Abstract
In this study, 2,4-dihydroxybenzaldehyde hydrazone (CELL@HBH) adsorbent was prepared from microcrystalline cellulose powder (CELL) through a simple and effective method. The investigated materials were characterized by Fourier Transform Infrared spectroscopy (FT-IR), Scanning electron microscopy (SEM), elemental analysis, Proton Nuclear Magnetic Resonance (1HNMR), Thermogravimetric analysis (TGA), X-ray diffraction (XRD), and Brunauer-Emmett-Teller (BET) surface area measurements. The prepared (CELL@HBH) adsorbent was applied to remove anionic food colorants carmoisine (E122), ponceau 4R (E124), and Cr(VI) from polluted water samples. The effects of pH, initial concentration, contact time, dosage, temperature, and competing ions have been investigated to maximize the adsorption capacities to reach 476.709 mg/g, 338.789 mg/g, and 190.072 mg/g for E122, E124, and Cr(VI) ions, respectively, at the optimum conditions. The results showed that the adsorption of E122, E124, and Cr(VI) follows pseudo-2nd-order kinetic and Langmuir isotherm models due to the more subordinate error functions and increased correlation coefficient (R2 ≥ 0.999). Thermodynamic studies indicate the adsorption of E122 and E124, as well as Cr(VI) ions, by CELL@HBH adsorbent to be exothermic and spontaneous. DFT calculations were employed to verify the molecular structure, analysis of Frontier Molecular Orbitals (FMOs), molecular electrostatic potential (MEP), and geometry reactivity descriptors (GRDs) for all phases. The prepared adsorbent effectively removed E122, E124, and Cr(VI) from polluted water samples, synthetic mixtures, and colored soft drinks, with a recovery rate of ~ 97%. The CELL@HBH adsorbent has good recycling performance. Under five regeneration and adsorption cycles, it still has removal effect greater than 85% of E122, E124, and Cr(VI), which indicates its high structural stability. The adsorption mechanism of E122, E124, and Cr(VI) onto CELL@HBH adsorbent is elucidated. Ultimately, this study demonstrates that the fast-responsive CELL@HBH adsorbent can be effectively utilized to eliminate E122, E124, and Cr(VI) from a wide range of real water sources. Collectively, the results indicate that the as-prepared CELL@HBH adsorbent is promising for anionic pollutant adsorption and our mechanistic results are of guiding significance in environmental cleanup. This work contributes significantly to understanding how experimental conditions influence the mechanism of E122, E124, and Cr(VI) adsorption by CELL@HBH adsorbent, offering valuable and new insights for future applications and optimizations in the treatment of effluent-containing anionic species.
Similar content being viewed by others
Introduction
The availability of safe water is increasingly scarce in numerous regions of the world1. Many people all over the world struggle to get enough water to drink because of the agricultural needs, manufacturing demands, and population expansion2. In developing nations, about 6 million children per year die as a result of the water pollution problem, according to the WHO. Water can be polluted by organic matters such as herbicides, fuel, synthetic dyes, and hydrocarbons, in addition to inorganic ones, such as salts, acids, phosphates, sulfates, and hazardous heavy metals3. Synthetic dyes are classified in the category of organic chemical pollution. Dyes can be classified into three main classes (cationic, anionic, or non-ionic)4.
Anthraquinone, azo chromophores, are present in many anionic dyes5. Approximately 65% of azo dyes are utilized as food additives in various products, including soft drinks, jams, sweets, and pickles6. The beverage industry’s extensive use of artificial food dyes has immensely improved the aesthetic appeal of various consumables. Although there is an urgent need for sustainable and efficient ways to lessen these dyes’ negative effects on the environment and human health, particularly in children, as a result of strict environmental rules and growing awareness about the possible health concerns they pose7. Carmoisine (E122) and Ponceau 4R (E124) are common food anionic azo dyes8. Significant amounts of them end up in the water effluents, which increases the threat to our lives. The main danger may be due to the cleavage of the azo bond and the formation of carcinogenic aromatic amines. These amines are formed either by the assistance of intestinal microorganisms or due to the presence of azo reductase enzyme in the liver or intestinal wall9. Moreover, the generated aromatic amines in the colon and the liver are eliminated through the kidney10. Sulfanilic acid, which is produced in this reaction as a key product, has the ability to influence cell division, hence leading to carcinogenesis11. Food dyes are nonbiodegradable because of their physicochemical, thermal, and optical stability resulting from their symmetrical aromatic groups. So, their elimination is essential in order to overcome their adverse effects12. A value of 0–4 mg/kg body weight is the acceptable daily intake of E122 and E124 according to the WHO7.
Heavy metals are also classified as dangerous contaminants that pose a great threat to human health and the environment. Chromium (Cr), with two forms: hexavalent [Cr(VI)] and trivalent [Cr(III)], is considered to be one of these dangerous heavy metals. As Cr(III) is insoluble and primarily bonded to organic matter, it is less dangerous than Cr(VI), which is highly soluble, movable, and toxic13. According to the WHO guidelines, the limited Cr(VI)− in drinking water is ≤ 0.05 mg/L14.
Recently, numerous methods have been developed to reduce the levels of pollution in the wastewater such as membrane systems15, flotation16, adsorption17, chemical precipitation18, and ion exchange19.
From an economic and environmental standpoint of view, adsorption has gained favor over these traditional methods. Adsorption is a powerful technique for removing anionic, cationic, or organic contaminants from one phase and transporting them to the outside surface via complexation or ionic exchange and has numerous advantages for wastewater treatment, including inexpensiveness, flexibility, design simplicity, economic viability, effectiveness, and sensitivity to harmful contaminants without the production of secondary pollutants20. Many adsorbents for contaminants’ removal have been documented, including activated carbon21, clays22, chitosan23, cellulose and biomass24, as well as other compounds such as resin25.
Cellulose is an adsorbent that has a large potential for eliminating pollutants from wastewater because of its traditional nature, low cost, and high efficiency, remarkable adsorption capacities, unique structural features, renewable nature, availability, ability to regenerate, environmental friendliness, and biodegradability26.
The purpose of cellulose modifications is to add functional groups. The three hydroxyl groups that are present in every cellulose unit allow cellulose to be readily modified. Numerous methods, including oxidation27, esterification28, etherification29, and halogenation, are used to modify cellulose.
One of the most well-known ways for modifying cellulose is to convert the major C-6 hydroxyl groups to excellent leaving groups like tosylate, mesylate, chloride, bromide, or iodide, and then carrying out a nucleophilic displacement reaction. A variety of cellulose derivatives containing azides, amines, thiols, and heterocycles have been made using this method30,31.
Various adsorbents have been applied for the adsorption of the E122 and E124 food dyes. It was observed that some limitations in these investigations were that (i) the majority of these adsorbents were utilized for the removal of these dyes in a single system, (ii) most of the applied adsorbents were high-cost, low adsorption efficiency, and low reusing ability, and (iii) cellulose-based adsorbents presents an ecofriendly, low cost, available adsorbent but suffer from low adsorption capacity toward anionic species so need further modifications32,33. Consequently, there is a vital need for preparing an adsorbent with the following characteristics: reusable, high adsorption capability, cost-effective, and effectively applied in single and multiple systems in various water samples. Mostafa et al. prepared the bentonite modified with CTAB for the removal of E122 and E124 in single and binary systems12. Kieu et al. used zeolite modified with surfactant for the E124 removal34. Moreover, Zhu et al. applied the alkali-treated fish scales for the E124 adsorption35.
Various published investigations have utilized cellulose-modified adsorbents for the efficient removal of various pollutants. The cellulose modification can be carried out to achieve enhanced physical and chemical properties. As the introduction of polyethyleneimine to natural cellulose resulted in better adsorption of mercury36, as well as of Cr3+and Fe3+37 than unmodified cellulose. Also, cellulose modified with glycidyl methacrylate and functionalized by imidazole has revealed significant removal of Pb2+ from aqueous solutions38. Complexation between cellulose and ethylene-diamine-tetra-acetic dianhydride has also enhanced the adsorption behavior of cellulose towards Cu2+, Cd2+, and Pb2+ ions39. Also, the functionalized Flax fibers with the semicarbazide were utilized to eliminate both Cr(VI) and Alizarin Red S dye from various water samples12. Mostafa et al. modified the dialdehyde cellulose by utilizing cyanoacetohydrazide and CS2, respectively, and used the modified material for the Hg(II) selective removal40.
The novelty of the current investigation lies in the preparation of a novel, affordable, eco-friendly, and reusable adsorbent that has a high efficiency toward anionic species. Moreover, it has high stability in various pH media and at different temperature degrees. According to our knowledge, no research has been reported on the following points:- (i) The formation of CELL@HBH adsorbent through the modification of chlorinated cellulose with di-hydroxybenzaldehyde ligand as well as its characterization, (ii) the use of CELL@HBH for the effective removal of E122, E124, and Cr(VI) from different polluted water samples in single and mixed systems and (iii) studying the molecular structure, electronic properties, and the quantum mechanical calculations of CELL, CELL@HBH, CELL@HBH@E122, CELL@HBH@E124, and CELL@HBH@Cr(VI).
The objectives of the present study are presented in the following points: (i) Modification of native cellulose (CELL) to create CELL@HBH adsorbent; (ii) Characterization of CELL and CELL@HBH using different instrumental performances as elemental analysis, SEM, BET, FTIR, 1HNMR, XRD and TGA; (iii) Experimental investigation of anionic pollutants E122, E124, and Cr(VI) adsorption on the surface of CELL@HBH; (iv) Studying the adsorption of E122, E124, and Cr(VI) anionic species in in multi-contaminant systems; (v) Investigating the different experimental parameters affecting the adsorption process of E122, E124, and Cr(VI) onto CELL@HBH adsorbent, e.g., adsorbent dosage, pH, initial of E122, E124, and Cr(VI) concentrations, and ionic strength; (vi) Conducting adsorption isotherm and kinetic experiments to understand the adsorption mechanism and highest adsorption ability of CELL@HBH adsorbent; (vii) Application of the prepared CELL@HBH adsorbent on real water and soft drinks samples; (viii) Elucidation of the adsorption mechanism of E122, E124, and Cr(VI) onto CELL@HBH adsorbent.
Experimental
Materials
Cellulose powder, phosphorus oxychloride (POCl3), dimethyl formamide (DMF), NaOH (5%), Acetic Acid (5%), Hydrazine Hydrate (HH), Ethanol, Carmoisine dye (C20H12N2Na2O7S2, E122), Ponceau 4R dye (C20H11N2Na3O10S3, E124), Cr(VI), 2,4 dihydroxy benzaldehyde (DHB) and HCl were all analytical grade and they were purchased from Merk.
Preparations
Preparation of deoxycellulose chloride (CELL-Cl)
A sample of 1 g of cellulose powder was suspended in 20 mL of dimethyl formamide (DMF) for about 90 min. After that, 1 mL of phosphorus oxychloride (POCl3) was added to the reaction under mechanical stirring in a water bath at 90 °C for about 1 h. The brown product, obtained from this chlorination step, was filtered off, then washed with DMF, distilled water, and NaOH (5%), and finally with 5% acetic acid solution followed by distilled water. The CELL-Cl became white again after these washing steps, then dried in air for 24 h41.
Preparation of deoxy-cellulose hydrazide (CELL@HH) and estimation of the chloro group content
Firstly, a sample of 0.5 g CELL-Cl was directly reacted with 5 mL of HH without using any solvent. The reaction mixture was stirred under heating for 3 h at 80 °C. The product (CELL-HH) obtained was filtered off, washed with DDW, followed by ethanol, and then dried in air.
In the second step, the concentration of the amino group of (CELL-HH) was estimated using a volumetric method as follows: 40 mL of 0.05 M HCl solution was added to 0.1 g of (CELL-HH) and conditioned for 15 h on a Vibromatic Shaker. The residual concentration of HCl was estimated through the titration against 0.05 M NaOH solution and phenolphthalein as an indicator. The number of moles of HCl interacted with amine groups and consequently the amine group concentration (mmol/g) was calculated from the following Eq. (1).
where, M1 and M2 are the initial and final concentrations of HCl, respectively42.
Preparation of 2,4 di-hydroxy benzaldehyde hydrazone supported deoxycellulose (CELL@HBH) adsorbent
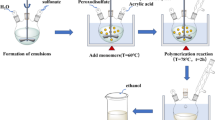
0.3 g of the CELL@HH was added to 0.1 g of the 2,4-dihydroxybenzaldehyde (DHB) in the presence of 20 mL of ethanol solvent. The reaction mixture was stirred in reflux at 80 °C for 6 h. After filtering out the desired material, it was washed with absolute ethanol, then with hot distilled water. And finally, after the washing steps, it was dried in air. The synthetic steps of CELL-HBH are illustrated in Fig. 1.
The synthesis and characterization of CELL@HBH adsorbent and its use for the removal of E122, E124, and Cr(VI) are graphically represented in Fig. 2.
Instrumentation
Fourier transform infrared (FT-IR) spectra and the Surface morphologies (SEM) of the prepared materials were recorded using a Perkin-ElmerSpectrum RX Iusing KBr pellets and Scanning Electron microscopy (A JSM-6510LV), respectively. A PAN analytical X’Pert PRO diffractometer was employed for measuring the X-ray diffraction (XRD) patterns of the CELL, CELL-Cl, and CELL@HBH samples at (4–70°) range of 2-theta (2θ). The specific surface area (SBET) of CELL, CELL-Cl, and CELL@HBH materials was investigated at a temperature of 77 K utilizing the Brunauer Emmet Teller (BET) analysis (Size Analyzer (QUANTACHROME—NOVA 2000 Series). Prior to the BET analysis to remove any moisture, 100 mg of the sample was degassed at a temperature of 120 οC under a 10− 3 mbar vacuum for 12 h. The SBET was calculated as presented in Fig. S1, corresponding to the N2 adsorption isotherm. The CHNO composition determination of the prepared materials was obtained by a Costech ECS-4010 elemental analyzer. The thermal stability of CELL-Cl and CELL@HBH materials was examined by thermogravimetric analysis (Berkin Elmer TGA 4000) at a heating rate of 15 °C/min from 30 to 900 °C. The 1HNMR spectra of the prepared materials were measured in a mixture of DMSO/trifluoroacetic acid (TFA) using a Joel 500 MHZ Japan.
In order to determine the pHPZC, 10 mL of a 0.1 M NaCl solution was added in a series of glasses. 0.1 M HCl or 0.1 M NaOH was added to create solutions with an initial pH (pHi) ranging from 2 to 12. After that, 0.01 g of adsorbent were added to each solution, and they were all stirred for 48 h. The final pH value (pHf) was obtained, and the difference between the pH values at the beginning and the end (ΔpH = pHi-pHf) was plotted against pHi. The value of pHpzc is the point at which the ΔpH vs pHi curve crosses the ΔpH = 0 line.
Adsorption studies
Batch adsorption
The adsorption behavior of E122, E124, and Cr(VI) using CELL@HBH adsorbent was investigated through batch adsorption experiments in 20 mL of each of them and a 0.0075 g of CELL@HBH adsorbent dose. Various parameters were investigated including pH from 2.0 to 12.0 for both E122 and E124 and from 2.0 to 8.0 for Cr(VI) ions, analyte concentration of (50–300 mg/L) for E122, Cr(VI) and (100–300 mg/L) for E124, adsorbent dose (0.005–0.02 g), and contact time (15–180 min) for E122 and E124 and (120 to 360 min) for Cr(VI). The pH was adjusted using diluted HCl (0.1 M) and NaOH (0.1 M) solutions. The removal (%) and adsorption capacity at equilibrium (qe) were determined using Eqs. (2) and (3), respectively.
Ci and Cf are the initial and equilibrium concentration (mg/L), respectively. While m (g) is the CELL@ HBH dose and V (L) is the adsorbate solution volume.
Effect of initial concentration and isotherm investigation
In order to evaluate the influence of initial concentration and investigate the isotherm studies, 20 mL solution of E122, E124, and Cr(VI) were added to a 0.0075 g of CELL@HBH adsorbent at pH of 2 and subjected to a thermostatic shaker at 150 rpm for 2 h for the two dyes and 5 h for Cr(VI) at a temperature of 25 °C.
Three isotherm models were studied, namely the Langmuir, Freundlich, and Dubinin–Radushkevich (D-R) models. The Langmuir isotherm postulates that once an adsorbate has occupied a spot, no more adsorption occurs there, creating a discriminating plateau in the curve, and there is no side commerce or steric interference between molecules that have been adsorbed43. The model equation and the Langmuir separation factor (RL), which is an important parameter that is used to calculate affinities between adsorbents and sorbates, are represented by Eqs. (4) and (5).
On the other hand, the Freundlich isotherm could be considered an empirical model in which there is an interaction between adsorbed molecules (multilayer adsorption) on heterogeneous surfaces with a uniform energy distribution. Also, this model proved that the adsorbate concentration on the adsorbent surface will be increased if there is a growth of adsorbate concentration in the solution without attaining saturation44. The Freundlich isotherm model is represented by Eq. (6). The D–R isotherm model assumes that biosorption is related to surface porosity and pore volume, and examines biosorption energetically. The mean free energy of biosorption (EDR) obtained from the D–R model determines whether the adsorption structure is chemical or physical. 8 < EDR < 16 kJ mol− 1 indicates that the adsorption has a chemical character. If EDR < 8 kJ mol− 1, it determines that the adsorption is physical. The model equation is represented by Eq. (7).
Where, Ce (ppm) is the initial concentration of the studied pollutant at equilibrium, qe (mg/g) is the capacity of the adsorbent for pollutant concentration at equilibrium, qm (mg/g) adsorption maximum amount, 1/n, KL, KF, and KDR are the heterogeneity factor, Langmuir coefficient (L/mg), Freundlich constant ((mg1−(1/n)⋅L1/n)/g), and the Dubinin–Radushkevich constant, respectively. While ε is the adsorption potential and is given by Eq. (8).
where, R (8.314 J/mol K) is the gas constant, and T is the temperature in kelvin.
Effect of contact time and kinetic studies
20 mL solution of 200 mg/L E122, 150 mg/L E124 and100 mg/L Cr(VI) were added to 0.0075 g of CELL@HBH adsorbent at pH 2 and subjected to a thermostatic shaker at 150 rpm at a temperature of 25 °C in order to evaluate the equilibrium time for the adsorption process and to investigate the kinetic studies.
The adsorption process is actually controlled by the slowest step, as the liquid solution is being adsorbed onto the solid adsorbent surfaces in multiple phases, so Pseudo-1st -order, pseudo-2nd -order, and intraparticle diffusion (IPD) kinetic models were studied45.
Pseudo-1st -order, pseudo-2nd -order, and IPD models are expressed in Eqs. (9), (10) and (11).
Where, qe (mg/g) and qt (mg/g) are the adsorption efficiency at equilibrium and at a specific time t (min), respectively. C is the intercept that reflects the boundary layer effect. Further, K1, K2, and Kdiff are pseudo-1st, pseudo-2nd order, and intra-particle diffusion constants, respectively.
Effect of temperature and thermodynamic studies
20 mL solution of 200 mg/L E122, 150 mg/L E124 and100 mg/L Cr(VI) were added to 0.0075 g of CELL@HBH adsorbent at pH 2 and subjected to a thermostatic shaker at 150 rpm at different temperatures between 30 and 45 °C in order to evaluate the equilibrium time for the adsorption process in and to investigate the kinetic studies.
In order to better understand the effect of rising temperature on the adsorption of the three pollutants onto the active sites of the CELL@HBH, three basic thermodynamic parameters were studied: the Gibbs free energy of adsorption (\(\:{{\Delta\:}\:\text{G}}_{\text{a}\text{d}\text{s}}^{\text{o}}\)), the enthalpy change (\(\:{{\Delta\:}\:\text{H}}_{\text{a}\text{d}\text{s}}^{\text{o}}\)), and the entropy change (\(\:{{\Delta\:}\:\text{S}}_{\text{a}\text{d}\text{s}}^{\text{o}}\))46. These parameters for the adsorption process were determined by using Eqs. (12), (13), and (14).
where Kc is the thermodynamic equilibrium constant, but Cad and Ce are the pollutant concentration taken by the adsorbent material at equilibrium (mg/g) and the pollutant concentration at equilibrium (mg/L), respectively. While R represents the universal gas constant, that is equivalent to 8.314 J/mol K.
Removal of E122, E124, and Cr(VI) in multi-contaminant systems
To examine the effectiveness of CELL@HBH in multi-contaminant systems of E122, E124, and Cr(VI), 0.0075 g of CELL@HBH were placed in 20 mL of each binary system solution containing 100 mg/L of each pollutant at pH 2 for the E122 + E124 combination and pH 4 for the other combinations, as Cr(VI) present in K2Cr2O7 solution worked as an inorganic oxidizing agent in very acidic medium and caused deceleration of E122 and E124 azo dyes47. The mixtures were then subjected to shaking at 120 rpm, and throughout a 4 h period, the maximum adsorption for each binary system was recorded hourly.
Error functional analysis
In order to analyze the error distribution between calculated values based on theoretical model correlations and experimental data, a variety of error functions were used to evaluate the isotherm models. The chi-square statistic (χ2), mean square error (MSE), and sum of squares error (SSE), which are explained in Eqs. (15), (16), and (17) respectively, were the three error functions that were utilized48.
where, n is the number of included observations. The subscript cal refers to theoretically calculated data while exp subscript represent experimental data.
Desorption and regeneration
The batch technique was employed to study the regeneration of CELL@HBH over five repeated cycles of the adsorption–desorption procedure. Adsorption experiments for Cr(VI), E122 and E124 were carried out by adding 0.0075 g of CELL@HBH adsorbent to 20 mL solutions of 200 mg/L E122, 200 mg/L E124 and 100 mg/L Cr(VI) for 2 h for both E122 and E124 and 5 h for Cr(VI) ions. After that, CELL@HBH was filtered and washed. For the investigation of the desorption process, 0.0075 g of CELL@HBH@E122, CELL@HBH@E124 were added to 20 mL EDTA (0.1 mol/L) and 0.0075 g of CELL@HBH@Cr(VI) in NaOH (0.5 M); then shaking for 2 h For CELL@HBH@E122, CELL@HBH@E124 and 5 h for CELL@HBH@Cr(VI). This process was repeated for five times. The desorption efficiency is the ratio between the amount desorbed to the solution to the amount adsorbed by CELL@HBH49.
The Desorption efficiency can be calculated using Eq. (18).
Application
In natural water samples
To investigate the applicability of CELL@HBH in removal of studied pollutants from real water samples (Tap, waste and seawater). Samples were spiked with 50, 100, 150, and 200 of E122, E124, and Cr(VI). In advance of the spiking of the pollutants, the natural water samples were digested by the addition of 0.5 g of K2S2O8 and 5 mL H2SO4 98% (w/w) to 1000 mL of water sample and heated for 120 min at 90 °C for complete digestion of organic materials50. Following the samples’ room temperature cooling, 0.0075 g of CELL@HBH was added. The samples were then continuously shaken for 120 min for E122, E124, and 300 min for Cr(VI) at pH 2. Using the appropriate wavelengths (518 nm for E122, 508 nm for E124, and 427 nm for Cr(VI)), a Unicam UV 2100 UV/Visible spectrometer was used to determine the remaining amounts of E122, E124, and Cr(VI).
In colored drinks and industrial samples
CELL@HBH was used to extract E122 from degassed carbonated soft drinks, gum, as well as extraction of E124 from jelly. The carbonated soft drinks (flavored with pomegranate and dyed with E122) were first degassed by exposing them to the air at 25 °C for 120 min. Following digestion (i.e., the breakdown of undesirable substances in the sample) with 4% acetic acid, the E124 dye, strawberry flavoured, gum and jelly were dissolved in distilled water51,52.
0.0075 g of CELL@HBH adsorbent were added to each sample for E122 and E124, and continuous shaking was used for 120 min at pH 2 for each dye. The remaining E122 and E124 were determined using the Unicam UV 2100 UV/Visible spectrometer at the appropriate wavelengths (518 nm for E122, 508 nm for E124, and 427 nm for Cr(VI)).
DFT calculations
Geometry optimizations and other DFT calculations were performed on the CELL, HBH, CELL@HBH, E122, E124, Cr(VI), CELL@HBH@E122, CELL@HBH@E124 and CELL@HBH@Cr(VI). DFT is considered a cost-effective method to approximate electron correlation effects. All DFT calculations were performed by using B3LYP level of theory, Becke’s three parameter (B3) nonlocal exchange with the correlation functional of Lee, Yang, and Parr (LYP)53. Nowadays, the B3LYP level is currently widely used to study organic electronic compounds because the predicted geometries are very reliably and provides good estimations for HOMO–LUMO gaps, in a good agreement with experimental values54,55,56,57. All the calculations, for geometry optimizations were carried out at the B3LYP/6–31 g (d, p) for all atoms. All computations were carried out by using Gaussian09 suite of program58. and full natural bond orbital (NBO) analyses were made to calculate the charge distribution for all molecules by using NBO version 3.159. Gauss View 5.0 package60 was used to obtain various graphic views of molecular shapes of distinctive molecular orbitals.
Highest Occupied Molecular Orbitals (HOMO), and Lowest Unoccupied Molecular Orbitals (LUMO) are very considerable elements of theoretical molecular design61. The electronic properties and reactivity definers such as ionization potential (IP), electron affinity (EA), hardness (\(\:\eta\:)\), softness (\(\:\sigma\:\)), electronegativity (\(\:\chi\:\)), chemical potential \(\:\left(\mu\:\right)\), and electrophilicity index (ω) can be determined from the HOMO and LUMO orbital energies62,63,64,65,66 through Koopman’s theorem67. Equations used to calculate these reactivity descriptors are presented in Table S1.
Results and discussion
Characterization
Elemental analysis
Cl-content analysis
Since modification of cellulose occurred at Cl atom, it is necessary to calculate the Cl content in CELL-Cl. This was carried out by substituting Cl with NH2 group, then using the volumetric method to estimate the NH2 content which is equivalent to Cl content. The analysis revealed that CELL-Cl contains 3.34 mmol-Cl/g).
CHNO analysis of materials
The elemental analysis was evaluated for CELL, CELL-Cl, CELL@HH and CELL@HBH materials to prove the modification steps. The results obtained in Table 1 indicate that the carbon, oxygen and hydrogen content decreased from 46.51%, 46.46%, and 7.03–42.65%, 44.19% and 6.16%, respectively and this may be due to the modification that occurred through the cellulose chlorination.
In the second step, nitrogen content of CELL@HH became 5.58% due to the hydrazine groups introduced instead of chlorine atoms.
In final step of modification, carbon content increased to 48.23% as a result of the insertion of ligand with an aromatic ring rich with carbon atoms to form CELL-HBH. These results confirm that CELL-Cl, CELL@HH and CELL@HBH are successfully formed.
N2 adsorption/desorption isotherm Brunauer–Emmett–Teller (BET) analysis
Using the BET method, the surface area of CELL-Cl and CELL@HBH was examined (Fig. S1) and the findings, which are displayed in Table S2, demonstrate that the BET surface area dropped from 41.007 to 0.390 m2/g for CELL and CELL@HBH respectively. The significant decrease in the surface area after the modification step with the DHB ligand may be due to numerous reasons, like: (i) the occupation of the surface pores by the ligand particles, which lowers the adsorption of N2 molecules used in the surface area measurement process12, (ii) the coating of the surface with the ligand, leading to a decrease in the surface roughness68, and (iii) the reduction of external surface area due to the partial agglomeration of the cellulose69.
FTIR spectra
FTIR spectra of native cellulose, its derivatives CELL-Cl, CELL@HH, and CELL@HBH are represented in Fig. 3.
The FTIR of CELL showed a number of distinctive peaks as the C–O stretching vibrations that appeared in the range of 1000–1200 cm− 1. Moreover, the peaks of C–H stretching vibrations that are present between 2700 and 3000 cm− 170.
FTIR spectrum of CELL-Cl exhibits a new peak at about 818 cm− 1 which may be assigned to the C-Cl bond formed through the chlorination reaction71. Modification with hydrazide-hydrate results in appearance of new peak at 1320 cm− 1 which may be due to C-N stretching72, in addition to disappearing of peak of C-Cl at 818 cm− 1.
In CELL@HBH FT-IR spectrum, the broadness of 1645 cm− 1 peak increased which may be returned to C = N formation73 between the ligand’s aldehyde group and NH-NH2 group of CELL@HH. Moreover, the peak between 3005 and 3708 cm− 1 became broader due.
to increasing of OH groups resulted from interaction between ligand (DHB) and CELL@HH.
SEM
Scanning electron microscope had been used to visualize the morphological appearances of each of CELL (Fig. 4a), CELL-Cl (Fig. 4b), CELL@HH (Fig. 4c), and CELL@HBH (Fig. 4d). The images of SEM are displayed at magnifications of 5000X. The presence of both roughness and pores on the surface of the chlorinated cellulose may be due to chlorination action of the native cellulose with POCl3. Increasing in the surface roughness without any cracking in cellulose structure itself after the treatment of chlorinated cellulose by hydrazine-hydrate and this may be returned to the insertion of hydrazine-hydrate instead of Cl atom. The surface then becomes rougher which may be attributed to the chemical reaction between the CELL-Cl and the CELL@HH to form CELL@HBH composite74. Figure 4e, f shows the SEM images of the CELL@HBH@E122 at two different magnification powers (5000x and 10000x). The observed full covering of the CELL@HBH surface with the E122 particles after the adsorption step confirms the adsorption process.
XRD
Diffraction peaks were shown in XRD patterns in Fig. 5 at roughly 13.18º, 19.5º, 22.36º, and 31.18º. The primary peaks associated with crystallographic planes of (11̅0), (110), (200), and (004), respectively and these peaks were discovered to be related to cellulose75.
The locations of the studied peaks in CELL-Cl and CELL@HBH materials remain similar with a few minor adjustments. The crystallinity indices (CrI) of the samples were determined according to Eq. (19) using the Segal method76.
CrI% was found to be 92.7, 89.6, and 88.3% for native cellulose, CELL-Cl, CELL@HBH. A slight decrease in the crystallinity is observed which may be returned to that the main structure of cellulose doesn’t break down but only Cl atom replace OH group of cellulose in first step and then Cl atom replaced with hydrazine group in second step and finally ligand attached to N atom of hydrazine after removal of water molecule.
Where, \(\:{\text{I}}_{200}\) is the maximum intensity of the 002-lattice diffraction and \(\:{\text{I}}_{\text{a}\text{m}}\)is the intensity of diffraction at 2θ.
1H NMR
The 1HNMR of CELL, CELL@HH and CELL@HBH were investigated by utilizing the DMSO/Trifluoro-acetic acid mixture. Figure represents the 1H NMR of CELL, CELL@HH and CELL@HBH. The 1HNMR of CELL present in (Fig.S2a) showed a peak at 2.03 ppm related to the proton that is present on C2 or C340. 1HNMR of CELL@HH is represented in (Fig.S2b) that showed a new prominent peak, broad signal near 6 ppm that could be related to protons of NH and near 9 ppm may be related to NH277. (Fig.S2c) represents the 1HNMR of CELL@HBH showed a new peak 6.5 to 7.5 ppm related to phenyl ring of induced ligand78.
TGA
The thermal degradation of CELL-Cl and CELL@HBH materials was investigated using thermogravimetry, as seen in Fig. 6a and b, respectively. The curve for CELL-Cl shows three mass losses corresponding to: (i) loss of water adsorbed on the surface in the 30–225 °C interval, (ii) mass losses attributed to loss of hydrochloric acid and condensation of hydroxyl groups present on carbons 2 and 3 in the 225 to 315 °C range, and (iii) decomposition of the organic framework above 315 to 800 °C. The CELL@HBH material also showed three mass.
losses, which were ascribed to water loss, pendant group degradation with condensation of DHB ligand hydroxyl groups and finally, breakdown of the organic support, respectively. At 800 °C, weight content decreased when the DHB ligand was loaded, passing from 29.409% for CELL-Cl to 14.77% for CELL@HBH material. proving that the DHB ligand accelerates the material’s degradation79.
DFT calculations
Molecular geometry
The optimized geometry of the CELL, HBH, CELL@HBH, E122, E124, Cr(VI), CELL@HBH@E122, CELL@HBH@E124, CELL@HBH@Cr(VI) are shown in Fig. 7.
Molecular orbital properties and global reactivity descriptors
The Global Reactivity Parameters of a compound can be predicted from the HOMO-LUMO gap80,81,82. The HOMO is electron donor and LUMO the electron acceptor sites are shown in Fig. 8 for CELL, HBH, CELL-HBH, E122, E124, Cr(VI), CELL@HBH@E122, CELL@HBH@E124, CELL@HBH@Cr(VI).
Energy gap, ΔEgap, measures the reactivity; as the energy gap decreases the reactivity increases. The higher reactivity of CELL@HBH@E122, CELL@HBH@E124, CELL@HBH@Cr(VI) over CELL@HBH over HBH over CELL is cleared from the results of their ΔEgap and the amount of electronic charge transferring as shown in Table S3.
Hard molecules (\(\:\eta\:)\) exhibit a significant energy gap, while soft molecules (\(\:\sigma\:\)) possess a minor energy gap83,84. A soft molecule exhibits greater reactivity than a hard molecule due to its lower ΔE(LUMO−HOMO). From the (σ) and (η) values shown in Table S3, CELL@HBH@E122, CELL@HBH@E124, and CELL@HBH@Cr(VI) are softer than CELL@HBH than HBH than CELL and this confirms the reactivity sequence of these molecules as mentioned above.
The \(\:\chi\:\) is a measure of power of atom(s) to attract the electrons from the other molecules85. A high value of electronegativity (χ) for E122, E124, Cr(VI) suggest strong ability to attract electrons from CELL@HBH, which leads to greater interaction to form their products with CELL@HBH.
The ionization potential (IP) and the electron affinity (EA) can be expressed as negative values of EHOMO and ELUMO, respectively. Greater ionization energy values signify increased stability and chemical inertness, whereas lower ionization energy values suggest greater reactivity of atoms and molecules86. The low ionization energy of CELL@HBH@E122, CELL@HBH@E124, and CELL@HBH@Cr(VI) than CELL@HBH than HBH than CELL indicates their higher reactivity in this order.
According to the definition electrophilicity index ( ω ) it measures the tendency of chemical species to acquire electrons. The greater electrophilicity values of CELL@HBH@Cr(VI), CELL@HBH@E122, CELL@HBH@E124 over CELL@HBH over HBH over CELL suggest strong ability of E122, E124, Cr(VI) to acquire electrons from CELL@HBH to form these products.
Finally, the dipole moment (µ) is a factor that can also provide information about interaction between molecules. The value (µ) of E122, E124, Cr(VI) product with CELL@HBH is higher than (µ) of E122, E124, Cr(VI) or CELL@HBH, this suggested the stronger interactions between them and CELL@HBH to form the compounds.
Molecular electrostatic potential (MEP)
A molecule’s electrostatic potential (MEP) map or surface illustrates the partial charge distribution that exists on it. Molecular electric potential surfaces, also known as electrostatic potential maps or electrostatic potential energy maps, offer a three-dimensional depiction of the charge distributions within molecules.
These maps allow us to visualize variably charged regions of a molecule. Knowledge of the charge distributions are very useful to help determine molecular polarity and can be used to determine how molecules interact with one another. The color spectra are used to facilitate the interpretation of the electrostatic potential energy data, assigning red to represent the lowest value and blue for the highest, effectively illustrating the different intensities of these energy values. The calculated ESP maps of the Cellulose, CELL, HBH, CELL@HBH, E122, E124, and Cr(VI) and their product with CELL@HBH are shown in Fig. 9.
Electrostatic potential (ESP) surface maps visualization for (a) CELL, (b) HBH, (c) CELL@HBH, (d) E122, (e) E124, (f) Cr(VI), (g) CELL@HBH@E122, (h) CELL@HBH@E124, (i) CELL@HBH@Cr(VI) based on the DFT/ B3LYP/6–31 g (d, p) methodology based on the DFT/ B3LYP/6–31 g (d, p) methodology. Blue, green and red correspond to ESP varying from min to max level, the blue and red spheres correspond to ESP surface minima and maxima, respectively. ESP ranges are included in the legend at each figure panel.
Batch adsorption
Adsorption efficiency of raw cellulose and CELL@HBH adsorbent
Preliminary experiments were performed to study the removal efficiency of raw cellulose and CELL@HBH adsorbent towards the removal of E122, E124, and Cr(VI) from aqueous solution at the optimum condition to demonstrate the benefits of the modification and the overall improvement in performance. The results are presented in Fig. 10. Under the same conditions for the investigated pollutants, the findings indicated that the effectiveness of the modification step with the ligand. The removal % utilizing raw cellulose (CELL) was 14%, 15.6%, and 9.1% for E122, E124, and Cr(VI), respectively. On the other hand, for CELL@HBH adsorbent, it was observed that the removal % increased to be 100%, 91%, and 87% for E122, E124, and Cr(VI), respectively. A finding that demonstrates the benefits of the modification and the overall improvement in performance.
Point of zero charge (pHPZC)
In order to understand the E122, E124, and Cr(VI) adsorption mechanism by the prepared composite, the point of zero charge of CELL@HBH material was studied. This investigation was obtained by measuring the pH at the point of zero charge (pHPZC). Commonly, the chelating agent will show greater affinities for anions at a pH value lower than the value of its pHPZC and greater affinities for cations at a pH value higher than the value of its pHPZC. The pHPZC value obtained for the CELL@HBH composite was approximately 5.92 as shown in Fig. S3. So, E122, E124, and Cr(VI) adsorption by the CELL@HBH was expected to be enhanced at a pH value lower than the pHPZC value87.
Effect of pH
By adding 0.0075 g of CELL@HBH to 20 mL of 200 mg/L and 150 mg/L for E122 and E124 aqueous solutions at 25 °C for 2 h, the pH parameter was investigated. The uptake of E122 and E124 was investigated in the pH range (2–12), as shown in Fig. 11. The maximum adsorption capacity for both E122 and E124 was found to be at pH 2 then drops from pH 4 to pH 12. pH parameter was also studied for Cr(VI) metal ion by adding 0.0075 g of CELL@HBH adsorbent to 20 mL of 100 mg/L Cr(VI) ion at 25° C for 5 h and it was discovered that pH 2 is the optimal pH value for the process of adsorption for Cr(VI) metal ion, followed by a fast drop from pH 4 to pH 6, then gradual decrease until it reaches the minimum value of adsorption capacity at pH 8.
The experiment results indicate that pH is a controlling factor in the adsorption mechanism as the uptake of the three pollutants increased whenever pH decreased. This can be explained by the fact that a decrease in solution pH leads to a rise in H+ ion concentration88.
While the anionic food colorant E122 and E124 and dichromate anion have a negative charge on their surfaces and pH produces a positive charge on the adsorbent surface, electrostatic attraction between the negative adsorbate and the positive adsorption sites occurred.
The pHPZC of the CELL@HBH adsorbent, which is 5.92, is another finding that supports this conclusion. When pH of the solution is lower than this pHPZC, the adsorbent surface will have a positive charge, making it a surface that anions may adsorb onto.
Effect of dose
The adsorbent dosage is one of the important factors because it represents the capacity of the adsorbent for a given initial concentration of the adsorbate. It was investigated by adding various weights of CELL@HBH adsorbent in 20 mL aqueous solution of 200 mg/L E122, 150 mg/L E124 and 100 mg/L Cr(VI) at 25 °C for 2 h for both E122 and E124 and 5 h for Cr(VI) at pH 2 for all pollutants.
It was found that at low adsorbent dosage, the dispersion of CELL@HBH particles in the bulk solution is better; that is, all of the active sites on the adsorbent surface are completely uncovered which may accelerate the approachability of pollutants’ molecules to a large number of the adsorbent active sites. Therefore, the adsorption on the surface-active sites reaches a saturated point, performing a high removal capacity. However, at higher adsorbent dosage, the accessibility of adsorbent active sites with higher energy decreases and a larger portion of active sites with lower energy become occupied, leading to a decrease in adsorption process.
It was found that the ideal adsorbent dosage was around 0.0075 g for all of them as shown in Fig. S4 and the acquired results demonstrated that CELL@HBH is a highly effective adsorbent, even at extremely low dosages.
Effect of initial concentration and isotherm studies
Adsorption capabilities increased from 100 to 460 mg/g for E122 and from 266.7 to 339.4 mg/g for E124 as the initial concentration increased from 50 to 250 mg/L for E122 and from 100 to 200 mg/L for E124 and decreased from 266.667 to 198.986 mg/g for Cr(VI) as the initial concentration increased from 50 to 100 mg/L and then increased again from 198.896 to 300.065 mg/g between concentration of 100 to 250 mg/L. Furthermore, as the initial concentration grew from 250 to 300 mg/L for E122 and from 200 to 300 mg/L for E124 and 250 to 300 mg/L for Cr(VI), the adsorption abilities remained fairly stable. The results of the experiments varying the initial concentrations of the studied pollutants over the CELL@HBH samples are illustrated in Fig. S5.
When the initial concentrations of pollutants were increased, the adsorption capacities increased until they reached a maximum level at 200 mg/L, 150 mg/L, and 100 mg/L for E122, E124, and Cr(VI), respectively. The increase in the loading capacities of CELL@HBH adsorbent with increasing pollutants concentrations is due to the interaction between pollutants and the adsorbent which provides the vital driving force to defeat the resistances to the mass transfer of pollutants between the aqueous solution and the bone samples. Moreover, this phenomenon could be explained by the following reasons: at low pollutant CELL@HBH ratios, pollutant adsorption takes place on the high-energy sites, but upon increasing the pollutant CELL@HBH ratios ratio, the higher energy sites are saturated and adsorption commences on the lower energy sites, concluding in a decrease in adsorption efficiency89,90,91.
According to the obtained results in Table S4, R2 values revealed a strong connection (0.99, close to 1) between the Langmuir model predicted and the experimental values, but the reverse was obtained by carrying out Freundlich model. The results showed that the adsorption of E122, E124, and Cr(VI) follows the Langmuir isotherm model due to the more subordinate error functions and more increased correlation coefficient (R2 ≥ 0.999). Hence, these results reflected that the experimental values of pollutants adsorption have a stronger correlation with the Langmuir isotherm model, as shown in Fig. 12.
The RL values observed for all starting concentrations of Cr(VI), E122, and E124 are between 0 and 1. This means that the used adsorbent is suitable to be utilized for the removal of studied species.
The EDR of adsorption from the Dubinin–Radushkevich model was calculated as 9.207, 8.935, and 9.532 kJ. mol− 1for E122, E124, and Cr(VI), respectively. These values suggested that the adsorption process of the three pollutants onto the CELL@HBH was carried out by a chemical mechanism in nature because the sorption energy lies within 8–16 kJ.mol− 1, showing that the adsorption process of E122, E124, and Cr(VI) on the CELL@HBH is chemisorption.
Effect of contact time and kinetic studies
As shown in Fig. S6 it was clear that the removal efficiency increased rapidly with the increase of contact time from 15 min to 120 min for both dyes, E122 (39.681 to 89.383%), E124 (49.122 to 84.711%), and from 120 to 300 min for Cr(VI) (62.567 to 73.262%). The efficiency remains constant when the shaking time is increased to more than 120 min for E122, E124, and more than 300 min for Cr(VI). This indicates that the removal efficiency reached equilibrium at 120 min for E122, E124, and 300 min for Cr(VI).
The initial rapid adsorption declines to a much lower rate approaching equilibrium and gradually levels off (120 min for E122, E124, and 300 min for Cr(VI)) towards the end of the experiment (a dynamic equilibrium is reached between CELL@HBH and the pollutants remaining in the liquid phase). In fact, the fast adsorption at the initial stage is probably due to the increased concentration gradient between the adsorbate in solution and in the adsorbent, as there must be an increased number of vacant active sites available at the beginning.
As in Fig. 13, three kinetic models including, pseudo-1st -order, pseudo-2nd -order, and intraparticle diffusion model (IPD) have been examined. Table 2 represents the estimated 1st -, 2nd -order, and IPD model parameters (K1, K2, Kdiff, qe1, and qe2) and correlation coefficient R2 values obtained from each investigated model nonlinear form.
The calculated adsorption capacities in the case of pseudo-2nd -order for E122, E124, and Cr(VI) were well fitted with the experimental values. Besides, R2 values of pseudo-1st -order were low: 0.96983 for E122, 0.96026 for E124, and 0.92890 for Cr(VI) in comparison with those of pseudo-2nd -order: 0.99827 for E122, 0.99889 for E124, and 0.99756 for Cr(VI). Therefore, pseudo-1st -order cannot be used for explaining the adsorption nature here. So, E122, E124, and Cr(VI) adsorption onto CELL@HBH adsorbent is well-fitted to pseudo-2nd -order.
The intraparticle diffusion plots for E122 and E124 indicated that the adsorption process occurred in three main stages. In stage 1, the slope was very sharp, as the dye molecules were quickly adsorbed onto the outer surface of the adsorbent as many active sites are present. This phase is called boundary layer diffusion, where the dye moves from the solution to the surface of the adsorbent. In stage 2, the line became straighter with a lower slope. This stage showed the dye molecules moving inside the pores of the adsorbent. This step is slower, as with time passing, the number of active sites on the CELL@HBH adsorbent decreases, and the diffusion of E122 and E124 into pores becomes more difficult. In stage 3, the slope became almost flat. This means the system was reaching equilibrium, and most of the active sites were full, and the adsorption slowed down a lot. But the intraparticle diffusion plot for Cr(VI) indicated that the adsorption process occurred in only two stages.
Effect of temperature and thermodynamic studies
It was noted that the adsorption capacity on CELL@HBH decreased with increasing temperature, as shown in Fig. S7 (a plot of ln KC versus (1/T) temperature in Kelvin). Table 3 shows that the negative values of ΔG°ads and ΔH°ads indicate that adsorption of three pollutants on the surface of CELL@HBH is spontaneous and exothermic. The negative value of ΔS°ads advertisements demonstrated an increase in order and a decrease in chaos.
Effect of ionic strength
A variety of species were utilized to investigate the ionic strength impact. This technique is critical because unfavorable ions exist in large amounts in industrial effluent. To investigate the influence of ionic strength, the following species were used: EDTA, CH3COONa, KCl, NaCl, and NaNO3 with concentration of 0.1 M and 1 M under optimal adsorption conditions by adding 0.0075 g of CELL@HBH to a 20 mL solution containing 200 mg/L of E122, 150 mg/L of E124, and 100 mg/L of Cr(VI) at 25 °C for 120 min for both E122 and E124 and 300 min for Cr(VI). As seen in (Fig. S8), the adsorption efficiency for the three pollutants increased as the concentrations of the studied electrolytes increased.
Desorption and regeneration studies
To test the reusability of CELL@HBH, the desorption procedure was performed under optimum parameters. Subsequently, we examined the result of applying adverse eluents like ethanol, EDTA(0.1 M), NaOH(0.5 M), HCl(0.5 M), Na2CO3(0.1 M) and CH3COONa(0.1 M) as presented in (Fig. S9) and it was observed that the effect of EDTA(0.1 M) achieved the best results for the desorption of E122 and E124 while NaOH (0.5 M) was the best eluent for Cr(VI). Hence, HCl, ethanol, Na2CO3, and CH3COONa are poorly affecting the desorption of the three investigated pollutants.
The CELL@HBH reuse was studied for five sorption–desorption cycles at the optimum conditions with sorption efficiency higher than 89% as shown in Fig. S10. It was predicted that CELL@HBH material could be a good sorbent for E122, E124, and Cr(VI) removal from aqueous solutions.
Removal of E122, E124, and Cr(VI) in multi-contaminant systems
Figure 14a-d reflected that a new λmax for each of the studied mixtures appeared that is not far away from the λmax of the single pollutants (518 nm for E122, 508 nm for E124, and 427 nm for Cr(VI)), but in between, which emphasized the equivalent selectivity toward the removal of the three pollutants in the binary and multicomponent systems at equilibrium time as shown in Fig. 15a-d without forming an intermediate.
This behavior not only revealed the greater affinity for these three pollutants but also proved the absence of selectivity of the adsorbent toward adsorbing these pollutants in the bi- and multi-adsorbate systems. This might be attributed to the similarity in their anionic nature. In addition, the elimination capabilities of different combined contaminants under the same conditions were similar to qe of individual E122, E124, and Cr(VI) (476.709, 338.789, and 190.072 mg/g). The results show that the prepared adsorbent was highly effective in removing a mix of anionic pollutants, proving its suitability for water treatment, particularly in systems with multiple pollutants. New overlapped peaks appeared after the formation of binary systems at 518 nm for [E122 + E124], 506 nm for [E124 + Cr(VI)], 525 nm for [E122 + Cr(VI)], and 512 nm for [E122 + E124 + Cr(VI)].
Application
In natural water samples
To evaluate the efficacy of modified cellulose for the adsorption of various contaminants, optimum experimental conditions were applied to natural samples. Standard solutions were utilized to generate calibration curves. Standard solutions (1.0 L) were handled using the optimal experimental conditions described above. Several water samples, including tap water from our lab and sea water from Ras El-bar City in Egypt, were utilized for analysis. Table S5 represents the results of the analysis. The recoveries of spiked samples containing known amounts of each contaminant were studied. The recoverable values varied between 96.00 and 118.101% with RSD < 1.50. These results indicate that modified cellulose can reliably recognize E122, E124, and Cr(VI) in natural water.
In colored soft drinks and food industries
To evaluate modified cellulose performance for anionic dye adsorption, several samples containing E122 and E124 were exposed to the best experimental conditions for both. The calibration curves were created using standard solutions. The dietary samples consist of soft drinks and jelly. Fig.S11 reveals that over 97.5% of the E122 and E124 removal from the tested samples was successful. These data show that CELL@HBH adsorbent may be used to remove E122 and E124 from a variety of materials.
Performance of CELL@HBH adsorbent
The E122, E124, and Cr(VI) anionic species can be effectively separated and removed from a variety of water samples using the CELL@HBH adsorbent. A comparison of the CELL@HBH adsorbent performance with those of the other cited adsorbents is shown in Table 4. When comparing various adsorbents for the remediation of E122, E124, and Cr(VI), it was found that the sorption capacity, type of adsorbent, adsorbent dose, initial concentration, and equilibrium time at which the sorption is performed should be considered. Finally, Table 4 shows that, in comparison to the other adsorbents mentioned, the CELL@HBH has high capacities and efficiencies for the E122, E124, and Cr(VI).
Plausible mechanism of adsorption onto CELL@HBH
The plausible mechanism can be discussed based on the optical images of CELL@HBH before and after adsorption of pollutants; the digital photographs of CELL, CELL-Cl, CELL@HH, and CELL@HBH adsorbent are shown in Fig. 16, respectively. An obvious color change after each step, it converted from the white color of the CELL (Fig. 16a) to the pale-yellow color after modification with hydrazide hydrate (Fig. 16b) and to brown after modification with 2,4 Di-Hydroxy Benzaldehyde (DHB) ligand as in (Fig. 16c). The color of the CELL@HBH changed into greenish yellow after the adsorption of Cr(VI) as in (Fig. 16d). And to dark red and red color after the adsorption of E122 and E124, respectively (Fig. 16e), (Fig. 16f).
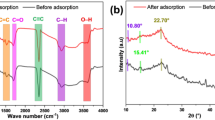
The prediction of the adsorption mechanism of the anionic species was based on the functional group present on the surface of CELL@HBH, as illustrated in Fig.S12a, b, and c. This functional group is the hydroxyl group (-OH). In Fig.S12a, it can be seen that Cr(VI) is adsorbed by CELL@HBH in an acidic environment through the electrostatic attraction between the negatively charged oxygen in Cr(VI) and the positively charged groups (-OH2+) present in the CELL@HBH adsorbent. Figure S12 b, c shows that the adsorption of anionic food dyes (E122 and E124) wasn’t attributed only to electrostatic attraction reactions but also to the n-π interactions between electron-donating groups, such as oxygen groups in the adsorbent and aromatic rings in both dyes100,101. The FT-IR spectra after loading of E122, E124, and Cr(VI) are shown in Fig.S12d. Decreasing in the broadness of the hydroxyl group can be observed in the IR spectra of CELL@HBH@Cr(VI), CELL@HBH@E122, and CELL@HBH@E124 indicating the reaction between + ve charge on (-OH2+) of the CELL@HBH adsorbent and -ve charge on E122, E124 and Cr(VI) through electrostatic attraction in addition to slight shift of this OH group band from 3216 cm− 1 to 3362 cm− 1 for both CELL@HBH@E122 and CELL@HBH@E124 and to 3421 cm− 1 for CELL@HBH@Cr(VI). In the IR spectra of CELL@HBH@E122 and CELL@HBH@E124, new peaks appeared at 1282 cm− 1, attributed to (S = O) stretching of the (SO3) group. Another new peak can be observed at 1505 cm− 1 refers to the C = C of the alkene of aromatic structure102. In the IR spectra of CELL@HBH@Cr(VI), the peak of Cr(VI) was supposed to be found in the range of 500–600 cm− 1, but it wasn’t visible individually due to the presence of overlapping bands in this region (fingerprint region of cellulose material).
Future prospectives
The current investigation demonstrated the preparation of cellulose-based adsorbents for the removal of both organic (E122 and E124) and inorganic (Cr(VI)) species in single and multiple systems. Moreover, several aspects should be taken into consideration for more advanced research:
-
1.
The adsorption of pollutants in multiple systems containing both organic and inorganic contaminants is a vital issue, as this simulates the actual case investigation of real water treatment.
-
2.
Error functions calculation for the isotherm models should be obtained for evaluating the adsorption process with well-fitted models.
-
3.
The application to real water samples should be taken into consideration.
-
4.
Throwing light on the characterization of the adsorbent after the adsorption process, for further confirmation of the adsorption process.
-
5.
Giving more attention to the adsorbent reusability.
Advantages and limitations of the current investigation
The current investigation demonstrates various advantges, including (i) the removal of organic and inorganic pollutants effectively in single and multiple systems, (ii) the characterization of the CELL@HBH before and after the adsorption step is obtained, (iii) the high adsorption capacity for the investigated pollutants, (iv) the application of the prepared material on spiked real water samples, and (v) the cost affordability.
Any water treatment process may be accompanied by various limitations, like selectivity, sensitivity, adsorption efficiency, adsorbent stability, regeneration, cost, and environmental challenges103. The limitations of the current study are (i) this investigation lacks a long-term biodegradability study, (ii) it is limited to batch adsorption, lacks a column investigation, and (iii) the regeneration investigation is limited to 5 cycles; an extended regeneration investigation is needed.
Conclusion
Here, the CELL@HBH adsorbent is a highly effective material for the adsorption of E122, E124, and Cr(VI) from aqueous solutions. The CELL@HBH adsorbent achieved high adsorption capacity at optimal conditions of 476.709 mg/g, 338.789 mg/g, and 190.072 mg/g for E122, E124, and Cr(VI) , respectively. It was discovered that the adsorption of the investigated pollutants depended on oscillating time, initial concentration, CELL@HBH dose, initial solution pH, and temperature. The results showed that the adsorption of E122, E124, and Cr(VI) follows pseudo-2nd-order kinetic and Langmuir isotherm models due to the more subordinate error functions and more increased correlation coefficient (R2 ≥ 0.999) indicating that the adsorption of E122, E124, and Cr(VI) onto CELL@HBH adsprbent is chemisorption in a monolayer patteren. Thermodynamic investigations indicate that the adsorption mechanism is exothermic and spontaneous from -ve ΔG° and ΔH° values. In contrast to earlier research that concentrated on single pollutant systems, this investigation is unique in that it applies CELL@HBH for the simultaneous removal of several pollutants (E122, E124, and Cr(VI)) in single and mixed systems. This makes the substance a flexible option for real-world wastewater treatment situations requiring convoluted pollutant blends. The CELL@HBH adsorbent has good recycling performance. Under five regeneration and adsorption cycles, it still has removal effect greater than 85% of E122, E124, and Cr(VI), which indicates its high structural stability. The adsorption mechanism of E122, E124, and Cr(VI) onto CELL@HBH is elucidated. Ultimately, this study demonstrates that the fast-responsive CELL@HBH can be effectively utilized to eliminate E122, E124, and Cr(VI) from a wide range of real water sources. Collectively, the results indicate that the as-prepared CELL@HBH is promising for anionic pollutant adsorption and our mechanistic results are of guiding significance in environmental cleanup.This work contributes significantly to understanding how experimental conditions influence the mechanism of E122, E124, and Cr(VI) adsorption by CELL@HBH adsorbent, offering valuable and new insights for future applications and optimizations in the treatment of effluent-containing anionic species. Eventually, the eco-friendly CELL@HBH adsorbent can be a viable option for eliminating anionic pollutants from aquatic systems and industrial samples.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Jamshaid, A. et al. Cellulose-based materials for the removal of heavy metals from wastewater–an overview. ChemBioEng Reviews. 4(4), 240–256 (2017).
Mekonnen, M. M. & Hoekstra, A. Y. Four billion people facing severe water scarcity. Sci. Adv. 2(2) (2016).
Kurwadkar, S., Kanel, S. R. & Nakarmi, A. Groundwater pollution: occurrence, detection, and remediation of organic and inorganic pollutants. Water Environ. Res. 92(10), 1659–1668 (2020).
Singhee, D. Review on natural dyes for textiles from wastes. Chem. Technol. Nat. Synth. Dyes Pigments. 99–122. (2020).
Barciela, P., Perez-Vazquez, A. & Prieto, M. A. Azo dyes in the food industry: features, classification, toxicity, alternatives, and regulation. Food Chem. Toxicol. 178, 113935 (2023).
Silva, M. M., Reboredo, F. H. & Lidon, F. C. Food colour additives: A synoptical overview on their chemical properties, applications in food products, and health side effects. Foods 11(3), 379 (2022).
Şenol, Z. M. et al. Removal of food dyes using biological materials via adsorption:a review. Food Chem. 450, 139398 (2024).
Turak, F., Dinç, M., Dülger, Ö. & Özgür, M. U. Four derivative spectrophotometric methods for the simultaneous determination of carmoisine and ponceau 4R in drinks and comparison with high performance liquid chromatography. Int. J. Anal. Chem. 2014(1), 650465 (2014).
Chung, K. T., Stevens, S. E. & Cerniglia, C. E. The reduction of azo dyes by the intestinal microflora. Crit. Rev. Microbiol. 18(3), 175–190 (1992).
Elbanna, K. et al. Microbiological, histological, and biochemical evidence for the adverse effects of food azo dyes on rats. J. Food Drug Anal. 25(3), 667–680 (2017).
Kiziltan, T. et al. Effects of the food colorant carmoisine on zebrafish embryos at a wide range of concentrations. Arch. Toxicol. 96(4), 1089–1099 (2022).
Mostafa, A. G., El-Hamid, A., Akl, M. A. & A. I., & Surfactant-supported organoclay for removal of anionic food dyes in batch and column modes: adsorption characteristics and mechanism study. Appl. Water Sci. 13(8), 163 (2023).
Prasad, S. et al. Chromium contamination and effect on environmental health and its remediation: A sustainable approaches. J. Environ. Manag. 285, 112174 (2021).
Rauf, A. U., Mallongi, A., Daud, A., Hatta, M. & Astuti, R. D. P. Ecological risk assessment of hexavalent chromium and silicon dioxide in well water in Maros Regency, Indonesia. Gaceta Sanitaria. 35S4–S8 (2021).
Munirasu, S., Haija, M. A. & Banat, F. Use of membrane technology for oil field and refinery produced water treatment—A review. Process Saf. Environ. Prot. 100, 183–202 (2016).
Akl, M. & Ahmad, S. A. Ion flotation and flame atomic absorption spectrophotometric determination of nickel and cobalt in environmental and pharmaceutical samples using a thiosemicarbazone derivative. Egypt. J. Chem. 62(10), 1917–1931 (2019).
Hambisa, A. A., Regasa, M. B., Ejigu, H. G. & Senbeto, C. B. Adsorption studies of methyl orange dye removal from aqueous solution using anchote peel-based agricultural waste adsorbent. Appl. Water Sci. 13(1), 24 (2023).
Gupta, V. K., Kumar, R., Nayak, A., Saleh, T. A. & Barakat, M. A. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv. Colloid Interface Sci. 193, 24–34 (2013).
Hussain, S. T. & Ali, S. A. K. Removal of heavy metal by ion exchange using bentonite clay. J. Ecol. Eng. 22(1), 104–111 (2021).
Nayl, A. A. et al. A novel method for highly effective removal and determination of binary cationic dyes in aqueous media using a cotton–graphene oxide composite. RSC Adv. 10(13), 7791–7802 (2020).
Moura, F. C., Rios, R. D. & Galvão, B. R. Emerging contaminants removal by granular activated carbon obtained from residual Macauba biomass. Environ. Sci. Pollut. Res. 25, 26482–26492 (2018).
Barakan, S. & Aghazadeh, V. The advantages of clay mineral modification methods for enhancing adsorption efficiency in wastewater treatment: a review. Environ. Sci. Pollut. Res. 28(3), 2572–2599 (2021).
Akl, M. A., Mostafa, A. G., Abdelaal, M. Y. & Nour, M. A. K. Surfactant supported chitosan for efficient removal of cr (VI) and anionic food stuff dyes from aquatic solutions. Sci. Rep. 13(1), 15786 (2023).
Krim, L., Nacer, S. & Bilango, G. Kinetics of chromium sorption on biomass fungi from aqueous solution. Am. J. Environ Sci. 2(1), 27–32 (2006).
Atia, A. A. Studies on the interaction of mercury (II) and uranyl (II) with modified chitosan resins. Hydrometallurgy 80(1–2), 13–22 (2005).
Gupta, V. K., Carrott, P. J. M., Singh, R., Chaudhary, M. & Kushwaha, S. Cellulose: a review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 216, 1066–1076 (2016).
Yan, W., Liu, J., Zheng, X., Zhang, J. & Tang, K. Wood-derived high-performance cellulose structural materials. e-Polymers 23(1), 20230010 (2023).
Ma, J. et al. Fast adsorption of heavy metal ions by waste cotton fabrics based double network hydrogel and influencing factors insight. J. Hazard. Mater. 344, 1034–1042 (2018).
Rol, F., Belgacem, M. N., Gandini, A. & Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 88, 241–264 (2019).
Aoki, N., Furuhata, K. I., Saegusa, Y., Nakamura, S. & Sakamoto, M. Reaction of 6-bromo‐6‐deoxycellulose with thiols in lithium bromide‐N, N‐dimethylacetamide. J. Appl. Polym. Sci. 61(7), 1173–1185 (1996).
Fox, S. C. & Edgar, K. J. Staudinger reduction chemistry of cellulose: synthesis of selectively O-acylated 6-amino-6-deoxy-cellulose. Biomacromolecules 13(4), 992–1001 (2012).
Ali, M., Rahman, M. M., Islam, M. A. & Khan, M. A. Adsorptive removal of synthetic food dyes from aqueous solution using surfactant-modified bentonite. Appl. Water Sci. 13(3), 98 (2023).
Nayl, A. A. et al. Advances in the modification and applications of cellulosic-based materials for wastewater remediation: a review. Appl. Water Sci. 15(7), 1–36 (2025).
Kieu, T. T. et al. Surface modification of zeolite by cationic surfactant and the application on adsorptive removal of azo dye Ponceau 4R. J. Mol. Struct. 1304, 137619 (2024).
Zhu, K. et al. Adsorption of Ponceau 4R from aqueous solutions using alkali boiled Tilapia fish scales. RSC Adv. 3(47), 25221–25230 (2013).
Navarro, R. R., Sumi, K., Fujii, N. & Matsumura, M. Mercury removal from wastewater using porous cellulose carrier modified with polyethyleneimine. Water Res. 30(10), 2488–2494 (1996).
He, Z. et al. Porous spherical cellulose carrier modified with polyethyleneimine and its adsorption for cr (III) and Fe (III) from aqueous solutions. Chin. J. Chem. Eng. 22(9), 984–990 (2014).
O’Connell, D. W., Birkinshaw, C. & O’Dwyer, T. F. Removal of lead (II) ions from aqueous solutions using a modified cellulose adsorbent. Adsorpt. Sci. Technol. 24(4), 337–348 (2006).
Júnior, O. K., Gurgel, L. V. A., de Freitas, R. P. & Gil, L. F. Adsorption of Cu (II), cd (II), and Pb (II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane Bagasse chemically modified with EDTA dianhydride (EDTAD). Carbohydr. Polym. 77(3), 643–650 (2009).
Mostafa, A. G., Gaith, E. A. & Akl, M. A. Aminothiol supported dialdehyde cellulose for efficient and selective removal of hg (II) from aquatic solutions. Sci. Rep. 13(1), 19507 (2023).
Krylova, R. G. Halogenodeoxy-derivatives of cellulose. Rus. Chem. Rev. 56(1), 97 (1987).
Smits, J. & Van Grieken, R. Synthesis of a chelating cellulose filter with 2, 2′-diaminodiethylamine functional groups. Die Angewandte Makromolekulare Chemie: Appl. Macromolecular Chem. Phys. 72(1), 105–113 (1978).
Nguyen, K. T. et al. Advances in as contamination and adsorption in soil for effective management. J. Environ. Manage. 296, 113274 (2021).
Dabhade, M. A., Saidutta, M. B. & Murthy, D. V. R. Adsorption of phenol on granular activated carbon from nutrient medium: equilibrium and kinetic study. (2009).
Hameed, B. H., Mahmoud, D. K. & Ahmad, A. L. Sorption of basic dye from aqueous solution by pomelo (Citrus grandis) peel in a batch system. Colloids Surf., A. 316(1–3), 78–84 (2008).
Rahman, M. A., Lamb, D., Kunhikrishnan, A. & Rahman, M. M. Kinetics, isotherms and adsorption–desorption behavior of phosphorus from aqueous solution using zirconium–iron and iron modified biosolid biochars. Water 13(23), 3320 (2021).
Sibiescu, D. & Vizitiu, M. Cochenille red dye decoloration and chromate reduction simultaneously in acid medium. In Advanced Topics in Optoelectronics, Microelectronics, and Nanotechnologies IX, Vol. 10977, 98–105 (SPIE, 2018).
Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59(1), 171–177 (2004).
Shakoor, M. B. et al. Exploring the arsenic removal potential of various biosorbents from water. Environ. Int. 123, 567–579 (2019).
Akl, M. A., Dawy, M. B. & Serage, A. A. Efficient removal of phenol from water samples using sugarcane bagasse based activated carbon. J. Anal. Bioanal Tech. 5(2), 1–12 (2014).
Yayayürük, O., Yayayürük, A. E., Özmen, P. & Karagöz, B. PDMAEMA grafted microspheres as an efficient adsorbent for the removal of sunset yellow from pharmaceutical preparations, beverages and waste water. Eur. Polymer J. 141, 110089 (2020).
Zahedi, M., Shakerian, A., Rahimi, E. & Sharafati Chaleshtori, R. Determination of synthetic dyes in various food samples of iran’s market and their risk assessment of daily intake. Egypt. J. Veterinary Sci. 51(1), 23–33 (2020).
Becke, A. D. Density-functional thermochemistry. I. The effect of the exchange‐only gradient correction. J. Chem. Phys. 96(3), 2155–2160 (1992).
Ammar, H. Y. & El-Gharkawy, E. S. R. Adsorption of NO molecule on oxygen vacancy-defected MgO nanotubes: DFT study. Eur. J. Sci. Res. 120, 401–415 (2014).
El-Gharkawy, E. S. R. & Ammar, H. Y. Adsorption of CO on TM-deposited (MgO) 12 nano-cage (TM = Ni, Pd and Pt): a study on electronic properties. J. Nanoelectronics Optoelectron. 13(4), 546–553 (2018).
Abd El-Maksoud, S. A., El-Dossoki, F. I., Migahed, M. A., Gouda, M. M. & El-Gharkawy, E. S. R. New Imidazol-1-ium bromide derivative surfactants as corrosion inhibitors for carbon steel in 1 M HCl solutions: experimental and theoretical studies. J. Bio-and Tribo-Corrosion. 7, 1–15 (2021).
Akl, M. A., El-Mahdy, N. A. & El-Gharkawy, E. S. R. Design, structural, spectral, DFT and analytical studies of novel nano-palladium schiff base complex. Sci. Rep. 12(1), 17451 (2022).
Frisch, M. J. et al. (Gaussian, Inc., 2013).
Glendening, E. D., Reed, A. E., Carpenter, J. E. & Weinhold, F. NBO 3.0 Program Manual (Theoretical Chemistry Institute, University of Wisconsin, 1990).
Dennington, R., Keith, T. & Millam, J. GaussView Semichem Inc. Shawnee Mission KS, Version, 5(8). (2009).
Shoba, D., Periandy, S., Karabacak, M. & Ramalingam, S. Vibrational spectroscopy (FT-IR and FT-Raman) investigation, and hybrid computational (HF and DFT) analysis on the structure of 2, 3-naphthalenediol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 83(1), 540–552 (2011).
Koopmans, T. Über Die Zuordnung Von Wellenfunktionen Und Eigenwerten Zu Den Einzelnen Elektronen Eines Atoms. Physica. 1(1–6), 104–113 (1934).
Wang, H., Wang, X., Wang, H., Wang, L. & Liu, A. DFT study of new bipyrazole derivatives and their potential activity as corrosion inhibitors. J. Mol. Model. 13, 147–153 (2007).
Pauling, L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals: an Introduction To Modern Structural Chemistry Vol. 18 (Cornell University Press, 1960).
Senet, P. Chemical hardnesses of atoms and molecules from frontier orbitals. Chem. Phys. Lett. 275(5–6), 527–532 (1997).
Parr, R. G., Szentpály, L. V. & Liu, S. Electrophilicity index. J. Am. Chem. Soc. 121(9), 1922–1924 (1999).
Chattaraj, P. K., Sarkar, U. & Roy, D. R. Electrophilicity index. Chem. Rev. 106(6), 2065–2091 (2006).
Aziz, T. et al. A review on the modification of cellulose and its applications. Polymers. 14(15), 3206 (2022).
Bayramoglu, G., Tilki, S. & Arica, M. Y. Preparation of amine or carboxyl groups modified cellulose beads for removal of uranium (VI) ions from aqueous solutions. Cellulose 31(8), 5133–5149 (2024).
D’Souza, L., Devi, P., Divya Shridhar, M. P. & Naik, C. G. Use of fourier transform infrared (FTIR) spectroscopy to study cadmium-induced changes in Padina tetrastromatica (Hauck). Anal. Chem. Insights. 3, 117739010800300001 (2008).
Kumar, N. S. et al. Date palm fiber agro-waste biomass for efficient removal of 2, 4, 6-trichlorophenol from aqueous solution: characterization, kinetics, isotherms studies and cost-effective analysis. Desalin. Water Treat.100405 (2024).
Ehsanimehr, S. et al. Layer-by-layer polymer deposited fabrics with superior flame retardancy and electrical conductivity. React. Funct. Polym. 173, 105221 (2022).
Hamed, O. et al. Design, synthesis and antimicrobial properties of cellulose-based amine film. Polym. Bull. 1–15 (2022)..
Astrini, N., Anah, L. & Haryadi, H. R. Adsorption of heavy metal ion from aqueous solution by using cellulose based hydrogel composite. In Macromolecular Symposia, Vol. 353, no. 1, 191–197 (2015).
Moryganov, A. P., Zavadskii, A. E. & Stokozenko, V. G. special features of x-ray analysis of cellulose crystallinity and content in f lax fibres. Fibre Chem. 49(6), 382 (2018).
Darim, R. A., Azizan, A. & Salihon, J. Study of crystallinity index (CrI) of oil palm frond pretreatment using aqueous [EMIM][OAc] in a closed system. In IOP Conference Series: Materials Science and Engineering, vol. 358, no. 1, 012007 (2018).
Al-Mohammadi, N. A. H. A., Al-Fahdawi, A. S. & Al-Janabi, S. S. Design and characterization of new dinuclear macrocyclic dithiocarbamate complexes by the Preparation of a free ligand derived from isopropylamine. Iraqi J. Sci. 1–15 (2021).
Kim, C., Diring, S., Furukawa, S. & Kitagawa, S. Light-induced nitric oxide release from physiologically stable porous coordination polymers. Dalton Trans. 44(34), 15324–15333 (2015).
da Silva Filho, E., Santana, S., Melo, J., Oliveira, F. & Airoldi, C. X-ray diffraction and thermogravimetry data of cellulose, chlorodeoxycellulose and aminodeoxycellulose. J. Therm. Anal. Calorim. 100(1), 315–321 (2010).
Praveen, P. L., Ojha, D. P. & DS, R., & UV spectral characterization of a smectic-C liquid crystal: theoretical support to the experiment. Mol. Cryst. Liq. Cryst. 643(1), 76–82 (2017).
Veligeti, R., Ramakrishna, D. S., Madhu, R. B. & Anireddy, J. S. Synthesis of fluoro and trifluoromethyl substituents containing novel tetracyclic N-benzylated benzopiperazine fused acridone regioisomers using a greener solvent 2-MeTHF and their DFT studies. J. Fluorine Chem. 257, 109989 (2022).
Veligeti, R., Anireddy, J. S., Madhu, R. B. & Ramakrishna, D. S. One pot, three component synthesis of fluoro and trifluoromethyl substituted unsymmetrical dihydropyrazine fused acridine-3-carboxamide using renewable 2-MeTHF solvent and their DFT studies. J. Fluorine Chem. 261, 110019 (2022).
Pearson, R. G. The principle of maximum hardness: chemical hardness, 99–124. (2005).
Reed, A. E., Curtiss, L. A. & Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 88(6), 899–926 (1988).
Parr, R. G., Donnelly, R. A., Levy, M. & Palke, W. E. Electronegativity: the density functional viewpoint. J. Chem. Phys. 68(8), 3801–3807 (1978).
Chakraborty, T., Gazi, K. & Ghosh, D. C. Computation of the atomic radii through the conjoint action of the effective nuclear charge and the ionization energy. Mol. Phys. 108(16), 2081–2092 (2010).
Ouachtak, H. et al. Highly efficient and fast batch adsorption of orange G dye from polluted water using superb organo-montmorillonite: Experimental study and molecular dynamics investigation. J. Mol. Liq. 335, 116560 (2021).
Largo, F. et al. Adsorptive removal of both cationic and anionic dyes by using sepiolite clay mineral as adsorbent: Experimental and molecular dynamic simulation studies. J. Mol. Liq. 318, 114247 (2020).
Arshadi, M., Shakeri, H. & Salvacion, J. W. L. A green adsorbent for the removal of BTEX from aqueous media. RSC Adv. 6(17), 14290–14305 (2016).
Carvalho, M. N., Motta, D., Benachour, M., Sales, M., Abreu, C. A. M. & D. C. S., & Evaluation of BTEX and phenol removal from aqueous solution by multi-solute adsorption onto smectite organoclay. J. Hazard. Mater. 239, 95–101 (2012).
Chin, C. J. M., Shih, L. C., Tsai, H. J. & Liu, T. K. Adsorption of o-xylene and p-xylene from water by SWCNTs. Carbon 45(6), 1254–1260 (2007).
Jamhour, R. M., Al-Msiedeen, A. M., Al-Sharaydeh, R. Z., Jamhour, M. R. & Altweiq, A. M. Zn-Al layered double hydroxide nanoparticles for efficient removal of food dyes from wastewater. Desalination Water Treat. 317, 100036 (2024).
Labiod, K. et al. Removal of Azo dye carmoisine by adsorption process on diatomite. Adsorpt. Sci. Technol. 2022, 9517605 (2022).
Hamada, M. S. & Jabal, R. A. Doped (Ag) ZnO nanoparticles for removal of azo dyes from aqueous solutions. Int. J. Chem. Biochem. Sci. 21, 210–217 (2022).
Toutounchi, S., Shariati, S. & Mahanpoor, K. Application of magnetic ordered mesoporous carbon nanocomposite for the removal of Ponceau 4R using factorial experimental design. Silicon 13(5), 1561–1573 (2021).
Sojoudi, M., Shariati, S. & Khabazipour, M. Amine functionalized Kit-6 mesoporous magnetite nanocomposite as an efficient adsorbent for removal of Ponceau 4R dye from aqueous solutions. Anal. Bioanalytical Chem. Res. 3(2), 287–298 (2016).
Gorzin, F., Bahri, R. & Abadi, M. M. Adsorption of cr (VI) from aqueous solution by adsorbent prepared from paper mill sludge: kinetics and thermodynamics studies. Adsorpt. Sci. Technol. 36(1–2), 149–169 (2018).
Dewa, L., Tichapondwa, S. M. & Mhike, W. Adsorption of hexavalent chromium from wastewater using polyaniline-coated microcrystalline cellulose nanocomposites. RSC Adv. 14(10), 6603–6616 (2024).
Mzinyane, N. N., Mnqiwu, K. M. & Moukangoe, K. M. Adsorption of hexavalent chromium ions using pine sawdust cellulose fibres. Appl. Sci. 13(17), 9798 (2023).
DAS, A. & Singh, S. K. The n→ π* interaction: a rapidly emerging non-covalent interaction. Phys. Chem. Chem. Phys. 17(15), 9596–9612 (2015).
Uddin, M. J., Ampiaw, R. E. & Lee, W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere 284, 131314 (2021).
Ziane, S., Bessaha, F., Marouf-Khelifa, K. & Khelifa, A. Single and binary adsorption of reactive black 5 and congo red on modified dolomite: performance and mechanism. J. Mol. Liq. 249, 1245–1253 (2018).
Akl, M. A., Elawady, D. M., Mostafa, A. G. & El-Gharkawy, E. R. Biogenic nano-silver doped grapefruit peels biocomposite for biosorptive photocatalytic degradation of organic pollutants. Sci. Rep. 15(1), 1–28 (2025).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Magda A Akl: Conceptualization, Methodology, Investigation, Writing – original draft, review, SupervisionAzza AH Fahim: Methodology, Investigation, Writing – original draft.Elsayed RH El-Gharkawy : Conceptualization, Writing and explanation of DFT studies, review, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akl, M.A., Fahim, A.A.H. & El-Gharkawy, ES.R.H. Novel dihydroxybenzohydrazide grafted deoxycellulose for efficient removal of anionic food colorants and hexavalent chromium from wastewater. Sci Rep 15, 29751 (2025). https://doi.org/10.1038/s41598-025-14609-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14609-5