Abstract
Central nervous system development influences the neurological prognosis of premature infants, and their management may be better guided by the evaluation of the oxygen supply-demand balance in brain tissue. Near-infrared time-resolved spectroscopy (NIR-TRS) can measure cerebral oxygen metabolism indices, including cerebral blood volume (CBV), as absolute values. Cerebral hemoglobin oxygen saturation (ScO2) and the cerebral tissue oxygen extraction rate (cFTOE), but not CBV, have been reported in premature infants. Here, we used NIR-TRS to evaluate the longitudinal changes in these indices, including CBV, in 26 extremely premature infants born at < 30 weeks’ gestation without intracranial hemorrhage. ScO2 was very slightly negatively correlated with postnatal week (r = − 0.19, p < 0.001). The cFTOE increased with postnatal week (r = 0.37, p < 0.001) and postconceptional week (r = 0.81, p < 0.001) while CBV decreased with postnatal week (r = − 0.34, p < 0.001) and postconceptional week (r = − 0.90, p < 0.001). This study is the first to prospectively evaluate changes in CBV after birth in preterm infants without intracranial hemorrhage. Our results show that the cerebral oxygen demand of preterm infants increases with postnatal week but that the growth of the cerebral vascular bed may be delayed.
Similar content being viewed by others
Introduction
Near-infrared spectroscopy (NIRS) allows evaluation of the oxygen metabolism balance in the target organs1. Because the neurological prognosis of premature infants is strongly influenced by the development of the central nervous system, their management might benefit from assessment of the oxygen supply-demand balance in brain tissue. Monitoring of cerebral oxygenation has recently been proposed to alert clinicians to the risk of reduced cerebral blood flow and cerebral ischemia2. The only cerebral oxygen metabolism indices that can be measured using conventional NIRS are cerebral tissue hemoglobin (Hb) oxygen saturation (ScO2), which is generally used as an indicator of the balance between oxygen supply and demand in brain tissue, and the cerebral tissue oxygen extraction rate (cFTOE)3, which is an indicator of cerebral ischemia. However, near-infrared time-resolved spectroscopy (NIR-TRS), a Japanese-developed NIRS modality, can measure not only ScO2 and cFTOE but also cerebral blood volume (CBV)4,5. CBV reflects cerebral venous congestion and the cerebral vascular bed and is considered an indicator of cerebral hemodynamics.
A low CBV may suggest underdeveloped vascular structures or cerebral hypoperfusion, whereas a high CBV may indicate vasodilation or venous stasis. Unlike conventional NIRS, which only provides relative measurements and cannot quantify CBV, NIR-TRS permits the absolute quantification of all three indices. This capability enables more accurate longitudinal assessments of cerebral circulation and oxygen metabolism in preterm infants. We hypothesize that cerebral development (cell proliferation, myelination, and vascularization) continues not only during the fetal period but also after birth under the influence of cerebral hemodynamics and oxygen metabolism and that poor cerebral development after birth may have adverse effects on cognitive and motor function in later life.
The combined evaluation of ScO2, cFTOE, and CBV enables a more integrated understanding of cerebral hemodynamics and oxygen metabolism. Moreover, the ability to obtain absolute values allows for fixed-point measurements over time, which is particularly advantageous in longitudinal monitoring. We have published multiple studies of cerebral hemodynamics and oxygen metabolism in newborns using NIR-TRS6,7,8,9. We have also evaluated changes in CBV from birth to term-equivalent ages as an indicator of cerebral vascular bed development in premature infants, including those with intracranial hemorrhage10. Some other recent studies have reported longitudinal changes in ScO2 and cFTOE in premature infants11,12, but no reports have included extremely premature infants born at 22–23 weeks of gestation without intracranial hemorrhage. Further investigation in this highly vulnerable population is warranted.
Although the mortality rate for premature infants is decreasing, the issue of neurological sequelae persists13,14. An appropriate oxygen supply must be maintained for the healthy development of the immature organs of premature babies, who continue to undergo hemodynamic changes from birth15. Percutaneous arterial Hb oxygen saturation (SpO2) has been used for many years as a management indicator for premature infants16,17,18,19. However, because SpO2 merely indicates the Hb oxygen saturation level of the peripheral arterial blood, it does not necessarily reflect the oxygen supply to the target organs. Understanding the longitudinal changes in ScO2, cFTOE, and CBV may contribute to the development of evidence-based monitoring strategies and early interventions to improve neurodevelopmental outcomes in preterm infants. Hence, we conducted the present study to investigate longitudinal changes in ScO2, cFTOE, and CBV as parameters of cerebral hemodynamics and oxygen metabolism in premature infants born at less than 30 weeks’ gestation without intracranial hemorrhage.
Results
The present study included 30 premature infants born at less than 30 weeks’ gestation at Tokyo Medical University Hospital between April 2019 and March 2021. Four infants with missing data were excluded; the remaining 26 infants were included in the analysis. None of these infants had an intracranial hemorrhage; this was a coincidental feature of the enrolled cohort and was not the result of pre-recruitment exclusion criteria. The mean gestational age and birth weight were 26 weeks and 2 days and 813 g, respectively (Table 1). Chorioamnionitis was confirmed pathologically in six cases (23%). Antenatal steroids were administered in 16 cases (62%). A cyclooxygenase inhibitor was administered to nine infants (35%) to treat symptomatic patent ductus arteriosus. Inotropes were administered during the acute phase in seven infants (27%). The raw clinical data for each of the 26 subjects is shown in the supplemental table.
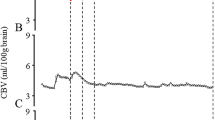
ScO2 showed a slight negative correlation with postnatal week (r = − 0.19, p < 0.001; Fig. 1A) and postconceptional week (r = − 0.17, p = 0.003; Fig. 1B). On the other hand, there was a positive correlation of the cFTOE with the postnatal week (r = 0.37, P < 0.001; Fig. 2A) and postconceptional week (r = 0.81, p < 0.001; Fig. 2B), with the correlation increasing with postconceptional week. CBV decreased with increasing postnatal week (r = − 0.34, p < 0.001; Fig. 3A) and postconceptional week (r = − 0.90, p < 0.001; Fig. 3B), and the correlation increased with postconceptional week. At all measurement time points, the range of CBV values in this cohort was 2.12 ± 0.39 mL/100 g brain (mean ± SD). These values were higher than the reference range of 1.85 ± 0.30 mL/100 g brain (mean ± SD) reported by Wolfsberger et al.20 for stable preterm infants during the first week of life. However, it is important to note that we extended measurements to the term-equivalent age, while the previous study included a higher proportion of term infants and used different NIR-TRS devices. Blood Hb levels also declined with postnatal week (r = − 0.37, p < 0.001; Fig. 4A) and postconceptional week (r = − 0.30, p < 0.001; Fig. 4B).
Postnatal changes in cFTOE. There was a significant positive correlation of the cFTOE with the number of weeks after birth (r = 0.37, p < 0.001) (A) and the corrected weeks (r = 0.81, p < 0.001) (B). The cFTOE showed a stronger association with corrected weeks than with the number of weeks after birth. Statistical analysis was performed using Spearman’s rank correlation coefficient.
Postnatal changes in CBV. There was a significant negative correlation of CBV with the number of weeks after birth (r = − 0.34, p < 0.001) (A) and the corrected weeks (r = − 0.90, p < 0.001) (B). CBV showed a stronger association with corrected weeks than with the number of weeks after birth. Statistical analysis was performed using Spearman’s rank correlation coefficient.
For both ScO2 and cFTOE, the correlation was stronger with postconceptional week than with postnatal week, underscoring the physiological relevance of gestational maturity. Similarly, the negative correlation between CBV and postconceptional week was more pronounced, suggesting that cerebral vascular development is more closely tied to gestational progression than to chronological age. In addition, we performed multivariable linear regression analyses with postconceptional week and Hb levels as independent variables. ScO2 showed a significant positive association with Hb (p = 0.003), but not with postconceptional week (p = 0.199). The cFTOE increased with postconceptional week (p < 0.001) and decreased with Hb (p < 0.001). CBV showed significant negative associations with both postconceptional week (p < 0.001) and Hb levels (p < 0.001). These findings indicate that Hb level is a significant factor affecting these indices, particularly CBV.
Discussion
This is the first longitudinal study to evaluate the changes in ScO2, cFTOE, and CBV from birth to the term-equivalent age in preterm infants without intracranial hemorrhage born at less than 30 weeks’ gestation. The study showed that, as the number of weeks after birth and corrected gestational age increased, the mean blood Hb decreased and the ScO2 very slightly decreased. Meanwhile, the cFTOE increased and the CBV decreased as the postnatal week and postconceptional age increased. Although cFTOE and CBV changed over time in opposite directions, they represent distinct physiological mechanisms and were not directly compared in this study. These findings suggest the possibility of a “mismatch” between increased brain oxygen demand and delayed vascular development in extremely preterm infants. Decreased ScO2 and CBV, along with increased cFTOE, reflect a state where oxygen consumption is compensating for restricted oxygen supply, and such imbalances are considered risk factors for chronic hypoxia and neurological impairments. Therefore, monitoring of brain oxygen status not only immediately after birth but also over time is important.
Postnatal changes in ScO2 and the blood Hb level
Ijichi et al.4 found a negative correlation between the postconceptional week and ScO2 in preterm infants born at 30 weeks’ or more gestation, based on measurements of ScO2 within 2 days of birth. As the weeks pass after conception in the uterus, the brain and nervous system develop, and this is thought to increase the oxygen consumption of the brain, causing a decrease in ScO2. However, longitudinal cerebral oxygenation studies in premature infants are limited. Mohamed et al.11 measured ScO2 and cFTOE in premature infants born at < 34 weeks’ gestation up to 28 days after birth. In that report, as the postconceptional age increased, the ScO2 decreased and cFTOE increased. However, the observation period was only 4 weeks.
Similar to our study, Vesoulis et al.12 conducted a longitudinal cerebral oxygenation study in which ScO2 and cFTOE were measured to the term-equivalent age for premature infants born at < 30 weeks’ gestation. They reported that, as the postnatal week increased for premature infants, the ScO2 decreased and cFTOE increased. In infants with intracranial hemorrhage, who represented about 30% of the subjects, there was a decrease in the ScO2 and an increase in the cFTOE. These changes were particularly prominent in infants with grade III or higher intracranial hemorrhage. In addition to the possibility that extravascular blood might decrease the apparent ScO2, another possible cause of these changes is the disruption of cerebral autoregulation by the development of intracranial hemorrhage in preterm infants with immature cerebral autoregulation. In the present study, ScO2 was very slightly negatively correlated with the postnatal week and postconceptional week. The reason for the lack of a strong correlation compared to the previous study may be differences in the blood Hb level in the subjects, the presence or absence of intracranial hemorrhage, the degree of intracranial hemorrhage, or the administration of inotropic drugs in the acute phase.
Previous studies have suggested a positive correlation between ScO2 and blood Hb levels: as anemia progresses, ScO2 decreases12. Although that study did not analyze their direct correlation, a similar relationship is likely given the underlying physiology. The preterm infants in the present study showed a tendency for a decrease in the blood Hb level over time due to iron deficiency anemia or anemia of prematurity, but they were being managed with a higher blood Hb level than in previous reports. It is possible that the difference in the degree of anemia affected the degree of the decrease in ScO2.
The presence of extravascular blood accumulation due to cerebral hemorrhage can also cause a decrease in ScO212. As none of the preterm infants in this study had intracranial hemorrhage, it is possible that we observed the oxygenation status of the brain tissue of healthy preterm infants without intracranial hemorrhage. Inotropic drug administration to premature infants has been reported to suppress cerebral autoregulation21, but its duration is unclear. Although there was a low rate of inotrope administration in this study (27%), previous studies have not discussed the administration of inotropic drugs in the acute phase, and the frequency of their administration may have influenced the difference in the results.
Postnatal changes in the cFTOE
In recent years, the utility of the cFTOE as an indicator of tissue metabolic failure has been reported22,23. We previously reported that the cFTOE in preterm infants increased as the postconceptional week increased10. In the present study, the cFTOE tended to increase as the postnatal week increased, but a stronger positive correlation was seen with the postconceptional week (Fig. 2). The results showed that the increase in oxygen extraction in brain tissue is more strongly dependent on the time that has passed since conception than on the time that has passed since birth. There is a possibility that oxygen metabolic failure in cerebral tissue may more easily occur when there is a decreased oxygen supply due to respiratory dysfunction, circulatory failure, severe anemia, or sepsis in preterm infants who have progressed some weeks, rather than in preterm infants who were in the early phase after birth, because of the higher oxygen demands at the later stage. The results suggest that clinicians should pay attention to cerebral oxygenation in extremely preterm infants not only in the early phase after birth but also in the long term.
Postnatal changes in CBV
Because a TRS system can measure the absolute value of total Hb (oxyHb and deoxyHb), it can also measure CBV. There are few reports on CBV in preterm infants. Nonetheless, Schwaberger et al.24 reported that CBV in the early postnatal period (within 15 min after birth) in newborns, including late preterm infants, decreased regardless of the presence or absence of respiratory support. This is thought to reflect a rapid adaptation of postnatal circulation and the degree of cerebral congestion. However, with the exception of one of our previous studies10, there have been no investigations of the CBV of premature infants over time. In that work, we found no relationship between the postconceptional week and CBV in extremely premature infants. However, some of the participants in our previous study had intracranial hemorrhage. In another non-longitudinal study4, CBV in preterm infants born at > 30 weeks’ gestation and measured within 2 days of birth was positively correlated with the number of weeks of gestation, suggesting that the cerebral vascular bed develops as the gestational period increases. In the present study, which included neonates without intracranial hemorrhage, CBV decreased with the postnatal week and, particularly, the postconceptional age (Fig. 3). This trend contrasts with our previous findings, possibly due to differences in study population. We speculate that intracranial hemorrhage may have affected the relationship between the postconceptional age and CBV changes in our previous studies. CBV is inversely correlated with the blood Hb level25 and generally increases in the presence of anemia. In the current study, the blood Hb levels in premature infants decreased with the postnatal week and were influenced by anemia while CBV showed a decreasing trend.
The development of the cerebral vascular bed in preterm infants born at < 30 weeks’ gestation is not clear. However, the secretion of vascular endothelial growth factor (VEGF) increases under conditions of low arterial blood oxygen saturation, and vice versa26. Previous studies using neonatal rats have reported that cerebral vascularization is promoted when the developing brain is exposed to a hypoxic environment27,28. Although arterial blood oxygen saturation is maintained at about 70% during fetal development29, preterm infants are managed after birth with a target arterial blood oxygen saturation of around 90–95%30,31,32, so the development of cerebral blood vessels may be delayed due to a decrease in VEGF secretion. The development of the cerebral vascular bed in preterm infants who are managed in the neonatal intensive care unit with relatively high arterial blood oxygen saturation may be more delayed than in full-term infants who have spent their time in the uterus with low arterial blood oxygen saturation. We hypothesize that preterm infants experience delayed development of the cerebral vasculature relative to their gestational age. Early intervention is recommended when events causing hypoxemia occur.
The target management values of cerebral oxygen metabolism indicators for improving the neurological prognosis of premature infants are unclear30,31,32. If we can clarify the relationship between the changes over time in ScO2, cFTOE, and CBV in preterm infants and their neurological prognosis, we will be able to get closer to determining the target values for managing cerebral oxygen metabolism indicators. Some recent studies examined the relationship among decreasing ScO2, increasing cFTOE3, and neurological prognosis in premature infants in the acute phase33,34,35,36, but consensus is lacking. These studies are limited as they only measured cerebral oxygenation during the acute phase (72 h after birth). Premature infants might develop various complications, such as chronic lung disease, necrotizing enterocolitis, periventricular leukomalacia, and infections, even beyond their acute phase. These complications have a notable impact on neurological prognosis. Thus, the link with neurological prognosis cannot be clarified without measurements of the cerebral oxygenation of premature infants over a long period from birth to their term-equivalent age. The results of the present study suggest that the cerebral oxygen demand of preterm infants increases with the number of weeks after birth while the growth of the cerebral vascular bed lags. We believe that preterm infants born at < 30 weeks’ gestation have a different cerebral oxygen metabolism and cerebral vascular development to full-term infants. The unique cerebral oxygen metabolism in preterm infants may contribute to the development of neurological sequelae. Because ScO2, cFTOE, and CBV are influenced by Hb levels, we additionally performed regression analyses adjusted for Hb. The results showed that Hb was a significant independent predictor of all three indices. In particular, CBV was negatively associated with Hb, supporting the notion that cerebral blood volume is affected by compensatory mechanisms related to anemia. These findings highlight the need to consider Hb levels when interpreting cerebral hemodynamic parameters measured by NIR-TRS.
The present study has several limitations. As the ideal study method, it would be desirable to continuously monitor cerebral oxygenation from birth to discharge. However, premature infants are vulnerable and have thin and fragile skin. This makes continuous monitoring impossible. The measurement method used in the present study balanced patient safety and data collection. Moreover, the sample size was relatively small (n = 26), and all infants were enrolled from a single center, which may limit the generalizability of the results. Finally, this was a single-center pilot study that did not examine the relationship between cerebral oxygen metabolism indices and long-term prognosis. In future work, we will clarify the relationship between changes in the cerebral oxygenation of premature infants over time using brain magnetic resonance imaging findings and developmental examinations performed at a later time. Additionally, variability in head circumference among infants may have affected CBV measurements, and a subset of participants met the criteria for extrauterine growth retardation. These factors may have influenced the CBV and should be further examined in future longitudinal studies.
In conclusion, the present study of preterm infants found that the correlation of ScO2 and the cFTOE with postnatal age was as previously reported, with a stronger association between the cFTOE and the postconceptional week rather than the postnatal week. There was also a negative correlation of CBV with the postnatal week and postconceptional week. NIR-TRS enables objective and reproducible data acquisition without operator training, minimizing inter-examiner variability. This makes it a promising tool for future integration into neonatal care. Our results may serve as a basis for considering individualized oxygen management strategies, early transfusions when necessary, and promoting vascular development through the administration of hematopoietic factors. Further studies are needed, such as multicenter, large-scale cohort studies, as well as evaluations of specific interventions for delayed vascular development.
Methods
Study design and measurements
This prospective observational study was conducted at Tokyo Medical University Hospital. The study was approved by the Regional Committee for Medical Research Ethics of Tokyo Medical University (approval number: T2019-0026) and conducted in accordance with the Declaration of Helsinki. The parents of all neonates enrolled in the study provided written informed consent after delivery and after receiving a full explanation of the research. The study included 26 premature infants born at < 30 weeks’ gestation between April 2019 and March 2021. We excluded infants with a chromosomal abnormality detected on amniocentesis or a chromosome test performed after birth or with congenital heart disease, excluding patent ductus arteriosus.
We used NIRS and collected data on indicators of brain tissue oxygen metabolism in the 26 subjects. To obtain the measurements, we used a portable three-wavelength TRS system (TRS-20; Hamamatsu Photonics K.K., Hamamatsu, Japan). A neonatologist attached the TRS optical probe to the newborn’s forehead and obtained measurements continuously for 10 min (300 s). At the same time, a transcutaneous pulse oximeter (Nellcor; Covidien, Mansfield, MA) was applied to one of the extremities for the simultaneous measurement of SpO2. The newborns were placed supine or prone, and measurements were taken under conditions of adequate rest and in the absence of body movements or crying. Because ScO2 and cFTOE are influenced by oxygen supply, as well as factors such as cardiac output, heart rate, blood pressure, and circulating blood volume, and because CBV is primarily affected by Hb concentration and potentially by circulating volume, we ensured that all NIRS measurements were performed when the infants were at rest, free of apnea or agitation, and with stable vital signs. This protocol minimized variability due to hemodynamic instability and ensured reliable data collection.
We also measured the blood Hb level using a blood gas analyzer (ABL-90 FLEX PLUS; Radiometer Medical ApS, Brønshøj, Denmark) within 3 h of the data collection. No additional blood sampling beyond standard clinical care was performed for this study. The same neonatologist measured NIRS once a week until the infant reached the term-equivalent age (37 weeks’ postconceptional age). Because many clinically stable preterm infants are discharged at around 37 weeks’ postconceptional age in Japan, this was chosen as the upper limit for longitudinal measurements. All cranial ultrasound examinations were performed by neonatologists. To screen for intraventricular hemorrhage (IVH), cranial ultrasound examinations were conducted every 8 h within the first 72 h after birth, once daily until postnatal day 7, and once weekly thereafter. The absence of IVH in all participants was confirmed through this protocol. The first NIRS measurement in each infant was conducted during the first postnatal week, although the exact postnatal day depended on clinical stability.
Measurements were taken only when patients were still and resting and not within 10 min of an apnea attack. In a previous study, the cerebral blood velocity in infants measured by Doppler ultrasound showed cyclical fluctuations with a frequency ranging from 1.5 to 5 cycles/min37. NIRS studies have also shown oscillations of the Hb oxygenation state with a frequency ranging from 3 to 5 cycles/min38. Therefore, the use of average values over a 10-min period in each neonate seemed sufficient for estimating cerebral Hb in a steady state. Each measurement session lasted 10 min (300 s). ScO2, cFTOE, and CBV were measured using NIR-TRS once every 6 s, with 10 plots per minute and 100 plots per 10 min. The ScO2, cFTOE, and CBV values are the averages of these plots. No artificial extraction or exclusion of data was conducted. Subjects were generally transfused with packed red blood cells in 15 mL/kg aliquots when the Hb level was less than 10 g/dL for mechanically ventilated or sick infants or less than 8 g/dL for extubated infants with a more stable clinical status. NIRS was not measured during transfusion therapy. The management target value of SpO2 for subjects was 90–95% for a post-menstrual age of less than 32 weeks and at least 95% for a post-menstrual age of 32 weeks or more30,31,32.
Near‑infrared time‑resolved spectroscopy
TRS uses a time-correlated single-photon counting technique for detection. The system was controlled by a computer through a digital I/O interface that consisted of a three-wavelength (761, 801, and 834 nm) picosecond light pulser as the pulse light source, a photon-counting head for single-photon detection, and signal-processing circuits for time-resolved measurement. The re-emission profiles observed at each measurement point were fitted with the photon diffusion equation proposed by Patterson et al.39 to calculate the absorption coefficient (µa) and the reduced scattering coefficient (µ’s) values of the head at wavelengths of 761, 801, and 834 nm. In each iterative calculation, the photon diffusion equation was calculated in reflectance mode and was convoluted with the instrumental response; it was then fitted to the observed re-emission profile. After determination of the µa and µ’s values at the three wavelengths, the oxy-Hb and deoxy-Hb concentrations were calculated from their respective absorption coefficients using the following Eqs. (1)–(3), based on the assumption that the background absorption was due to 85% (by volume) water40.
In these equations, \($$\varepsilon^x_{{\lambda}\text{nm}}$$\)εx λnm is the extinction coefficient at λnm and [oxyHb] and [deoxyHb] are the concentrations of oxyHb and deoxyHb, respectively. We used a source optical fiber bundle with a diameter of 1 mm and a detector optical fiber bundle with a diameter of 3 mm, both with a 90° bent tip and numerical aperture of 0.29. The light emission and detection optodes were positioned on the frontal region at an interoptode distance of 30 mm. The total cerebral Hb (totalHb) concentration, ScO2, cFTOE, and CBV were obtained using the following equations:
[totalHb] = [oxyHb]+[deoxyHb]
ScO2 (%) = ([oxyHb]/([oxyHb]+[deoxyHb]) × 100
cFTOE = (SpO2 − ScO2)/SpO2
CBV (mL/100 g brain tissue) = [totalHb]×MWHb×10−6/(tHb×10−2×Dt×10)
where [] indicates the Hb concentration (µM), MWHb is the molecular weight of Hb (64,500), tHb is the venous Hb concentration (g/dL), and Dt is the brain tissue density (1.05 g/mL). All neonates underwent blood gas analysis, and CBV was calculated from the concentration of blood Hb within 2 h of measurement.
Statistical analysis
The mean ScO2, cFTOE, and CBV values were calculated at 10-s intervals, and the SpO2 data were measured at 15-s intervals during NIRS measurement. The relationships of the NIR-TRS parameters (ScO2, cFTOE, CBV, and blood Hb) with the postnatal age and postconceptional age were examined using Pearson’s correlation coefficient and Spearman’s rank correlation coefficient. For each estimate, the least squares mean and its 95% confidence interval (CI) were calculated. A two-sided p value < 0.05 was considered statistically significant. All statistical analyses were performed with EZR version 2.6 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) that has also been modified to include statistical functions used in biostatistics41.
Data availability
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Boushel, R. et al. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand. J. Med. Sci. Sports. 11, 213–222 (2001).
Sortica da Costa. Changes in hemodynamics, cerebral oxygenation and cerebrovascular reactivity during the early transitional circulation in preterm infants. Pediatr. Res. 86, 247–253 (2019).
Balegar, K. K., Stark, M. J., Briggs, N. & Andersen, C. C. Early cerebral oxygen extraction and the risk of death or sonographic brain injury in very preterm infants. J. Pediatr. 164, 475–480e1 (2014).
Ijichi, S. et al. Developmental changes of optical properties in neonates determined by near-infrared time-resolved spectroscopy. Pediatr. Res. 58, 568–573 (2005).
Ohmae, E. et al. Cerebral hemodynamics evaluation by near-infrared time-resolved spectroscopy: correlation with simultaneous positron emission tomography measurements. Neuroimage 29, 697–705 (2005).
Takami, T. et al. Changes in cerebral perfusion extremely LBW infants during the first 72 h after birth. Pediatr. Res. 68, 435–439 (2010).
Takami, T. et al. Umbilical cord milking stabilizes oxygenation and perfusion in infants born before 29 weeks of gestation. J. Pediatr. 161, 742–747 (2012).
Fujioka, T. et al. Difference in cerebral and peripheral hemodynamics among term and preterm infants during the first three days of life. Neonatology 106, 181–187 (2014).
Ishii, H. et al. Comparison of changes in cerebral and systemic perfusion between appropriate- and small-for-gestational-age infants during the three days after birth. Brain Dev. 36, 380–387 (2014).
Nara, S. et al. Chronological changes in cerebral perfusion and oxygenation parameters in preterm neonates measured using portable near-infrared time-resolved spectroscopy system. J. Tokyo Med. Univ. 71, 227–234 (2014).
Mohamed, M. A. et al. Changes in cerebral tissue oxygenation and fractional oxygen extraction with gestational age and postnatal maturation in preterm infants. J. Perinatol. 41, 836–842 (2021).
Vesoulis, Z. A., Whitehead, H. V., Liao, S. M. & Mathur, A. M. The hidden consequence of intraventricular hemorrhage: persistent cerebral desaturation after IVH in preterm infants. Pediatr. Res. 89, 869–877 (2021).
Stoll, B. J. et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics 126, 443–456 (2010).
Glass, H. C. et al. Outcomes for extremely premature infants. Anesth. Analg. 120, 1337–1351 (2015).
Obst, S. et al. Perinatal hyperoxia and developmental consequences on the lung-brain axis. Oxid. Med. Cell. Longev. 24, e20225784146 (2022).
Vaucher, Y. E. et al. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N Engl. J. Med. 367, 2495–2504 (2012).
Schmidt, B. et al. Canadian oxygen trial (COT) group. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA 309, 2111–2120 (2013).
Stenson, B. J. et al. Oxygen saturation and outcomes in preterm infants. N Engl. J. Med. 368, 2094–2104 (2013).
Schmidt, B., Whyte, R. K. & Roberts, R. S. Trade-off between lower or higher oxygen saturations for extremely preterm infants: the first benefits of oxygen saturation targeting (BOOST) II trial reports its primary outcome. J. Pediatr. 165, 6–8 (2014).
Wolfsberger, C. H. et al. Precision and normal values of cerebral blood volume in preterm neonates using time-resolved near-infrared spectroscopy. Acta Paediatr. 113, 677–683 (2024).
Eriksen, V. R., Hahn, G. H. & Greisen, G. Dopamine therapy is associated with impaired cerebral autoregulation in preterm infants. Acta Paediatr. 103, 1221–1226 (2014).
Andersen, C. C., Hodyl, N. A., Kirpalani, H. M. & Stark, M. J. A theoretical and practical approach to defining adequate oxygenation in the preterm newborn. Pediatrics 139, e20161117 (2017).
Ronco, J. J. et al. Identification of the critical oxygen delivery for anaerobic metabolism in critically ill septic and nonseptic humans. JAMA 270, 1724–1730 (1993).
Schwaberger, B. et al. Cerebral blood volume during neonatal transition in term and preterm infants with and without respiratory support. Front. Pediatr. 132, e201800132 (2018).
Gupta, A. K. et al. Non-invasive measurement of cerebral blood volume in volunteers. Br. J. Anaesth. 78, 39–43 (1997).
Liu, Y., Cox, S. R., Morita, T. & Kourembanas, S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5’ enhancer. Circ. Res. 77, 638–643 (1995).
Ogunshola, O. O. et al. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Brain Res. Dev. Brain Res. 119, 139–153 (2000).
Rosenstein, J. M., Mani, N., Silverman, W. F. & Krum, J. M. Patterns of brain angiogenesis after vascular endothelial growth factor administration in vitro and in vivo. Proc. Natl. Acad. Sci. U S A. 95, 7086–7091 (1998).
Rudolph, A. M. Congenital Disease of the Heart: Clinical-Physiological Considerations 3rd edn (Wiley-Blackwell, 2009).
The BOOST II United Kingdom, Australia, and New Zealand Collaborative Groups. Oxygen saturation and outcomes in preterm infants. N Engl. J. Med. 368, 2094–2104 (2013).
SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. N Engl. J. Med. 362, 1959–1969 (2010).
Schmidt, B. et al. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA 309, 2111–2120 (2013).
Hyttel-Sorensen, S., Greisen, G., Als-Nielsen, B. & Gluud, C. Cerebral near-infrared spectroscopy monitoring for prevention of brain injury in very preterm infants. Cochrane Database Syst. Rev. 9, CD011506 (2017).
Plomgaard, A. M. et al. No neurodevelopmental benefit of cerebral oximetry in the first randomised trial (SafeBoosC II) in preterm infants during the first days of life. Acta Paediatr. 108, 275–281 (2019).
Alderliesten, T. et al. Low cerebral oxygenation in preterm infants is associated with adverse neurodevelopmental outcome. J. Pediatr. 207, 109–116e2 (2019).
Hansen, M. L. et al. Cerebral oximetry monitoring in extremely preterm infants. N Engl. J. Med. 388, 1501–1511 (2023).
Anthony, M. Y., Evans, D. H. & Levene, M. I. Cyclical variations in cerebral blood flow velocity. Arch. Dis. Child. 66, 12–16 (1991).
Taga, G. et al. Spontaneous Oscillation of oxy- and deoxy-hemoglobin changes with a phase difference throughout the occipital cortex of newborn infants observed using non-invasive optical topography. Neurosci. Lett. 282, 101–104 (2000).
Patterson, M. S., Chance, B. & Wilson, B. C. Time resolved reflectance and transmittance for the non-invasive measurement of tissue optical properties. Appl. Opt. 28, 2331–2336 (1989).
Cooper, C. E. et al. The noninvasive measurement of absolute cerebral deoxyhemoglobin concentration and mean optical path length in the neonatal brain by second derivative near infrared spectroscopy. Pediatr. Res. 39, 32–38 (1996).
Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 48, 452–458 (2013).
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 20K16907.
Author information
Authors and Affiliations
Contributions
S.Nara., and S.G. were involved in the initial study design, and S. Nara. wrote the main text. S.Nara. obtained the necessary financial support for this project and provided study materials. M.N., M.N., and N.H. contributed to the data analysis and performed the statistical analysis. H.O., G.Y., S. Nakamura. and T.K. proofread the main text and interpret the data. All members drafted the article and critically revised it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and informed consent
The study was approved by the Regional Committee for Medical Research Ethics of Tokyo Medical University (approval number: T2019-0026) and conducted in accordance with the Declaration of Helsinki. The parents of all neonates enrolled in the study provided written informed consent after delivery, after receiving a full explanation of the research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nara, S., Nishibata, M., Nishibukuro, M. et al. Measuring cerebral hemodynamics and oxygen metabolism indices using NIR-TRS in premature infants from birth to term-equivalent age. Sci Rep 15, 30464 (2025). https://doi.org/10.1038/s41598-025-15548-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15548-x