Abstract
Improving prevention and treatment strategies for invasive fungal infections (IFI) is essential to reduce associated morbidity and mortality. We performed a systematic review and meta-analyses to assess factors associated with IFI in adult patients with hematological malignancies and/or hematopoietic stem cell transplantation (HSCT). Data sources. We searched PubMed, Embase, CENTRAL and the grey literature from 01/01/2002 to 08/02/2024. Study eligibility criteria. Eligible studies were case-control or cohort studies including adult patients with hematological malignancies and/or HSCT and reporting risk factors for IFI. Participants. Adult patients with hematological malignancies and/or HSCT. Assessment of risk of bias. Risk of bias assessment was assessed independently using the Critical Appraisal Skills Programme checklists for cohort studies and case-control studies. Methods of data synthesis. Study selection and data extraction were done independently. Adjusted estimates were pooled using random effects models. Among 12 624 references identified, 69 studies (reporting 2917 IFI) were included in the systematic review, 20 of which were included in meta-analyses. Factors independently associated with IFI included previous allo-HSCT (pooled aHR 3.21 [95%CI 1.54–6.71]), especially with a haploidentical donor (pooled aHR 2.41 [95%CI 1.27–4.57]), acute graft vs. host disease (aGvHD) ≥ 2 (pooled aHR 2.59 [95%CI 1.36–4.90]), corticosteroids (pooled aOR 2.84 [95%CI 1.42–5.70]) or T-cell depleting agents (pooled aOR 2.73 [95%CI 1.61–4.64]). Antifungal prophylaxis was a protective factor for IFI (pooled aOR 0.20 [95%CI 0.13–0.28]). The identification of factors independently associated with IFI may help to stratify IFI risk among hematological patients.
Registration: PROSPERO, CRD42023429103.
Similar content being viewed by others
Introduction
Invasive fungal infections (IFI) remain at high risk for morbidity and mortality1,2,3. Even in the era of antifungal prophylaxis, individuals with hematological malignancies remain particularly vulnerable. In particular, those undergoing allogeneic hematopoietic stem cell transplant (HSCT) or diagnosed with acute leukemia exhibit the highest risk of IFI1,4,5. For instance, between 1999 and 2003, among 11 802 Italian patients with newly diagnosed hematological malignancies, acute myeloid leukemia (AML) exhibited the highest IFI incidence (12%, 373/3 012), followed by acute lymphoid leukemia (ALL) (6.5%, 77/1 073)5. Similarly, in Brazil, data from 2007 to 2009 revealed a 1-year cumulative IFI incidence of 18.7% in AML/myelodysplastic syndrome (MDS) and 11.3% in allo-HSCT recipients, with an overall mortality rate of 47%4.
Standardized definitions for IFIs, classifying them into proven, probable and possible categories, were first introduced by the Infectious Diseases Group of the European Organization for Research and Treatment of Cancer and the Mycoses Study Group (EORTC/MSG) in 2002, facilitating interstudy comparisons. These definitions were updated in 2008 and most recently in 2020, taking into account progress in diagnostic tests and improving the reliability and applicability of these criteria6,7,8.
While standardized definitions provide a framework for understanding IFI, identifying risk factors remains crucial for prevention and management. Risk factors can be broadly categorized into host characteristics, nature of the underlying hematological malignancy, treatment regimens, and environmental fungal exposure9,10.
Existing systematic reviews and meta-analyses on risk factors for IFI have predominantly focused on specific subgroups of hematological patients (e.g. patients receiving HSCT or in pediatric population)11,12. These targeted approaches offer valuable insights but fail to capture the broader landscape of IFI across all hematological malignancies.
In this study, we conducted a systematic review to identify factors associated with IFI in adult patients with hematological malignancies and/or HSCT. Additionally, we performed meta-analyses using consensus definitions for IFI to ensure robust and standardized results.
Methods
This systematic review was reported according to the PRISMA-2020 (Additional file 1) and the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) statements13,14. The protocol was registered with PROSPERO on June 3rd, 2023 (Reference: CRD42023429103). Modifications made to the protocol are detailed in Additional file 2.
Eligibility criteria
A study was included if: (1) it was a cohort or a case-control study, (2) at least 90% of patients were aged 18 years and older, (3) at least 90% of patients had a hematological malignancy and/or had received a HSCT, (4) the study was published after January 1st, 2002, (5) the number of patients with IFI and risk factors were specified in the study report. We chose to restrict the search from January 1st, 2002 to have more homogeneous definitions for IFI because it is the date of first publication of EORTC/MSG definitions. Studies where patients were older than 16 years old were included and considered as including adults and children when we could not obtain more information from the authors.
We excluded studies with (1) no assessment of factors associated with IFI; (2) less than 10 patients in any group ; (3) insufficient reporting of patient characteristics; (4) genes as risk factor, (5) only specific IFIs considered (e.g. invasive mould infections, candidiasis).
No restriction on the language of publication was applied. Authors were contacted when the publication was not available.
Search strategy
We conducted an electronic search in Medline through PubMed, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL). A search equation was made for each database combining Medical Subject Headings (MeSH)/Embase Medical headings (EMTREE) and free text words for observational studies, IFI, hematological malignancies or HSCT in adult patients (Additional files 3–5). To identify grey literature, we also searched ClinicalTrials.gov, the International Clinical Trials Registry Platform and screened the abstracts of congresses of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID, 2002–2023) and of the Infectious Disease Society of America (IDSA, 2003–2023). Then, we screened the reference list of the identified studies and of available reviews. The initial search was conducted on February 1st, 2023 and updated on February 8th, 2024.
Study selection
One reviewer (EG) screened the articles based on title and abstract after removing duplicates. Two reviewers independently selected studies based on full text following eligibility criteria (EG reviewed all the articles and LA and PMG equally shared the work). Any discrepancy was resolved by reaching a consensus between the two reviewers or by adjudication with the third reviewer. The Rayyan software was used to perform selection15.
Data extraction
Two reviewers (EG extracted all the articles and LA and PMG equally shared the work) independently extracted data from reports using a dedicated standardized data extraction form. The definition used for IFI (EORTC/MSG or use of another definition) and degree of certainty, namely proven and/or probable IFI (PP-IFI) or proven, probable and/or possible IFI (PPP-IFI) was assessed. Other parameters evaluated included article general characteristics, population characteristics at baseline (inclusion and exclusion criteria, demographic characteristics, comorbidities), hematological history (type and status of the hematological malignancy and/or HSCT, biological parameters, treatment, and factors related to the hospital stay). Additionally, results of the univariate and multivariate analyses were extracted, including the measure effect and adjusted measure effect for the association between expected risk factors and IFI, along with their 95% confidence interval (CI), and the factors used for adjustment of the analysis. Of note, a study could use patients or episodes as denominator. An episode could be a cycle of chemotherapy, a respiratory viral infection etc., and a patient could then be included in both IFI and no-IFI groups based on the episode’s characteristics. Additionally, for patients having received a HSCT, we extracted the period for which a risk factor was assessed (e.g. early, late or very late periods post-transplant corresponding to 1–39 days, 40–100, 101–365 days post-transplant, respectively).
At any stage of the process, in case of missing information, the authors of the publication were contacted by email for further details.
Risk of bias assessment
Two reviewers independently assessed the methodological quality of included studies with the Critical Appraisal Skills Programme (CASP) checklists for cohort studies and case-control studies (EG reviewed all the articles and LA and PMG equally shared the work) (additional file 2)16,17.
Analyses
For the qualitative synthesis, all studies meeting the inclusion criteria were analyzed, irrespective of the IFI definition used. When needed, we calculated OR and 95%CI based on available data in each group (IFI vs. no-IFI) and calculated the 95%CI based on measure effect and p-value18. We reported all variables significantly associated with IFI in univariate analysis and the results for all variables introduced in multivariate models whatever their statistical significance.
For the main quantitative analysis, we included studies assessing PP-IFI according to the EORTC/MSG classifications and reporting adjusted measure effects, regardless of whether they were based on patients or episodes6,7,8. A secondary quantitative analysis including the studies also reporting possible IFI based on 2002 and 2008 EORTC/MSG definitions was performed (reported as PPP-IFI)6,7. Hazard-ratio (HR), sub-distribution HR (SHR) and relative risk (RR) were pooled together. OR and HR were pooled separately. For each potential risk factor, when relevant, we specified the specific population for which it was studied (e.g. AML). Risk factors studied for a specific period of time (e.g. post-transplant periods) were not pooled with those assessed for an overall period. As we expected a high between-study heterogeneity, we used random-effects models. We estimated between-study heterogeneity based on visual examination of the forest plots, by the Q Cochran heterogeneity test and I2.
Sensitivity analyses were planned beforehand: excluding studies at high risk of bias (for both PP-IFI and PPP-IFI analyses), studies with children, and studies with an episode as the denominator for PP-IFI.
Analyses were performed with the R software version 4.4.0 using the meta package and the metagen function19.
Grading of evidence
Grading of evidence for each potential risk factor was assessed using the GRADE approach20.
Results
Selection process and characteristics of included studies
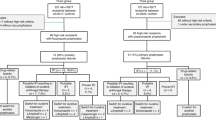
Among 12 624 references identified, we assessed 578 studies on full-text to finally include 69 studies (Fig. 1; Table 1), reporting 2 917 IFI in 35 781 episodes. Reasons for exclusion based on full-text assessment are listed in additional file 6. The studies included were mainly single-center (n = 56, 81%) retrospective (n = 57, 90%) cohorts (n = 63, 91%) describing IFI based on 2008 EORTC/MSG criteria (n = 49, 71%) (Table 1). Publications mostly included patients from North America, Asia and Europe (n = 24, 35%, n = 23, 33%, and n = 18, 26%, respectively). The studies concerned mainly patients with allo-HSCT (n = 20, 29%), followed by myeloid malignancies (n = 18, 26%) (Table 1). Three studies with adjusted measure effects were performed on large databases (with more than 3 000 patients included each), but used other definitions than EORTC/MSG and, as a consequence, were not included in the meta-analysis (additional file 7). Eight studies reported results on episodes rather than patients. These episodes were based on chemotherapy courses for seven and on respiratory viral infections for one.
Methodological quality assessment for cohort and case control studies is described in additional files 8 and 9, respectively. Of the 69 included studies, 31 (45%) were considered at low risk of bias, 30 (43%) at unclear risk of bias and 8 (12%) at high risk of bias.
For the meta-analysis on PP-IFI, among 26 studies fulfilling criteria for potential inclusion, 20 were finally included (additional file 7 and Table 1), including 1 014 IFI in 9 270 episodes. For the PPP-IFI meta-analysis, 36 of the 41 considered studies were included, corresponding to 1 348 IFI in 11 247 episodes (Table 1). In additional file 10, we reported methods and variables on which the analyses were adjusted. In the PP-IFI meta-analysis, 16 (80%) studies were considered at low risk of bias, 2 (10%) at intermediate risk of bias and 2 (10%) at high risk of bias. In the PPP-IFI meta-analysis, 26 (72%) studies were considered at low risk of bias, 3 (8%) at unclear risk of bias and 7 (20%) at high risk of bias (additional files 8 and 9).
Qualitative synthesis
Overall, a total of 62 variables were assessed in at least one study. Age, neutropenia, corticosteroids and antifungal prophylaxis were the most frequently studied parameters (adjusted measure effects for 14, 17, 13 and 12 studies, respectively).
Having a pulmonary comorbidity was associated with IFI in the 3 studies assessing this variable in multivariate analysis. Namely, having an underlying pulmonary disease was associated with an increased risk of PP-IFI occurring in the first 40 days after HSCT (HR 7.57 [95%CI 2.05–27.77]) and with IFI regardless of a time period (HR 1.66 [95%CI 1.03–2.67]) and chronic obstructive pulmonary disease (COPD) was associated with PPP-IFI (OR 3.28 [95%CI 1.48–7.28]) (additional file 11). Interestingly, in five studies reporting diabetes (two in univariate analysis and three in multivariate analysis), none showed a statistically significant association with IFI (additional files 11 and 12). Regarding treatment, an intensified GvHD therapy was statistically associated with PP-IFI in multivariate analysis (OR 3.60 [95%CI 1.30–9.90]), both in the late period and very late period after HSCT (41–100 days after HSCT HR 3.45 [95%CI 1.15–10.31] and 101–365 days after HSCT HR 37.93 [95%CI 3.76-379.45], respectively) (additional file 11).
A synthesis of factors associated with IFI assessed in the studies, along with their definitions as reported by the original study authors, are displayed in additional files 11 and 12, based on univariate and multivariate analyses, respectively.
Quantitative synthesis
Patients’ characteristics
In the meta-analysis including PP-IFI, no patients’ characteristics were significantly associated with IFI (Fig. 2).
When considering PPP-IFI, age more than 60 years old was significantly associated with IFI (pooled OR with 3 studies 3.46 [95% CI 1.48–8.08]) as well as history of IFI (in the allo-HSCT population, pooled HR with 2 studies 5.49 [95% CI 2.52–11.97])21,22 (additional file 13). Nonetheless, with OR as the measure effect, with one study reporting multiple myeloma patients23 and the other reporting allo-HSCT patients24, history of IFI was not significantly associated with IFI (pooled OR with 2 studies 5.39 [95% CI 0.52–55.80]) (additional file 13).
Hematological malignancy or HSCT characteristics
AML was not significantly associated with PP-IFI or PPP-IFI based on 3 and 5 studies, respectively (Fig. 3 and additional file 14) but was compared to other high-risk populations, namely transfusion-dependent MDS or ALL patients25,26. Moreover, this variable displayed moderate heterogeneity (I2 = 60%, phet = 0.08).
Forest plots of hemopathy or hematogenous stem cell transplantation status’ characteristics evaluated as risk factors for proven and/or probable invasive fungal infections. Abbreviations: AML acute myeloid leukemia; CI confidence interval; GvHD graft vs. host disease; HR hazard ratio; HSCT hematogenous stem cell transplant; OR odds-ratio; SE standard error, TE treatment effect. *High risk for mortality was defined in the studies according to the European Group for Blood and Marrow Transplantation or the HSCT-comorbidity index risk scores.
In hypomethylating agents-treated AML patients, the relapsed and/or refractory status of the hemopathy was significantly associated with PP-IFI (pooled OR 6.64 [95% CI 3.72–11.84], based on 2 studies) (Fig. 3).
Previous allo-HSCT was significantly associated with IFI (pooled HR with 2 studies 3.21 [95% CI 1.54–6.71]).
Allo-HSCT at higher risk for mortality according to consensus risk scores27,28 showed discrepant results based on association measure (pooled HR with 2 studies 2.52 [95% CI 1.36–4.67], and pooled OR with 2 studies 0.52 [95% CI 0.19–1.44]). Haploidentical donor was significantly associated with PP-IFI (pooled HR with 2 studies 2.41 [95% CI 1.27–4.57]).
Higher grades of acute GvHD (≥ 2) was significantly associated with PP-IFI whereas severe chronic GvHD was not (pooled HR 2.59 [95% CI 1.36–4.90] and pooled HR 1.49 [95% CI 0.55–4.07], with substantial heterogeneity, I2 = 66%, phet = 0.08, respectively, with 2 studies each, Fig. 3). When including PPP-IFI, GvHD regardless of its acute or chronic status, was significantly associated with IFI (pooled OR with 3 studies 4.84 [95% CI 2.34–10.05]) (additional file 14).
Biological parameters
Prolonged neutropenia (> 10 days) was significantly associated with PP-IFI (pooled HR with 2 studies 2.71 [95% CI 1.35–5.44]), although when OR was the association measure the results were not significant but with a high heterogeneity (pooled OR with 2 studies 6.72 [95%CI 0.71–63.55], with substantial heterogeneity, I2 = 79%, phet = 0.03) (Fig. 4).
In addition, neutropenia, regardless of duration, was significantly associated with PPP-IFI (pooled OR with 3 studies 2.79 [95% CI 1.53–5.09]) (additional file 15).
Liver dysfunction was significantly associated with PPP-IFI (OR 3.16 [95%CI 1.14–8.74] with 2 studies) (additional file 15).
Treatment-related risk factors
Decitabine was not significantly associated with PP-IFI, although it should be noted that there was considerable heterogeneity (OR 0.81 [95% CI 0.05–14.55] with 2 studies, I2 = 91%, phet < 0.01). Corticosteroids were significantly associated with PP-IFI (OR 2.84 [95% CI 1.42–5.70] with 2 studies) (Fig. 5). High dose corticosteroids were significantly associated with PP-IFI and PPP-IFI when considering OR as the association measure (pooled OR with 2 studies 1.98 [95% CI 1.13–3.48]; pooled OR with 5 studies 2.25 [95% CI 1.59–3.19]) but not with PP-IFI when considering HR (pooled HR with 2 studies 3.66 [95% CI 0.42–32.12], I2 = 89%, phet < 0.01). T-cell depleting agents were significantly associated with PP and PPP-IFI (pooled OR 2.73 [1.61–4.64], and pooled HR 2.00 [95% CI 1.07–3.76], with 2 studies respectively) (Fig. 5 and additional file 16).
Antifungal prophylaxis was a protective factor for PP-IFI (pooled OR with 2 studies 0.20 [95% CI 0.13–0.28]), including mould-active prophylaxis (pooled OR with 2 studies 0.24 [95% CI 0.14–0.42]) (Fig. 5).
Factors related to the hospital stay
Deep vein catheterization was statistically associated with PPP-IFI (pooled OR with 2 studies 3.11 [95%CI 1.27–7.63]) (additional file 17).
Sensitivity analyses and quality of evidence
Sensitivity analyses after exclusion of studies at high risk of bias did not significantly modify the results (additional file 18–25). Other sensitivity analyses performed, excluding studies with children and studies using episodes, gave similar results when data could be pooled (additional files 26–33).
Quality of evidence is summarized in additional file 34. There was a moderate quality of evidence for the association between IFI and corticosteroids and antifungal prophylaxis while the quality of evidence was low for haploidentical donor, acute GvHD, neutropenia, and T-cell depleting agents and very low for age > 60 years old, history of IFI, relapsed or refractory hemopathy, previous HSCT, a HSCT at high risk for mortality, and liver dysfunction.
Discussion
In this study, we found that, in the hematological population, previous HSCT, prolonged neutropenia and corticosteroids were significantly associated with IFI. In AML patients, relapsed or refractory status was significantly associated with IFI. Among the HSCT population, haploidentical donors, acute GvHD grade ≥ 2, and T-cell depleting agents were significantly associated with IFI. Antifungal prophylaxis was a protective factor for IFI.
As expected, high dose corticosteroids and allo-HSCT were significantly associated with IFI, while antifungal prophylaxis was protective12,29,30. Other risk factors have been less consistently recognized, yet are crucial to consider. These include the relapsed or refractory status of hematologic malignancies—namely AML in our meta-analysis—, HSCT with a haploidentical donor, acute GvHD as opposed to chronic GvHD, and the use of T-cell-depleting agents.
We found a significant association between the advanced status of the hemopathy and PP-IFI consistent with Offidani et al. (OR 1.30; 95% CI, 1.10–1.60)31. In contrast, Corzo-Leon et al. and Choi et al. found no such significant association in the HSCT population, hence our findings may be influenced by our focus on AML patients32,33.
Haploidentical HSCT was significantly associated with IFI, in accordance with higher infection rates found in a study comparing unmanipulated haploidentical transplants with other alternative donor and matched sibling grafts34. Haploidentical transplantation requires approaches that counteract an intense bidirectional alloreactivity of host and donor T-cells against HLA allo-antigens. The three main strategies include the infusion of T-cell depleted mega-dose of CD34 + cells, or high-dose post-transplant cyclophosphamide or another GvHD prophylaxis regimen (namely, anti-thymocyte globulin, calcineurin inhibitor, methotrexate, mycophenolate mofetil +/- basiliximab) resulting in slower immune reconstitution in these patients as compared to matched siblings34,35. T-cell depleting agents were also found to be significantly associated with PP-IFI. This association can likely be attributed to the critical role of T-cells in the immune response to fungal infections and the prolonged recovery time required for lymphocyte reconstitution36,37.
We found that, as opposed to acute GvHD, there was no significant association between extensive chronic GvHD and the risk for IFI. In contrast, Biyun et al., which included pediatric and adult populations, found a statistical association between IFI and chronic GvHD (HR 2.74; 95%CI 2.05–3.67)12. The discordant results between acute and chronic GvHD may be explained by the more aggressive immunosuppression given to patients with acute GvHD compared to those with chronic GvHD, moreover traditionally occurring in patients in the early period post HSCT, with an immune system not fully recovered38,39. Intensified GvHD therapy have been found to be associated with increased risk of IFI32.
Our findings align with cooperative group recommendations, with posaconazole recommended for AML patients receiving intensive chemotherapy and post-HSCT patients treated with corticosteroids for acute GvHD40. Interestingly, we were able to show that haplotransplantation is significantly associated with IFI, which raises the question of prophylaxis in this population.
Our systematic review and meta-analysis followed a robust method. To enhance the reliability of study comparisons, we restricted our search to studies published after the first publication of standard definitions in 20026,7,8. Indeed, in a review published in 2001, including 173 studies with 485 distinct definitions for IFI, overall agreement between the definitions was only fair, even slighter when considering the degree of IFI certainty41. In addition, we conducted two separate analyses: one focusing exclusively on PP-IFI and the other incorporating possible IFIs based on the EORTC/MSG definitions from 2002 to 2008. This approach allows us to highlight risk factors associated with IFI with a high degree of certainty while also accounting for potential undiagnosed cases of IFI prior to the adoption of the latest diagnostic tools. However, despite these precautions, we encountered several limitations. Notably, although a high number of patients and studies were included in the systematic review, few study results could be pooled together in meta-analyses. Some risk factors exhibited substantial heterogeneity, further increased by the variety of confounding factors considered in each study. As we chose to assess risk factors for IFI without restricting to a specific hematological population, presenting the results of the meta-analysis proved challenging. Several studies focused on specific populations (e.g. HSCT or AML patients) while others assessed risk factors more broadly in the hematological population. Consequently, we decided to specify the population for which a given risk factor was evaluated, rather than forming pre-defined subgroups. It is important to note that recently available treatments were not assessed in the studies included in this systematic review (e.g., chimeric antigen receptor (CAR) T-cells and bispecific antibodies). While IFIs do not appear to be the most pressing concern among infections in this context, a meta-analysis of infections in CAR T-cell therapy reported a pooled incidence rate of 2.0% (95%CI 1.1–3.0) across 27 studies involving 1 565 patients42. In addition, although we did not restrict our search on specific geographic regions, no studies from Africa and only 2 studies from South America were included, limiting the possibility to generalize our results to these continents.
To conclude, physicians should closely monitor hematological patients with a previous allo-HSCT, prolonged neutropenia, corticosteroids use, and a relapsed or refractory AML. In the allo-HSCT population, increased vigilance is necessary for those with haploidentical donors, acute GvHD grade ≥ 2, or receiving T-cell depleting agents.
Data availability
Data is provided within the manuscript or supplementary information files. Emmanuelle Gras (corresponding author) will make data available upon reasonable request.
Change history
28 November 2025
The original online version of this Article was revised: In the original version of this Article Affiliation 10 was incorrectly given as ‘Département de Santé Publique, Sorbonne Université, INSERM, Institut Pierre Louis d’Epidémiologie et de Santé Publique, AP-HP, Hôpital Pitié-Salpêtrière, Centre de Pharmacoépidémiologie de l’AP-HP (Cephepi), Paris, France’. The correct affiliation is listed as ‘Sorbonne Université, INSERM, Institut Pierre Louis d'Épidémiologie et de Santé Publique, AP-HP, Sorbonne Université, Hôpital Pitié-Salpêtrière, Département de Santé Publique, Paris, France’.
References
Denning, D. W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 24 (7), e428–e438. https://doi.org/10.1016/S1473-3099(23)00692-8 (2024).
Webb, B. J. et al. Epidemiology and clinical features of invasive fungal infection in a US health care network. Open. Forum Infect. Dis. 5 (8), ofy187. https://doi.org/10.1093/ofid/ofy187 (2018).
Bitar, D. et al. Population-based analysis of invasive fungal infections, france, 2001–2010. Emerg. Infect. Dis. 20 (7), 1149–1155. https://doi.org/10.3201/eid2007.140087 (2014).
Nucci, M. et al. Invasive fungal diseases in Haematopoietic cell transplant recipients and in patients with acute myeloid leukaemia or myelodysplasia in Brazil. Clin. Microbiol. Infect. Off Publ Eur. Soc. Clin. Microbiol. Infect. Dis. 19 (8), 745–751. https://doi.org/10.1111/1469-0691.12002 (2013).
Pagano, L. et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 91 (8), 1068–1075 (2006).
Ascioglu, S. et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. Off Publ Infect. Dis. Soc. Am. 34 (1), 7–14. https://doi.org/10.1086/323335 (2002).
De Pauw, B. et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the National Institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin. Infect. Dis. Off Publ Infect. Dis. Soc. Am. 46 (12), 1813–1821. https://doi.org/10.1086/588660 (2008).
Donnelly, J. P. et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin. Infect. Dis. Off Publ Infect. Dis. Soc. Am. 71 (6), 1367–1376. https://doi.org/10.1093/cid/ciz1008 (2020).
Pagano, L. et al. Risk stratification for invasive fungal infections in patients with hematological malignancies: SEIFEM recommendations. Blood Rev. 31 (2), 17–29. https://doi.org/10.1016/j.blre.2016.09.002 (2017).
Douglas, A. P. & Slavin, M. A. Risk factors and prophylaxis against invasive fungal disease for haematology and stem cell transplant recipients: an evolving field. Expert Rev. Anti Infect. Ther. 14 (12), 1165–1177. https://doi.org/10.1080/14787210.2016.1245613 (2016).
Fisher, B. T. et al. Risk factors for invasive fungal disease in pediatric cancer and hematopoietic stem cell transplantation: A systematic review. J. Pediatr. Infect. Dis. Soc. 7 (3), 191–198. https://doi.org/10.1093/jpids/pix030 (2018).
Biyun, L., Yahui, H., Yuanfang, L., Xifeng, G. & Dao, W. Risk factors for invasive fungal infections after Haematopoietic stem cell transplantation: a systematic review and meta-analysis. Clin. Microbiol. Infect. Off Publ Eur. Soc. Clin. Microbiol. Infect. Dis. 30 (5), 601–610. https://doi.org/10.1016/j.cmi.2024.01.005 (2024).
Moher, D. et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4 (1), 1. https://doi.org/10.1186/2046-4053-4-1 (2015).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283 (15), 2008–2012. https://doi.org/10.1001/jama.283.15.2008 (2000).
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 5 (1), 210. https://doi.org/10.1186/s13643-016-0384-4 (2016).
Critical Appraisal Skills, Programme. CASP Cohort Study Checklist [Internet]. [cited 2023 Jan 9]. Available from: https://casp-uk.net/casp-tools-checklists/ (2018).
Critical Appraisal Skills, Programme. CASP Case-Control Study Checklist [Internet]. [cited 2023 Jan 9]. Available from: https://casp-uk.net/casp-tools-checklists/(2018).
Altman, D. G. & Bland, J. M. How to obtain the confidence interval from a P value. BMJ 343, d2090. https://doi.org/10.1136/bmj.d2090 (2011).
R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing. Available from: https://www.R-project.org (2024).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336 (7650), 924–926. https://doi.org/10.1136/bmj.39489.470347.AD (2008).
Liu, Y. C. et al. Incidence and risk factors of probable and proven invasive fungal infection in adult patients receiving allogeneic hematopoietic stem cell transplantation. J. Microbiol. Immunol. Infect. 49 (4), 567–574. https://doi.org/10.1016/j.jmii.2015.01.002 (2016).
Christen, D. et al. Outcome of non-mold effective anti-fungal prophylaxis in patients at high-risk for invasive fungal infections after allogenic stem cell transplantation. Leuk. Lymphoma. 60 (8), 2056–2061. https://doi.org/10.1080/10428194.2018.1553303 (2019).
Liu, J. et al. Epidemiology and treatment of invasive fungal diseases in patients with multiple myeloma: findings from a multicenter prospective study from China. Tumor Biol. 37 (6), 7893–7900. https://doi.org/10.1007/s13277-015-4441-8 (2016).
Ozyilmaz, E. et al. Risk factors for fungal pulmonary infections in hematopoietic stem cell transplantation recipients: the role of iron overload. Bone Marrow Transpl. 45 (10), 1528–1533. https://doi.org/10.1038/bmt.2009.383 (2010).
Apostolidi, E. A. et al. Bone marrow iron stores are not associated with increased risk for invasive fungal infections in patients with newly diagnosed acute leukemia or myelodysplastic syndrome in transformation: is there a relationship?? J. Fungi. 9 (7), 748. https://doi.org/10.3390/jof9070748 (2023).
Oh, S. M. et al. Incidence of invasive fungal infection in acute lymphoblastic and acute myelogenous leukemia in the era of antimold prophylaxis. Sci. Rep. 11 (1), 22160. https://doi.org/10.1038/s41598-021-01716-2 (2021).
Sorror, M. L. et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106 (8), 2912–2919. https://doi.org/10.1182/blood-2005-05-2004 (2005).
Gratwohl, A. et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic leukemia working party of the European group for blood and marrow transplantation. Lancet Lond. Engl. 352 (9134), 1087–1092. https://doi.org/10.1016/s0140-6736(98)03030-x (1998).
Wang, J. et al. Comparison of antifungal prophylaxis drugs in patients with hematological disease or undergoing hematopoietic stem cell transplantation. JAMA Netw. Open. 3 (10), e2017652. https://doi.org/10.1001/jamanetworkopen.2020.17652 (2020).
Zeng, H. et al. Network meta-analysis of triazole, polyene, and Echinocandin antifungal agents in invasive fungal infection prophylaxis in patients with hematological malignancies. BMC Cancer. 21 (1), 404. https://doi.org/10.1186/s12885-021-07973-8 (2021).
Offidani, M. et al. Diagnostic value of C-reactive protein in discriminating fungal from nonfungal pulmonary infiltrates in patients with hematologic malignancies. Support Care Cancer. 14 (8), 874–877. https://doi.org/10.1007/s00520-006-0030-0 (2006).
Choi, J. K. et al. Epidemiology and risk factors for invasive fungal diseases among allogeneic hematopoietic stem cell transplant recipients in korea: results of RISK study. Biol. Blood Marrow Transpl. 23 (10), 1773–1779. https://doi.org/10.1016/j.bbmt.2017.06.012 (2017).
Corzo-León, D. E. et al. Epidemiology and outcomes of invasive fungal infections in allogeneic Haematopoietic stem cell transplant recipients in the era of antifungal prophylaxis: a single‐centre study with focus on emerging pathogens. Mycoses 58 (6), 325–336. https://doi.org/10.1111/myc.12318 (2015).
Raiola, A. M. et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol. Blood Marrow Transpl. J. Am. Soc. Blood Marrow Transpl. 20 (10), 1573–1579. https://doi.org/10.1016/j.bbmt.2014.05.029 (2014).
Chang, Y. J. et al. Immune reconstitution following unmanipulated HLA-mismatched/haploidentical transplantation compared with HLA-identical sibling transplantation. J. Clin. Immunol. 32 (2), 268–280. https://doi.org/10.1007/s10875-011-9630-7 (2012).
Romani, L. Cell mediated immunity to fungi: a reassessment. Med. Mycol. 46 (6), 515–529. https://doi.org/10.1080/13693780801971450 (2008).
Hill-Cawthorne, G. A. et al. Long term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 83 (3), 298–304. https://doi.org/10.1136/jnnp-2011-300826 (2012).
Penack, O. et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European society for blood and marrow transplantation. Lancet Haematol. 7 (2), e157–e167. https://doi.org/10.1016/S2352-3026(19)30256-X (2020).
Storek, J. et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood 97 (11), 3380–3389. https://doi.org/10.1182/blood.v97.11.3380 (2001).
Maertens, J. A. et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J. Antimicrob. Chemother. 73(12), 3221–30. https://doi.org/10.1093/jac/dky286 (2018).
Ascioglu, S., de Pauw, B. E., Donnelly, J. P. & Collette, L. Reliability of clinical research on invasive fungal infections: a systematic review of the literature. Med. Mycol. 39 (1), 35–40. https://doi.org/10.1080/mmy.39.1.35.40 (2001).
Telli Dizman, G., Aguado, J. M. & Fernández-Ruiz, M. Risk of infection in patients with hematological malignancies receiving CAR T-cell therapy: systematic review and meta-analysis. Expert Rev. Anti Infect. Ther. 20 (11), 1455–1476. https://doi.org/10.1080/14787210.2022.2128762 (2022).
Acknowledgements
E.G. received a grant « mobilité internationale de recherche dans le domaine de l’infectiologie de la Réunion Interdisciplinaire de Chimiothérapie Anti-Infectieuse », 2023 and a grant from the Collège des Universitaires de Maladies Infectieuses et Tropicales, 2023.The authors would like to thank Guillaume Martin for his help in adjusting the code for presenting the forest plots of the meta-analysis.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
EG, AD, PMG, LA, LS, KL, FL, CGV, EB and JG designed the study. EG, LA and PMG selected the studies to be included in the systematic review and extracted the data from the studies. EG performed the statistical analysis. EG wrote the first draft with input from KL, LS and AD. All authors contributed to the interpretation of the data, revised the draft critically for important intellectual content and approved the final version. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
C.G.-V. has received honoraria for talks on behalf of Gilead Sciences, MSD, Pfizer, Jansen, Novartis, Basilea, GSK, Shionogi, AbbVie and Advanz Pharma, and grant support from Gilead Sciences, Pfizer, GSK, MSD and PharmaMar. KL has received honoraria and grant support outside of the topic of this manuscript by Gilead, MSD and ViiV Healthcare. All other authors do not declare any conflict of interest.
All other authors do not declare any conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gras, E., Monzo-Gallo, P., Azoyan, L. et al. Risk factors for invasive fungal infections in adult patients with hematological malignancies and/or stem cell transplant: a systematic review and meta-analysis. Sci Rep 15, 30724 (2025). https://doi.org/10.1038/s41598-025-16066-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16066-6