Abstract
Iodine, an essential trace element, may play a role in reproductive health through its extrathyroidal actions. This study aimed to investigate the effects of iodine supplementation on oocyte apoptosis and proliferation in women with diminished ovarian reserve. A quasi-experimental study was conducted on ten women undergoing two cycles of ovarian stimulation. In the first cycle, they followed the standard protocol, while in the second, they received a daily 150 µg iodine supplement for two months. Urinary iodine levels, cumulus cell apoptosis and necrosis, and PCNA expression were evaluated before and after supplementation. We found that iodine supplementation significantly improved women’s iodine status, increasing it from borderline sufficiency to optimal levels. This improvement was accompanied by a significant increase in the number of metaphase II oocytes. Following iodine supplementation, we observed a marked increase in cumulus cell viability and a reduction in both early and late apoptosis. Additionally, the expression of PCNA was upregulated, showing a positive association with the percentage of live cells and a reduction in total apoptosis. These findings suggest that iodine supplementation could be a promising therapeutic strategy for improving oocyte quality and enhancing fertility outcomes, particularly in women with diminished ovarian reserve.
Similar content being viewed by others
Introduction
Infertility is defined as the inability to conceive after 12 months or more of regular, unprotected sexual intercourse. It is a multifactorial condition that can result from male factors, female factors, a combination of both, or unexplained causes1. Among female-related causes, diminished ovarian reserve (DOR) is a significant contributor, posing a substantial challenge to fertility treatments, particularly in assisted reproductive technologies (ART). DOR is characterized by a reduced quantity and quality of oocytes, often leading to poor in vitro fertilization (IVF) outcomes. It is observed in approximately 10–15% of women undergoing IVF and is one of the major limiting factors in ART success2. Women with DOR typically exhibit a lower number of retrieved oocytes per IVF cycle, a reduced number of viable embryos, and consequently, a decreased pregnancy rate. Additionally, DOR is associated with a higher incidence of miscarriages in ART cycles3. While oocyte quantity is a critical determinant of fertility potential, accumulating evidence suggests that oocyte quality plays an equally important role in reproductive success4.
One of the key modulators of female reproductive health is micronutrient intake, particularly iodine, an essential trace element required for human growth, development, and metabolism. Iodine is essential for thyroid hormone synthesis, which regulates key reproductive processes such as ovulation, follicular development, and ovarian function5. Inadequate iodine intake can lead to hypothyroidism, which negatively affects metabolism, weight regulation, and fertility6. Thyroid hormones, particularly thyroid-stimulating hormone, play a critical role in follicular growth and maturation by interacting with follicle-stimulating hormone receptors. This interaction stimulates the development of pre-antral follicles, further supporting ovarian function7. Beyond its indirect effects through thyroid function, iodine is directly absorbed by ovarian tissues and the endometrium. Research indicates that iodine is crucial for the secretory activity of granulosa cells, which are essential for follicular development and oocyte maturation5. Furthermore, iodine deficiency has been linked to anovulatory cycles, underscoring its critical role in female fertility5.
Given the crucial role of iodine in ovarian function, oocyte quality, and overall female fertility, further research is needed to elucidate its precise mechanisms. While iodine’s significance in reproductive health is well established, studies directly examining the impact of dietary iodine on oocyte apoptosis and proliferation remain limited. Therefore, this study aims to assess the effect of iodine supplementation on oocyte quantity and quality, as well as apoptosis and proliferation of cumulus cells in women with DOR.
Materials and methods
Study design and participants
The present study was a quasi-experimental (before-after) study conducted with 10 women diagnosed with DOR. These women sought treatment at the Infertility Clinic of Tehran University of Medical Sciences, Tehran, Iran, between September 2024 and January 2025. The inclusion criteria were as follows: (1) women aged between 25 and 42 years with no history of smoking, alcohol consumption, or drug abuse, (2) women with no history of recurrent miscarriage (≥ 2), (3) women free from underlying medical conditions, including endometriosis or thyroid disorders, (4) women who were candidates for IVF or oocyte banking, and (5) women who were willing to undergo two consecutive ovarian stimulation cycles. Information on weight, height, body mass index (BMI), medical history, and fertility history was collected using an interviewer-administered questionnaire. Written informed consent was obtained from all participants after a comprehensive explanation of the study protocol and objectives. The study was approved by the Ethics Committee of Tehran University of Medical Sciences.
Procedures and samples collection
Following the diagnosis of DOR, women were evaluated after undergoing two cycles of ovarian stimulation as part of the IVF procedure or oocyte banking. According to the Bologna criteria, DOR is defined by an antral follicle count of 3–5 on transvaginal ultrasound and an anti-Müllerian hormone concentration ranging from 0.1 to 1.5 ng/mL8. In the first cycle (pre-intervention), participants followed the standard ovarian stimulation protocol. In the second cycle (post-intervention), they were instructed to take one iodine supplement (150 µg daily) for two months while avoiding any other iodine-containing supplements during the study period. Notably, the prescription of iodine supplementation complied with Iran’s national guidelines for women planning to conceive. To assess iodine status, participants provided a spot urine sample before and after the intervention. All samples were immediately refrigerated and subsequently frozen at -20 °C until iodine concentration measurements were performed.
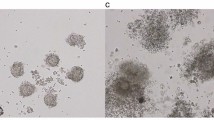
Ovarian stimulation was conducted using the antagonist protocol9. Gonadotropins were administered subcutaneously at a minimum daily dose of 300 IU, starting on days 2–3 of the menstrual cycle, based on the patient’s condition. After five days, a transvaginal ultrasound was performed. If follicles measured 13 mm or larger, a subcutaneous injection of Ganirelix (Orgalutran 500 mcg/0.5 cc) was administered for two days while gonadotropin treatment continued. A follow-up ultrasound was conducted 48 h later by an infertility specialist. When follicle size reached 17–18 mm, a human chorionic gonadotropin trigger injection (10,000 IU) was administered. Oocyte retrieval was performed 36 h later in the operating room. The total number of retrieved oocytes and their quality were documented. Oocytes were classified based on their maturity and morphology, which were key indicators of their developmental potential. The classifications included germinal vesicle (GV), metaphase I (M1), metaphase II (M2), and degenerated (Deg) oocytes, as well as empty follicles10. To separate the cumulus cells from the oocyte, the cumulus-oocyte complex was treated with hyaluronidase. This enzyme breaks down the hyaluronic acid in the extracellular matrix, allowing the cumulus cells to be gently removed from the oocyte. Then, all samples were sent to the genetics laboratory for the assessment of apoptosis and proliferation in cumulus cells.
Laboratory measurements
Iodine measurement in urine samples
Iodine concentration in the urine samples was analyzed using the Sandell-Kolthoff (acid-digestion) reaction and the results were expressed as micrograms of iodine per litre (µg/L) of urine11. According to the World Health Organization, iodine sufficiency is defined as a urinary iodine concentration (UIC) ≥ 100 µg/L in the studied population12.
Assessment of apoptosis and necrosis in cumulus cells
Apoptosis and necrosis in cumulus cells were assessed using flow cytometry. To detect apoptotic cells, Annexin V-FITC staining (Sigma-Aldrich, Germany) was performed, while propidium iodide staining was used to distinguish necrotic cells from apoptotic ones. For this procedure, cumulus cells were first washed with phosphate-buffered saline and centrifuged to remove debris. The cell pellet was then resuspended in 100 µL of binding buffer, followed by the addition of 5 µL of Annexin V-FITC. The sample was incubated in the dark at 4 °C to allow Annexin V binding. After incubation, the cells were washed again, centrifuged, and 10 µL of PI solution (10 µL/100 µL PBS) was added to stain necrotic cells. Finally, flow cytometry analysis was performed using a Partec PAS flow cytometer (Partec GmbH, Germany) to quantify apoptotic and necrotic cell populations13.
Proliferating cell nuclear antigen (PCNA) expression analysis
PCNA is a key marker of cell proliferation, playing a crucial role in DNA replication and repair. It acts as a processivity factor for DNA polymerase δ, ensuring efficient DNA synthesis during the S-phase of the cell cycle. PCNA expression is widely analyzed to evaluate cell proliferation activity in various biological and pathological contexts, including ovarian function and follicular development. In the present study, for gene expression analysis, total RNA was extracted from cumulus cells using an RNA extraction kit (Yekta Tajhiz Azma, Tehran, Iran) following the manufacturer’s protocol. The quality and quantity of the extracted RNA were assessed using gel electrophoresis and a NanoDrop spectrophotometer. Subsequently, complementary DNA (cDNA) was synthesized using a cDNA synthesis kit (Yekta Tajhiz Azma, Theran, Iran) according to the manufacturer’s instructions. Primers for the PCNA gene were designed using Gene Runner software. The specific primer sequences used for amplification are listed in Table 1, with β-Actin serving as the internal control gene. PCNA gene expression was assessed using real-time polymerase chain reaction under thermal cycling conditions adapted from a previous study13. The relative expression levels of PCNA were analyzed using the ΔΔCt method, with β-Actin as the reference gene.
Statistical analyses
Descriptive statistics for categorical and continuous variables are presented as frequency distributions (percentages), means ± standard deviations (SD), or medians with interquartile ranges (IQR). The normality of data distribution was evaluated using the Kolmogorov-Smirnov and Shapiro–Wilk tests. To compare fertility related parameters before and after the intervention, paired t-tests were applied for normally distributed data, while the Wilcoxon test was used for non-normally distributed variables. A linear regression analysis was performed to assess the association between PCNA gene expression with various cumulus cell populations. Statistical analyses were conducted using IBM SPSS for Windows (version 20.0, 2011, Armonk, NY: IBM Corp), with a P-value < 0.05 considered statistically significant.
Results
The mean ± SD age and BMI of women with diminished ovarian reserve were 37.8 ± 4.0 years and 21.7 ± 1.0, respectively. The mean ± SD UIC was 104 ± 67 µg/L before iodine intervention and increased to 256 ± 126 µg/L after the intervention (P = 0.001). Table 2 presents changes in oocyte parameters before and after iodine supplementation. Although the total number of retrieved oocytes increased from 2.8 ± 2.3 to 4.1 ± 2.8 following supplementation, this difference was not statistically significant (P = 0.093). Regarding oocyte quality, the proportion of GV oocytes slightly increased after supplementation (0.2 ± 0.4 vs. 0.0 ± 0.0), though the change was not significant (P = 0.345). Similarly, the number of M1 oocytes remained unchanged (0.5 ± 0.5 vs. 0.5 ± 0.7, P = 1.000). However, a significant increase was observed in the number of M2 oocytes, which rose from 1.9 ± 1.4 to 3.2 ± 2.2 (P = 0.021), suggesting a potential positive effect of iodine supplementation on oocyte maturation. No significant differences were found in the number of Deg oocytes (0.3 ± 0.7 vs. 0.1 ± 0.3, P = 0.586) or empty follicles (0.3 ± 0.5 vs. 0.4 ± 0.5, P = 1.000) before and after supplementation.
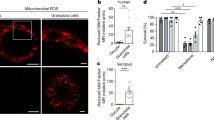
Figure 1 illustrates the distribution of cumulus cell populations before and after iodine supplementation. Following iodine supplementation, a significant increase in the percentage of live cumulus cells was observed, rising from 26.4% ± 2.8 to 67.9% ± 6.5 (P < 0.001). Additionally, early apoptosis was significantly reduced from 19.3% ± 0.4 to 13.7% ± 3.4 (P < 0.001), while late apoptosis showed a more substantial decrease from 44.7% ± 1.9 to 9.0% ± 1.8 (P < 0.001). As a result, total apoptosis decreased significantly from 64.0% ± 2.0 to 22.7% ± 5.1 (P < 0.001). In contrast, the percentage of necrotic cells remained unchanged (P = 0.725), suggesting that iodine supplementation predominantly impacted apoptotic pathways rather than necrosis. Further details can be found in Table 3.
The fold change in PCNA gene expression before and after iodine supplementation was assessed. Before the intervention, the average PCNA expression was normalized to 1.000 (reference value). After iodine supplementation, PCNA gene expression significantly increased, with a mean fold change of 3.38 ± 1.10 (P < 0.001). This indicates a notable upregulation in PCNA expression following supplementation, suggesting that iodine has a positive effect on the proliferation activity of cumulus cells in women with DOR. Figure 2 shows the results of the linear regression analyses examining the relationship between PCNA expression and various cell populations in cumulus cells, including live cells, early apoptosis, late apoptosis, and total apoptosis. PCNA expression was significantly positively associated with the percentage of live cells (Slope (SE) = 7.33 (1.8); P = 0.0009). Additionally, it was significantly associated with a decrease in both early (Slope (SE)= -1.09 (0.3); P = 0.005) and late apoptosis (Slope (SE)= -6.18 (1.5); P = 0.0009), with a pronounced negative correlation with total apoptosis (Slope (SE)= -7.28 (1.8); P = 0.0008). However, no significant association was observed between PCNA expression and necrotic cell death.
Linear regression analysis of the association between PCNA expression and live cells (A), early apoptosis (B), late apoptosis (C), and total apoptosis (D). Blue squares indicate cumulus cells prior to iodine supplementation, and red circles represent cumulus cells following iodine supplementation. PCNA: proliferating cell nuclear antigen.
Discussion
To the best of our knowledge, this is the first study to provide the preliminary data on the effects of iodine supplementation on oocyte quantity and quality, cumulus cell apoptosis, and PCNA gene expression in women with DOR. Our findings indicated that two months of iodine supplementation significantly improved oocyte quality and positively affected various aspects of cell function, including a reduction in cumulus cell apoptosis and upregulation of PCNA expression. These results suggest that iodine supplementation may have a beneficial effect on ovarian health and fertility potential.
To date, only a limited number of studies have investigated the potential role of dietary iodine in fertility outcomes. For example, a large cross-sectional study conducted in China found that women with iodine deficiency were significantly more likely to experience delayed conception, with prolonged time to pregnancy and reduced fecundability compared to those with adequate iodine levels14. Similarly, a study in the United States observed that moderate-to-severe iodine deficiency was associated with a 46% decrease in fecundability, further reinforcing the link between insufficient iodine levels and impaired fertility15. Despite recommendations from leading international health organizations, including the American Thyroid Association and the Endocrine Society16,17, for iodine supplementation in pregnant and lactating women, or those planning pregnancy, the impact of iodine supplementation on fertility outcomes remains inconclusive18,19. In our study, we found that iodine supplementation significantly improved women’s iodine status, shifting it from borderline sufficiency to optimal levels. While the national program for the elimination of iodine deficiency in Iran advises iodine supplementation for all pregnant and lactating women, women planning to become pregnant are not routinely recommended to take iodine-containing supplements. We observed the same trend in women seeking treatment for fertility-related issues, where iodine supplementation is not routinely incorporated into their treatment plans, despite its potential positive effects on reproductive health. In the present study, we demonstrated the iodine supplementation can positively influence the reproductive system by modulating both cellular and molecular pathways.
Cumulus cells play a crucial role in oocyte maturation by facilitating direct communication, nutrient transfer, and the regulation of key signaling pathways. These cells establish a bidirectional relationship with the oocyte through gap junctions, enabling essential metabolic and molecular exchanges necessary for oocyte development20. Additionally, cumulus cells regulate cell survival and apoptosis, processes that are fundamental to maintaining oocyte quality and fertility. A delicate balance between pro-apoptotic and anti-apoptotic factors within these cells is essential for successful ovulation and embryo development21. Disruptions in this balance, leading to increased apoptosis in cumulus cells, have been associated with poor oocyte quality, lower fertilization rates, and reduced oocyte yield22. In our study, iodine supplementation significantly enhanced cumulus cell viability while reducing both early and late apoptosis. These improvements were accompanied by a notable increase in the number of metaphase II oocytes, suggesting a positive impact on oocyte maturation and overall reproductive potential.
The molecular mechanisms underlying these effects may involve the modulation of key proteins, such as PCNA, which plays a crucial role in cell cycle progression and DNA repair. The observed upregulation of PCNA expression following iodine supplementation suggests that iodine may enhance the proliferative capacity of cumulus cells, creating a more favorable microenvironment for oocyte maturation. In our study, PCNA expression was significantly positively associated with the percentage of live cells. Additionally, it was significantly correlated with a decrease in both early and late apoptosis, showing a marked negative association with total apoptosis. This finding was consistent with previous studies suggesting that iodine’s antioxidant and anti-apoptotic properties help preserve cellular integrity, ultimately contributing to improved oocyte quality23,24.
It is important to note that the effects of iodine on reproductive health remain a topic of ongoing research. Although our study offers promising preliminary evidence for the beneficial effects of iodine on cumulus cells, further investigations using double-blind randomized controlled trial designs are essential to validate these findings. Such studies are necessary to isolate the specific effects of iodine supplementation and to minimize potential confounding factors, including natural biological variation over time and the influence of repeated ovarian stimulation cycles. One of the key limitations of our study is the small sample size (N = 10), which may reduce the statistical power to detect significant differences—particularly in outcomes where non-significant trends were observed, such as the increase in the total number of retrieved oocytes (P = 0.093). This suggests that the study may be underpowered to identify smaller, yet potentially clinically meaningful effects. However, as this study was designed as a pilot, the primary aim was to assess feasibility and explore preliminary effects rather than draw definitive conclusions. Accordingly, the findings should be considered hypothesis-generating, and caution is warranted in generalizing them to broader populations. Importantly, the chosen sample size aligns with widely accepted recommendations for pilot studies, which typically suggest enrolling 10–12 participants per group to estimate variability and inform the design of future, adequately powered trials25. These findings should be interpreted with caution, as the observed increase in M2 oocytes and improvements in cumulus cell parameters following iodine supplementation represent surrogate markers that may not directly translate into improved clinical outcomes such as pregnancy or live birth rates. Future studies should be designed to evaluate whether these biological improvements lead to better reproductive outcomes in women with DOR.
In conclusion, dietary iodine plays a significant role in the regulation of cellular and molecular processes in cumulus cells, and its supplementation may offer a therapeutic strategy for improving oocyte quality and fertility outcomes, particularly in women with DOR. Our findings suggest that iodine supplementation, through the upregulation of PCNA expression, enhances cumulus cell survival and proliferation, reduces apoptosis, and may foster a healthier ovarian microenvironment, ultimately contributing to improved reproductive health. Given the growing evidence supporting iodine’s role in fertility, it may be worthwhile for clinicians to consider iodine supplementation as part of fertility management, especially in women with iodine deficiency or insufficiency.
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
References
Carson, S. A. & Kallen, A. N. Diagnosis and management of infertility: A review. JAMA 326(1), 65–76 (2021).
Zhu, S. et al. Effect of diminished ovarian reserve on the outcome of fresh embryo transfer in IVF/ICSI cycles among young women: A retrospective cohort study. BMC Womens Health. 24(1), 230 (2024).
An, N. et al. Metabolomic analysis reveals association between decreased ovarian reserve and in vitro fertilization outcomes. Metabolites 14(3) (2024).
Scantamburlo, V. M. et al. Association between decreased ovarian reserve and poor oocyte quality. Obstet. Gynecol. Sci. 64(6), 532–539 (2021).
Mathews, D. M. et al. Iodine and fertility: do we know enough? Hum. Reprod. 36(2), 265–274 (2021).
Kuehn, B. Iodine deficiency May impair fertility. JAMA 319(8), 760 (2018).
Brown, E. D. L., Obeng-Gyasi, B., Hall, J. E. & Shekhar, S. The thyroid hormone axis and female reproduction. Int. J. Mol. Sci. 24(12) (2023).
Ferraretti, A. P. & Gianaroli, L. The Bologna criteria for the definition of poor ovarian responders: is there a need for revision? Hum. Reprod. 29(9), 1842–1845 (2014).
Gui, J., Ni, Y., Liu, Q., Wang, X. & Xie, Q. Comparison of clinical effects between early follicular prolonged GnRH agonist protocol and GnRH antagonist protocol in 3310 cycles: a retrospective study. BMC Pregnancy Childbirth. 22(1), 942 (2022).
Lemseffer, Y., Terret, M. E., Campillo, C. & Labrune, E. Methods for assessing oocyte quality: A review of literature. Biomedicines 10(9) (2022).
Hedayati, M. et al. Rapid microwave digestion and microplate reading format method for urinary iodine determination. Clin. Chem. Lab. Med. 49(2), 281–284 (2011).
Zimmermann, M. B. & Andersson, M. Global Endcrinology: Global perspectives in endocrinology: coverage of Iodized salt programs and iodine status in 2020. Eur. J. Endocrinol. 185(1), R13–R21 (2021).
Hashemi, M., Masoumi, M., Salehi, M. & Angaji, S. A. Effects of titanium dioxide nanoparticles and coenzyme Q10 on testicular Ischemia-Reperfusion injury: role of the mitochondrial apoptosis pathway. Galen Med. J. 11:e2334–e. (2022).
Xing, M. et al. Low iodine intake may decrease women’s fecundity: A population-based cross-sectional study. Nutrients 13(9) (2021).
Mills, J. L. et al. Delayed conception in women with low-urinary iodine concentrations: a population-based prospective cohort study. Hum. Reprod. 33(3), 426–433 (2018).
Alexander, E. K. et al. 2017 guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27(3), 315–389 (2017).
De Groot, L. et al. Management of thyroid dysfunction during pregnancy and postpartum: an endocrine society clinical practice guideline 2017 guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. J. Clin. Endocrinol. Metab. 97(8), 2543–2565 (2012).
Bradbury, R. A., Christie-David, D., Smith, H. C., Byth, K. & Eastman, C. J. Prior iodine exposure and impact on thyroid function during controlled ovarian hyperstimulation: A prospective study. Aust N Z. J. Obstet. Gynaecol. 62(1), 133–139 (2022).
Ku, C. W. et al. Dietary supplement intake and fecundability in a Singapore preconception cohort study. Nutrients 14(23) (2022).
Xie, J., Xu, X. & Liu, S. Intercellular communication in the cumulus-oocyte complex during folliculogenesis: A review. Front. Cell. Dev. Biol. 11, 1087612 (2023).
Sayutti, N., Abu, M. A. & Ahmad, M. F. PCOS and role of cumulus gene expression in assessing oocytes quality. Front. Endocrinol. (Lausanne). 13, 843867 (2022).
Lee, K. S. et al. Cumulus cells apoptosis as an indicator to predict the quality of oocytes and the outcome of IVF-ET. J. Assist. Reprod. Genet. 18(9), 490–498 (2001).
Abadjieva, D., Petkova, M., Grigorova, S. & Kistanova, E. Iodine supplementation activates folliculogenesis in rabbit ovary. Pol. J. Vet. Sci. 21(3), 559–566 (2018).
Aceves, C., Anguiano, B. & Delgado, G. The Extrathyronine actions of iodine as antioxidant, apoptotic, and differentiation factor in various tissues. Thyroid 23(8), 938–946 (2013).
Kunselman, A. R. A brief overview of pilot studies and their sample size justification. Fertil. Steril. 121(6), 899–901 (2024).
Acknowledgements
This study was supported by financial grants from Tehran University of Medical Sciences, Tehran, Iran (grant number 1402-4-418-69402).
Author information
Authors and Affiliations
Contributions
Masoumeh Masoumi and Maryam Bagheri contributed to design the study concept, data collection, interpretation, and writing, reading and final approval of the manuscript; Sedighe Hantoushzadeh contributed to data analysis and interpretation and writing, reading and final approval of the manuscript; Mina Jafarabadi, Masoumeh Dehghan Tarazjani, and Fedyeh Haghollahi contributed to data collection and writing, reading and final approval of the manuscript; Mehdi Hedayati and Farnush Naseri contributed to laboratory measurements, and writing, reading and final approval of the manuscript; Pantea Nazeri contributed to design the study concept, data interpretation, and writing, reading and final approval of the manuscript. All authors contributed to the writing, review, and final approval of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and informed consent
All procedures were conducted in accordance with the ethical standards of the responsible committee on human experimentation and the principles of the 1964 Helsinki Declaration and its subsequent amendments. Informed consent was obtained from all participants prior to their inclusion in the study. This study was approved by the Ethics Committee of Tehran University of Medical Sciences.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Masoumi, M., Bagheri, M., Hantoushzadeh, S. et al. The effect of iodine supplementation on oocyte apoptosis and proliferation in women with diminished ovarian reserve: a pilot study. Sci Rep 15, 34501 (2025). https://doi.org/10.1038/s41598-025-16545-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16545-w