Abstract
Systemic Sclerosis (SSc) is a complex autoimmune disorder characterized by vascular dysfunction, immune dysregulation, and progressive fibrosis of the skin and internal organs. Despite substantial research, its pathophysiology remains enigmatic, necessitating the identification of altered metabolic pathways to improve disease management. This study utilized NMR-based serum metabolomics to identify distinctive metabolic signatures and deranged pathways in SSc. 1D 1H CPMG spectra of sera from 83 SSc patients and 43 age/sex matched HC were measured using 800 MHz NMR. Spectral binning and concentration profiling data were analyzed using multivariate statistical analysis to identify discriminatory metabolic features based variable importance in projection (VIP) scores and mean decrease accuracy (MDA). Univariate statistical analysis and receiver operating characteristic (ROC) curve analysis further evaluated their diagnostic potential. Multivariate analysis revealed clear differentiation between SSc and HC. SSc patients showed decreased levels of alanine, valine, myo-inositol, creatinine, pyruvate, lactate, PUFA, LDL/VLDL, and lipids, alongside elevated levels of acetate, 3-hydroxybutyrate, and mannose. Twelve key metabolites demonstrated high sensitivity and specificity in distinguishing SSc from HC. Pathway analysis revealed disruptions in sugar metabolism, branched-chain amino acids, fatty acid metabolism, and energy metabolism. These findings underscore NMR-based metabolomics as a promising tool for improving SSc diagnosis and understanding its pathogenesis, paving the way for targeted therapeutic strategies.
Similar content being viewed by others
Introduction
Systemic Sclerosis (SSc) is an autoimmune disease characterized by multifaceted pathophysiological mechanisms including endothelial dysfunction, microvascular damage, and progressive fibrosis affecting the skin and visceral organs1. SSc typically manifests in individuals aged between 30 and 50 years, with a global prevalence estimated at 1.87 per 100,000. In the Indian population, the prevalence ranges from 2.3 to 10 per million, highlighting regional variability influenced by genetic, environmental, and socioeconomic factors2,3. Notably, women are disproportionately affected, being approximately five times more susceptible to the disease than men, underscoring potential hormonal or genetic predispositions. The rarity of SSc poses significant challenges in diagnosis and therapeutic intervention. Current diagnostic methods and treatments often lack sensitivity, robustness, and precision, primarily due to the high interpatient variability in clinical presentation and disease progression. Furthermore, the financial burden associated with managing SSc, coupled with its severe outcomes, including marked impairment in patient’s quality of life, underscores the urgent need for innovative strategies for effective disease assessment and clinical management.
Given the devastating nature of SSc outcomes, there is immense clinical interest in uncovering the underlying molecular and biochemical alterations that drive its pathogenesis. Such insights could pave the way for targeted biomarkers and therapeutic approaches to better assess disease severity and manage its progression. A growing body of evidence suggests that disease development and progression are influenced by changes at multiple omics levels—including genomics, transcriptomics, proteomics, and epigenomics. However, among these, metabolomics offers a unique advantage as it provides a functional readout of biochemical and physiological changes occurring during disease development4.
Advantageously, the metabolic changes often precede the clinical manifestation of typical symptoms; thus offering promising prospects for early disease detection, the assessment of therapeutic efficacy and safety for potential drug candidates in a timely manner5. The present NMR based clinical metabolomics study is an attempt to identify distinctive metabolic alterations in systemic sclerosis by analyzing serum samples from patients with the condition and comparing them to those from healthy individuals6. The metabolic alterations will provide insights into disease progression and metabolic pathway perturbations underlying pathophysiological of systemic sclerosis. Various previous studies have shown insulin sensitivity and abnormal sugar metabolism might contribute to altered protein glycosylation and mannosylation, which is the key pathophysiological hallmark of immune-mediated diseases like SSc7,8,9. However, the potential involvement of abnormal sugar metabolism, specifically pertaining to mannose and myo-inositol, in the development and progression of SSc has not been explored in majority of previous clinical metabolomics studies.
Results
Clinical investigations
The present study involved eighty-three SSc patients (N = 83) and forty-three healthy controls (HC, N = 43). The clinical and demographic characteristics of recruited patients is summarized in Table 1. The male-to-female ratio for both the study groups group was ~ 1:10 (i.e. 7:76 for SSc and 4:39 for HC). The mean age was also comparable between the study groups (35.06 ± 1.07 years for SSc and 34 ± 6.03 years for HC group). SSc disease have different clinical subtypes and in present study, 48 SSc patients were diagnosed with diffuse cutaneous systemic sclerosis (dcSSc) and 35 with limited cutaneous systemic sclerosis (lcSSc). The average disease duration for SSc patients was 55.26 ± 8.02 months, while the duration of Raynaud’s phenomenon was 51.30 ± 8.04 months. Cutaneous involvement, reflected by skin thickening duration, averaged 40.88 ± 8.25 months. The modified Rodnan Skin Score (mRSS), indicative of skin fibrosis severity, had a mean value of 19.8 ± 2.24. Visceral organ involvement in the SSc group is included GERD (81), PAH (1), ILD (22). As this study primarily focused on identifying the distinctive metabolic pathway alterations in SSc, we considered all SSc patients of this study as one group and their serum metabolic profiles were compared with HC subjects.
Quantitative serum metabolic profiling by NMR
The quantitative metabolic profiles on SSc and HC serum samples were measured using 1D 1H CPMG (Carr − Purcell − Meiboom − Gill) NMR spectra and the stacking of cumulative spectra representing SSc and HC sera are shown in supporting Figure S1. The spectral peaks corresponding to specific serum metabolites were assigned (details are described in the methods section) and labeled according to chemical shift assignments of metabolites (see supporting Table S1 of electronic supplementary material abbreviated as ESM). (The NMR spectra of serum samples showed signals of various organic acids (including lactate, pyruvate, acetate, succinate, and formate), amino acids (such as leucine, isoleucine, valine, glutamine, glutamate, phenylalanine, and tyrosine), energy metabolites (including glucose, creatine, creatinine), ketone bodies like acetone and 3-hydroxybutyrate), and specific membrane metabolites such as Choline and sn-glycero3-phosphocholine (snG3P). Several well-characterized lipid signals observed and labeled, corresponding to various structural elements of fatty acids and lipoproteins. The terminal methyl groups (–CH₃) of fatty acid chains appeared around 0.85 ppm, while the bulk methylene protons (–CH₂–) of saturated chains resonated at approximately 1.25 ppm. Methylene groups adjacent to carbonyls (β-CH₂) were detected near 1.57 ppm, and allylic methylene protons (–CH₂–CH=) were visible around 2.02 ppm. Additionally, signals corresponding to bis-allylic methylene protons of polyunsaturated fatty acids (PUFAs) were observed at ~ 2.8 ppm, while olefinic protons (–CH = CH–) appeared near 5.28 ppm. A distinct signal at ~ 3.2 ppm was assigned to the choline headgroup of phosphatidylcholines and other choline-containing lipids. Broad resonances in the 0.85–1.5 ppm region were attributed to lipid protons associated with High density lipoproteins, low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL) particles Figure S1, reflecting their complex lipid-rich envelope10,11. Visual inspection of the spectra reveals subtle differences in peaks corresponding to various metabolites (Figure S1). Therefore, to substantiate the underlying metabolic alterations, the multivariate statistical analysis was applied.
Multivariate analysis
The NMR spectral features of serum samples were subjected to multivariate data analysis for characterizing the metabolic disparity between the SSc and HC study groups. In present study, the serum metabolic profiles were estimated both in terms of normalized spectral features as well as concentration profiles estimated for 41 serum metabolites (as per Table S1) using NMR suite of commercial software CHENOMX (see details in the method section and more details in the Annexure -I of ESM. The reason for performing this elaborative analysis is well justified in some of our very recent studies12,13,14. First, the serum metabolic features/profiles were subjected to unsupervised principal component analysis (PCA) method, for evaluating inherent grouping trends, explore data quality, and overall variance patterns without bias from class labels. (Figure S2A for normalized spectral features and Figure S2B for concentration profiling). PCA score plots in both the cases (Figure S2A and S2B) indeed indicated a visible trend of separation between SSc and HC study groups, suggesting underlying metabolic disparities. However, this separation was not fully optimized or quantified.
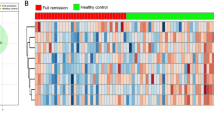
Multivariate Partial least square -discriminatory analysis (PLS-DA) based on (A, C) Normalized spectral features (bin size 0.01 ppm) and metabolite concentration profiling respectively extracted from 1D 1H CPMG NMR spectra of sera from systemic sclerosis (SSc) patients (N = 83) and healthy control (HC) subjects (N = 43); (A, C) PLS-DA scatter plot with Ellipse representing the 95% confidence region based on Hotelling’s T2. (B, D) Variable Importance in Projection (VIP) scores derived from PLS-DA highlighting key normalized spectral features contributing to group differentiation.
To achieve a more improved discriminative model that maximizes inter-group separation, we employed Partial Least Squares Discriminant Analysis (PLS-DA), a supervised multivariate analysis method that explicitly incorporates class labels. Unlike PCA, PLS-DA is designed to identify key spectral/metabolic features most responsible for class discrimination (as it incorporates class information to maximize group separation), making it suitable for biomarker discovery and diagnostic model development. The resulting 2D score scatter plots are shown in Fig. 1A and C. The PLS-DA models based on both normalized spectral features (Fig. 1A) and metabolite concentration profiles (Fig. 1C) revealed distinctive metabolic disparities between SSc and HC groups. The model parameters R2X[1] = 0.206 and R2X[2] = 0.151 for normalized spectral features (Fig. 1A) and R2X[1] = 0.557 and R2X[2] = 0.0585 for concentration profiles (Fig. 1C) suggested better explained variance for the primary component in the latter case. As PLS-DA tends to overfit the dataset the Model validation is performed through a permutation test with 500 permutations and summary of fit along with CV-ANOVA (results are also shown in Table S2, see ESM). Cumulative R2Y and Q2 values reflect the goodness-of-fit and predictive accuracy of the models. The normalized spectral features PLS-DA model achieved cumulative R2Y = 0.879 and Q2 = 0.814 for four components (Fig. S2C), while the concentration profiling model yielded cumulative R2Y = 0.691 and Q2 = 0.646 for two components (Fig. S2D), both models having significantly higher cummulative R2Y (> 0.65) and Q2 (> 0.50) ensuring the robustness and reliability. In other words, the metabolic disparity is significant and not random in both models in distinguishing SSc and HC. Next, the Variable Importance in Projection (VIP) score analysis was used to highlight the key spectral bins and metabolic profiles contributing to the discrimination between SSc and HC (Fig. 1B and D). For normalized spectral features, Lipids, myo-inositol, and glucose bins were among the top contributors, while for concentration profiling, acetate, mannose, lactate, myo-inositol and pyruvate showed the highest discriminatory power. Oftenly cut off value for selecting genuine discriminatory feature from 100 normalized features is 1.015. However in our study, we selected 69 bins and profiled 41 concentration profiles, out of which metabolic features with discriminatory potential were considered after applying a cutoff value of 1.0 for selection.
To address the potential confounding factor of sex—given the known female predominance in SSc—we performed a separate multivariate analysis restricted to female SSc and HC subjects only. The PCA using normalized spectral features (Figure S3A) showed slightly better group dispersion than in the full cohort, with R2X[1] = 0.254 and R2X[2] = 0.183, while the PLS-DA (Figure S3C) still achieved group separation but with reduced explained variance (R2X[1] = 0.181, R2X[2] = 0.131) and model fit. Similarly, concentration profiled dataset based PCA and PLS-DA on females (Figure. S3B & S3D) displayed slightly lower component-wise explained variance compared to the total dataset. Additionally, VIP score analysis performed on the female subgroup revealed metabolic feature trends to be consistent with those obtained from the total cohort, in both normalized spectral feature and concentration profiling datasets (see Figure S3E and S3F in ESM). Despite SSc being more prevalent in females, these results suggest that statistical analysis using the total population yields stronger and more stable multivariate models, with higher explained variance and predictive power. Importantly, the inclusion of male participants did not introduce noise or variability that compromised model integrity. Therefore, the total cohort was retained for further statistical analysis, ensuring maximum sample power and generalizability.
We also applied machine learning Random Forest (RF) classification method to evaluate the ability of normalized spectral features and concentration profiling data in differentiating SSc patients from HC subjects. The results are shown in Figure S4 of ESM. For the normalized spectral features, the model achieved an out-of-bag (OOB) error of merely 3.17%, with class-specific errors of 4.65% for HC and 2.41% for SSc, indicating robust classification performance (Fig. S4A). Next, the RF classification model was used to derive the Mean Decrease Accuracy (MDA) Plot illustrating the significant metabolic features in discriminating SSc from HC (Fig. S4B). Key discriminatory features included creatine (3.02 ppm), acetate (1.90 ppm), LDL (0.84 ppm), and myo-inositol (4.04 ppm), glucose and lipids i.e., LDL, LDL/VLDL and lipids (5.20 ppm and 1.57 ppm) exhibiting particularly high importance (Fig. S4A). In comparison, the concentration profiling model yielded a slightly lower OOB error of 2.48%, with class-specific errors of 6.98% for HC and 2.41% for SSc (Fig. S4C). The corresponding MDA plot highlighted key discriminatory metabolites included myo-inositol, pyruvate, valine, creatinine, and mannose, with mannose and acetate showing significant contributions to classification accuracy (Fig. S4D).
Volcano plots depicting differential expression of circulatory metabolic features in Systemic Sclerosis (SSc) patients compared to Healthy Controls (HC). (A) Normalized metabolic features derived from binned NMR data (bin size: 0.01 ppm). (B) Metabolic features derived from concentration profiling data. The x-axis represents the log2 (fold change) of metabolite levels between SSc and HC groups, while the y-axis shows the -log10(p-value). Metabolites with statistically significant changes (p < 0.05) highlighted, with a color gradient indicating upregulated metabolites in SSc (red) and downregulated metabolites in SSc (blue).
Univariate statistical analysis
The key discriminatory metabolic features were further evaluated for statistical significance using volcano plot statistics and the resulting volcano plots are shown in Fig. 2 and parameters are listed in Table S3 and S4. The univariate statistics for normalized spectral features suggested NMR signals of creatine, acetate, mannose, and LDL are significantly upregulated in SSc patients (Fig. 2A). Conversely, the NMR signals of lipids, PUFA and LDL/VLDL were notably downregulated in SSc patients. The univariate statistics for concentration profiles revealed the circulatory levels of 3-HB, acetate and mannose are upregulated, while the circulatory levels of metabolites such as pyruvate, myo-inositol, alanine, valine, lactate, betaine, choline, tyrosine and glutamine were significantly downregulated in SSc (Fig. 2B). Overall, 41 spectral bins and 35 metabolic features were significantly altered as identified with an FDR-adjusted p-value < 0.05 through unpaired Student’s t-tests (Table S5 and S6) and further corroborated by fold change analysis (Table S7 and S8). Finally, the metabolic changes, found consistently significant across volcano plots and univariate analyses, are visualized in box-cum-whisker plots (See ESM, Fig. S5).
Diagnostic panel of metabolic biomarker
The diagnostic potential of discriminatory normalized spectral features and circulatory metabolites were evaluated using Receiver Operating Characteristics (ROC) curve analysis and the area under the ROC (AUROC) values greater than 0.85 were used as the criteria for the diagnostic effectiveness. As noted, normalized spectral bins and metabolite concentrations reflect different levels of data abstraction. While normalized spectral data provide a comprehensive view of the metabolic fingerprint, concentration profiling offers metabolite-specific quantification. We performed ROC analysis on both to explore whether discriminatory features converge at both levels. Though some markers differ between the two datasets, overlap (e.g., myo-inositol, acetate) reinforces biological relevance. Further, compared to normalized spectral features, the metabolic concentration profiles demonstrated very high sensitivity and specificity and the generated ROC plots for top six features are shown in Fig. 3. The various parameters derived from ROC analysis are listed in Table 2 and Table S9 of ESM. The diagnostic panel of top six normalized spectral features includes: creatine@3.02, LDL@0.84, acetate@1.90, glucose@3.22, ornithine@3.04, and mannose@5.17 (Fig. 3A). The diagnostic panel of top six metabolic concentration profiles includes: myo-inositol, valine, lactate, creatinine, alanine and pyruvate (Fig. 3B). Further, we also performed multivariate exploratory ROC analysis to evaluate the diagnostic accuracy of multiple sets of normalized spectral features and metabolic concentration features. The comparative ROC analysis of six panels with different sets of metabolic features is shown in ESM (see Figure S6A and S6B). The analysis demonstrates that as the number of metabolites increases, the discriminatory power of the model in differentiating HC from SSc also improves. The multivariate ROC curves based on top ten discriminatory metabolic features demonstrated AUROC value of 0.985 [95% CI = 0.957–1] for normalized spectral features (Fig. S6C) and 0.984 [95% CI = 0.952–1] for concentration profiled features (Fig. S6D), respectively, indicating their exceptional diagnostic potential. Predictive accuracy of the multivariate ROC models as shown in ESM (Fig. S6E and Fig. S6F) remained robust across various feature subsets, signifying the model’s reliability and generalizability, and illustrating that as the number of metabolic features increases, the predictive accuracy improves (Fig. S6E and Fig. S6F). Further we also checked for Top ten most important metabolic features ranked from most important to least important for the best selected biomarker model (Figure S7). This overall analysis highlights the value of using a comprehensive metabolic signature rather than relying on single metabolites for disease diagnosis. These findings establish a strong foundation for developing metabolomics-based diagnostic tools, which could transform the clinical management of SSc by enabling early, accurate, and personalized treatment.
The Receiver Operating Characteristics (ROC) curve plots for top six discriminatory spectral features (panel A) and top six concentration profiles (panel B) exhibiting potential to distinguish Systemic Sclerosis (SSc) and Healthy Control (HC) group. The AUROC (Area Under the Receiver Operating Characteristic Curve) values indicates diagnostic ability of metabolic features and set of metabolic features. The computed 95% Confidence Intervals (CI) are displayed in a faint blue background, providing a statistical measure of the precision associated with the diagnostic potential of these discriminative metabolites.
Metabolite set enrichment analysis
The set of metabolites significantly altered in SSc patients were further subjected to Metabolite Set Enrichment Analysis (MSEA) to extract meaningful biological insights or their involvement in metabolic pathways. Quantitative enrichment analysis was performed using KEGG human metabolic pathway library and an overview of top 25 enriched metabolite sets is shown in Figure S8. Based on the criteria of observed hits > 25 and enrichment ratio > 35 (Table S10), the metabolic pathways expected to be affected in SSc includes glycolysis (gluconeogenesis), pyruvate metabolism, cysteine and methionine metabolism, ascorbate and aldarate metabolism, inositol phosphate metabolism, phosphatidylinositol signaling, tyrosine metabolism, pantothenate and CoA biosynthesis and valine, leucine and isoleucine degradation and biosynthesis. These metabolic pathway alterations observed in SSc including disruptions in glycolysis/gluconeogenesis, amino acid, lipid, and energy metabolism were found partly in concordance with previous preclinical and clinical metabolomics studies suggesting potential of NMR based metabolomics in unraveling the intricate pathobiology of SSc and thus offering new insights for therapeutic targeting16,17,18,19,20,23,24,−25.
Discussion
The SSc presents varied clinical manifestations caused by the associative effect of vasculopathy and fibrosis, leading to a highly variable prognosis26. The clinical features of SSc include Raynaud’s phenomenon, digital ulcers, gangrene, joint pains and contractures, other internal organ complications are such as pulmonary hypertension, cardiac fibrosis, renal crisis, lung fibrosis, and gastrointestinal dysfunction. Individuals with SSc are classified based on the extent of skin fibrosis; diffuse cutaneous subtype (skin fibrosis proximal to elbows or knee), limited subtype (skin fibrosis distal to elbows and knee), and Sine SSc subtype (without skin involvement but develop clinical features such as Raynaud’s phenomenon, pulmonary hypertension and autoantibodies that are specific to systemic sclerosis). Additionally, some individuals may develop SSc overlap syndrome when they have another connective tissue disease such as arthritis, myositis, and lupus1,27. The present study aimed to reveal the distinctive metabolic signatures and metabolic pathways perturbed in SSc. The summary of metabolic disturbances observed in SSc has been depicted in Fig. 4 and explicit metabolic pathway alterations have been discussed below with reference to previous clinical/preclinical studies. We have compared the metabolic changes observed in this study with earlier metabolomics studies in Table 2 as well.
Altered sugar and energy metabolism
Our study revealed significant alterations in altered sugar and energy metabolism in SSc patients, particularly focusing on the roles of mannose, glucose and myo-inositol, contributing to the understanding of its complex pathogenesis. The circulatory levels of glucose, pyruvate, and lactate were significantly lower in SSc patients compared to healthy controls. This decreased circulatory levels of glucose along with pyruvate and lactate reflects a fundamental metabolic shift, likely driven by increased glycolytic activity to meet the heightened energy demands of activated fibroblasts. This pathological manifestation, akin to the Warburg effect, suggests that glucose is rapidly consumed to fuel excessive glycolysis20, contributing to systemic energy depletion. Furthermore, elevated expression of the glucose transporter GLUT-1 in the skin of SSc patients, as reported in previous studies, indicates increased glucose uptake by the skin and redirection of glucose metabolism from the liver to cutaneous tissues7. Positron emission tomography (PET) studies indicate increased skin glucose avidity in SSc, correlating with skin involvement. Higher whole body insulin sensitivity was observed in SSc patients with more extensive skin involvement (dcSSc vs. lcSSc)7. This metabolic reprogramming supports the hypothesis of aberrant energy metabolism, underscores the critical role of glycolysis, and its downstream pathways in the pathogenesis of SSc.
Mannose dysregulation and glycosylation alterations
One of the most striking findings was the elevated circulatory levels of mannose in SSc patients. As Mannose, an essential sugar metabolite and a key component of N-glycans, plays a pivotal role in glycosylation processes (graphical explanation is provided in Figure S9). The accumulation of mannose in SSc may disrupt glucose metabolism by inhibiting glucose phosphate isomerase, particularly in the form of mannose-6-phosphate. This inhibition can impair multiple metabolic pathways, including glycolysis, the tricarboxylic acid (TCA) cycle, and the pentose phosphate pathway, resulting in a cascade of metabolic disturbances (Fig. 4).
The elevated circulatory mannose levels also point to altered glycosylation patterns, which are implicated in autoimmune diseases like SSc. Glycan remodeling, including changes in galactosylation, fucosylation, and sialylation, may influence immune cell activity and antibody stability, potentially driving chronic inflammation and immune dysregulation ultimately contributing to the development and progression of autoimmune diseases8,9,28. These findings establish mannose as a central player in the metabolic derangements observed in SSc and highlight its potential role in disease pathogenesis.
Myo-Inositol and endothelial dysfunction
Circulatory levels of myo-inositol were significantly decreased in SSc patients, shedding light on its critical role in endothelial dysfunction. Myo-inositol is a key substrate in the inositol pathway, which stimulates Ca++/calmodulin-dependent nitric oxide (NO) synthase leading to nitric oxide (NO) production. Reduced levels of myo-inositol may impair NO synthesis, leading to increased vasoconstriction (Figure S9), vascular remodeling, and thrombotic signaling - hallmarks of SSc29. These vascular complications are compounded by oxidative stress, as myo-inositol is known to mitigate reactive oxygen species (ROS) and reduce endothelial cells inflammation30. The observed decrease in myo-inositol aligns with the vascular abnormalities seen in SSc and suggests a potential therapeutic role for myo-inositol in addressing endothelial dysfunction. The interplay between myo-inositol depletion and elevated oxidative stress further highlights the systemic impact of metabolic imbalances in SSc.
Aberrant utilization of branched chain amino acids
In our study, we observed significantly decreased circulatory levels of BCAAs including valine, leucine, and isoleucine in systemic sclerosis (SSc) patients compared to healthy controls. BCAAs play vital roles in protein metabolism, energy production, and immune regulation31. Reduction in circulatory levels of BCAAs can be attributed to the heightened oxidative stress and inflammation associated with SSc, which increases the body’s energy demands. In response, BCAAs are utilized at an accelerated rate to meet the elevated energy requirements in the diseased state. This aberrant utilization of BCAAs may contribute to their depletion in circulation, further exacerbating metabolic imbalances in SSc. These findings underscore the role of BCAA metabolism in SSc pathophysiology and suggest that restoring BCAA homeostasis could represent a potential therapeutic avenue.
Altered lipid metabolism
Earlier Studies on metabolic alterations in systemic sclerosis have highlighted disturbances in lipid metabolism, including fatty acid synthesis. Research has shown dysregulation in fatty acid and carnitine metabolism in systemic sclerosis patients, indicating abnormalities in fatty acid processing32. Furthermore, Metabolomic analyses have further identified changes in metabolites linked to fatty acid metabolism and the tricarboxylic acid (TCA) cycle, reflecting distinct metabolic profiles associated with fibrosis and endothelial dysfunction in SSc subtypes18.
In our study, increased circulatory levels of acetate and 3-hydroxybutyrate (Fig. 4 and S5), key metabolites involved in lipid synthesis and beta-oxidation of fatty acids. These findings point to a metabolic shift in SSc, where these metabolites may accumulate due to impaired downstream pathways or altered cellular demands. Simultaneously, levels of low-density lipoprotein (LDL)/very-low-density lipoprotein (VLDL), and other lipids were significantly downregulated (Fig. 2A), further indicating the disruptions in lipid metabolism. This pattern reflects a failure in lipid homeostasis, potentially linked to systemic inflammation, endothelial dysfunction, and oxidative stress that hallmark SSc.
The clinical implications of these metabolic disturbances are particularly striking in the context of vascular health. Despite the impaired ability of SSc patients to produce functional fat cells, the disease is associated with a severe burden of atherosclerotic plaques. For example, carotid intima-media thickness (IMT) in a 30-year-old SSc patient can be comparable to that of an 80-year-old individual, highlighting the accelerated vascular aging seen in SSc. This profound vascular involvement, coupled with impaired lipid metabolism, underscores the critical role of fatty acid alterations in the disease’s progression.
By unraveling these metabolic disruptions, our study not only sheds light on the role of altered lipid and fatty acid metabolism in SSc but also identifies potential metabolic biomarkers for early diagnosis and therapeutic intervention. These findings underscores the need for further research into lipid-processing pathways and their interplay with fibrosis and endothelial dysfunction in SSc, paving the way for targeted strategies to mitigate the disease’s impact.
Concluding remarks
Here our study suggests the potential involvement of perturbed sugar metabolism, branched-chain amino acid (BCAA) metabolism, fatty acid metabolism, and mannose glycosylation in the pathophysiology of Systemic Sclerosis. Specifically, alterations in myo-inositol and mannose metabolism suggest a novel insight into their roles in the pathogenesis of SSc. Mannose is a critical monosaccharide involved in glycoprotein synthesis, while myo-inositol is essential for cellular signaling, mitigating oxidative stress and inflammation. The observed abnormalities in circulating levels of glucose, myo-inositol, and mannose indicate significant disruptions in sugar metabolism, which, along with altered levels of lactate and pyruvate, may contribute to changes in energy metabolism, endothelial dysfunction, and glycosylation patterns. These metabolic disruptions could influence the stability and function of antibodies and modulate immune cell activities, which are key factors in the autoimmune processes associated with SSc.
Additionally, we propose that aberrant utilization of BCAAs, leading to decreased circulatory levels of valine, leucine, and isoleucine, may enhance a pro-inflammatory phenotype, further contributing to the disease’s pathobiology. Alterations in circulatory levels of 3-HB, acetate key metabolites of fatty acid metabolism along with reduced levels of lipids, indicating disruptions in lipid metabolism and beta-oxidation pathways, underscore the importance of lipid alterations in SSc. These findings point to significant underlying metabolic pathway disruptions that warrant further investigation and could provide novel targets for therapeutic intervention. Collectively, our results offer valuable insights into the complex metabolic dysregulation in SSc and its potential impact on disease development and progression, providing a basis for future research into targeted metabolic therapies for SSc.
Materials and methods
Ethical considerations
This present study involved two groups: patients with systemic sclerosis (SSc) and healthy control subjects (HC). The research was conducted in adherence to the ethical principles outlined in the Helsinki Declaration, following approval from the Institutional Human Ethics Committee (IHEC) of SGPGIMS, Lucknow (IEC Code: 2017-54-OTHER-96, File No. PGI/BE/94/2018; dated 19 March 2018). All procedures were carried out in accordance with the Standard Operating Procedures (SOPs) established by the IHEC, as detailed on the official institutional website (https://sgpgims.org.in/Home/1_SOP.html). Prior to blood sample collection, informed written consent obtained from all enrolled participants.
Patient recruitment and sample collection
The patients for the present study were enrolled from the OPD of Department of Clinical Immunology and Rheumatology. All patients, between ages of 18 and 60 years, fulfilled Systemic Sclerosis diagnosis as per ACR2013/ Leroy criteria33,34. Individuals with active infection, malignancy, end stage renal disease, end stage lung disease, pregnant or lactating women and those receiving glucocorticoids or had received immunosuppressive medications within the preceding 6 months were excluded. Demographic and clinical details retrieved from the clinic case files and laboratory tests from the electronic hospital information system. All the detailed information collected on case report form. Healthy control samples were collected from age, sex, and race matched volunteers and were non-smokers. Inclusion criteria was individuals having normal health parameters, no prior clinical diagnosis of acute or chronic illness and not receiving any medication. Blood samples were collected from both patients and healthy controls using serum separator vials. To allow clotting, the samples were incubated at room temperature for 30–40 minutes. Subsequently, the vials were centrifuged at 3000 g for 15 min. The resulting serum was carefully separated and transferred into 1.5 ml microcentrifuge tubes (MCTs), followed by immediate storage at -80 °C until further processing. For NMR experiments, sample preparation was done with slight modifications in earlier published procedure35.
NMR sample Preparation and NMR data collection
The NMR experiments were performed using a saline sodium phosphate solution (buffer strength, 200 mM; 100% D2O; containing 0.9% NaCl; 0.1 mM TSP - sodium salt of 3-trimethylsilyl-(2,2,3,3-d4)-propionic acid) serving as an external reference. The stored serum samples were thawed at room temperature and subsequently, the NMR sample was prepared by mixing 300 µl of serum with an equal volume (300 µl) of phosphate buffer. The mixture was then vortexed for 1 min and centrifuged at 4000 rpm for 2 min. Following centrifugation, 550 µl of the supernatant was carefully transferred to a 5 mm NMR tube (Wilmad glass, USA) for subsequent NMR spectral acquisition. The quantitative profiles of the serum metabolites were measured using 800 MHz high-resolution NMR spectrometre (Bruker, Avance III, with Cryoprobe) employing 1D1H CPMG (Carr–Purcell–Maiboom–Gill) spin–echo pulse sequence i.e., standard Bruker pulse sequence (cpmgpr1d), along with water presaturation and a T2 filter (water suppression during the recycle delay (RD) of 5.0 s), at 300 K, effectively suppressing broad signals originating because of proteins and other macromolecules10,36. NMR data acquisiton, data processing and spectral binning details are provided in the Electronic Supplementary material (ESM) as Annexure-.
Serum metabolite assignment
Metabolite identification in the 1D 1H CPMG NMR spectra was achieved through a combination of literature-referenced chemical shift data10,11,37 and confirmatory NMR spiking experiments12. The assignment of the broad signal at 5.17 ppm to mannose was initially based on its characteristic chemical shift pattern and previously reported spectral data. To experimentally validate this annotation, NMR spiking experiments were conducted using a pure mannose standard (Supporting Figure S10A), which confirmed the identity of this resonance. The identity of another important serum metabolite i.e. myo-inositol was confirmed through NMR spiking experiments as exemplified in Supporting Figure S10B. To further increase the confidence in metabolite assignments—particularly in crowded or overlapping spectral regions—we acquired two-dimensional (2D) NMR spectra on representative serum samples. These included ¹H–¹H TOCSY and 1H–13C HSQC experiments (Figure S10C), as well as ¹H-1H J-resolved (Jres) spectra (Figure S10D). These multidimensional approaches provided crucial spin-coupling and heteronuclear correlation information that enabled unambiguous peak assignments. Finally, the data quality was checked overlaying the unsegmented CPMG NMR spectra of SSc and HC serum samples (Figure S10E) and exquisite clustering of NMR peaks confirmed consistency across samples and no-significant inter-individual variability observed the data set.
Concentration profiling of serum metabolites
For explicit concentration profiling of serum metabolites, we used the PROFILER module of the NMR Suite of commercial software CHENOMX (v8.6, Edmonton, Canada) containing 800 MHz chemical shift database. First, the PROCESSOR module of CHENOMX software was used for manual baseline correction of each 1D1H CPMG NMR spectrum and for calibration w.r.t. endogenous formate by setting its concentration to 0.03 mM in concordance with previous reports38,39,40. Profiled concentration of metabolites were then exported in CSV (comma separated value) format for further multivariate and statistical analysis as per the details provided in the Annexure-I of ESM.
Data availability
The NMR spectral Data has been deposited to ZENODO https://zenodo.org/records/7792273 and will be available for future validation studies on request to the corresponding authors.
Abbreviations
- ACR:
-
American College of Rheumatology
- ANOVA:
-
Analysis of Variance
- AUROC:
-
Area under ROC curve
- BCAA:
-
Branched Chain Amino Acids
- CI:
-
Confidence interval
- CPMG:
-
Carr–Purcell–Meiboom–Gill
- CV-ANOVA:
-
Cross validation analysis of Variance
- dcSSc:
-
diffuse cutaneous systemic sclerosis
- ESM:
-
Electronic Supplementary Material
- EULAR:
-
European League Against Rheumatism
- GERD:
-
Gastroesophageal reflux disease
- GLUT-1:
-
Glucose transporter
- HC:
-
Healthy control
- HDL:
-
High Density Lipoproteins
- ILD:
-
Interstitial lung disease
- INR:
-
International Normalized Ratio
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- lcSSc:
-
limited cutaneous systemic sclerosis
- LDL/VLDL:
-
Low Density Lipoproteins/Very Low Density Lipoproteins
- MCTs:
-
Micro-centrifuge tube
- MDA:
-
Mean Decrease accuracy
- mRSS:
-
Modified Rodnan Skin Score
- MSEA:
-
Metabolite Set Enrichment Analysis
- NMR:
-
Nuclear Magnetic Resonance
- NO:
-
Nitric Oxide
- OOB:
-
Out-of-bag error
- PAH:
-
Pulmonary Arterial Hypertension
- PCA:
-
Principal component analysis
- PET:
-
Positron Emission Tomography
- PLS-DA:
-
Projection to least-squares discriminant analysis
- PUFA:
-
Poly-unsaturated Fatty Acids
- RD:
-
Recycle delay
- RF:
-
Random forest
- ROC:
-
Receiver operating characteristic curve
- ROS:
-
Reactive Oxygen Species
- SD:
-
Standard deviation
- SSc:
-
Systemic Sclerosis
- TCA:
-
Tricarboxylic acid
- TCA:
-
Tricarboxylic acid cycle
- VIP:
-
variable importance in projection
References
Denton, C. P. & Khanna, D. Systemic sclerosis. Lancet 390, 1685–1699 (2017).
Tian, J. et al. Global, regional, and National incidence and prevalence of systemic sclerosis. Clin. Immunol. 248, 109267 (2023).
Ghosh, S. K., Bandyopadhyay, D., Saha, I. & Barua, J. K. Mucocutaneous and demographic features of systemic sclerosis: A profile of 46 patients from Eastern India. Indian J. Dermatology. 57, 201–205 (2012).
Guleria, A., Kumar, A., Kumar, U., Raj, R. & Kumar, D. NMR based metabolomics: an exquisite and facile method for evaluating therapeutic efficacy and screening drug toxicity. Curr. Top. Med. Chem. 18, 1827–1849 (2018).
Gowda, G. N. et al. Metabolomics-based methods for early disease diagnostics. Expert Rev. Mol. Diagn. 8, 617–633 (2008).
Shanaiah, N., Zhang, S., Desilva, M. A. & Raftery, D. NMR-based metabolomics for biomarker discovery. In Biomarker Methods Drug Discovery Development. Methods in Pharmacology and Toxicology™. (ed. Wang, F.) 341–368 (Humana Press, 2008).
Ciaffi, J. et al. Whole body insulin sensitivity is increased in systemic sclerosis. PloS One. 18, e0283283 (2023).
Zhou, X. et al. Antibody glycosylation in autoimmune diseases. Autoimmun. Rev. 20, 102804 (2021).
Morel, M. et al. Abnormal B cell glycosylation in autoimmunity: A new potential treatment strategy. Front. Immunol. 13, 975963 (2022).
Beckonert, O. et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2, 2692 (2007).
Ala-Korpela M. 1H NMR spectroscopy of human blood plasma. Progress Nucl. Magn. Reson. Spectrosc. 27, 475–554 (1995).
Dubey, D. et al. Metabolite assignment of ultrafiltered synovial fluid extracted from knee joints of reactive arthritis patients using high resolution NMR spectroscopy. Magn. Reson. Chem. 57, 30–43 (2019).
Singh, A. et al. Serum metabolic disturbances in lung cancer investigated through an elaborative NMR-based serum metabolomics approach. ACS Omega. 7, 5510–5520 (2022).
Kumar, U. et al. An elaborative NMR based plasma metabolomics study revealed metabolic derangements in patients with mild cognitive impairment: a study on North Indian population. Metab. Brain Dis. 36, 957–968 (2021).
Akarachantachote, N., Chadcham, S. & Saithanu, K. Cutoff threshold of variable importance in projection for variable selection. Int. J. Pure Appl. Math. 94, 307–322 (2014).
Smolenska, Z., Zabielska-Kaczorowska, M., Wojteczek, A., Kutryb-Zajac, B. & Zdrojewski, Z. Metabolic pattern of systemic sclerosis: association of changes in plasma concentrations of amino acid-related compounds with disease presentation. Front. Mol. Biosci. 7, 585161 (2020).
Bogl, T. et al. Plasma metabolomic profiling reveals four possibly disrupted mechanisms in systemic sclerosis. Biomedicines 10, 607 (2022).
Murgia, F. et al. Metabolomic profile of systemic sclerosis patients. Sci. Rep. 8, 7626 (2018).
Sun, C. et al. Serum metabolite differences detected by HILIC UHPLC-Q-TOF MS in systemic sclerosis. Clin. Rheumatol. 42, 125–134 (2023).
Guo, M. et al. Serum metabolomic profiling reveals potential biomarkers in systemic sclerosis. Metabolism 144, 155587 (2023).
Bengtsson, A. A. et al. Metabolic profiling of systemic lupus erythematosus and comparison with primary sjögren’s syndrome and systemic sclerosis. PloS One. 11, e0159384 (2016).
Meier, C. et al. Serum metabolites as biomarkers in systemic sclerosis-associated interstitial lung disease. Sci. Rep. 10, 21912 (2020).
Ottria, A. et al. Fatty acid and carnitine metabolism are dysregulated in systemic sclerosis patients. Front. Immunol. 11, 822 (2020).
O’Reilly, S. Metabolic perturbations in systemic sclerosis. Curr. Opin. Rheumatol. 34, 91–94 (2022).
Henderson, J., Duffy, L., Stratton, R., Ford, D. & OGÇÖReilly, S. Metabolic reprogramming of Glycolysis and glutamine metabolism are key events in myofibroblast transition in systemic sclerosis pathogenesis. J. Cell. Mol. Med. 24, 14026–14038 (2020).
Allanore, Y. et al. Systemic sclerosis. Nat. Reviews Disease Primers. 1, 1–21 (2015).
Katsumoto, T. R., Whitfield, M. L. & Connolly, M. K. The pathogenesis of systemic sclerosis. Annu. Rev. Pathol. 6, 509–537 (2011).
Marth, J. D. & Grewal, P. K. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8, 874–887 (2008).
Flavahan, N. A. A vascular mechanistic approach to Understanding Raynaud phenomenon. Nat. Rev. Rheumatol. 11, 146–158 (2015).
Tomo, D. & Pietro, D. Myoinositol reduces inflammation and oxidative stress in human endothelial cells exposed in vivo tochronic hyperglycemia. Nutrients 13(7), 2210 (2021).
Zhenyukh, O. et al. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Biol. Med. 104, 165–177 (2017).
Morales-González, V. et al. Metabolic fingerprinting of systemic sclerosis: a systematic review. Front. Mol. Biosci. 10, 1–21 (2023).
Jordan, S., Maurer, B., Toniolo, M., Michel, B. & Distler, O. Performance of the new ACR/EULAR classification criteria for systemic sclerosis in clinical practice. Rheumatology 54, 1454–1458 (2015).
LeRoy, E. C. et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J. Rheumatol. 15, 202–205 (1988).
Gowda, G. N. & Raftery, D. Can NMR solve some significant challenges in metabolomics? J. Magn. Reson. 260, 144–160 (2015).
Wishart, D. S. Quantitative metabolomics using NMR. TRAC Trends Anal. Chem. 27, 228–237 (2008).
Wurtz, P. et al. Quantitative serum nuclear magnetic resonance metabolomics in large-scale epidemiology: a primer on-omic technologies. Am. J. Epidemiol. 186, 1084–1096 (2017).
Ando, I. et al. Quantification of molecules in 1H-NMR metabolomics with formate as a concentration standard. J. Toxicol. Sci. 35, 253–256 (2010).
Kriat, M. et al. Quantitation of metabolites in human blood serum by proton magnetic resonance spectroscopy. A comparative study of the use of formate and TSP as concentration standards. NMR Biomed. 5, 179–184 (1992).
Rastogi, S., Verma, A., Trivedi, R., Shukla, A. & Kumar, D. Clinical metabolomics investigation of rheumatoid arthritis patients receiving ayurvedic whole system intervention. J. Ayurveda Integr. Med. 15, 101009 (2024).
Acknowledgements
The Authors acknowledge the Department of Medical Education, Govt. of Uttar Pradesh and DST SUPREME DST Project DST/SUPREME/2023/70 for supporting the maintenance, functioning and upgradation of High Field 800 MHz state-of-the art NMR Spectrometer at Centre of Biomedical Research, Lucknow, India. The research was partly supported by intramural funding from the Centre of Biomedical Research (CBMR), Lucknow (Project No. CBMR/IMR/0010/2021). GS acknowledges the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for his fellowship under CSIR Senior Research Fellowship (SRF) scheme (No. F. 09/0916(14014)/2022-EMR-I).
Author information
Authors and Affiliations
Contributions
G.S. and D.K.: Conceptualization of idea and study design, performed the spectral binning and concentration profiling, NMR based metabolomics data analysis, preparing the figures, and manuscript drafting, proof reading and submission, S.A. and V.A.: Involved in the clinical screening of patients, imaging analysis and clinical data collection. M.K.R.: Collection of blood samples, extraction of serum and compiling the clinical and demographic details. D.D. and A.R.: Prepared NMR buffer and NMR samples, recorded the NMR spectra. G.S. and D.K.: NMR based metabolomics data analysis, preparing the figures, and manuscript drafting, proof reading and submission. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The Institute Ethics Committee of SGPGIMS, Lucknow (IEC Code: 2017-54-OTHER-96, File No. PGI/BE/94/2018; dated 19 March 2018), approved the study. All the study participants provided written informed consent. The samples were obtained from Department of Clinical Immunology and Rheumatology SGPGIMS, Lucknow.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Singh, G., Ahmed, S., Dubey, D. et al. NMR based clinical metabolomics revealed altered lipid and sugar metabolism in systemic sclerosis with implications for diagnosis and therapeutic strategies. Sci Rep 15, 30676 (2025). https://doi.org/10.1038/s41598-025-16493-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16493-5