. 17, 3215–3221 (2019).
Kulkarni, S. S. & Canto, C. The molecular targets of Resveratrol. Biochim. Biophys. Acta. 1852, 1114–1123 (2015).
Fu, Y. et al. Resveratrol inhibits ionising irradiation-induced inflammation in MSCs by activating SIRT1 and limiting NLRP-3 inflammasome activation. Int. J. Mol. Sci. 14, 14105–14118 (2013).
Neves, A. R., Queiroz, J. F. & Reis, S. Brain-targeted delivery of Resveratrol using solid lipid nanoparticles functionalized with Apolipoprotein E. J. Nanobiotechnol. 14, 27 (2016).
Woodman, C., Vundu, G. & George, A. Applications and strategies in nanodiagnosis and nanotherapy in lung cancer. Semin Cancer Biol. 69, 349–364 (2021).
Zhou, J., Zhao, W. Y. & Ma, X. The anticancer efficacy of Paclitaxel liposomes modified with mitochondrial targeting conjugate in resistant lung cancer. Biomaterials 34, 3626–3638 (2013).
Liu, S., Wei, W. & Wang, J. Theranostic applications of selenium nanomedicines against lung cancer. J. Nanobiotechnol. 21, 96 (2023).
Suarez-Arnedo, A., Torres, F. F. & Clavijo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS One. 15, e0232565 (2020).
Zhang, X., Guo, S. & Fan, R. Dual-functional liposome for tumor targeting and overcoming multidrug resistance in hepatocellular carcinoma cells. Biomaterials 33, 7103–7114 (2012).
Zhuang, J., Tan, J. & Wu, C. Extracellular vesicles engineered with valency-controlled DNA nanostructures deliver CRISPR/Cas9 system for gene therapy. Nucleic Acids Res. 48, 8870–8882 (2020).
Kuerban, K., Gao, X. & Zhang, H. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm. Sin B. 10, 1534–1548 (2020).
Robinson, K., Mock, C. & Liang, D. Pre-formulation studies of Resveratrol. Drug Dev. Ind. Pharm. 41, 1464–1469 (2015).
Qiu, Q. et al. Activation of NLRP3 inflammasome by lymphocytic microparticles via TLR4 pathway contributes to airway inflammation. Exp. Cell. Res. 386, 111737 (2020).
Esteves, A. R. et al. LPS-induced mitochondrial dysfunction regulates innate immunity activation and alpha-synuclein oligomerization in parkinson’s disease. Redox Biol. 63, 102714 (2023).
Tengesdal, I. W. et al. Targeting tumor-derived NLRP3 reduces melanoma progression by limiting MDSCs expansion. Proc. Natl. Acad. Sci. U S A. 118, e2000915118 (2021).
Marshall, E. A. et al. Emerging roles of T helper 17 and regulatory T cells in lung cancer progression and metastasis. Mol. Cancer. 15, 67 (2016).
Guan, X. Cancer metastases: challenges and opportunities. Acta Pharm. Sin B. 5, 402–418 (2015).
Gal, R. et al. Resveratrol improves heart function by moderating inflammatory processes in patients with systolic heart failure. Antioxid. (Basel). 9, 1108 (2020).
Jang, M. et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275, 218–220 (1997).
Fu, X. et al. Targeting of cancer cell death mechanisms by resveratrol: a review. Apoptosis 26, 561–573 (2021).
Li, W. et al. Resveratrol inhibits hexokinases II mediated Glycolysis in non-small cell lung cancer via targeting Akt signaling pathway. Exp. Cell. Res. 349, 320–327 (2016).
Robertson, I. et al. The science of resveratrol, formulation, Pharmacokinetic barriers and its chemotherapeutic potential. Int. J. Pharm. 618, 121605 (2022).
Moossavi, M. et al. Role of the NLRP3 inflammasome in cancer. Mol. Cancer. 17, 158 (2018).
Arjsri, P. et al. Suppression of inflammation-induced lung cancer cells proliferation and metastasis by Exiguaflavanone A and Exiguaflavanone B from sophora Exigua root extract through NLRP3 inflammasome pathway Inhibition. Front. Pharmacol. 14, 1243727 (2023).
Li, Z. et al. Effect of NLRP3 inflammasome on lung cancer immune microenvironment activation and its mechanism. Altern. Ther. Health Med. 30, 86–91 (2024).
Xue, Y. et al. Correlation between the NLRP3 inflammasome and the prognosis of patients with LSCC. Front. Oncol. 9, 588 (2019).
Paik, S. et al. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 18, 1141–1160 (2021).
Hsieh, W. Y. et al. Toll-Like receptors induce Signal-Specific reprogramming of the macrophage lipidome. Cell. Metab. 32, 128–143e125 (2020).
Yang, J., Wise, L. & Fukuchi, K. I. TLR4 Cross-Talk with NLRP3 inflammasome and complement signaling pathways in alzheimer’s disease. Front. Immunol. 11, 724 (2020).
Das, S. et al. Tumor Cell-Derived IL1beta promotes desmoplasia and immune suppression in pancreatic cancer. Cancer Res. 80, 1088–1101 (2020).
Li, Y. et al. IL-1beta promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol. Cancer. 11, 87 (2012).
Mantovani, A., Barajon, I. & Garlanda, C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol. Rev. 281, 57–61 (2018).
Demaria, O. et al. Harnessing innate immunity in cancer therapy. Nature 574, 45–56 (2019).
Amin, M. A. et al. Interleukin 18 induces angiogenesis in vitro and in vivo via Src and Jnk kinases. Ann. Rheum. Dis. 69, 2204–2212 (2010).
Li, S. et al. TLR2 limits development of hepatocellular carcinoma by reducing IL18-mediated immunosuppression. Cancer Res. 75, 986–995 (2015).
Acknowledgements
This research was supported by funds from the National Natural Science Foundation of China (81960026).
Author information
Authors and Affiliations
Contributions
Chunxia Chen wrote the main manuscript text, contributed to review and editing, conceptualization, validation, methodology, investigation, and formal analysis. Huan Huang contributed to investigation, methodology, visualization, formal analysis, data curation, conceptualization, and wrote the original draft, as well as participated in review and editing. Haofeng Zhang wrote the original draft, contributed to review and editing, investigation, conceptualization, and data curation. Junli Huang contributed to visualization, methodology, investigation, and data curation. Xing Zhou contributed to investigation, visualization and formal analysis. Xixiang Xie contributed to investigation, visualization and formal analysis. Liao contributed to investigation, methodology, and formal analysis. Yao Zhou contributed to validation, methodology, and formal analysis. Fangqi Cai contributed to validation and methodology. Guirong Chen contributed to formal analysis and methodology. Yuping Xie contributed to methodology and data curation. Luying Huang contributed to review and editing, visualization, supervision, resources, conceptualization, funding acquisition, investigation, and project administration. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and informed consent
This study was approved by the Animal Care & Welfare Committee of Guangxi Medical University Institutional (No. 202310001).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, C., Huang, H., Zhang, H. et al. In vivo mechanisms of resveratrol liposomes in targeting the TLR4/NLRP3 pathway in lung cancer. Sci Rep 15, 31737 (2025). https://doi.org/10.1038/s41598-025-14587-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14587-8