Abstract
The world is facing new challenges related to climate change and agriculture and is called to ensuring higher crop yield and quality following the concept of food safety, security and efficient resource use. One of the possible strategies could be the valorisation of agro-industrial by-products for the production of biostimulants. The aim of the present work was to assess the action of different protein hydrolysates (PHs) derived by residues of Chlorella vulgaris biomass as biostimulant on lettuce (Lactuca sativa L.), monitoring the agronomic, physiological and qualitative traits, from seed germination to the harvest. The application of biostimulants derived by PHs had interesting results on lettuce, highlighting several positive effects on the investigated parameters. Additionally, the metabolite profiles of baby leaf lettuce were investigated. In particular, was recorded an improvement of the qualitative traits, as flavonoids and anthocyanins contents, and physiological parameters such as the reduction of stomatal conductance. Our results demonstrated that plants treated with PHs obtained through trypsin-induced hydrolysis of Chlorella vulgaris biomass (TPH-1000) exhibited the highest content of bioactive compounds and antioxidant activity compared to those treated with water alone or with a commercial biostimulant. Additionally, plants treated with pepsin protein hydrolysates (PPH-1000) showed significant improvements in agronomic, physiological, and qualitative performance. These results suggest that biostimulants derived from agro-industrial by-products represent a promising and resource-efficient approach to support agricultural productivity and food quality in the context of climate change and the need for more sustainable practices.
Similar content being viewed by others
Introduction
Modern agriculture faces the dual challenge of ensuring global food security and implementing it in a sustainable manner. However, abiotic and biotic stresses make it difficult to achieve these goals and are becoming significant threats to plant survival, growth and development1.
The demand for raw or minimally processed vegetables is expected to increase, and consequently, one of the main goals of the farms that produce baby leaf vegetable like lettuce, rocket, etc. is to maintain high yield and quality, in the same time preserving the agricultural sustainably and overcoming the issue related to climate change. Baby leaf vegetables are used for the preparation of the fourth (IV) range sector and one of the main goal is to obtain products that are qualitatively excellent, safety, healthy and at the same time environmentally sustainable, tending to reduce the use of synthetic chemical fertilisers so as to cope with the major problem of nitrate accumulation2.
In accordance with the European Regulation on fertilisers (2019/1009) ratified by the European Parliament, a plant biostimulant is defined as a product that enhances key plant nutrition processes without directly supplying nutrients. The main purpose is to improve certain characteristics of the plant or the plant rhizosphere, such as nutrient use efficiency, tolerance to environmental stress, quality traits, or the availability of essential nutrients in the soil or rhizosphere3. Biostimulants have no direct effect on pests and pathogens and therefore do not fall into the category of pesticides. Biostimulants, instead of acting as biocidal agents, might induce systemic resistance responses such as Induced Systemic Resistance (ISR) or Systemic Acquired Resistance (SAR). These processes help the plant to better overcome biotic stress caused by pests maintaining or even improving productivity and quality by supporting overall plant health and metabolic resilience, rather than by directly eliminating harmful organisms4. From a nutritional point of view, biostimulants promote plant growth, improve seed germination, fruit set and fruit production, and post-harvest shelf life5,6. Biostimulants in general can influence root development, either by improving lateral root formation7,8 or by increasing the total volume of the root system9.
Biostimulants can be classified into microbial and not microbial biostimulant and might prepared using algae extracts, compost, humic and fulvic acids, beneficial chemicals (e.g. silicon, selenium, sodium, cobalt, aluminium), chitin and chitosan derivatives, beneficial microorganisms, inorganic salts (phosphite) and protein-based biostimulants (peptides and amino acids)10.
Due to their biostimulant and biofertilising properties, microalgae are attracting the interest of agrochemical industries and farmers11. Several species of microalgae are present, however nowadays only a few microalgae species are used for the production of the biostimulants such as: Isochrysis spp., Chaetoceros spp., Chlorella spp., Arthrospira spp. and Dunaliella spp12. Among these species, the most cultivated and widely marketed ones are: Arthrospira spp. and Chlorella spp. Chlorella vulgaris (C. vulgaris) is a unicellular microalga that can grow in both fresh and brackish water and in various environmental conditions that, when applied to soil or leaves, has multiple biostimulant properties13.
Microalgae might enhance nutrient assimilation and when used as biostimulants, helping fertiliser uptake and assimilation, and in turn with a less environmental impact14. Worldwide, interest in microalgae has recently increased due to their economic and commercial relevance as well as their great versatility11.
Moreover, integrated biorefinery approaches have gained traction, enabling the sequential extraction of multiple valuable compounds from a single biomass source. This strategy improves the economic viability of microalgal processes and aligns with circular bioeconomy goals by maximizing resource use and minimizing waste 15,16. Chlorella vulgaris stands out in this context due to its rich content of lipids, proteins, carbohydrates, and pigments. Cascade biorefinery schemes are increasingly seen as essential for sustainable and profitable microalgal production. These sequential protocols enable the targeted recovery of biomolecules under mild conditions that preserve their functionality, for example, extracting polysaccharides with hot water or alkaline media, pigments with solvents, and proteins via alkaline solubilization or enzymatic hydrolysis17. Notably, the remaining biomass, still rich in cell wall components, structural polysaccharides, proteins, and lipids, can serve as feedstock for further recovery of bioactives, effectively closing the loop in a sustainable process18. In this regard, the present study aims to contribute to this model by valorising the residual biomass remaining after polysaccharide extraction. This biomass, still rich in bioactive compounds, is used to produce protein hydrolysates (PHs), thereby aligning with a circular bioeconomy approach and minimizing resource waste. The use of residual biomass after polysaccharide extraction, as opposed to whole algal biomass or virgin feedstocks, enhances the efficiency of resource use. This approach is consistent with recent trends in microalgal biorefinery, which emphasize the importance of fully exploiting algal biomass through integrated, low-impact extraction processes19. Life Cycle Assessment (LCA) studies also indicate that such strategies can lead to significant environmental benefits, including reduced CO₂ emissions and energy demand20. Although a full LCA is not included in the present study, the methodological choice to use a by-product aligns with the principles of sustainable agriculture and resource-efficient biostimulant production. In this context, water-based extraction of polysaccharides from C. vulgaris was conducted in parallel with other research activities. To build on this process and further valorise the residual biomass after polysaccharide extraction, also considering that microalgae are a valuable source of proteins in this study we evaluated the biostimulant effects of protein hydrolysates (PHs) derived from residual Chlorella biomass after polysaccharide extraction. This approach aligns with the biorefinery concept, aiming to maximize resource use by recovering multiple high-value products from a single biomass source. In this context, PHs exhibit distinct agronomic advantages. Their high bioavailability, ability to trigger specific metabolic pathways, and demonstrated effectiveness in promoting plant growth, enhancing tolerance to abiotic stress, and improving nutrient uptake efficiency position them as promising tools for sustainable and resource-efficient agriculture21.
Lactuca sativa L. is one of the most widely used vegetable crops in the human diet and in IV range due to its nutritional properties rich in vitamins, fiber and polyphenols and is an important crop in several countries, including Italy, Spain, Japan and Mexico22. Khan et al.5 tested treatments with protein hydrolysates and algae extracts on lettuce and found an auxin-like effect that led to an increase in secondary root development and increased nutrient utilization efficiency. Ergun et al.23 used C. vulgaris as a biofertiliser for lettuce grown in hydroponics, achieving a saving in mineral nutrients. C. vulgaris also increased the quality properties of the lettuce as it promoted an increase in total soluble solids and vitamin C. La Bella et al.24 evaluated the foliar application of a C. vulgaris extract in lettuce seedlings to improve the final yield. The results showed that the C. vulgaris extract increased lettuce weights and freshness as well as chlorophyll, carotenoids, protein and ash contents. Puglisi et al.25 evaluated the effects of a methanolic extract of C. vulgaris applied by root spraying or by foliar spray on lettuce by monitoring the morpho-biometric and biochemical effects. Root application influenced the enzyme activities involved in primary carbon metabolism while foliar application influenced the activities of enzymes involved in primary nitrogen metabolism. The aim of the present study was to assess the agronomic effects of PHs derived from residual C. vulgaris biomass as a sustainable agricultural biostimulant on lettuce (Lactuca sativa L.) genotype “Eden”, aiming to improve both yield and quality. Specifically, the PHs were subjected to a process that removed the polar fraction, rich in polysaccharides, in order to evaluate the biostimulant effects of the protein component alone on agronomic, physiological, and qualitative performance during, from seed germination to the harvest.
Materials and methods
Recovery of post aqueous extraction of Chlorella vulgaris residual biomass after polysaccharide extraction
The microalga used in this work, Chlorella vulgaris f. viridis Chodat (strain SAG 211 − 12), belongs to the Division Chlorophyta and the Class Trebouxiophyceae. It was purchased from the Algal Culture Collection of the University of Göttingen (SAG, Germany) and was cultivated in a system of integrated photobioreactors using a modified BG-11 growth medium26. Harvesting at the end of the exponential growth phase involved a two-step process—initial sedimentation to concentrate the cells followed by continuous centrifugation with the Extreme Algae Centrifuge (Sacramento, CA, USA). The collected biomass was then rapidly dried using a Büchi B-290 Mini Spray Dryer (Büchi Laboratoriums-Technik, Flawil, Switzerland) at operational conditions reported elsewhere27. The spray-dried biomass was subsequently subjected to a green extraction process to isolate a polar fraction rich in polysaccharides. This extraction employed hot water at 80 °C over three consecutive 2-hour maceration cycles. The supernatants from the three cycles were combined and subjected to alcoholic precipitation, yielding a polysaccharide-rich extract (CHL-P). The remaining biomass was freeze-dried as the starting point for protein extraction.
Protein hydrolysate preparation
Proteins were isolated from the residual biomass after polysaccharide extraction pellet of C. vulgaris, following the protocol previously described by Chronakis et al.28, with slight modifications. Briefly, 10 g of freeze-dried biomass were suspended in 500 mL of 2 N NaOH (pH 12) and stirred for 2 h at 40 °C. The mixture was centrifuged at 6.000 × g for 10 min at 4 °C (Mikro 220R centrifuge, Hettich, Germany). The resulting supernatant was adjusted to pH 3 using 0.1 M HCl to induce protein precipitation, followed by a second centrifugation step. Then the protein pellet was subsequently neutralised to pH 7 using 0.01 M NaOH. PHs were prepared via enzymatic hydrolysis. For this purpose, the isolated proteins were incubated overnight at 37 °C with trypsin and pepsin, using a substrate-to-enzyme ratio of 1:10 (w/w) for each enzyme. Following incubation, the enzymes were inactivated by heating the mixture at 95 °C for 15 min. Subsequently PHs were then purified by ethanol precipitation. The supernatants obtained after centrifugation were collected, evaporated to dryness under vacuum at 40 °C using a rotary evaporator, and finally lyophilised for 24 h at − 80 °C at 0.001 mPa (Manifold Freeze Dryer MFDQ 2002, Laboquest Inc., Westchester USA). The protein content was determined using the Bradford method29 with bovine serum albumin (BSA) as the standard. The protein content was found to be 38.2 ± 3.5% w/w, corresponding to a protein yield of 0.382 ± 0.035 g of protein per g of Chlorella vulgaris biomass30.
Protein hydrolysates and extracts
In the present study, two lyophilised PHs derived from residuals of C. vulgaris were used for the preparation of the aqueous extracts. For each extract, 2.5 mg of lyophilised powder was suspended in 50 mL of distilled and sterilised water. The resulting solution was sonicated for 30 min (GRANT ULTRASONIC BATH XUBA3).
The product obtained after sonication was placed in a centrifuge (THERMO IEC CL30R) at 2000 rpm for 5 min. Lastly, the supernatant was removed to obtain solutions diluted with water at 1:10, 1:100, 1:1000 respectively, which were used as foliar biostimulants on the experimental crop.
Seed germination and phytotoxicity test
To test the phytotoxicity of PHs the method suggested by Zucconi et al.31 was performed. The first tests were performed on twenty seeds of Lactuca sativa L. (genotype “Eden” purchased from Maraldi Srl, Italy). The seeds were incubated at 20 °C in Petri dishes (three replicates per treatment), each containing sterile filter paper and moistened with 4 mL of aqueous extract. Three biological replicates were carried out for each treatment. Negative control, consisting of sterile distilled water without any added substances, was used for comparison. After 36 h, the germination index percentage (GI%) was calculated for both roots and shoots using a digital calliper. For roots, the following formula was used:
where G1 and G2 are germinated seeds in the sample and control and R1 and R2 are the average root length for the sample and control, respectively.
For shoots, a formula like that adopted for roots was used:
where G1 and G2 are germinated seeds in the sample and control and S1 and S2 are the mean shoot length of the sample and control, respectively.
Greenhouse experiment
The pot experiment was carried out in the greenhouse at University of Salerno under controlled conditions, monitoring the main climatic parameters, using the best dilutions achieved in the GI test. The temperature and humidity inside the greenhouse were measured by a mobile meteorological station (PCE instruments PCE-FWS 20 N) placed inside the structure at a height of 130 cm from the ground. The temperature was kept constant in a range of 28 –25 °C by means of an air conditioner with a relative humidity (RH) ranging from 37 to 50%. Regarding the light factor, illumination was provided by lamps (Indium art 1158 Sap-T 400 Cnr-L Graphite Disano, 250 W, 230 V, 50 Hz) with a photoperiod of 16 h of light and 8 h of darkness. The selected extracts were evaluated as foliar spray treatment on the crop and compared to two controls (one treated with only water, and one treated with a microalgal commercial biostimulant Agrialgae (using the dose reported in the label). Five biological replicates were used for each treatment. In each pot (10 cm diameter and 500 mL capacity), 15 seeds of lettuce (genotype “Eden” purchased from Maraldi Srl, Italy) were sown using a neutral, unfertilised peat (Mannaflor Tray 80/20 0.4) as growth medium. Throughout the experimental trial, the pots were irrigated with 125 mL of H2O (three days per week) and fertilised twice with 0.13 g of nitrogen using NH4NO3 at sowing and 10 days after sowing. The water used for irrigation had a pH of 6.8 and an electrical conductivity of 1.07 dS m− 1. The foliar treatment was carried out weekly from the development of the first true leaf until harvest using a hand sprayer. Harvesting carried out 28 days after germination. The experiment was carried out according to the following treatment: T1: control-1 (CTRL); T2: control-2 (commercial biostimulant “Agrialgae®”); T3: TPH-100 (Trypsin Protein Hydrolysates diluted 1:100); T4: TPH-1000 (Trypsin Protein Hydrolysates diluted 1:1000); T5: PPH-100 (Pepsin Protein Hydrolysates diluted 1:100); T6: PPH-1000 (Pepsin Protein Hydrolysates diluted 1:1000).
Agronomic surveys
During the crop cycle, the height of the plant was measured using a ruler and, the soil water content was measured through the TDR (Time Domain Reflectometry) sensor from Fieldscout. At harvest aboveground fresh weight (g pot− 1) and the belowground fresh weight (g pot− 1) were weighed using an electronic balance. Root length and leaf number were also measured, and crop water productivity (CWP) and nitrogen agronomic efficiency (NAE) were calculated according to Ronga et al.32. After measurement of the fresh weight, the samples were placed in an oven at 65 °C for 48 h to obtain the shoot and root dry weights.
Physiological surveys
Physiological surveys were conducted both during the growing cycle and at harvest. In particular, the main leaf pigments such as chlorophyll, flavonoids, anthocyanins and NFI index (Chlorophyll/Flavonoid) related to the plant’s primary and secondary metabolisms were monitored using the MPM100 instrument (Opti-sciences). Canopy temperature was monitored using the Flir Ex-Series thermal imaging camera, stomatal conductance was monitored using the Delta T Service AP4 Porometer and finally, the Normalized Difference Vegetation Index (NDVI) and Photochemical Reflectance Index (PRI) were recorded using Decagon sensors.
Qualitative surveys
At harvest, qualitative investigations were carried out. Leaf area was determined with the Li-cor Model Li-3000 portable leaf area meter, leaf colour was determined with the CR-210 Chroma Meter (Minolta Corp., Osaka, Japan) equipped with a standard D65 illuminator and, finally, the nitrate content (NO3−) was determined with the Horiba LAQUAtwin instrument.
Extraction of polyphenols
Polyphenols were extracted from the harvested leaf samples following the protocol previously reported by Materska et al.33, with slight modifications. Briefly, a lyophilised lettuce sample was used and extracted with 70% aqueous methanol for 30 min at 25 °C. The mixture was then centrifuged at 6.000 rpm for 15 min at 4 °C. The resulting supernatant was collected, and the fresh extracts were stored at 4 °C in the dark until further analysis. Each extraction process was performed in triplicate.
Determination of total phenolic content
The total phenolic content (TPC) was determined using the Folin–Ciocalteau method as described by Way et al.34 with slight adjustments. Reagent A was prepared by combining 5 mL of 2 M Folin-Ciocalteu reagent to 45 mL of distilled water. For reagent B, 2.87 g of sodium carbonate was dissolved in distilled water in a 25 mL volumetric flask. For each sample, 2 µL of extract was added to 100 µL of reagent A in a microplate, mixed, and left for 5 min before adding 70 µL of reagent B and mixing. Then, the microplate was incubated for 1 h at 40 C. The absorbance of the solution was then evaluated at 765 nm using a Multiskan SkyHigh Microplate Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). A blank solution was prepared and gallic acid was selected as the standard. Stock solution (1 mg mL− 1) was prepared in MeOH, and the calibration curve was obtained in a concentration range of 5–500 µg mL− 1, with six concentration levels (y = 895.24x – 5.73) and the linearity of the standard curve was 99.91%. The solution was measured in triplicate. The total phenolic content was calculated and expressed as milligrams of gallic acid equivalents per gram of dry weight (mg GAE/g− 1 DW).
Determination of total flavonoids content
The total flavonoids content (TFC) was determined by applying the method reported by Imeneo et al.35 appropriately modified. An aliquot of extract (25 µL), 100 µL of deionised water, and 7.5 µL of NaNO2 (5%, w/v) were placed in a microplate and incubated at room temperature (5 min). Then, 7.5 µL of AlCl3 (10%, w/v) were added and incubated for 6 min; after that, 100 µL of NaOH (4%, w/v) was mixed. Simultaneously a blank solution was prepared. The reaction mixture was left to settle in the dark for 15 min; afterward, the absorbance was measured at 510 nm using a Multiskan SkyHigh Microplate Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and compared to a rutin calibration standard curve (10–500 µg mL− 1; y = 1.568,90x – 11,02; R2 = 99.92%). The results were expressed as milligrams of rutin equivalents per gram of dry weight (mg RE g− 1 DW).
Polyphenol analysis via UHPLC-HRMS
UHPLC-HRMS/MS analysis was performed on a Thermo Scientific™ Vanquish™ UHPLC system, equipped with a VF-P10-A binary solvent delivery system, a VH-C10-A column compartment and VF-A10-A autosampler. The UHPLC system was coupled online to a Orbitrap Exploris™ 120 mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with a heated electrospray ionization probe (HESI II) operating in negative mode.
The chromatographic separation was performed on a Kinetex® 2.6 μm EVO C18 100 Å, LC Column 150 × 2.1 mm (Phenomenex, Bologna, Italy). The column temperature and the flow rate were set at 45 °C and 0.4 mL/min, respectively. The mobile phases were: H2O (A) and ACN (B) both acidified with 0.1% HCOOH (v/v) with the following gradient: 0.01–2.00 min, isocratic to 2% B; 2.01–14.00 min, 2–98% B; 14.01–16.00 min, isocratic to 98% B; 16.01–17.00 min, 98 − 2% B; then five minutes for column re-equilibration.
Full MS (100–1400 m/z) and data-dependent MS/MS were performed at a resolution of 30,000 and 15,000 FWHM respectively; Normalized Collision Energy (NCE) value of 30 was used. Source parameters: Sheath gas pressure, 35 arbitrary units; auxiliary gas flow, 10 arbitrary units; spray voltage, + 3.4 kV, -2.8 kV; capillary temperature, 320 °C; auxiliary gas heater temperature, 320 °C.
The identification of analytes was carried out by comparing their retention times and MS/MS data with those present in the literature. Data analysis and processing were performed using FreeStyle™ 1.8 SP2 and the commercial software Compound Discoverer v. 3.3.1.111 SP1 (Thermo Fisher Scientific, Bremen, Germany).
For the quantification of the main compounds the chromatographic separation was conducted under the same conditions as previously described, and chlorogenic acid (CGA) and luteolin 7-O-glucoside (LUT-G) were used as external standards for the quantification of phenolic acids and flavonoids, respectively. Stock solution (1 mg mL− 1) was prepared in MeOH, and the calibration curve was obtained in a concentration range of 0.0625–10 µg mL− 1 for CGA and 0.0313–0.5 µg mL− 1 for LUT-G. An equal amount of internal standard (quercetin-d3) was added to each level to obtain a final concentration of 2 µg mL− 1. Linear regression was used to generate the calibration curve with R2 values ≥ 0.998. Peak areas of the standard were plotted against corresponding concentrations (µg mL− 1). The compound content in the sample was expressed as milligram per gram of dried extract.
Limits of detection (LOD) and quantification (LOQ) were calculated by using the standard deviation (SD) and the slope of the calibration curve, multiplied by 3.3 and 10, respectively. The repeatability of the chromatographic system was assessed in terms of intraday and interday precision by performing three injections at three different concentration levels. The data obtained demonstrated an acceptable degree of accuracy and precision for the analytical method developed (Table S1).
Determination of total chlorophyll and carotenoids content
The chlorophyll content was determined using the methods previously described by Wang et al.36 and Zhang et al.37 while the carotenoid content was determined using the method reported by Šamec et al.38 with appropriate modifications. Briefly, 10 mg of extract was mixed with 1.25 mL of 80% acetone and vortexed for 5 min. The mixture was then centrifuged at 14,000 rpm for 10 min. The absorbance of the supernatant was measured at 646.8 nm, 663.2 nm, and 452.5 nm using a Multiskan SkyHigh Microplate Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The chlorophyll concentration was calculated using the following formula:
where V was the extract volume (mL) and m was the sample dry weight (mg).
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay
The free radical scavenging ability of the extracts was tested using the DPPH radical scavenging assay. The DPPH test was conducted with slight modifications to the conditions reported by Noreen et al.39. Briefly, 20 µL of extracts, antioxidant standards, and methanol (as control) were mixed with 150 µL of a methanolic DPPH solution (0.08 mM). Trolox was used as the analytical standard (2–12 µM; y = 52.3041x + 0.2186; R2 = 0.9869). All the samples were prepared in triplicate, shaked and incubated in dark for 30 min at 37 °C. Changes in the absorbance of the samples were measured against methanol as blank at 517 nm using a microplate reader (Multiskan SkyHigh Microplate Spectrophotometer, Thermo Fisher Scientific, Waltham, MA, USA). The percentage inhibition activity on DPPH radical was calculated and the result of the DPPH reduction in the sample was reported as IC50 value, a greater IC50 value implies less antioxidant capacity in extracts.
Ferric reducing antioxidant potential (FRAP) assay
The FRAP method is based on the reduction of ferric ion (Fe3+) to ferrous ion (Fe2+). The assay was conducted with slight modifications to the conditions previously described by Noreen et al.39. FRAP reagent was prepared by mixing three solutions: A, 300 mM sodium acetate buffer, pH = 3.6; solution B, 10 mM TPTZ solution in 40 mM HCl; and solution C, 20 mM ferric chloride (FeCl3) in a volumetric ratio of 10:1:1 v/v/v, respectively. The reagent was kept in darkness for 30 min to complete the reaction. Briefly, 5 µL of extracts were mixed with 145 µL of FRAP reagent. FeSO4 was used as reference (0.025–1 mM; y = 0.7142x + 0.0029; R2 = 0.9999). All the samples were prepared in triplicate, shaked and incubated in dark for 30 min at 37 °C. Changes in the absorbance of the samples were measured against blank at 593 nm using a microplate reader. FRAP activity was calculated as millimoles of ferrous equivalents per gram of dry weight (mM Fe(II)E g− 1 dw).
2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS)
The scavenging activity was determined according to the method described by Noreen et al.39. The ABTS radical cation (ABTS•+) was prepared by mixing 7 mM ABTS solution (Solution A) and 2.45 mM potassium persulfate (K2S2O8) solution (Solution B) in ratio of 1:1 (v/v) and kept in darkness at room temperature for 12–16 h to complete the reaction. The stock solution was diluted with ethanol to get the ABTS working solution until absorbance reached values of 0.70 (± 0.02) AU at 734 nm. For the assay, different concentration levels of plant extracts and standard trolox (2.5–25 µM) were mixed with 200 µL of ABTS working solution. An equal amount of methanol (100 µL) was used as a blank (control). All the samples were prepared in triplicate and vortexed for 1 min. After 5 min incubation, the decrease in absorbance of each sample was measured against methanol as blank on UV–Visible spectrophotometer at 734 nm. The percentage ABTS inhibition was calculated, and the results were reported as IC50 value.
Statistical analysis
Experimental data results were analyzed by one way ANOVA and means were compared by Tukey’s HSD (Statgraphics Centurion 18, Stat Point Technologies, Inc., Warrenton, VA, USA). Significance was accepted at p < 0.05.
Results
C. vulgaris was cultivated in a system of integrated photobioreactors, the collected biomass was dried and polysaccharides removed. Biomass post-polysaccharide extraction was freeze-dried and proteins were isolated following a previously described protocol28 through enzymatic hydrolysis with trypsin and pepsin. Two lyophilised PHs (TPH and PPH) were prepared and tested.
Preliminary phytotoxicity test on lettuce seeds
The preliminary germination tests did not indicate any phytotoxicity problems for the dilutions of the PHs tested. In fact, as shown in Table 1, the values were above 50%, which can be considered as a threshold value for phytotoxicity31. In particular, the GIs (%), for shoot was the highest in preliminary treatment 3 (undiluted chl PH pepsin) while the GIr (%), for root was the highest in preliminary treatment 7 (chl PH pepsin 1:10) and in preliminary treatment 9 (chl PH pepsin 1:1000).
Greenhouse treatment
Considering the results of the germination test, the best concentrations of the PHs extract tested in vivo on lettuce were TPH-100 (T7), TPH-1000 (T6), PPH-100 (T8) and PPH-1000 (T9).
Results of agronomic and physiological not destructive surveys during the crop cycle
Table 2 shows the results of the agronomic and the physiological not destructive surveys carried out during the crop cycle. In particular, plant height was statistically higher in control compared to the other ones, while the volumetric water content was the highest in treatment 4 (TPH-1000). Treatment 5 (PPH-100), followed by treatments 4 (TPH-1000) and 6 (PPH-1000) showed higher chlorophyll index compared to the other treatments. Treatment 5 (PPH-100) highlighted the highest value of flavonol index by about + 25% compared to the two controls, while the untreated control and the commercial biostimulant showed the highest NFI index. Canopy temperature, stomatal conductance and NDVI index were higher in lettuce plants treated with treatment 6 (PPH-1000) compared to the other ones.
Results of agronomic and physiological not destructive surveys during the harvest
At harvest, the foliar treatments showed no statistically significant differences in plant height compared to the untreated control and compared to the commercial biostimulant (Table 3). The volumetric water content was the highest in treatment 5 (TPH-100) by about 20% compared to the untreated control. Treatment 6 (PPH-1000) reported the highest number of leaves. The chlorophyll index was higher in all the treatments treated with the extracts and the commercial biostimulant than in the untreated control. The lettuce plants treated with treatment 6 (PPH-1000) reported the highest value of the flavonol index, while anthocyanins were higher in the lettuce plants treated with the commercial biostimulant and in those treated with treatment 5 (PPH-100) compared to other ones (Table 3). The control and treatment 6 (PPH-1000) reported the highest canopy temperature while stomatal conductance was the highest in treatment 4 (TPH-1000).
Results of production and quality surveys at harvest
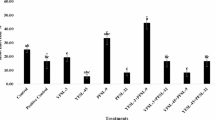
Table 4 shows the results of production and quality surveys carried out at harvest. In particular, root length was the highest in control and treatment 5 (PPH-100). T6 increased the aboveground fresh weight by about + 61% compared to the untreated control and by about + 22% compared to the commercial biostimulant. In addition, treatment 6 (PPH-1000) reported aboveground dry weight value that was about + 70% higher than the untreated control and about + 22% higher than the commercial biostimulant (Table 4; Fig. 1). Regarding the belowground fresh weight, treatment 6 (PPH-1000) reported a + 167% higher weight than the untreated control and a + 116% higher weight than the commercial biostimulant. Treatment 6 (PPH-1000) increase belowground dry weight compared to the untreated control and compared to the commercial biostimulant. Crop water productivity (CWP) and nitrogen agronomic efficiency (NAE) were also the highest in treatment 6 (PPH-1000) increasing both water and nutrient efficiency of the lettuce plants. In mean time with the physiological and agronomic surveys, qualitative surveys were also carried out during harvest. As shown in Table 4, leaf area was the highest in treatment 6 (PPH-1000). Regarding the parameters determining color, the brightness index (L), green index (a) and yellow index (b) did not differ significantly between treatments. Regarding leaf nitrate, the control reported the highest values while the tested extracts reduced the nitrate leaf content.
Polyphenolic profile and antioxidant activity of lettuce samples
The phenolic and flavonoid content of extracts from baby leaf lettuces was analysed. The results (Table 5) showed a significant increase of approximately 23% in both phenolic acids and flavonoid content in treatment 4 (3314.23 ± 137.16 µg GAE g⁻¹ dw; 7185.08 ± 39.44 µg RE g⁻¹ dw) compared to the control (2666.67 ± 335.51 µg GAE g⁻¹ dw; 5828.85 ± 221.88 µg RE g⁻¹ dw). Conversely, the treatment with the commercial biostimulant (T2) resulted in a 33% decrease in polyphenol content relative to treatment 1 (1777.40 ± 166.70 µg GAE g⁻¹ dw), while no significant changes were observed in flavonoid content (5783.53 ± 586.74 µg RE g⁻¹ dw).
Interestingly, chlorophyll and carotenoid levels also increased significantly in treatment 4 (TPH-1000) compared to all other treatments, suggesting enhanced photosynthetic pigment accumulation. To further evaluate the nutraceutical potential of baby leaf lettuces treated with PHs, antioxidant activity was assessed.
using DPPH and ABTS assays. Consistent with the increased levels of bioactive compounds, treatment 4 exhibited superior radical-scavenging activity compared to all other treatments, underscoring its substantial antioxidant potential (Table 5).
These findings were corroborated by the FRAP assay, which measures reducing capacity expressed as µM Fe(II) equivalents per gram of dry weight. Treatment 4 demonstrated the highest reducing power, showing approximately twice the activity of the commercial biostimulant and a significantly enhanced ability to reduce Fe(III)-TPTZ to Fe(II)-TPTZ.
Identification and quantification of flavonoids and phenolic compounds in the extract of baby leaf lettuce through LC-MS/MS
UHPLC-Orbitrap-HRMS analysis was performed to identify and quantify flavonoids and phenolic acids in the hydroalcoholic extract of baby leaf lettuces. The analysis focused on treatment 4 (TPH-1000), which exhibited the highest content of bioactive compounds and the strongest antioxidant activity, and on treatment 6 (PPH-1000), which proved to be the most effective in enhancing several key parameters, particularly in terms of production, compared to both the control (T1) and the commercial biostimulant (T2).
A total of 12 phenolic compounds were identified in the analysed samples (Table 6). All experiments were conducted using electrospray ionisation in negative mode (ESI−).
The most abundant compound identified in the lettuce extracts was associated with chromatographic peak 2. Peak 2, with an MS parent ion at m/z 353, was identified as chlorogenic acid. The precursor [M − H]⁻ ion generated product ions at m/z 191, indicating the loss of a hexoside moiety and resulting in a quinic acid ion, and at m/z 179, corresponding to the caffeic acid ion. Chlorogenic acid was notably the most abundant compound in lettuce leaf extracts, with a concentration of 909.79 ± 12.22 µg g⁻¹ dw in treatment 4, compared to 626.38 ± 59.59 µg g⁻¹ dw in the control, 354.78 ± 13.46 µg g⁻¹ dw in treatment 6, and 205.33 ± 33.64 µg g⁻¹ dw in treatment 2.
A similar fragmentation pattern was observed for peak 10, tentatively identified as dicaffeoylquinic acid (DCQA). The [M − H]⁻ ion was detected at m/z 515, and the MS/MS fragmentation pattern revealed a base peak at m/z 353 and m/z 323, corresponding to the loss of a caffeoyl moiety ([M − H−162]⁻) and the loss of a quinic moiety ([M − H−192]⁻), respectively.
Peak 11 was tentatively identified as dicaffeoyltartaric acid (DCTA) and was quantified as the most abundant compound in treatment 4 with a concentration of 921.68 ± 12.84 µg g⁻¹ dw. This value was significantly higher compared to Treatment 1 (714.36 ± 68.7 µg g⁻¹ dw), treatment 6 (575.64 ± 18.99 µg g⁻¹ dw), and treatment 2 (243.78 ± 26.02 µg g⁻¹ dw).
The MS/MS fragmentation pattern of DCTA was characterised by the loss of a hexoside moiety, yielding an ion at m/z 311, followed by the loss of a water molecule, resulting in an ion at m/z 293. Additionally, caffeic acid and tartaric acid ions were observed in the MS/MS spectra at m/z 179 and m/z 149, respectively40.
Several flavonoids were identified, primarily corresponding to quercetin and luteolin glycosides. Peak 6 exhibited an MS spectrum characterised by a deprotonated molecular ion at m/z 447, consistent with the molecular formula C₂₁H₁₈O₁₃. The fragmentation pattern showed the loss of a glucoside moiety (162 Da), resulting in a fragment at m/z 285 ([M − H−162]⁻), followed by the loss of a CHO group (29 Da), producing a fragment at m/z 255 ([M − H−162 − 29]⁻). This fragmentation profile confirmed the identity of peak 6 as luteolin 7-O-glucoside. Its concentration was comparable across the four treatments, averaging approximately 1.14 ± 0.14 mg g⁻¹ dw. Additional luteolin glycoside derivatives were tentatively identified based on similar fragmentation patterns. These included luteolin glucuronide (peak 8, m/z 461, C₂₁H₁₈O₁₂) and luteolin O-malonylglucoside (peak 12, m/z 533, C₂₄H₂₂O₁₄). Both compounds exhibited the characteristic m/z 285 fragment, corresponding to the luteolin aglycone. For luteolin glucuronide, this fragment resulted from the loss of the glucuronide moiety (176 Da), whereas for luteolin O-malonylglucoside, it resulted from the sequential loss of malonyl (86 Da) and glucoside (162 Da) residues41.
Peaks 5, 7, and 9 were tentatively identified as quercetin glycoside derivatives. Peak 5 was identified as quercetin hexoside, exhibiting a molecular ion at m/z 463 ([M − H]⁻) and a fragment at m/z 301, resulting from the loss of a sugar moiety (162 Da). Similarly to peaks 8 and 12, quercetin glucuronide (peak 7, m/z 477, C₂₁H₁₈O₁₃) and quercetin O-malonylglucoside (peak 9, m/z 549, C₂₄H₂₂O₁₅) were tentatively identified based on the loss of the glucuronide (176 Da) and the loss of the malonyl-glucoside residues (86 + 162 Da), respectively, both leading to a quercetin aglycone fragment ion at m/z 301.
Quercetin O-malonylglucoside was identified as the most abundant flavonoid in both treatment 1 (22.71 ± 3.43 µg g⁻¹ dw) and treatment 4 (21.99 ± 1.32 µg g⁻¹ dw), with no significant difference between them. However, a statistically significant difference was observed when compared to treatment 6 (12.02 ± 1.54 µg g⁻¹ dw) and treatment 2 (9.31 ± 0.43 µg g⁻¹ dw).
Discussion
PHs are a category of plant biostimulants defined as ‘mixtures of polypeptides, oligopeptides and amino acids that are manufactured from protein sources using partial hydrolysis’42. Interest in PHs has increased in recent years due to their beneficial effects on crop performance, particularly under conditions of environmental stress.
PHs are primarily produced through the chemical and/or enzymatic hydrolysis of proteins contained in agroindustrial by-products derived from both animal and plant sources, as well as in the biomass of dedicated legume crops43,44,45.
Chemical hydrolysis breaks down all peptide bonds in proteins, resulting in a high level of hydrolysis and a significant content of free amino acids. However, this process also destroys certain amino acids (e.g., tryptophan, cysteine, serine, threonine) and converts others, such as asparagine and glutamine, to their acidic forms. Additionally, thermolabile compounds like vitamins are often lost. A key issue with chemical hydrolysis is racemisation, where L-amino acids convert to D-forms, which plants cannot metabolise, reducing the effectiveness of the PH and potentially making it toxic46. Furthermore, the extensive use of acids and alkalis during chemical hydrolysis increases the salinity of PHs.
Enzymatic hydrolysis is commonly used for producing plant-based PHs. This process employs proteolytic enzymes derived from animal organs, plants or microorganisms. These enzymes hydrolyse proteins more gently than acids or alkalis, operating at lower temperatures and targeting specific peptide bonds. The resulting PHs are a mixture of amino acids and peptides of varying lengths, with low salinity and a stable composition over time. Additionally, enzymatic hydrolysis is more environmentally friendly than chemical methods due to lower energy consumption and reduced carbon dioxide emissions47.
Given these benefits, this study investigated the biostimulant effects of two PHs derived from the enzymatic hydrolysis of proteins isolated from the residual biomass of C. vulgaris, obtained after a green extraction process aimed at isolating a polysaccharide-rich polar fraction.
For this purpose, we used two different animal-derived proteolytic enzymes: trypsin and pepsin. Trypsin is derived from porcine pancreas, while pepsin is obtained from porcine gastric mucosa. These enzymes cleave peptide bonds at various sites and operate at different pH values. Pepsin preferentially cleaves at the C-terminus of tyrosine, tryptophan, or leucine. In contrast, trypsin cleaves the peptide bond between the carboxyl group of arginine or lysine and the amino group of the adjacent amino acid. The optimal pH ranges for the activity of pepsin and trypsin are 1–3 and 7–9, respectively.
The foliar application with the different concentrations of PHs tested showed biostimulant effects on the treated lettuce seedlings increasing both leaf pigment, fresh and dry biomass, and water and nutrient agronomic efficiency compared to the untreated control and to the commercial biostimulant, in according with the reports of Silambarasan et al.48 in which the authors, tested the foliar application of 60% Nostoc sp. LS04 cell extracts obtaining increased plant height, root length, fresh dry biomass, chlorophyll a and b and carotenoids. In the present study, plants treated with PPH-1000 increased aboveground fresh and dry weight by 61% and 70%, respectively, in according with the reports of La Bella et al.24 in which foliar application of Scenedesmus quadricauda resulted in an increase of about 23% in aboveground fresh weight and about 20% in aboveground dry weight compared to the control. In addition, the belowground weight results are in according with those reported by Hajnal-Jafari et al.49 in which the treatment with C. vulgaris on lettuce positively influenced root growth, particularly weight. In the present work, the belowground fresh weight of the plants treated with treatment 6 (PPH-1000) was approximately 167% higher than that of the control plants. This value is also in according with that obtained by Ammaturo et al.13 in which belowground weight of plants treated with C. vulgaris (15%) appeared to be 36.9% higher than control plants. Comparing the results in the literature with those obtained in the present work, PHs increased the number of leaves as reported by Supraja et al.50 in which treatment with microalgae increased the number of leaves in tomato plants treated with foliar sprays. In this regard and according to previous studies, the leaf pigment content was higher in lettuce plants treated with PHs extracts than in the control. The foliar application influenced nitrogen metabolism by increasing the NAE. This result is justified by the action of PHs extracts, considering that, when applied as foliar sprays, they influence nitrogen metabolism in plants11. The nitrate content is an increasingly discussed parameter and subject to Regulation (EU) No 1258/2011 concerning maximum admissible nitrate levels in lettuce. In this trial, the lettuce plants treated with the extracts significantly reduced the nitrate content compared to the control, which also improved the quality aspect. Considering the ability of microalgal biostimulants to increase the nutrient and water uptake efficiency of plants, this study reports useful information for the reduction of mineral fertilisation and irrigation water in lettuce production. However, further trials are needed to determine the efficacy of PHs extracts in improving yields and reducing the use of synthetic chemical fertilisers and irrigation water on a real farm, while maintaining high quality standards and agronomic effects as well as on other crops.
Interestingly, in previous researches PHs have been shown to enhance not only plant nutrition but also the quality of vegetables and fruits produced, particularly by increasing the concentration of nutraceutical compounds21,51,52,53. In the current investigation, PHs treatment 4 (TPH-1000) led to a significant increase in polyphenolic compounds of baby leaf lettuce compared to both the control and the commercial biostimulant, alongside enhancing antioxidant activity, as demonstrated by the lower sample concentration required to inhibit 50% of ABTS radicals.
On the other hand, PHs treatment 6 (PPH-1000) exhibited enhanced antioxidant properties, which were associated with a higher concentration of polyphenolic compounds compared to baby leaf lettuce treated with the commercial biostimulant.
This observation suggests that the increased content of phenolic compounds may be linked to the activation of secondary metabolism, particularly through the upregulation of the phenylalanine (tyrosine) ammonia-lyase enzyme, which plays a crucial role in the phenylpropanoid pathway46,54,55.The stimulation of the phenylpropanoid pathway by PHs could be due to the improvement of N assimilation, although an alternative metabolic pathway that couples the proline biosynthesis to the oxidative pentose phosphate pathway has been proposed21,56.
High levels of polyphenols are essential for prolonging the plants’ shelf life and improving human nutrition by mitigating oxidative stress, a factor frequently associated with numerous inflammatory conditions related to neurodegenerative diseases and cancer57,58.
Conclusion
The treatments carried out with PHs extracts from C. vulgaris had very interesting results on the tested crop. This study demonstrates how the foliar application of PHs extracts is to be considered as an innovative and safe agricultural practice for the environment and agricultural sustainability, enabling ever higher yields in a global agricultural scenario that is increasingly moving towards a circular economy. Each of the examined treatments showed different effects on various parameters: some enhanced crop quality by increasing antioxidants like flavonoids and anthocyanins, while others improved physiological traits such as reducing stomatal conductance. Treatment 4 (TPH-1000) has been shown to enhance the health-related properties of plants subjected to foliar application. This improvement is attributed to the increased concentration of bioactive compounds, such as polyphenols, which contribute to the plant’s overall health and resilience. Additionally, the enhanced antioxidant activity observed in plants treated with TPH-1000 further supports their improved health status. Treatment 6 (PPH-1000) was generally the most effective in enhancing several detected parameters, particularly in relation to production, concurrently enhancing water and nutritional efficiency in lettuce plants, making it a viable biostimulant. In conclusion, the use of PHs extracts from C. vulgaris on leafy vegetables destined for IV range has proven to be an excellent eco-sustainable solution for increasing physiological and agronomic performance as well as final yields. Further studies on other types of crops are needed to provide further insights into the efficacy of PHs to reduce the use of synthetic fertilisers and improve environmentally friendly farming practices.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Ramegowda, V. & Senthil-Kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: mechanistic Understanding from drought and pathogen combination. J. Plant. Physiol. 176, 47–54. https://doi.org/10.1016/j.jplph.2014.11.008 (2015).
Al-Chalabi, M. Agricoltura verticale: sostenibilità dei grattacieli? Sustain. Cities Soc. 18, 74–77. https://doi.org/10.1016/j.scs.2015.06.003 (2015).
Rouphael, Y. & Colla, G. Biostimulants in agriculture. Front. Plant Sci. 11, 40. https://doi.org/10.3389/fpls.2020.00040 (2020).
Nehul, J. N. et al. Biostimulants and their role in enhancing plant resistance to pests and pathogens. UPJOZ 45 (24), 213–225. https://doi.org/10.56557/upjoz/2024/v45i244726 (2024).
Khan, S., Khan, M. A., Hanjra, M. A. & Mu, J. Pathways to reduce the environmental footprints of water and energy inputs in food production. Food Policy. 34, 2, 141–149. https://doi.org/10.1016/j.foodpol.2008.11.002 (2009).
Calvo, P., Nelson, L. & Kloepper, J. W. Agricultural uses of plant biostimulants. IJPS 383 (1), 3–41. https://doi.org/10.1007/s11104-014-2131-8 (2014).
Atzmon, N. & Van Staden, J. The effect of seaweed concentrate on the growth of Pinus Pinea seedlings. New. For. 8, 3, 279–288 (1994).
Vernieri, P., Borghesi, E., Ferrante, A. & Magnani, G. Application of biostimulants in floating system for improving rocket quality. JFAE 3 (3/4), 86 (2005).
Thompson, B. Five years of Irish trials on biostimulants: the conversion of a skeptic. USDA Serv. Proc. 33, 72–79 (2004).
Franzoni, G., Cocetta, G., Prinsi, B., Ferrante, A. & Espen, L. Biostimulants on crops: their impact under abiotic stress conditions. Hortic 8, 189. https://doi.org/10.3390/horticulturae8030189 (2022).
Ronga, D. et al. Microalgal biostimulants and biofertilisers in crop productions. Agron 9 (4), 192. https://doi.org/10.3390/agronomy9040192 (2019).
Acién Fernández, F. G. et al. The role of microalgae in the bioeconomy. N Biotechnol. 61, 99–107. https://doi.org/10.1016/j.nbt.2020.11.011 (2021).
Ammaturo, C. et al. Use of Chlorella vulgaris and Ulva lactuca as biostimulant on lettuce. Appl. Sci. 13, 16, 9046. https://doi.org/10.3390/app13169046 (2023).
Lafarga, T., Villaró-Cos, S., Rivera-Sánchez, E., Salinas-García, M. & Acién, G. Chapter 9 microalgae as a source of agricultural products. Sustainable Industrial Processes Based Microalgae Chap. 9, 185–207. https://doi.org/10.1016/B978-0-443-19213-5.00009-1 (2024).
Fal, S., Smouni, A. & Arroussi, E. Integrated microalgae-based biorefinery for wastewater treatment, industrial CO2 sequestration and microalgal biomass valorization: A circular bioeconomy approach. Environ. Adv. Vol. 12, 2666–7657. https://doi.org/10.1016/j.envadv.2023.100365 (2023).
Prabha, S., Vijay, A. K., Paul, R. R. & George, B. Cyanobacterial biorefinery: towards economic feasibility through the maximum valorization of biomass. Sci. Total Environ. 827, 154297. https://doi.org/10.1016/j.scitotenv.2022.154297 (2022).
Hammann, W., Ross, A. & Seames, W. Sequential extraction of carbohydrates and lipids from chlorella vulgaris using combined physical and chemical Pre-Treatments. Chem. Eng. 8, 11. https://doi.org/10.3390/chemengineering8010011 (2024).
Sunday Okeke, E. et al. Microalgae biorefinery: an integrated route for the sustainable production of high-value-added products. Energy Convers. Manag X. 16 https://doi.org/10.1016/j.ecmx.2022.100323 (2022). 100323, ISSN 2590 – 1745.
Hu, L. et al. Microalgal proteins for a circular bioeconomy: nutritional, material, and chemical valorization. Bioresour Technol. 133049. https://doi.org/10.1016/j.biortech.2025.133049 (2025).
Sousa, V., Novais, R., Mata, T. M., Martins, A. A. & Pereira, R. N. Life cycle assessment of protein extraction from microalgae biomass using ohmic heating. Algal Res. 86, 103962. https://doi.org/10.1016/j.algal.2025.103962 (2025).
Colla, G. et al. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 196, 28–38. https://doi.org/10.1016/j.scienta.2015.08.037 (2015).
Sularz, O., Smoleń, S., Koronowicz, A., Kowalska, I. & Leszczyńska, T. Chemical composition of lettuce (Lactuca sativa L.) biofortified with iodine by KIO3, 5-Iodo-, and 3.5-diiodosalicylic acid in a hydroponic cultivation. Agron 10 (7), 1022. https://doi.org/10.3390/agronomy10071022 (2020).
Ergun, O., Dasgan, H. Y. & Isık, O. Effects of microalgae Chlorella vulgaris on hydroponically grown lettuce. Acta Hortic. 1273, 169–176. https://doi.org/10.17660/ActaHortic.2020.1273.23 (2020).
La Bella, E., Baglieri, A., Rovetto, E. I., Stevanato, P. & Puglisi, I. Foliar spray application of Chlorella vulgaris extract: effect on the growth of lettuce seedlings. Agron 11 (2), 308. https://doi.org/10.3390/agronomy11020308 (2021).
Puglisi, I. Morpho-biometric and biochemical responses in lettuce seedlings treated by diferent application methods of Chlorella vulgaris extract: foliar spray or root drench? J. Appl. Phycol. 34, 889–901. https://doi.org/10.1007/s10811-021-02671-1 (2022).
Rippka, R., Deruelles, J., Waterbury, J. B., Herdman, M. & Stanier, R. Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiol 111 (1), 1–61. https://doi.org/10.1099/00221287-111-1-1 (1979).
Sansone, F. et al. Exploring microencapsulation potential: multicomponent spray dried delivery systems for improvement of chlorella vulgaris extract preservation and solubility. Powder Technol. 429, 118882. https://doi.org/10.1016/j.powtec.2023.118882 (2023).
Chronakis, I. S., Galatanu, A. N., Nylander, T. & Lindman, B. The behaviour of protein preparations from blue-green algae (Spirulina platensis strain Pacifica) at the air/water interface. Colloids Surf. A: Physicochem Eng. Asp. 173 (1–3), 181–192. https://doi.org/10.1016/S0927-7757(00)00548-3 (2000).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 (1–2), 248–254. https://doi.org/10.1016/0003-2697(76)90527-3 (1976).
Del Prete, F. et al. Extract from Chlorella vulgaris: Production, characterization, and effects on the germination, growth and metabolite profile of Eruca sativa microgreens. Ind. Crop. Prod. 233, 121490, https://doi.org/10.1016/j.indcrop.2025.121490 (2025).
Zucconi, F., Pera, A., Forte, M. & De Bertoldi, M. Evaluating toxicity of immature compost. BioCycle 22, 54–57 (1981).
Ronga, D., Parisi, M., Pentangelo, A., Mori, M. & Di Mola, I. Effects of nitrogen management on biomass production and dry matter distribution of processing tomato cropped in Southern Italy. Agron 9, 12, 855. https://doi.org/10.3390/agronomy9120855 (2019).
Materska, M. et al. Polyphenolic profiles in lettuce (Lactuca sativa L.) after CaCl2 treatment and cold storage. Eur. Food Res. Technol. 245, 733–744. https://doi.org/10.1007/s00217-018-3195-0 (2019).
Way, M. L., Jones, J. E., Nichols, D. S., Dambergs, R. G. & Swarts, N. D. A. Comparison of laboratory analysis methods for total phenolic content of cider. Beverages 6 (3), 55. https://doi.org/10.3390/beverages6030055 (2020).
Imeneo, V., De Bruno, A., Piscopo, A., Romeo, R. & Poiana, M. Valorization of ‘rossa Di tropea’ onion waste through green recovery techniques of antioxidant compounds. Sustainability 14 (8), 4387. https://doi.org/10.3390/su14084387 (2022).
Wang, H. et al. The effect of microwave radiation on the green color loss of green tea powder. Foods 11 (16), 2540. https://doi.org/10.3390/foods11162540 (2022).
Zhang, M., Choe, J., Bu, T., Liu, S. & Kim, S. Comparison of antioxidant properties and metabolite profiling of Acer Pseudoplatanus leaves of different colors. Antioxidants 12 (1), 65. https://doi.org/10.3390/antiox12010065 (2022).
Šamec, D., Bogović, M., Vincek, D., Martinčić, J. & Salopek-Sondi, B. Assessing the authenticity of the white cabbage (Brassica Oleracea var. Capitata f. alba) cv. ‘Varaždinski’ by molecular and phytochemical markers. Int. Food Res. 60, 266–272. https://doi.org/10.1016/j.foodres.2013.07.015 (2014).
Noreen, H., Semmar, N., Farman, M. & McCullagh, J. S. Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopus Didymus. Asian Pac. J. Trop. Med. 10 (8), 792–801. https://doi.org/10.1016/j.apjtm.2017.07.024 (2017).
Assefa, A. D. et al. Identification and quantification of selected metabolites in differently pigmented leaves of lettuce (Lactuca sativa L.) cultivars harvested at mature and bolting stages. BMC Chem. 13, 56. https://doi.org/10.1186/s13065-019-0570-2 (2019).
Papetti, A., Maietta, M., Corana, F., Marrubini, G. & Gazzani, G. Polyphenolic profile of green/red spotted Italian cichorium intybus salads by RP-HPLC-PDA-ESI-MSn. J. Food Compos. Anal. 63, 189–197. https://doi.org/10.1016/j.jfca.2017.08.010 (2017).
Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 63 (10), 1161–1168 (2009).
Maini, P. The experience of the first biostimulant, based on amino acids and peptides: a short retrospective review on the laboratory researches and the practical results. Fertilitas Agrorum. 1, 29–43 (2006).
Schiavon, M., Ertani, A. & Nardi, S. Effects of an Alfaalfa protein hydrolysate on the gene expression and activity of enzymes of TCA cycle and N metabolism in Zea Mays L. J. Agric. Food Chem. 56 (24), 11800–11808 (2008). (2008).
Halpern, M. et al. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 130, 141–174. https://doi.org/10.1016/bs.agron.2014.10.001 (2005).
Ertani, A. et al. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. JPNSS 172 (2), 237–244. https://doi.org/10.1002/jpln.200800174 (2009).
Bernabei, G. Biostimolanti a base di idrolizzati proteici: costo energetico ed impatto ambientale del processo produttivo, Doctoral dissertation, Università degli studi della Tuscia, (2015).
Silambarasan, S. et al. Cultivation of Nostoc Sp LS04 in municipal wastewater for biodiesel production and their deoiled biomass cellular extracts as biostimulants for lactuca sativa growth improvement. Chemosphere 280, 130644. https://doi.org/10.1016/j.chemosphere.2021.130644 (2021). (2021).
Hajnal-Jafari, T. I., Đurić, S. S. & Stamenov, D. R. Influence of green algae chlorella vulgaris on initial growth of different agricultural crops. Zb Matice Srp Prir. Nauke. 130, 29–33. https://doi.org/10.2298/ZMSPN1630029H (2016).
Supraja, K. V., Behera, B. & Balasubramanian, P. Efficacy of microalgal extracts as biostimulants through seed treatment and foliar spray for tomato cultivation. Ind. Crops Prod. 151, 112453. https://doi.org/10.1016/j.indcrop.2020.112453 (2020).
Ertani, A. et al. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: chemical and metabolomic approaches. Front. Plant. Sci. 5, 375. https://doi.org/10.3389/fpls.2014.00375 (2014).
Gutiérrez-Gamboa, G., Romanazzi, G., Garde‐Cerdán, T. & Pérez‐Álvarez, E. P. A review of the use of biostimulants in the vineyard for improved grape and wine quality: effects on prevention of grapevine diseases. J. Sci. Food Agric. 99 (3), 1001–1009. https://doi.org/10.1002/jsfa.9353 (2019).
Rouphael, Y., Carillo, P., Cristofano, F., Cardarelli, M. & Colla, G. Effects of vegetal-versus animal-derived protein hydrolysate on sweet Basil morpho-physiological and metabolic traits. Sci. Hortic. 284, 110123. https://doi.org/10.1016/j.scienta.2021.110123 (2021).
Schiavon, M. et al. High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea Mays L). J. Chem. Ecol. 36, 662–669. https://doi.org/10.1007/s10886-010-9790-6 (2010).
Ertani, A., Schiavon, M., Altissimo, A., Franceschi, C. & Nardi, S. Phenol-containing organic substances stimulate phenylpropanoid metabolism in Zea Mays. JPNSS 174 (3), 496–503. https://doi.org/10.1002/jpln.201000075 (2011).
Shetty, K. & Wahlqvist, M. A model for the role of the proline-linked pentose-phosphate pathway in phenolic phytochemical bio-synthesis and mechanism of action for human health and environmental applications. APJCN 13 (1), 0964–7058 (2004).
Hollman, P. C. H. Evidence for health benefits of plant phenols: local or systemic effects? J. Sci. Food Agric. 81 (9), 842–852. https://doi.org/10.1002/jsfa.900 (2001).
Rashmi, H. B. & Negi, P. S. Phenolic acids from vegetables: A review on processing stability and health benefits. Int. Food Res. 136, 109298. https://doi.org/10.1016/j.foodres.2020.109298 (2020).
Acknowledgements
The authors would like to thank the students that collaborated with the activities carried out.
Funding
This study was carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them.
Author information
Authors and Affiliations
Contributions
A.DS., G.A., D.R., G.P., F.DP. wrote the main manuscript text, All authors reviewed the manuscript and carried out lab activities. G.P. received funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Di Serio, A., Aquino, G., Prete, F.D. et al. Protein hydrolysates derived from residual after polysaccharide extraction of Chlorella vulgaris biomass improves yield and quality of baby leaf lettuce. Sci Rep 15, 29612 (2025). https://doi.org/10.1038/s41598-025-15748-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15748-5