Abstract
The Weight-adjusted Waist Index (WWI) is a novel anthropometric indicator for assessing obesity. Given the well-established association between obesity and periodontitis, this cross-sectional clinical study aimed to investigate the relationship between WWI and severe periodontitis while evaluating its potential as a simple predictive marker for periodontitis. The study analyzed periodontal examination data and WWI measurements from participants in the 2009–2014 National Health and Nutrition Examination Survey (NHANES), employing weighted logistic regression, smoothed curve fitting, and subgroup analyses. Among the 10,307 eligible participants, results demonstrated a significant positive correlation between WWI and the prevalence of stage III/IV periodontitis. After comprehensive adjustment for all confounding factors, each 1-unit increase in WWI was associated with a 1.19-fold higher risk of stage III/IV periodontitis (OR = 1.19, 95% CI 1.06–1.32, P = 0.007977). Subgroup analysis revealed that this association showed statistically significant variation only in the hypertension subgroup, indicating that the WWI-severe periodontitis relationship is particularly applicable to non-hypertensive individuals. These findings confirm that WWI can serve as a novel obesity-based predictive indicator for severe periodontitis.
Similar content being viewed by others
Introduction
Both obesity and periodontitis are common health issues that pose a considerable economic and social burden worldwide1. Although studies have confirmed the relationship between periodontitis and obesity2, it is still necessary to identify an indicator, one that reflects both obesity status and association with periodontitis. This will help the public to understand the obesity conditions that predispose them to periodontitis, thereby better preventing the occurrence and development of periodontitis.
Periodontitis is an inflammatory oral disease and one of human’s six most common diseases3. As the disease progresses, the attachment of periodontal soft tissue and supporting tissues (such as alveolar bone) are gradually destroyed. It can eventually progress to the stage of loosening and loss of teeth. Various factors, such as microorganisms, cytokines, and host reactions, influence the progression of inflammation4.
Research has shown that over 70 types of oral microbiota are associated with periodontal disease, and the microbiota’s dysbiosis can lead to periodontitis and cause systemic diseases5. It has been confirmed that periodontitis is indeed closely related to a variety of systemic diseases, such as hypertension, diabetes, and obesity, as well as adverse pregnancy consequences, atherosclerosis, rheumatoid arthritis, etc.6,7,8. Among these, the correlation between obesity and the prevalence of periodontitis is now a hot research topic.
The World Health Organization (WHO) defines obesity as a disease that is considered to be a systemic, chronic inflammatory disorder caused by an increase in adipose tissue9. Also, the causes include genetic factors, chronic infections, changes in circadian rhythms, and so on10. In 2016, the American Academy of Clinical Endocrinologists (AACE) released guidelines for the treatment of obesity, which included waist circumference (WC) and body mass index (BMI) as the gold criteria for diagnosing obesity11. WC is often a direct reflection of the accumulation of abdominal fat12. It has been argued that relying on BMI does not accurately reflect the amount of muscle and fat mass. BMI is always affected by differences in age, gender, and ethnicity13,14. Therefore, WC is often chosen over BMI as a good indicator of body fat content in individuals when exploring obesity-related diseases. However, there is an undeniable correlation between WC and BMI, which directly leads to the fact that WC is not suitable for use as an indicator of obesity alone. In 2018, Park et al. proposed a new obesity index, the WWI, which makes WC and weight standardized, making obesity status easy to capture15. In conjunction with the previous description, WWI extracts the benefits of WC while weakening its correlation with BMI and reflects centripetal obesity independent of body weight16.
Regarding the impact of abdominal obesity (i.e., centripetal obesity) on systemic health status, existing studies have confirmed its frequent association with poor metabolic status17, making it a high-risk factor for metabolic syndrome18. In studies examining the relationship between abdominal obesity and periodontitis, research has clearly demonstrated an association between abdominal obesity and both attachment loss and bleeding on probing, whereas no such association was observed with generalized obesity19.
In summary, given the established correlation between obesity and periodontitis, coupled with the unique representation of abdominal obesity by the novel obesity index WWI, we contend that investigating the relationship between WWI and periodontitis holds significant importance. Accordingly, we designed this cross-sectional study based on the NHANES, aiming to elucidate the profound association between WWI and periodontitis, particularly severe periodontitis, through large-scale population data analysis.
Materials and methods
Survey description
Data for this clinical cross-sectional study were obtained from the United States NHANES. The NHANES project is implemented by the Centers for Disease Control and Prevention in the United States to use stratified, multi-stage probability surveys to evaluate the health and nutritional status of noninstitutionalized Americans20. NHANES is authorized by the National Center for Health Statistics (NCHS) Ethics Review Board, which requires all participants to sign a written informed consent form before participation. No additional approval from the Institutional Review Board was required for the secondary analysis. The participants completed home interviews to provide demographic, socioeconomic, and health-related information. The physical examination and laboratory tests were performed at a mobile examination center (MEC). The detailed design and data on the NHANES study are publicly available at http://www.cdc.gov/nchs/nhanes/.
Study population
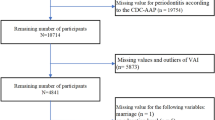
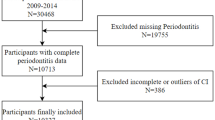
The data for this study were derived from participants in NHANES 2009–2014. Initially, a total of 30,468 participants were enrolled in the study. Subsequently, rigorous screening was performed based on the following inclusion/exclusion criteria. The inclusion criteria required participants to be: (1) participants aged ≥ 30 years; (2) those with complete periodontal examination records; and (3) individuals with available WWI-related anthropometric measurements. Participants failing to meet any of these criteria were excluded. After applying these selection criteria, a final cohort of 10,307 eligible participants was included for analysis, with the detailed screening process illustrated in Fig. 1.
Assessment of weight-adjusted waist index
WWI is an index used to assess obesity, with higher values indicating more significant obesity. Data on WC and body weight were collected from each subject at the MEC by technicians with standardized training. The WWI was obtained by further calculation: WC in centimeters divided by the square root of weight in kilograms. Therefore, the WWI obtained was a continuous variable, and in this study, all WWI data were grouped according to quartiles (Q1, Q2, Q3, Q4) to better analyze its relationship with periodontitis.
The diagnosis of periodontitis
Periodontal probing examinations were carried out (at all teeth except third molars and six sites per tooth) in the MEC by skilled and calibrated examiners with a periodontal probe following the NHANES examination protocol21. Adults aged 30 years and older were eligible for a full-mouth periodontal examination (FMPE) that included measurements of gingival recession and pocket depth. The primary clinical parameters obtained included: probing pocket depth (PPD) and gingival recession (GR). The clinical attachment loss (CAL) is calculated as the sum of PPD and the absolute value of GR.
Following the recommendations of the American Academy of Periodontology, this study employed the 2018 classification system jointly established by the American Academy of Periodontology and the European Federation of Periodontology (AAP/EFP) for the diagnosis of periodontitis22.
The diagnostic criteria for periodontitis are as follows: either (1) interdental CAL ≥ 3 mm detected in ≥ 2 non-adjacent teeth, or (2) buccal/lingual CAL ≥ 3 mm with PPD > 3 mm observed in ≥ 2 teeth. The staging of periodontitis is determined by the maximum interdental CAL: Stage I (1–2 mm attachment loss), Stage II (3–4 mm attachment loss), and Stage III/IV (≥ 5 mm attachment loss). Cases initially classified as Stage II should be reclassified as Stage III when the maximum PPD is ≥ 6 mm; Stage III cases should be reclassified as Stage IV when fewer than 20 teeth (10 pairs) remain.
Definition of covariates
The demographic covariates involved in the study include gender, age, race, education level, marital status, household poverty income ratio (PIR), alcohol consumption, smoking status, sleep disorder, physical activity, mental health (depression), hypertension, diabetes, the frequency of using dental floss/devices over the past 7 days, and BMI. Among them, race is divided into Mexican Americans, other Hispanics, non-Hispanic whites, non-Hispanic blacks, non-Hispanic Asians, or the other race. Education level is divided into less than high school, high school or equivalent, and some college or higher. Marital status is divided into married, living with a partner, widowed/divorced/separated /never married. PIR was divided into three levels: PIR ≤ 1 as low, PIR 1–3 as medium, and PIR > 3 as high23. Alcohol consumption is divided into never, mild-to-moderate, and heavy24,25. Smoking status is divided into never, former, and now26,27. Physical activity is divided into never/mild, moderate, and intense intensity28. Sleep disorders are divided into yes or no. Mental health is mainly evaluated against depression; according to the questionnaire, the PHQ-9 score is subdivided into no depression and depression. Hypertension and diabetes are divided into yes and no. The frequency of using dental floss/devices over the past 7 days is divided into 0 to 7 days. BMI is a continuous variable, which is further subdivided into normal weight (BMI < 25), overweight (BMI 25–30), and obese (BMI > 30)29.
At the same time, for partial loss of covariates, we use multiple imputations based on five replicates and chain equations to maximize statistical ability and minimize possible bias caused by missing data.
Statistical analysis
Given the complex multistage whole population survey, all of our statistical analyses were conducted using appropriate NHANES sampling weights in accordance with CDC guidelines. Therefore, we used NHANES 2009–2014 sampling weights to ensure that the analyses provide unbiased, accurate performance estimates for the entire population.
Descriptive statistics were performed on the overall data of the included population to summarize and generalize the distributional characteristics of the study sample. Continuous variables in covariates were presented as survey-weighted means (95% confidence interval) (95% CI), while categorical variables were presented as survey-weighted percentages (95% CI).
In this study, WWI was analyzed as the independent variable in two distinct forms: the continuous form designated as “WWI (continuous)” and the categorical form categorized by quartiles (Q1–Q4) and uniformly termed “WWI (quartile)”. Periodontitis was specified as the dependent variable, with stage III/IV periodontitis serving as the primary outcome of interest, while participants without stage III/IV periodontitis constituted the reference group.
Survey-weighted linear regression was used for continuous variables, and the survey-weighted chi-square test was used for categorical variables. The P-values of their distributions were also tested. Logistic regression models were then fitted by adjusting for covariates (unadjusted model, model I (adjusting for covariates such as age, gender, and race), and model II (adjusting for all covariates)) to reveal the possible relationship between the two thoroughly. The results were expressed as odds ratio (OR), 95% CI, and P-value.
Smoothed curves were also fitted for WWI (continuous) and “periodontitis” to analyze whether there was a linear correlation. Finally, saturation effect analysis was conducted to identify inflection points in their dose-response relationship.
In addition to the above analysis, this study also conducted the subgroup analysis and interaction test to study the potential impact of gender, BMI, hypertension and diabetes, mental health, alcohol consumption, smoking status, sleep disorders, frequency of using dental floss/devices over the past 7 days, and physical activity on the relationship between WWI and severe periodontitis.
All the statistical analyses of this study were performed using SPSS software version 26 (https://www.ibm.com/products/spss-statistics), Stata software version 16 (https://www.stata.com), R software version 4.2.2 (http://www.r-project.org), and the EmpowerStats software version 4.2 (http://www.empowerstats.com).
P < 0.05 was considered statistically significant.
Results
Baseline characteristics of participants
A total of 10,307 participants were recruited for this study. After survey weighting, the mean age of all participants was 50.69 years, with males accounting for 49.15% and females for 50.85%. The prevalence of obesity was 37.38%, while the prevalence rates of non/stage I/II and stage III/IV periodontitis were 62.71% and 37.29%, respectively. The baseline distribution of covariates for all participants is detailed in Table 1.
The columns in the table present stratified groups based on WWI quartiles, which were defined as follows: Quartile 1(Q1): 8.62–10.55, Quartile 2(Q2): 10.55–11.07, Quartile 3(Q3): 11.07–11.60, Quartile 4(Q4): 11.60–14.79.
As demonstrated in the table, significant differences were observed in the distribution of various factors across WWI quartiles (Q1–Q4), including gender, age, race, education level, marital status, PIR, alcohol consumption, smoking status, physical activity, mental health (depression), hypertension, diabetes, frequency of using dental floss/devices over the past 7 days, BMI, and periodontitis. Notably, the prevalence rates of stage III/IV periodontitis across Q1–Q4 WWI groups were 28.27%, 35.41%, 41.26%, and 46.79%, respectively.
The association between WWI and stage III/IV periodontitis
Logistic regression analysis
The interrelationship between WWI and stage III/IV periodontitis was determined using logistic regression analysis, and the results are presented in Table 2, including the OR, 95% CI, and P-value.
Analysis of the relationship between WWI and stage III/IV periodontitis revealed significant positive associations in both unadjusted and adjusted models. For continuous WWI, the odds ratios were as follows: unadjusted model (OR = 1.50, 95% CI 1.40–1.62, P < 0.0001), Model I (OR = 1.29, 95% CI 1.19–1.40, P < 0.0001), and Model II (OR = 1.19, 95% CI 1.06–1.32, P = 0.007977).
Similarly, significant associations were observed between WWI (quartiles) and stage III/IV periodontitis. Compared to Q1, the Q4 group showed:
-
1.
1.23-fold higher risk in the unadjusted model (OR = 2.23, 95% CI 1.93–2.58, P < 0.0001, P for trend < 0.0001).
-
2.
0.58-fold higher risk in Model I (OR = 1.58, 95% CI 1.33–1.87, P = 0.000004, P for trend < 0.0001).
-
3.
0.27-fold higher risk in Model II (OR = 1.27, 95% CI 1.03–1.57, P = 0.041239, P for trend = 0.0228).
Non-linearity and saturation effect analysis
The smoothed curve fitting results (Fig. 2) confirmed a nonlinear relationship between WWI (continuous) and stage III/IV periodontitis. Furthermore, saturation effect analysis identified inflection points at 9.83 and 12.38 (Table 3). Specifically, when WWI was below 9.83, each 1-unit increase was associated with a 1.26-fold higher risk of stage III/IV periodontitis (OR = 2.26, 95% CI 1.21–4.19, P = 0.0101). For WWI values between 9.83 and 12.38, each 1-unit increase corresponded to a 12% elevated risk (OR = 1.12, 95% CI 1.02–1.23, P = 0.0148), while when WWI exceeded 12.38, each additional unit led to a more substantial 1.68-fold increased risk (OR = 2.68, 95% CI 1.37–5.21, P = 0.0038). The log-likelihood ratio tests demonstrated statistically significant threshold effects (P < 0.001), confirming the robustness of this non-linear association. These findings reveal a clear dose–response relationship between WWI levels and severe periodontitis (stage III/IV), with progressively stronger effect sizes observed at higher exposure levels.
Smooth curve fitting for WWI and stage III/IV periodontitis. A nonlinear relationship between WWI and stage III/IV periodontitis was detected by the generalized additive model.The solid and dashed line denoted the estimated values and their corresponding 95% confidence interval, respectively. The adjusted factors involved gender, age, race, education level, marital status, PIR, alcohol consumption, smoking status, sleep disorder, physical activity, mental health (depression), hypertension, diabetes, frequency of using dental floss/devices, and BMI.
Subgroup analysis and sensitivity analysis
The subgroups were divided according to hypertension, diabetes, sleep disorder, mental health, alcohol consumption, smoking status, BMI, physical activity status, frequency of using dental floss/devices, and gender. The analysis results are shown in Fig. 3. The effect of WWI on stage III/IV periodontitis was consistent across subgroups of diabetes, sleep disorder, mental health, alcohol consumption, smoking status, BMI, physical activity status, frequency of using dental floss/devices, and gender, which means that these stratified conditions had no significant effect on the positive association between WWI and severe periodontitis. An inconsistent interaction was demonstrated only in the hypertension subgroup (P for interaction = 0.0003), suggesting that this association applies more to those without hypertension.
Subgroup analysis for the association between WWI and stage III/IV periodontitis. The effect of WWI on severe periodontitis was consistent across subgroups of gender, BMI, diabetes, mental health, alcohol consumption, smoking status, sleep disorder, frequency of using dental floss/devices and physical activity status. An inconsistent interaction was demonstrated only in the hypertension subgroup (P for interaction = 0.0003).
Discussion
This cross-sectional study included 10,307 participants, with strict control of major covariates to accurately elucidate the relationship between WWI and severe periodontitis. Logistic regression analyses demonstrated a significant positive association between WWI and stage III/IV periodontitis risk after comprehensive adjustment for confounding factors: each 1-unit increase in WWI was associated with a 19% higher risk of stage III/IV periodontitis (OR = 1.19, 95% CI 1.06–1.32, P = 0.007977). When analyzed by WWI quartiles, participants in the highest quartile (Q4) had a 27% increased risk of stage III/IV periodontitis compared to those in the lowest quartile (Q1) (OR = 1.27, 95% CI 1.03–1.57, P = 0.041239), with a significant dose–response trend (P for trend < 0.05). Subgroup analyses further confirmed the stability of this association across most stratified factors, including gender, BMI, and diabetes status. However, a significant interaction was observed in the hypertension subgroup (P for interaction = 0.0003), suggesting this association may be clinically more relevant for non-hypertensive populations. These findings indicate that controlling WWI growth may help reduce periodontitis risk, particularly in non-hypertensive individuals where this preventive strategy might be most effective.
Periodontitis, a chronic inflammatory oral disease, is accompanied by typical clinical manifestations, including loss of periodontal tissue support, bleeding on probing, gingival recession, loss of clinical adhesion, loss of bone tissue, and reduced tooth mobility30. The presence of periodontal pathogens, their metabolic byproducts, and the elevation of pro-inflammatory bioactive molecules in microcirculation cause local inflammation of oral supporting tissues. At the same time, periodontal disease affects the level of immune response throughout the body, thus affecting general health. Periodontitis is, therefore, closely related to diseases such as type 2 diabetes, cardiovascular disease, and obesity.
Obesity, as a chronic disease, it has been confirmed that obesity-related persistent systemic chronic inflammatory state has a specific, close correlation with periodontitis in terms of pathogenesis and prognosis31,32. An animal experiment confirmed that the expression levels of inflammatory mediators associated with periodontitis were significantly higher in obese rats33. Meanwhile, compared to non-obese individuals, obese individuals secrete higher tumor necrosis factor (TNF- α) levels in their adipose tissue and interleukin-6(IL-6), leading to systemic inflammation34,35. TNF-α can induce destruction of alveolar bone and connective tissue by stimulating osteoclast formation. Therefore, TNF-α triggers early periodontitis in obese populations36. In addition, another possible mechanism leading to the link between obesity and periodontitis is the presence of specific periodontal pathogens in obese populations. This pathogen specifically recognizes obese people and is also found in periodontal biofilms. This pathogen is nodular Selenomonas noxia (S. noxia), which accounts for 41.05% of the total bacterial count in saliva. S. noxia belongs to the phylum Firmicutes, and an elevated relative proportion of this bacterium has been reported in the gut microbiota of obese individuals37,38.
The relationship between obesity and periodontitis is well known, but how to clarify this relationship is still an urgent problem. In other words, we must find an indicator reflecting the relationship. Compared to participants with normal BMI, obese participants had a 1.45-fold higher risk of periodontal attachment loss19. Current research suggests this increased risk may be related to obesity-induced immune-inflammatory responses, where expansion of abdominal white adipose tissue promotes macrophage migration and neutrophil infiltration to infection sites while elevating production of inflammatory factors39. Another study demonstrated that each 5-unit increase in BMI or 12 cm increase in waist circumference corresponds to a significant elevation in disease risk40. However, BMI cannot differentiate between fat and muscle mass, potentially overlooking individuals with excessive body fat but normal overall weight. It also fails to distinguish between central and peripheral obesity while being influenced by age, gender and ethnic factors41,42. Also, some studies found BMI only associated with mild periodontitis without significant correlation to severe cases43,44.
Notably, while some studies have found significant associations between BMI/WC and periodontitis2. However, other researchers have suggested that periodontitis is not related to BMI and WC, giving the reason that the relationship between obesity and periodontal disease varies by region and ethnicity45. In contrast to traditional anthropometric measures like BMI which incompletely captures fat distribution patterns, or waist-to-height ratio (WHtR) that primarily considers height proportion without incorporating total body weight, WWI provides a balanced assessment of central obesity by simultaneously accounting for both WC and body weight parameters46. So, WWI is effective at capturing this critical change47. In other words, by considering WC (a direct measure of visceral fat), WWI improves on this shortcoming of BMI.
Addressing the relationship between WWI and systemic diseases, studies have shown that increased WWI may be an essential risk factor for hyperuricemia48; urinary albumin excretion16, and abdominal aortic calcification49. In addition to this, there is a positive correlation between WWI and new-onset hypertension, diabetes mellitus, and even all-cause and cardiovascular mortality50,51,52. As a result, WWI offers significant advantages in assessing individual health risk factors and overall health status.
Due to these contradictory views, clarifying the relationship between this indicator (WWI) and periodontitis is critical.
Therefore, our study hypothesized some correlation between WWI and severe periodontitis. Our findings suggested that WWI (which integrates measures of central obesity and body weight) may possess superior predictive value for periodontitis risk. This indicates that WWI could potentially serve as a key biomarker for monitoring the progression of periodontitis, particularly in cases of severe periodontitis (stage III/IV). These results further elucidate the association between obesity (especially central obesity) and periodontitis (particularly severe cases), providing a more comprehensive understanding of their relationship.
In our discussion of this association, we found that obesity may contribute to periodontitis through distinct pathological mechanisms, including altered adipokine release, induction of inflammatory responses, immune dysregulation, and oral microbiota disturbances. Concurrently, studies suggest that visceral fat reflected by WWI, secretes pro-inflammatory cytokines, and these inflammatory changes may modify the body’s immune response, increasing susceptibility to bacterial infections while exacerbating the inflammatory and destructive processes of periodontitis induced by oral micro-organisms53. Another study indicates that elevated WWI may also reflect adipose tissue dysfunction, leading to the synthesis and secretion of various pro-inflammatory cytokines54. Therefore, we posit that these findings collectively suggest a genuine correlation between WWI and the development of periodontitis, though the precise and unique intrinsic mechanisms underlying this association still require further investigation.
Based on the correlation between WWI and periodontitis, this study further revealed that WWI assessment holds particular clinical value for periodontitis in non-hypertensive populations. The underlying mechanisms may involve: the fact that WWI, as a sensitive indicator of central obesity, directly reflects visceral fat accumulation. Adipose tissue secretes pro-inflammatory cytokines, including TNF-α and IL-634, which may activate the NF-κB signaling pathway in periodontal tissues. This activation could subsequently upregulate matrix metalloproteinase expression, promote osteoclast differentiation, leading to alveolar bone resorption, and compromise gingival epithelial barrier function. In hypertensive patients, pre-existing vascular pathologies might obscure this direct inflammatory linkage. Therefore, the relationship between WWI and periodontitis may be more pronounced in non-hypertensive individuals.
However, the regulatory mechanisms among these three factors (WWI, periodontitis, and hypertension status) still require further investigation for confirmation.
Finally, our research group summarized the advantages and limitations of the study. The advantages of our study are as follows: Firstly, this study was based on the NHANES and spanned three research cycles. Therefore, the sample size was sufficiently large. We have adopted the weight standards recommended by the official database to ensure that the results have greater statistical power and reliability. Secondly, based on previous studies, this study included comprehensive covariates. Secondly, in terms of diagnostic criteria, this study innovatively adopted the new 2018 classification system for periodontitis to define and categorize the dependent variables. This approach has significantly enhanced the reliability of our analytical results. Additionally, by adjusting for potential confounding factors, multiple subgroup analyses were conducted to clarify the applicability of the conclusions. The limitations of our study are as follows: To begin with, a significant portion of the data collected in the study relies on participant self-reporting. Although previous studies have also confirmed that the data provided by this database has high accuracy and credibility, we still believe this may introduce some recall bias. In the second place, although all periodontal examinations were performed by calibrated examiners following standardized NHANES protocols, the lack of examiner calibration details means we cannot completely exclude potential measurement variability. This limitation should be considered when interpreting our findings. Thirdly, NHANES can only represent the population situation in the United States region, and the generalizability of the conclusion is slightly weaker in other countries and populations. In addition, the update of periodontal examination data available from NHANES as of 2014 has limited the validity of this study. Therefore, the latest data is needed to verify and supplement this conclusion.
Conclusion
This study demonstrates a significant association between WWI and periodontitis, with a progressive increase in periodontitis incidence—particularly for stage III/IV severe periodontitis—observed with rising WWI levels. These findings suggest that WWI, as a simple and cost-effective assessment metric, could effectively identify high-risk populations for periodontitis (e.g., patients in primary care settings), providing a basis for targeted screening. Future research should further investigate whether weight-loss interventions can improve periodontal health outcomes in individuals with elevated WWI, thereby fully realizing the clinical and public health value of this index. It must be emphasized that current results demonstrate correlation rather than causation, requiring validation through longitudinal cohort studies with expanded sample sizes. Additionally, in-depth investigations across diverse ethnicities and geographical populations will contribute to a more comprehensive understanding of the WWI-periodontitis relationship, ultimately providing a stronger theoretical foundation for global periodontitis prevention strategies.
Data availability
The NHANES data of our study are openly available at https://www.cdc.gov/nchs/nhanes/.
References
Zhao, P., Xu, A. & Leung, W. K. Obesity, bone loss, and periodontitis: The interlink. Biomolecules https://doi.org/10.3390/biom12070865 (2022).
Liu, L. et al. Association between obesity and periodontitis in US adults: NHANES 2011–2014. Obes Facts 17, 47–58. https://doi.org/10.1159/000534751 (2024).
Chen, M. X., Zhong, Y. J., Dong, Q. Q., Wong, H. M. & Wen, Y. F. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 48, 1165–1188. https://doi.org/10.1111/jcpe.13506 (2021).
Makkar, H. et al. Periodontal, metabolic, and cardiovascular disease: Exploring the role of inflammation and mental health. Pteridines 29, 124–163. https://doi.org/10.1515/pteridines-2018-0013 (2018).
Gao, L. et al. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 9, 488–500. https://doi.org/10.1007/s13238-018-0548-1 (2018).
Ahn, Y. B. et al. Periodontitis is associated with the risk of subclinical atherosclerosis and peripheral arterial disease in Korean adults. Atherosclerosis 251, 311–318. https://doi.org/10.1016/j.atherosclerosis.2016.07.898 (2016).
Hajishengallis, G. & Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 21, 426–440. https://doi.org/10.1038/s41577-020-00488-6 (2021).
Zhang, J. et al. The effects of Porphyromonas gingivalis on atherosclerosis-related cells. Front. Immunol. 12, 766560. https://doi.org/10.3389/fimmu.2021.766560 (2021).
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781. https://doi.org/10.1016/s0140-6736(14)60460-8 (2014).
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech. Rep. Ser. 894, i-xii, 1–253 (2000).
Mechanick, J. I., Hurley, D. L. & Garvey, W. T. Adiposity-based chronic disease as a new diagnostic term: The American association of clinical endocrinologists and American college of endocrinology position statement. Endocr. Pract. 23, 372–378. https://doi.org/10.4158/ep161688.Ps (2017).
Cornier, M. A. et al. Assessing adiposity: a scientific statement from the American Heart Association. Circulation 124, 1996–2019. https://doi.org/10.1161/CIR.0b013e318233bc6a (2011).
Jackson, A. S. et al. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int J Obes Relat Metab Disord 26, 789–796. https://doi.org/10.1038/sj.ijo.0802006 (2002).
Lam, B. C., Koh, G. C., Chen, C., Wong, M. T. & Fallows, S. J. Comparison of Body Mass Index (BMI), Body Adiposity Index (BAI), Waist Circumference (WC), Waist-To-Hip Ratio (WHR) and Waist-To-Height Ratio (WHtR) as predictors of cardiovascular disease risk factors in an adult population in Singapore. PLoS One 10, e0122985. https://doi.org/10.1371/journal.pone.0122985 (2015).
Park, Y., Kim, N. H., Kwon, T. Y. & Kim, S. G. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep 8, 16753. https://doi.org/10.1038/s41598-018-35073-4 (2018).
Qin, Z. et al. The association between weight-adjusted-waist index and increased urinary albumin excretion in adults: A population-based study. Front Nutr 9, 941926. https://doi.org/10.3389/fnut.2022.941926 (2022).
Rodríguez-López, C. P., González-Torres, M. C., Cruz-Bautista, I. & Nájera-Medina, O. Visceral obesity, skeletal muscle mass and resistin in metabolic syndrome development. Nutr. Hosp. 36, 43–50. https://doi.org/10.20960/nh.1889 (2019).
Bovolini, A., Garcia, J., Andrade, M. A. & Duarte, J. A. Metabolic syndrome pathophysiology and predisposing factors. Int. J. Sports Med 42, 199–214. https://doi.org/10.1055/a-1263-0898 (2021).
Nascimento, G. G. et al. Obesity and periodontal outcomes: A population-based cohort study in Brazil. J. Periodontol. 88, 50–58. https://doi.org/10.1902/jop.2016.160361 (2017).
Curtin, L. R. et al. National health and nutrition examination survey: Sample design, 2007–2010. Vital Health Stat. 2, 1–23 (2013).
Borrell, L. N. & Talih, M. Examining periodontal disease disparities among U.S. adults 20 years of age and older: NHANES III (1988–1994) and NHANES 1999–2004. Public Health Rep. 127, 497–506. https://doi.org/10.1177/003335491212700505 (2012).
Papapanou, P. N. et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 45(Suppl 20), S162-s170. https://doi.org/10.1111/jcpe.12946 (2018).
Fadeyev, K., Nagao-Sato, S. & Reicks, M. Nutrient and food group intakes among U.S. children (2–5 years) differ by family income to poverty ratio, NHANES 2011–2018. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph182211938 (2021).
Chen, X., Han, R., Liu, X. & Xu, J. Association between composite dietary antioxidant index and the prevalence of periodontitis: Results from NHANES 2009–2014. BMC Oral. Health 25, 779. https://doi.org/10.1186/s12903-025-06151-7 (2025).
Qiu, Z. et al. Associations of serum carotenoids with risk of cardiovascular mortality among individuals with type 2 diabetes: Results from NHANES. Diabet.Care 45, 1453–1461. https://doi.org/10.2337/dc21-2371 (2022).
Costa, S. A., Costa Ribeiro, C. C., Oliveira Moreira, A. R., Nascimento, G. G. & Carvalho Souza, S. F. Association between high serum ferritin and periodontitis: A population-based cross-sectional preliminary study. J. Periodontol. https://doi.org/10.1002/jper.24-0491 (2025).
Borrell, L. N., Burt, B. A. & Taylor, G. W. Prevalence and trends in periodontitis in the USA: The [corrected] NHANES, 1988 to 2000. J. Dent. Res. 84, 924–930. https://doi.org/10.1177/154405910508401010 (2005).
Piercy, K. L. et al. The physical activity guidelines for Americans. JAMA 320, 2020–2028. https://doi.org/10.1001/jama.2018.14854 (2018).
Ren, Z. et al. Association between probiotic consumption and periodontitis: Evidence from NHANES 2009–2014. J. Clin. Periodontol. 50, 1476–1486. https://doi.org/10.1111/jcpe.13865 (2023).
Armitage, G. C. Periodontal diagnoses and classification of periodontal diseases. Periodontol. 2000(34), 9–21. https://doi.org/10.1046/j.0906-6713.2002.003421.x (2004).
Chaffee, B. W. & Weston, S. J. Association between chronic periodontal disease and obesity: A systematic review and meta-analysis. J. Periodontol. 81, 1708–1724. https://doi.org/10.1902/jop.2010.100321 (2010).
Kawai, T., Autieri, M. V. & Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 320, C375-c391. https://doi.org/10.1152/ajpcell.00379.2020 (2021).
Zuza, E. P. et al. Influence of obesity on experimental periodontitis in rats: Histopathological, histometric and immunohistochemical study. Clin. Oral. Investig. 22, 1197–1208. https://doi.org/10.1007/s00784-017-2207-y (2018).
Suvan, J., D’Aiuto, F., Moles, D. R., Petrie, A. & Donos, N. Association between overweight/obesity and periodontitis in adults. A systematic review. Obes. Rev. 12, e381-404. https://doi.org/10.1111/j.1467-789X.2010.00808.x (2011).
Zuza, E. P. et al. The role of obesity as a modifying factor in patients undergoing non-surgical periodontal therapy. J. Periodontol. 82, 676–682. https://doi.org/10.1902/jop.2010.100545 (2011).
Akram, Z., Abduljabbar, T., Abu Hassan, M. I., Javed, F. & Vohra, F. Cytokine profile in chronic periodontitis patients with and without obesity: A systematic review and meta-analysis. Dis. Markers 2016, 4801418. https://doi.org/10.1155/2016/4801418 (2016).
Goodson, J. M., Groppo, D., Halem, S. & Carpino, E. Is obesity an oral bacterial disease?. J. Dent. Res. 88, 519–523. https://doi.org/10.1177/0022034509338353 (2009).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: Human gut microbes associated with obesity. Nature 444, 1022–1023. https://doi.org/10.1038/4441022a (2006).
Falagas, M. E. & Kompoti, M. Obesity and infection. Lancet Infect. Dis. 6, 438–446. https://doi.org/10.1016/s1473-3099(06)70523-0 (2006).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206. https://doi.org/10.1038/nature14177 (2015).
Kim, K. J., Son, S., Kim, K. J., Kim, S. G. & Kim, N. H. Weight-adjusted waist as an integrated index for fat, muscle and bone health in adults. J. Cachexia Sarcopenia Muscle 14, 2196–2203. https://doi.org/10.1002/jcsm.13302 (2023).
Prillaman, M. Why BMI is flawed—And how to redefine obesity. Nature 622, 232–233. https://doi.org/10.1038/d41586-023-03143-x (2023).
Linden, G., Patterson, C., Evans, A. & Kee, F. Obesity and periodontitis in 60–70-year-old men. J. Clin. Periodontol. 34, 461–466. https://doi.org/10.1111/j.1600-051X.2007.01075.x (2007).
Ylöstalo, P., Suominen-Taipale, L., Reunanen, A. & Knuuttila, M. Association between body weight and periodontal infection. J. Clin. Periodontol. 35, 297–304. https://doi.org/10.1111/j.1600-051X.2008.01203.x (2008).
Torrungruang, K. et al. Risk indicators of periodontal disease in older Thai adults. J. Periodontol. 76, 558–565. https://doi.org/10.1902/jop.2005.76.4.558 (2005).
Xu, N. et al. Association between five novel anthropometric indices and erectile dysfunction in US adults from NHANES database. Sci. Rep. 15, 1625. https://doi.org/10.1038/s41598-024-80878-1 (2025).
Wang, X., Xie, L. & Yang, S. Association between weight-adjusted-waist index and the prevalence of rheumatoid arthritis and osteoarthritis: A population-based study. BMC Musculoskelet. Disord. 24, 595. https://doi.org/10.1186/s12891-023-06717-y (2023).
Zhao, P. et al. Positive association between weight-adjusted-waist index and hyperuricemia in patients with hypertension: The China H-type hypertension registry study. Front. Endocrinol. (Lausanne) 13, 1007557. https://doi.org/10.3389/fendo.2022.1007557 (2022).
Qin, Z. et al. The association between weight-adjusted-waist index and abdominal aortic calcification in adults aged ≥ 40 years: Results from NHANES 2013–2014. Sci. Rep. 12, 20354. https://doi.org/10.1038/s41598-022-24756-8 (2022).
Li, Q. et al. Association of weight-adjusted-waist index with incident hypertension: The rural Chinese cohort study. Nutr. Metab. Cardiovasc. Dis. 30, 1732–1741. https://doi.org/10.1016/j.numecd.2020.05.033 (2020).
Ding, C. et al. Association of weight-adjusted-waist index with all-cause and cardiovascular mortality in China: A prospective cohort study. Nutr. Metab. Cardiovasc. Dis. 32, 1210–1217. https://doi.org/10.1016/j.numecd.2022.01.033 (2022).
Liu, Y. et al. Association of anthropometric indices with the development of diabetes among hypertensive patients in China: A cohort study. Front. Endocrinol. (Lausanne) 12, 736077. https://doi.org/10.3389/fendo.2021.736077 (2021).
Nascimento, G. G. et al. Relationship between periodontal disease and obesity: The role of life-course events. Braz. Dent. J. 25, 87–89. https://doi.org/10.1590/0103-6440201300019 (2014).
Fang, H., Xie, F., Li, K., Li, M. & Wu, Y. Association between weight-adjusted-waist index and risk of cardiovascular diseases in United States adults: A cross-sectional study. BMC Cardiovasc. Disord. 23, 435. https://doi.org/10.1186/s12872-023-03452-z (2023).
Acknowledgements
This study was supported by Zhejiang Science and Technology Program of Chinese Medicine (Grant No. 2024ZL724), the Health and Science Project of Hangzhou (Grant No. A20210056), Guided Project of Science and Technology of Hangzhou (Grant No. 20211231Y028), Hangzhou Biological Medicine and Health Industry Development Support Science and Technology Project (Grant No. 2021WJCY131), and Postgraduate scientific research innovation promotion project of Hangzhou Normal University (Grant No. 1115B20500515).
Author information
Authors and Affiliations
Contributions
Guiying He drafted the manuscript and conducted statistical analysis. Jiatong Zhang conducted statistical analysis and contributed to the writing of the methods section. Xinyue Liu and Zhengjie Qiu conceptualized the study and contributed to the background writing. Mengqing Yan, Mingxuan Zhang, Dongyang Wu, and Lipei Liu were responsible for data collection. Cheng Ding and Xing Chen were responsible for guiding and reviewing this article. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, G., Zhang, J., Liu, X. et al. Association between weight-adjusted waist index and severe periodontitis using NHANES 2009–2014. Sci Rep 15, 30536 (2025). https://doi.org/10.1038/s41598-025-14664-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14664-y