Abstract
The current study has been conducted to assess the influence of the morphology of bismuth ferrite perovskite (BiFeO3) on its photocatalytic performance. Nanorod and nanoparticle morphologies of BiFeO3 were prepared using the hydrothermal process and analyzed with several techniques. The photocatalysts’ effectiveness was appraised by MB photo-degradation in the presence of visible light. Various techniques were applied to analyze the structure, morphology, together with photoelectrochemical and optical properties of the photocatalyst. Optimum photo-degradation parameters were recognized via RSM, resulting in a maximum MB degradation efficiency of 98.2% (0.2021 min−1) in 20 min for nanorods. Whereas, the degradation yield for nanoparticles was achieved at 76% (0.075 min−1).From the radical trapping experiments, superoxide reactive radicals played a main role in MB photo-degradation. Also, BiFeO3 nanorods displayed superb stability during the five cycles of the photo-reaction procedure. This current survey suggests that the appropriate selection of nanomaterial morphology is crucial for achieving efficient photocatalysts.
Similar content being viewed by others
Introduction
Rising water pollution, crowd expansion, and climatic alteration are considered significant risks to the availability of purified water for residential, commercial, and agricultural applications. Consequently, the reclamation of processed municipal or industrial effluent has attracted considerable interest from researchers due to increasing concerns regarding water scarcity1. Given that organic contaminants in aquatic environments are predominantly extremely hazardous and poisonous, which may result in a variety of significant health concerns, the effective removal of these detrimental organic substances from the environment has emerged as a critical priority for the research community. Among the various industrial contaminants, pigmented substances are of particular concern in discussions about natural environments2,3. Latterly, advanced oxidation processes (AOPs) have been recognized as viable alternatives to customary procedures4. In the last few years, the organic contaminants’ photodegradation through a radical oxidative mechanism has been extensively researched as a safe and sustainable method for their removal2. Photocatalysis is a progressive oxidation method that involves the activation of a semiconductor under light exposure, resulting in the generation of active species that can degrade contaminant molecules5,6. Regrettably, the majority of commonly utilized photocatalysts predominantly react to UV-visible light exposure, which restricts their effectiveness during light exposure. As a result, the search for extremely effective and durable photocatalysts driven by visible light has become a prominent area of interest within the domain of photocatalytic processes2. Within the extensive range of materials, multiferroics represent a promising category for design and synthesis, owing to their applicability in multifunctional applications. They have garnered significant interest due to the intriguing physics that governs their behavior and their potential for innovative device applications7. Recently, BiFeO3 photocatalysts have garnered interest due to their low energy band gap, exceptional chemical durability at ambient temperature, and superior charge carrier transport characteristics. BiFeO3 demonstrates the potential for catalytic uses when exposed to visible illumination. Research indicates that BiFeO3 possesses a band gap of almost 2.12 ~ 2.4 eV7,8. It demonstrates a positive reaction to visible light and is also utilized for photodegradation of water contaminants, including various types of dye9,10. Bismuth (Bi) containing photocatalytic composites were selected for their superior absorption of visible radiation. These photocatalysts remove organic contaminants by producing reactive oxygen species and exhibit elevated chemical durability and resistance to photo-corrosive degradation, ensuring their stability11. A variety of synthesis techniques for BiFeO3 perovskite are available, providing different strategies for adjusting its characteristics. These synthesis methods include solution combustion12, co-precipitation13, sol-gel14, perching15, hydrothermal16, and solution evaporation17. Each method offers distinct benefits and obstacles, affecting parameters including particle dimensions, purity, degree of crystallinity, and shape18. Different synthesis methods generate products of diverse shapes and sizes, which influences the catalyst’s efficacy of efficacy of the catalyst is primarily determined by surface characteristics. Catalysts produced with exact regulation of the size and morphology of particles can effectively recognize specific catalytic sites19. The high temperature decomposition of bismuth salt compounds and the variations in the ionic chemical valence make it challenging to synthesize bismuth ferrite without impurities using typical solid phase reactions at elevated sintering temperatures20. Among these techniques, the hydrothermal method is a widely recognized and straightforward approach for synthesizing highly crystalline, phase-pure bismuth ferrite nanostructures. Also, this synthesis technique offers the advantage of producing nanostructures exhibiting adjusted morphology, narrow size distribution, and excellent dispersion, which can be achieved by modifying experimental parameters such as pH, duration, temperature, and the incorporation of surfactants, among others21. The hydrothermal technique enables improved management of the material’s purity, microstructure, and morphology by adjusting the reaction parameters22,23. Enhancing the morphology of the BiFeO3 bulk structure is a crucial approach to augment the specific surface area and the active site count24. The regulation of particle morphology and dimensions is crucial for optimizing photocatalytic efficiency, as it affects the reaction sites and the magnitude of energy of the band gap. In the last few years, various shapes and structures of bismuth ferrite have been synthesized, including wire25, tubes26, and Submicron spindle-shaped structures27. In this context, significant advancements have been achieved in the regulated synthesis and self-assembly of bismuth ferrite particles exhibiting controlled geometric configurations. Understanding how morphology and dimensions influence the band gap energy and photocatalytic effectiveness is essential for grasping catalytic mechanisms and improving semiconductor catalysts20. Previous research aimed at enhancing the multiferroic characteristics of BiFeO3 has noted an improvement in electrical and magnetic characteristics within single-phase BiFeO3nanostructures, which exhibit a pronounced dependence on size. Additionally, acid leaching is essential for achieving a pure phase, which results in the formation of coarser powders and reduced reproducibility28,29. The solubilization of bismuth (Bi) and ferric(Fe) precursors as their corresponding hydroxides through the reaction with a base, for example, potassium hydroxide (KOH), along with the formation of not soluble oxide powders through precipitation from highly concentrated hydrothermal solution is considered the process responsible for the phase formation of bismuth ferrite samples30. BiFeO3 with various morphologies has been successfully produced through a hydrothermal method30,31. Nevertheless, comprehensive studies examining the effect of nanostructure and morphology on the physicochemical characteristics of single-phase BiFeO3 have been minimally explored. The photocatalytic characteristics of nanostructures are significantly affected by the specific shape of the nanostructure being examined32,33. Size and shape are crucial for enhancing the photocatalytic characteristics of BiFeO3. Thus, in this research, the successful synthesis of BiFeO3 nanostructures was achieved. Moreover, their photocatalytic performance was examined for comparative purposes. Using analyses, the structural characteristics of the nanostructures were expounded, including DRS, XRD, TEM, PL, Mott-Schottky, and BET. The RSM-CCD was utilized to identify the optimal operating parameters, including pH, intensity time, LED power, catalyst dosage, and the MB concentration. This investigation also evaluated the influence of variables on the photodegradation effectiveness of MB dye using BiFeO3 nanorods as the catalyst. The current research involved the synthesis of photocatalysts, called BFO, with morphological structures of both particles andnanorods. To comprehend how BFO morphology influences optical properties, a detailed comparative study was carried out. The novelty of this study is the impact of the morphology of the bismuth ferrite nanorod on the MB dye degradation.
Experimental
Materials
Bismuth nitrate [Bi(NO3)3·5H2O, 98%] was procured from Alfa Aesar. Iron nitrate [Fe(NO3)3·9H2O, 99%], potassium hydroxide (KOH, 85%), cetyltrimethylammonium bromide (CTAB, 99%), sodium sulfate (Na2SO4, ≥ 99%), sodium carbonate (Ca (NO3)2 >99%), nitric acid (HNO3, 65%) and sodium chloride (NaCl, > 99%) were provided by Merck. All the chemical compounds were utilized as received without any additional purification. The solutions employed to identify species of active comprised 2 mM isopropyl alcohol (IPA, Scharlau), 1 mM p-benzoquinone (BQ, Merck), and 1 mM ethylenediaminetetraacetic acid (EDTA), which acted as scavengers for hydroxyl radicals (·OH), superoxide anions (·\(\:{\text{O}}_{2}^{-})\), and hole (\(\:{h}^{+}\)), respectively.
Synthesis of BiFeO3 (nanorods and nanoparticles)
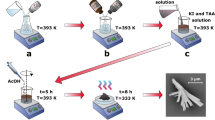
BiFeO3 nanorods were produced through a hydrothermal process as described by Remya et al.21, with certain modifications. In a usual method, the necessary molar concentrations of ferric nitrate nonahydrate [Fe(NO3)3·9H2O] and bismuth nitrate pentahydrate [Bi(NO3)3·5H2O] (in a 1.1 ratio) were dissolved individually. Bi (NO3)3·5H2O was dissolved in diluted HNO3, while Fe(NO3)3·9H2O was dissolved in double-distilled water. The Fe(NO3)3 solution was gradually combined with the Bi(NO3)3 solution while maintaining steady stirring. The prepared solution was subjected to ultrasonication for 30 min. Ultimately, KOH was combined gradually, drop by drop, in the aforementioned blended solution to facilitate the co-precipitation of Fe3+ and Bi3+ ions. Subsequently, the entire solution was placed in a 75 ml Teflon-lined autoclave, where it remained at 200 °C for 12 h and then allowed to cool to the temperature of the ambient naturally. The prepared precipitates were rinsed multiple times using distilled water until reaching a pH of 7. Thereafter, it was dried at 73 °C for 12 h. After drying, the brown powder was heated to calcination in a furnace at 400 °C for three hours to produce phase-pure BiFeO3 nanopowder. The conventional synthesis method implemented various modifications to generate nanostructures with distinct morphologies. CTAB and KOH were utilized to adjust the morphology of the synthesized catalyst further. Throughout this investigation, the BiFeO3 nano-catalyst was identified by the abbreviation BFO.
Characteristics
Using a variety of techniques, the prepared BFO powders were analyzed. The phase structure of the prepared photocatalytic samples was characterized througha powder X-ray diffractometer with high-intensity Cu Kα radiation (XRD, PW 1730, PHILIPS). Utilizing transmission electron microscopy (TEM, Zeiss EM900), the morphology and nanostructure of the synthesized photocatalytic samples were analyzed. Also, using the Brunauer-Emmett-Teller (BET) instrument (BET, BELSORP MINI II, BEL), the surface area specific to the photocatalytic powders was determined.PL (photo-luminescence) spectra were analyzed using a luminescence spectrophotometer (Varian Cary Eclipse, Varian) with an excitation wavelength of 483 nm. Using a UV–vis spectrophotometer (Avaspec-ULS3648-RS, Avantes) was measured Ultraviolet-visible diffuse reflectance spectra (DRS). Mott-Schottky (MS, ZIVE-SP1) curves were achieved using an electrochemical workstation. LED lamps with varying wattages were utilized as the light source for irradiation.
Photocatalytic study
The methylene blue (MB) decolorization from aqueous solution was conducted utilizing the photocatalyst samples under the influence of exposure to visible light from home LED (λ > 420 nm) and natural sunlight sources. 20 mg of BFO sample was introduced in a volume of 100 mL of methylene blue dye solution (5 mg/L). Before activating the LED lamp, to achieve the adsorption balance of the dye and the photocatalytic material, the obtained solution was stirred for 30 min under dark conditions. Subsequently, the photocatalytic activity was scrutinized in the presence of exposure to LED light (200 W) and sunlight. The lamp was located 12 cm distant from the surface of the solution, and it was constant for all tests. Throughout the dye degradation process, 5 mL of the suspended solution was extracted at predetermined periods and placed to centrifugation to isolate the photocatalysts. The concentration of methylene blue was determined utilizing the UV-vis spectrophotometer at a wavelength of 664 nm. The degradation rate was determined as follows:
In Eq. (1), \(C_{\circ}\) & \(\:C\) represent the initial concentrations and concentrations of the dye solution at time t.
Photoelectrochemical testing
Mott − Schottky (MS) tests were conducted on an electrochemical work station. Ag/AgCl and Pt were used as the reference electrode and counter electrodes, respectively, in a three-electrode system. Na2SO4 (0.5 mol/L) was used as electrolyte solution. The amount of 15 µl of nafion and 10 mg of BFO catalyst was dispersed in an isopropanol/water mixture (1 mL, 0.5:0.5 mL) for the preparation of the working electrode, and then 0.03 mL of suspension was transferred onto a 1 \(\:\times\:\) 2 cm2 ITO glass.
RSM-CCD design procedure
The response surface methodology (RSM) was employed using Design- Expert software (version 7, https://www.statease.com/software/design-expert/) for designing experiments and optimizing the influence of process variables. Notably, the optimization of the process was accomplished through the strategic implementation of Central Composite Design (CCD), which is acknowledged as a constituent of RSM. CCD was selected from various RSM approaches as the most efficacious and widely recognized technique for developing a response surface model with five levels for five independent variables: photocatalyst dosage, MB concentration, time, LED power, and pH34,35, as illustrated in Table 1. Furthermore, as outlined in Eq. (2), a second-order polynomial relationship was utilized to predict the correlation between the key factors and the efficiency of MB dye degradation.
Here, ε and \(\:{{\upbeta\:}}_{0}\) are the unanticipated errors and a constant amount, \(\:{X}_{i}\) and \(\:{X}_{j}\) are reaction variables. Furthermore, \(\:{{\upbeta\:}}_{\text{i}}\), \(\:{{\upbeta\:}}_{\text{i}\text{i}}\), and \(\:{{\upbeta\:}}_{\text{i}\text{j}}\) illustrate the constants of reaction36.
Results and discussions
The scrutiny of morphology formation
Singular phase bismuth ferrite samples exhibiting two distinct morphologies were produced through the hydrothermal method. Figure 1 presents a schematic representation of the empirical situation utilized to adjust the morphology of bismuth ferrite through the hydrothermal method. A spherical particle shape was achieved when potassium hydroxide (KOH) was employed as the structure-directing factor. The production of impurity-free perovskite oxides necessitates a crucial concentration of the alkaline cultivation environment. Throughout the synthesis, under elevated temperatures and pressures, the metal hydroxides were exposed to reactions with potassium hydroxide (KOH). The insoluble depositions composed of oxides of ceramic samples were obtained throughout this procedure. Effectuating the processes of dissolution and recrystallization that lead to the self-stabilization of the system and the production of impurity-free BFO nanoparticles, under regulated situations of temperature, pressure, and appropriate time. An increased molarity of potassium hydroxide (KOH) in the reaction ambient promotes the production of Bi25FeO4021.
The Reactions related to BFO nanostructure can be expressed as follows: Eqs. (3–7):
Based on the equations, \(\:{\text{F}\text{e}\left(\text{N}{\text{O}}_{3}\right)}_{3}\) and \(\:\text{B}\text{i}{\left(\text{N}{\text{O}}_{3}\right)}_{3}\) were converted into amorphous precipitate of \(\:\text{F}\text{e}{\left(\text{O}\text{H}\right)}_{3}\) and \(\:\text{B}\text{i}{\left(\text{O}\text{H}\right)}_{3}\). Then, KOH as a mineralizer interacts with the hydroxides to dissolve them and generate groups of ions. After achieving super saturation, groups of ions through nucleation and growth, resulting in the formation of BFO crystals37. During the nucleation trend, hydroxyl ions (OH) derived from potassium hydroxide (KOH) are adsorbed onto the surfaces of the nanoparticles, reducing their surface energy and resulting in the formation of spherical nanoparticles with minimal surface energy31,38,39. During the crystal growth procedure, the use of 3 molar potassium hydroxide (KOH) results in the formation of particles of tiny size, typically measuring nanometers in size, which are generally spheroid-shaped in appearance27. Achieving the phase of impurity-free bismuth ferrite (BFO) is accomplished by utilizing the precise concentration of potassium hydroxide (KOH), as it affects the solubility of the mediocre Bi6O66+ and Fe(OH)4, as well as the ratio of concentrations of bismuth (Bi) to iron (Fe). Until cetyltrimethylammonium bromide (CTAB) is employed as a cationic surfactant under identical conditions, BFO nanorods are produced. Surfactants create advantageous sites and also affect the processes of nucleation, formation, and agglomeration. It is well recognized that surfactant ions significantly impact the formation of various morphologies of nanostructures of oxide, which are compounds of polar direct interactions with the same substance particles. CTAB is a type of cationic surfactant characterized by a hydrophilic head and a hydrophobic tail. Cetyltrimethylammonium bromide operates through the action and reaction groups of the head CTA+, its positive charge with negatively charged surfaces of the BFO crystals, promoting a self-organization procedure of particles via molecular interactions. This interaction can facilitate the development of a template layer on specific crystal surfaces of BiFeO3, steering the growth of nanorods in particular crystallographic directions. Also, a decrease in the attraction of the surface of the solution leads to diminished energy needed for the growth of a novel phase. The adsorption of CTAB on the surfaces of nanoscale crystals aligned with the c-axis constrains growth kinetics along the lateral facets, which may prevent solute particle diffusion and lead to one-dimensional nucleus growth, resulting in rod-like structures21.
Surface structural studies
X-ray diffraction (XRD) is a laboratory method that gathers data non-destructively and precisely with various aspects, including crystal structure, chemical composition, crystal orientation, lattice strain, crystallite size, layer thickness, and preferred orientation. Investigators are utilizing this analysis to identify the impurities within the samples40,41. The phase of BFO nanostructures (JCPDS Card No. 86-1518) was exhibited by XRD analysis in Fig. 2. The major peaks at 2θ = 22.4, 31.7, 31.9, 39.6, 46.2, 51.4, 57.5, 57.6, and 67.4° corresponds to the reflections from (012), (104), (110), (202), (024), (116), (018), (214), and (220) planes respectively21,42. Distinctly, taper diffraction peaks demonstrate the growth of crystalline structures. In contrast, the broadening and fluctuations in the intensity of the diffraction patterns imply variations in the size of the synthesized nanostructures, which can be attributed to the specific experimental conditions during the synthesis process. The small peak at 28° in Fig. 2(b), marked with (*), indicates a small amount of the secondary phase Bi2Fe4O9, which may result from the formation kinetics and the metastable nature of BFO21.
To achieve morphological information, form, size, and structural specification, transmission electron microscopy (TEM) is employed. The particle and rod morphologies by transmission electron microscopy (TEM) in bright field mode are illustrated in Fig. 3. Can be observed in Fig. 3(a), BFO included zero-dimensional nanoparticles with an average size of ~ 48 nm, which exhibit a distribution with noticeable agglomeration, likely due to a shortage of surfactants. Also, adjacent spherical particles are joined together in a layered arrangement during the hydrothermal synthesis by KOH (Fig. 3a\(\:\left(iii\right)\)). The reduction of surface energy of nanoparticles through the adsorption of hydroxyl ions on the particle surfaces leads to spherical nanoparticles with the least surface energy37.
Figure 3(b) illustrates the 1D-nanorod structure. It seems that the nanorods were generated through the assembly of various irregularly shaped nanoparticles, as shown in Fig. 3b(ii). The growth of nucleation in a one-dimensional state and anisotropic in the presence of surfactants contributes to the formation of rod-shaped structures21. Furthermore, based on the Gibbs-Thomson law, the growth environment has a major impact on determining the shape of bismuth ferrite particles by dissolution and crystallization. Firstly, Crystals grow into bigger particles in an Alkaline environment. Subsequently, based on the concentration of alkaline, other nanocrystals are adsorbed onto the surface of the particles, which leads to anisotropic growth20.
Figure 4 illustrates that the assessment of the physical characteristics of the synthesized BFO nano-catalysts can be conducted through both BET and BJH techniques. The isotherms of the nitrogen (N2) adsorption-desorption for BFO nanoparticle and nanorod in diagrams correspond to type IV, featuring an H-type loop (H3-type hysteresis loop). In addition, the pore size distribution for the synthesized samples concentrated around 4–12 nm, which classifies them as mesoporous material25. As presented in Table 2, the area of the surface for the BFO nanoparticle was 5.2 m2/g, where as it increased to 12.8 m2/g for the BFO nanorod after morphological changes, and the total pore volume for the nanoparticle was 0.0213 nm, which was increased to 0.0411 nm for the nanorod. These outcomes imply that nanorods could have greater photocatalytic effectiveness than nanoparticles. Similarly, other photocatalytic studies have shown high degradation rates (100%), despite a low value of BET (13m2/g)43.
Optical properties
An investigation of the optical properties was carried out using DRS and PL analyses for BFO with various morphologies. The prepared photocatalysts’ UV-Vis DRS spectra (Reflectance), as illustrated in Fig. 5(a), both BFO nanorod and nanoparticle, illustrate the light reflectance within the visible light spectrum. However, the reflectance intensity for the nanorod structure was decreased compared to nanoparticles, demonstrating the enhancement in light absorption1. Compared to the BFO nanoparticle (absorption edge around 415 nm), the BFO nanorod shows a red shift (absorption edge around 520 nm), indicating higher photocatalytic performance in the region of visible light8. Figure 5(b) demonstrates that, from the Tauc plots, the band gap values for the nanorod and nanoparticle samples are determined at 2.10 eV and 2.12 eV, respectively. Although there was no significant change in the band gap, the BET analysis confirmed that the area of the surface of the nanorods has increased by more than twofold concerning the nanoparticles. Numerous studies indicate that the form of semiconductors has an impact on both the absorption of light and the energy of the band gap23,44. The slight variation in the band gap is likely attributed to the minor differences in the nanostructure of the produced BFO particles. Consequently, particles of the BFO may be a beneficial choice as a photocatalytic material for removal tasks that require the utilization of a wider UV–visible light range20.
Also, the energy corresponding to the band gaps of the BFO nanostructures was determined by applying the equation (Eq. (8):
Here, α, hυ, \(\:{E}_{g}\), A, and represent the absorption coefficient, the frequency of the incident light, the band gap energy, and a constant parameter1. Additionally, the quantity of n is contingent upon the type of optical transition occurring in a semiconductor, and for BFO, it is identified as 2. The quantity of the band gap for the produced particles is calculated by graphing (αhυ)n against hυ24. Furthermore, the energy of the band gap (\(\:{E}_{g}\)) is 2.10 eV for the BFO nanorod and 2.12 eV for the BFO nanoparticle, as determined by the DRS analysis results, which is less than the formerly recorded quantities of 2.27 eV and 2.25 eV45. The positive slopes of the Mott-Schottky curves for BFO in Fig. 5(c, d) confirm that it is an n-type semiconductor46. The conduction band potential (CB) in n-type semiconductors is 0.1–0.3 eV less than the potential of the flat band (Ef). Following the information from Mott-Schottky diagrams, the flat-band potentials (Ef) of BFO nanorod and nanoparticle are − 0.74 and − 0.59 V (vs. Ag/AgCl, pH = 7). The flat-band potential (Ef) is convertible to the NHE, which was calculated as follows46:
Hence, for BFO nanorod and nanoparticle, the conduction band energy (ECB) was determined approximately − 0.60 and − 0.40 eV (vs. NHE). Subsequently, using Eq. (9), the VB potential of BFO nanorod and nanoparticle can be determined, resulting in values of 1.5 and 1.72 eV4.
To analyze the efficacy of charge carrier capture, transport, migration, and separation in semiconductor materials, photoluminescence (PL) emission spectra are employed. PL occurs due to electron-hole pairs (free charge carriers) recombination in the valence band (VB). Consequently, a great photoluminescence (PL) intensity in a semiconductor indicates increased recombination of excited charge carriers47,48,49. Figure 6 displays a strong emission peak at 605 nm is observed for BFO nanoparticles, while the intensity of this peak decreases for the nanorods. Compared to the nanoparticles, a notable reduction in charge carrier recombination occurs for BFO nanorods. This implies that the BFO nano-catalyst morphology can influence the process of charge-carrier recombination.
Statistical assessment of the RSM procedure
The RSM was utilized to assess the effect of response factors on the MB degradation rate. An experimental design was developed using CCD to achieve optimal efficiency for MB degradation in aqueous solutions through the adjustment of reaction parameters. As presented in Table 3, an experimental design consisting of 50 runs was created through the application of CCD50,51,52. The expected yield of MB photodegradation can be determined by the following Eq. (11) via the RSM approach, based on the key actual factors.
MB degradation (%) = \(\:-\) 188.54 \(\:\:+\) 0.86 A \(\:+\) 1.64 \(\:B\:+\:\) 1.8\(\:\:C\:+\:\:\)31.9\(\:\:D\:-\:\)3.34\(\:\:E-\)0.002\(\:\:AB+\) 0.006\(\:\:AC\:-\) 0.02\(\:\:AD\:+\:\)0.008\(\:\:AE-\) 0.01\(\:\:BC\:-\) 0.05\(\:\:BD\:+\) 0.004\(\:\:\:BE\:-\:\:\)0.28\(\:\:\:CD-\:\)0.1\(\:\:CE-\)0.41\(\:\:DE-\:\)0.002\(\:\:\:A\)2\(\:\:-\) 0.0036\(\:\:B\)2\(\:+\) 0.0058
The parameters are represented as: (\(\:A\)) LED lamp, (\(\:B)\:\)Irradiation time, (\(\:C\)) Dose catalyst, (\(\:D\)) pH, (\(\:E\)) Initial MB concentration.
As illustrated in Table 4, an Analysis of Variance (ANOVA) was conducted to validate the influence of significant parameters within the model. F-value of 20.08, low P-value (< 0.0001), and a high sum of square values of 28,454 demonstrated that the quadratic model is the most appropriate choice. The software indicated that the high regression coefficients (R2 = 0.93, R2adj = 0.87) between the variables and responses reflect a high level of accuracy in estimating the experimental data using the quadratic model. Additionally, a coefficient of variation (CV) of 14.12% suggests that the model is statistically significant. The value of the R-squared, anticipated to be 0.75, aligns well with the Adjusted R-squared of 0.87. Adequate Precision, which assesses the signal-to-noise ratio, ideally should exceed 4. In this case, an Adequate precision of 17.50 for the degradation of MB indicates a strong response51.
The influence of photocatalytic variables on the MB degradation
To demonstrate the interplay among the five parameters, the RSM was employed using Design- Expert software. In Fig. 7(a), the impact of MB concentration (5–25 mg/L) and catalyst amount (5–25 mg) on the degradation effectiveness during the photocatalytic activity is presented. The factor of catalyst dose has a significant impact on the economic efficiency, so a lower dose could be more appropriate. As the photocatalyst dosage is increased from 5 to 25 mg, the effectiveness of degradation exhibits trend of decline, from 87.86 to 58.71%. A decline in the effectiveness of degradation might result in the agglomeration of catalyst particles, which reduces the active site count on the surface available for the absorption of photons. Further, this phenomenon diminishes the intensity of light that reaches the photocatalyst53. An increase in the MB concentration molecules prevents the adsorption of irradiated photons on the catalyst particles, which leads to a decrease in.
photocatalytic yield. Figure 7(b) indicates that the MB degradation improves when the time of exposure to irradiation is raised from 20 to 100 min. When the time of illumination is short, the light may not produce species of reactivity on the surface of the photocatalyst, leading to a low photodegradation efficiency of MB dye. Prolonged light exposure enhances the absorption of photons, resulting in the production of species of reactive species. The degradation process and overall efficiency are enhanced by the effective interaction between MB molecules and reactive species54. Additionally, Fig. 7(b) illustrates the impact of light intensity by LED lamps on the MB degradation. An increase in the power of the LED lamp (50 to 250 W) improved the percentage of MB degradation. The highest efficiency of MB degradation, recorded at 64.26%, was observed at a light power of 250 W.The rise in intensity of irradiation improves electron excitation, resulting in the production of a greater number of active radicals36. Moreover, pH is a pivotal factor in the process of photodegradation. According to Fig. 7(c), a rise in pH (from 2 to 10) leads to an improvement in the effectiveness of MB degradation. The highest efficiency of MB degradation was recorded, 0.64%, 67.5%, and 96.6%, at pH = 2, 6, and 10. According to the relevant figure, under alkaline pH, at the presence of hydroxide ions (\(\:{OH}^{-}\)) photocatalyst surface attracted cationic MB dye. In contrast, under acidic conditions, the positive charge (\(\:{H}^{+}\)) on the surface causes repulsion of the MB. As a result, it is expected that under alkaline environments, the adsorption of positive charge pigment molecules on the catalyst increases, and the degradation effectiveness will be enhanced55.
In Fig. 7(d), the normal probability plotsof the residuals are displayed.The graph illustrates a compact scatter of points. This correlation illustrates the model’s usefulness and a High level of precision. Based on the statistical analysis, the experimental data did not exhibit notable deviations from the predicted values56.
Optimization of the photodegradation process
The optimal conditions for each operational parameter are demonstrated in Fig. 8. The optimal degradation of MB was attained by following the reaction conditions. Under optimum conditions, pH: 8.97, MB concentration: 5.02 mg/L, dosage of catalyst: 19.52 mg/L, time of irradiation: 20 min, and lamp power: 195.18 W, the expected optimal removal rate is 100%. Additionally, the effectiveness of 98.2% was recorded under experimental conditions. This highest efficiency was achieved in the shortest optimal time.
(a) MB absorption spectra by BFO nanorod visible light (LED lamp), (b) sunlight exposure, (c) MB degradation via photocatalytic processes under visible light and (d) sunlight irradiation, (e) degradation kinetics under visible light (f) sunlight, (19.52 mg BFO, 100 ml MB, pH = 8.97, 5.02 mg/L, 195.18 W).
Photocatalytic performance
The effectiveness of BFO as a photocatalyst for MB degradation was evaluated under visible light (home LED lamp) and sunlight over time. In Fig. 9(a, b), the UV–vis absorption spectrum of MB dye demonstrates a gradual reduction in the peak intensity at approximately 664 nm for 60 min, which implies that the photocatalyst is causing the breakdown of MB dye. The control experiment (conducted without a photocatalyst) revealed that the concentration of MB dye remained virtually steady over time, implying the stability of the MB dye and the absence of a self-photolysis process43. Moreover, the required time to reach equilibrium of adsorption-desorption between the photocatalyst and the MB solution is 30 min in a dark environment. Also, during the dark time, very nominal decolorization was detected. It is clear that in Fig. 9(c), only 76% of MB dye was removed in 20 min when BFO nanoparticles were present, whereas the percentage of degraded MB dye rose to 98.2% in the presence of BFO nanorods. Moreover, as depicted in Fig. 9(d), the degradation of MB dye under natural sunlight with BFO nanorods achieved a rate of 91.3% (0.0277 min−1), following 90 min. Furthermore, in comparison to BFO nanoparticles (0.0715 min−1), BFO nanorods (0.2021 min−1) exhibited a greater rate of MB dye degradation when exposed to visible light (LED lamp), as depicted in Fig. 9(e, f) and Table 5. These conclusions imply that the high removal efficiency is influenced by the morphology of the nano-catalysts, as the BFO nanorods exhibited greater dye degradation under visible light and sunlight in comparison to the BFO nanoparticles.
Photodegradation mechanism
To determine the mechanism of MB dye photodegradation by BFO nanorods, active species trapping tests of photocatalytic, along with calculations of conduction band and valence band potentials (based on DRS analysis results), were conducted. Scavengers, including BQ, IPA, and EDTA, were utilized to capture ·\(\:{O}_{2}^{-}\), ·\(\:OH\), and \(\:{h}^{+}\) correspondingly. As presented in Fig. 10(a), the effectiveness of dye degradation decreased dramatically from 98.2% under no scavenger situation to 20.1% when BQ was used as ·\(\:{O}_{2}^{-}\) scavenger. Adding the BQ (a scavenger for ·\(\:{O}_{2}^{-}\)) decreased the dye degradation greatly, indicating that the charge carriers generated by light contribute significantly to this photocatalytic activity. Conversely, the incorporation of the IPA scavenger for ·\(\:OH\) had no significant impact on the photocatalytic dye degradation activity. However, adding EDTA (a scavenger for \(\:{h}^{+}\)) slightly impedes the degradation of dye, suggesting that \(\:{h}^{+}\) has a minimal impact on the destruction rate. Consequently, under visible light irradiation ·\(\:{O}_{2}^{-}\) had a most significant impact on the degradation of dye by BFO nanorods, afterwards \(\:{h}^{+}\) and ·\(\:OH\) locations in the second and third, respectively ·\(\:{O}_{2}^{-}\)>\(\:h\)+> ·\(\:OH\).
According to the conclusions obtained from scavenger experiments (active species), DRS, and Mott-Schottky analyses (the potentials of VB and CB), the proposed photocatalytic mechanism in the visible/BFO system for the MB photodegradation through the use of the BFO has been presented in Fig. 11. According to Eqs. (12–16), when the BFO photocatalyst is subjected to visible light, it is capable of absorbing photons that have energy identical to or greater than its energy of band gap (\(\:E\)≥2.1). This process leads to the excitation of a significant number of electron-hole pairs, and the generated charges (\(\:{e}_{CB}^{-}\)) move from the valence band (VB) to the conduction band (CB). Consequently, the generated electrons are capable of reacting with the O2 present on the surface to produce superoxide reactive radicals (·\(O_2\)⁻), subsequently facilitating the photodegradation of pollutants. Moreover, because the values of potential ·\(\:OH\)/\(\:OH\) and \(\:OH\)/H2O (+ 1.99 eV and + 2.27 eV, respectively) are greater than the BFO nanorod valence band energy in the Mott-Schottky diagram, the holes in the valence band \(\:{(h}_{VB}^{+}\)) of the photocatalyst cannot interact with water (H2O) and hydroxyl ions (\(\:O{H}^{-}\)) to generate hydroxyl radicals (·\(\:OH\)). Consequently, methylene blue dye molecules are oxidized by the holes present in the valence band of the BFO nanorod. Ultimately, the reactive species that significantly contribute to the decolorization of methylene blue are superoxide radicals (·\(O_2\)⁻) and holes (\(\:h\)⁺)24.
The impact of anions
The effect of three common anions (\(\:{SO}_{4}^{2-},{Cl}^{-},\:{NO}_{3}^{-}\)) on the MB photodegradation by BFO was investigated57,58. Anions were added to the MB solution at concentrations of 0.1 mM, and the relevant findings are illustrated in Fig. 10(b). After 20 min under visible light, the removal of the BFO catalyst decreased from 98.2 to 97%, 93%, and 90% in the presence of \(\:{NO}_{3}^{-}\), SO₄²⁻, and Cl⁻, respectively. Adding \(\:{NO}_{3}^{-}\) ions to the solution had no notable influence on MB degradation. In addition, the catalyst’s activity was minimally affected by sulfate ions \(\:{SO}_{4}^{2-}\)[58]. It is possible that \(\:{SO}_{4}^{2-}\) ions functioned as scavengers for ·\(\:OH\) radicals and holes via the reaction [Eqs. (17,18)]:
The reaction of \(\:{SO}_{4}^{2-}\) with ·\(\:OH\) and \(\:{h}^{+}\) can produce the formation of radicals (\(\:{\cdot\:SO}_{4}^{-}\)) that are less reactive. It is clear that chloride ions have a more significant effect on the removal than other ions. This can be explained through the trapping of hydroxyl radicals by the Eq. (19):
The lower reactivity of hydroxyl radicals compared to ions of chloride, adding \(\:{Cl}^{-}\) had a negative effect on the degradation. For the BFO catalyst, as discussed in the scavenger section, \(\:{h}^{+}\)and ·\(\:OH\) were not the dominant active species in the MB degradation. Generally, organic material degradation is not notably influenced by the mentioned ions59.
Reusability of BFO photocatalytic
Reusability tests were performed to evaluate the stability of BFO nanorods. A stability assessment of the photocatalytic sample was conducted over five consecutive cycles for the photodegradation of MB. Following each usage period, the BFO nanorod catalyst was reactivated, and its performance was assessed. Figure 12 depicts the BFO photocatalyst stable experiment in MB dye photodegradation. The figures imply that the BFO nanorod sample maintained significant photocatalytic activity after undergoing five consecutive cycles of MB dye photodegradation. The analysis of the first and last cycles of the stability test shows that the photocatalytic performance of the sample diminished by less than 20%, suggesting that the photocatalyst demonstrated commendable stability throughout the test. The decrease in process effectiveness is caused by the diminished amount of the catalyst during both its recovery and photodegradation. Furthermore, the agglomeration of particles after centrifugation, which causes a reduction in specific surface, can hinder photocatalysis53,60.
Table 6 displays the outcomes of pollutant photodegradation for different BFO composite photocatalysts, highlighting that nanorod (1D)-shaped BFO exhibits enhanced photocatalytic effectiveness at a lower time relative to earlier research findings.
Conclusions
Two different morphologies of bismuth ferrite perovskite, including nanorod and nanoparticle, were prepared using a hydrothermal technique. The as-synthesized photocatalysts were characterized using various analyses such as TEM, XRD, BET, DRS, Mott-Schottky, and PL. The TEM analysis demonstrated the successful synthesis of nanorod and nanoparticle morphologies. Following the BET analysis, the ratio of the surface area of nanorods (12.83 m²/g) to nanoparticles (5.23 m²/g) was more than doubled. Additionally, the total pore volume of the samples was 0.04 cm3/g for the nanorod and 0.02 cm3/g for the nanoparticle. The DRS analysis revealed that both morphologies of BFO are capable of responding to visible light for photocatalytic reactions. However, nanorod morphology displayed red shift (within the visible light range) and decreased reflectance intensity compared to nanoparticles, indicating higher photocatalytic activity. The band-gap values of the nanorods and nanoparticles were determined at 2.10 eV and 2.12 eV. The synthesized nanorods displayed reduced PL in comparison with the nanoparticles, suggesting efficient charge transfer within the bands and a delayed recombination electron-hole process. Performance of the photocatalytic samples was compared via the MB decolorization process. The optimization was applied through the RSM-CCD technique for variables: (pH: 8.97, MB concentration: 5.02 mg/L, catalyst dosage: 19.52 mg/L, time: 20 min, Lamp power: 195.18 W) under the noted optimal conditions. The optimal photodegradation yield for BFO nanorods and nanoparticles was obtained at 98.2% and 76% in 20 min, respectively. Also, the BFO nanorod removed 91.3% of the MB dye in 90 min under natural sunlight. The superoxide radicals and positive holes were vital for the photodegradation of MB by BFO nanorods under visible light. Further, the BFO nanorods illustrated excellent photo-stability after 5 cycles of the MB degradation process.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Tanzifi, M., Peyravi, M. & Jahanshahi, M. Investigation of photocatalytic and visible light self-cleaning performance of 2D/2D g-C3N4-ZrO2 based PES/PDA membranes for organiccontaminants degradation. J. Mol. Struct. 1315, 138877. https://doi.org/10.1016/j.molstruc.2024.138877 (2024).
Zhou, R., Tu, X., Zheng, P., Zhang, L. & Zeng Zh. In situ synthesis of Bi2S3/BiFeO3nanoflower hybrid photocatalyst for enhanced photocatalytic degradation of organic pollutants. Molecules 28, 8007. https://doi.org/10.3390/molecules28248007 (2023).
Abbasi, F., Mansouri, M., Tanzifi, M., Ebrahimi, F. & Sadeghizadeh, A. The modified pomegranate Peel is an economical and highly effective adsorbent for malachite green dye removal from wastewater. Colloids Surf. C: Environ. Aspects2, 100040. https://doi.org/10.1016/j.colsuc.2024.100040 (2024).
Pattnaik, A., Poonia, A. K. & Ghosh, P. Preparation of bismuth ferric oxide nanoparticles for the microwave-induced photodegradation of organic pollutants in Greywater. Environ. Res. 262, 119814. https://doi.org/10.1016/j.envres.2024.119814 (2024).
Alem, F. et al. Photocatalytic degradation of food colorant azo dyes using iron and fluorine co-doped TiO2/SiO2 nanocomposites: semi-pilot study cum density functional theory calculations. Nano-Struct. Nano-Object. 35, 100992–1010. https://doi.org/10.1016/j.nanoso.2023.100992 (2023).
Liu, T. et al. Dynamic photocatalytic membrane coated with ZnIn2S4 for enhanced photocatalytic performance and antifouling property. Chem. Eng. J. 379, 122379. https://doi.org/10.1016/j.cej.2019.122379 (2020).
Li, S., Lin, Y. H., Zhang, B. P., Wang, Y. & Nan, C. Controlled fabrication of BiFeO3 uniform microcrystals and their magnetic and photocatalytic behaviors. J. Phys. Chem. C. 114, 2903–2908. https://doi.org/10.1021/jp910401u (2010).
Gao, F. et al. Visible-light photocatalytic properties of weak magnetic BiFeO3 nanoparticles. Adv. Mater. 19, 2889–2892. https://doi.org/10.1002/adma.200602377 (2007).
Yang, X. et al. Phase and morphology evolution of bismuth ferrites via hydrothermal reaction route. Mater. Res. Bull. 48, 1694–1699. https://doi.org/10.1016/j.materresbull.2013.01.032 (2013).
Zhang, X. et al. Photocatalytic and magnetic behaviors observed in BiFeO3 nanofibers by electrospinning. J. Nanomater. 2013, 917948. https://doi.org/10.1155/2013/917948 (2013).
Pattnaik, A., Poonia, A. K. & Ghosh, P. Bi-tailored compounds for photocatalytic environmental applications: current trends, advancements, challenges, and future perspectives. Sustainable Mater. Technol. 38, e00769. https://doi.org/10.1016/j.susmat.2023.e00769 (2023).
Penalva, J. & Lazo, A. Synthesis of bismuth ferrite BiFeO3 by solution combustion method. J. Phy. 1143, 012025. https://doi.org/10.1088/1742-6596/1143/1/ (2018).
Liu, Z., Qi, Y. & Lu C.Highly efficient ultraviolet photocatalytic activity of BiFeO3 nanoparticles synthesized by a chemical coprecipitation process. J. Mater. Sci. Mater. Electron. 21, 380–384. https://doi.org/10.1007/s10854-009-9928-x. (2010).
Sinha, R. et al. Synthesis of bismuth ferrite nanoparticles by sol-gel method and their characterization. IOSR J. Appl. Phys. 7, 44–47. https://doi.org/10.9790/4861-07134447(2015).
García-Zaleta, D. S. et al. Solid solutions of La-doped BiFeO3 obtained by the pechini method with improvement in their properties. Ceramics International 40, 9225–9233. https://doi.org/10.1016/j.ceramint.2014.01.143(2014).
Xue, Z. et al. Degradation of tetracycline with BiFeO3 prepared by a simple hydrothermal method. Materials 8, 6360–6378. https://doi.org/10.3390/ma8095310 (2015).
Sharmila, M. et al. Visible light photocatalyst and antibacterial activity of BFO (Bismuth Ferrite) nanoparticles from honey. Water 14 (1545). https://doi.org/10.3390/w14101545 (2022).
Nkwachukwu, O. V., Muzenda, C. H., Koiki, B. & Arotiba, O. Perovskites in photoelectrocatalytic water treatment: bismuth ferrite-graphite nanoparticles composite photoanode for the removal of Ciprofloxacin in water. J. Photochem. Photobiol., A. 434, 114275. https://doi.org/10.1016/j. jphotochem.2022.114275 (2023).
Riente, P. & Noel, T. Application of metal oxide semiconductors in light-driven organic transformations. Catal. Sci. Technol. 9, 5186–5232. https://doi.org/10.1039/c9cy01170f (2019).
Duan, Q. et al. Synthesis and characterization of morphology controllable BiFeO. Mater. Res. Bull. 112, 104–108. https://doi.org/10.1016/j.materresbull.2018.12.012 (2019).
Remya, K. P. et al. Tailoring the morphology and size of perovskite BiFeO3 nanostructures for enhanced magnetic and electrical properties. Mater. Design. 192, 108694. https://doi.org/10.1016/j.matdes.2020.108694 (2020).
Dai, X. J., Luo, Y. S., Zhang, W. D. & Fu, S. Y. Facile hydrothermal synthesis and photocatalytic activity of bismuth tungstate hierarchical Hollow spheres with an ultrahigh surface area. Dalton Trans. 39, 3426–3432. https://doi.org/10.1039/b923443h (2010).
Zhang, J., Liu, Z. & Liu, Z. Novel WO3/Sb2S3 heterojunction photocatalyst based on WO3 of different morphologies for enhanced efficiency in photoelectrochemical water splitting. ACS Appl. Mater. Interfaces. 8, 9684–9691. https://doi.org/10.1021/acsami.6b00429 (2016).
Tanzifi, M., Jahanshahi, M., Peyravi, M. & Khalili, S. A. Morphological decoration of G-C3N4/ZrO2 heterojunctions as a visible light activated photocatalyst for degradation of various organic pollutants. J. Environ. Chem. Eng. 10, 108600. https://doi.org/10.1016/j.jece.2022.108600 (2022).
Gao, F. et al. Preparation and photoabsorption characterization of BiFeO3nanowires. Appl. Phys. Lett. 89, 102506. https://doi.org/10.1063/1.2345825 (2006).
Park, T. J., Mao, Y. & Wong, S. Synthesis and characterization of multiferroic BiFeO3nanotubes. J. Chem. Commun. 23, 2708–2709. https://doi.org/10.1039/B409988E (2004).
Han, J. T. et al. Tunable synthesis of bismuth ferrites with various morphologies. Adv. Mater. 18, 2145–2148. https://doi.org/10.1002/adma.200600072 (2006).
Valant, M., Axelsson, A. K. & Alford, N. Peculiarities of a Solid-State synthesis of multiferroic polycrystalline BiFeO3. Chem. Mater. 19, 5431–5436. https://doi.org/10.1021/cm071730+ (2007).
Bernardo, M. S., Jardiel, T., Peiteado, M., Caballero, A. C. & Villegas, M. Reaction pathways in the solid state synthesis of multiferroic BiFeO3. J. Eur. Ceram. Soc. 31, 3047–3053. https://doi.org/10.1016/j.jeurceramsoc.2011.03.018 (2011).
Chen, C., Cheng, J., Yu, S., Che, L. & Meng, Z. Hydrothermal synthesis of perovskite bismuth ferrite crystallites. J. Cryst. Growth. 291, 135–139. https://doi.org/10.1016/j.jcrysgro.2006.02.048 (2006).
Yang, Q., Li, Y., Yin, Q., Wang, P. & Cheng, Y. B. Hydrothermal synthesis of bismuth oxide needles. Mater. Lett. 55, 46–49. https://doi.org/10.1016/S0167-577X(01)00617-6 (2002).
Radovsky, G., Shalev, T. & Ismach, A. Tuning the morphology and chemical composition of MOS2nanostructures. J. Mater. Sci. 54, 7768–7779. https://doi.org/10.1007/s10853-019-03437-4 (2019).
Kumar, Y., Sharma, A. & Shirage, P. Impact of different morphologies of CoFe2O4nanoparticles for tuning of structural, optical and magnetic properties. J. Alloys Compd. 778, 398–409. https://doi.org/10.1016/j.jallcom.2018.11.128 (2019).
Hirad, A. H. Photocatalyst based on a composite of trimetallic metal-organic frameworks for efficient visible-light degradation in pharmaceutical pollutants abatement and process modeling using RSM. Surf. Interfaces. 52 https://doi.org/10.1016/j.surfin.2024.104909 (2024).
Nademi, A. H., Moradi, G. & Mansouri, M. A comprehensive study on the photocatalytic activity of CuO-doped ZrO2–ZnO nanocomposites under visible light. Inorg. Nano-Met. Chem. 52, 1383–1395. https://doi.org/10.1080/24701556.2022.2066127 (2022).
Maleki, B., Khalaif, T. H., Jasim, M. K. & Mansouri, M. Preparation and utilization of Zn-La oxide nanocatalyst as a binary composite for photocatalytic degradation of methylene blue dye: optimization through RSM-BBD. Arab. J. Chem. 17, 105667. https://doi.org/10.1016/j.arabjc.2024.105667 (2024).
Hojamberdiev, M. et al. Morphology-Controlled hydrothermal synthesis of bismuthferrite using various alkaline mineralizers. Ceram. Silik. 53, 113–117. https://www.researchgate.net/publication/229048364 (2009).
Cao, M. et al. Single-crystal dendritic micro-pines of magnetic α-Fe2O3: large-scale synthesis, formation mechanism, and properties. Angewandte Chemie Inter. Edition. 44, 4197–4201. https://doi.org/10.1002/anie.200500448 (2005).
Peng, X. et al. Shape control of cdsenanocrystals. Nature 404, 59–61. https://doi.org/10.1038/35003535 (2000).
Yadav, H. M. et al. Metal oxide-based composites: synthesis and characterization. In Advances in Metal Oxides and Their Composites for Emerging Applications, 57–96. https://doi.org/10.1016/B978-0-323-85705-5.00010-5 (2022).
Li, Y. et al. In situ synthesis of Au-induced hierarchical nanofibers/nanoflakes structured BiFeO3homojunction photocatalyst with enhanced photocatalytic activity. Front. Chem. 6, 649. https://doi.org/10.3389/fchem.2018.00649 (2018).
Katsina, A. U., Cursaru, D. L., Matei, D. & Mihai, S. Effect of morphology modification of BiFeO3 on photocatalytic efficacy of P-G-C(3)N(4)/BiFeO3composites. Int. J. Mol. Sci. 25, 4948. https://doi.org/10.3390/ijms25094948 (2024).
Verma, M. et al. Efficient and rapid sunlight-driven photocatalytic degradation of methylene blue dye using multiferroic BiFeO3 nanoparticles. J. Solgel Sci. Technol. 113, 356–373. https://doi.org/10.1007/s10971-024-06607-2 (2025).
Wang, X. et al. Pvp Assisted hydrothermal fabrication and morphology-controllable fabrication of BiFeO3 uniform nanostructures with enhanced photocatalytic activities. J. Alloys Comp. 677, 288–293. https://doi.org/10.1016/j.jallcom.2016.02.246 (2016).
Sukumar, M. et al. Structural, optical, and magnetic properties of Ca2+doped La2CuO4 perovskite nanoparticles. Vacuum 167, 407–415. https://doi.org/10.1016/j.vacuum.2019.06.036 (2019).
Yu, C. et al. Enhanced aquatic antibiotic removal via dual piezoelectric photocatalyst CdS/BiFeO3 S-scheme heterojunction: mechanism, degradation pathway, and toxicity evaluation. J. Colloid Interface Sci. 692, 137541. https://doi.org/10.1016/j.jcis.2025.137541 (2025).
Jiang, D. et al. Perovskite oxide ultrathin Nanosheets/G-C3n4 2d-2d heterojunction photocatalysts with significantly enhanced photocatalytic activity towards the photodegradation of Tetracycline. Appl. Catal. B. Environ. 201, 617–628. https://doi.org/10.1016/j.apcatb.2016.09.001 (2017).
Yu, J., Qi, L. & Jaroniec, M. Hydrogen production by photocatalytic water splitting over Pt /TiO2nanosheets with exposed (001) facets. J. Phys. Chem. C114, 13118–13125. https://doi.org/10.1021/jp104488b (2010).
Hashemi, Y. K., Yaraki, M. T., Ghanbari, S., Saremi, L. H. & Givianrad, M. H. Photodegradation of organic water pollutants under visible light using anatase F, N Co-doped TiO2/SiO2 nanocomposite: semi-pilot plant experiment and density functional theory calculations. Chemosphere 275, 129903. https://doi.org/10.1016/j.chemosphere.2021.129903 (2021).
Gadore, V., Singh, A. K. & Mishra, S. R. &Ahmaruzzaman, md. RSM approach for process optimization of the photodegradation of congo red by a novel NiCo2S4/chitosan photocatalyst. Sci. Rep. 14, 1118. https://doi.org/10.1038/s41598-024-51618-2 (2024).
Bhatti, M. Z. & Mohsin, M. I.A. Mineralization of fipronil using solar light-induced ZnO/Co3O4 photocatalyst@ceramic substrate: optimization of parameters by rsm,materials research. Bulletin 162, 112206. https://doi.org/10.1016/j.materresbull.2023.112206 (2023).
Afzalinia, A., Mirzaee, M. & Amani, M. A. Design of an S-scheme photo-catalyst utilizing a Cu-doped perovskite and MOF-5 for simultaneous degradation of organic pollutants under LED light irradiation: Application of EXRSM method for spectra separation and BBD-RSM modeling. Spectrochim. Acta A Mol. Biomol. Spectrosc. 287, 122116. https://doi.org/10.1016/j.saa.2022.122116 (2023).
Rasouli, J., Binazadeh, M. & Sabbaghi, S. Synthesis of a novel biomass waste-based photocatalyst for degradation of high concentration organic pollutants under visible light: optimization of synthesis conditions and operational parameters via RSM-CCD. Surf. Interfaces. 49, 104400. https://doi.org/10.1016/j.surfin.2024.104400 (2024).
Tri, N. L. M. et al. Ag-doped graphitic carbon nitride photocatalyst with remarkably enhanced photocatalytic activity towards antibiotics in hospital wastewater under solar light. J. Ind. Eng. Chem. 80, 597–605. https://doi.org/10.1016/j.jiec.2019.08.037 (2019).
Nazari, P., Sohrabi, S., Akhlaghian, F., Mansouri, M. & Malek Mohammadi, M. M. Activity, stability, and kinetic study of CuO/TiO2Janus photocatalyst for Rhodamine B degradation. Water Pract. Technol. 19, 1893–1907. https://doi.org/10.2166/wpt.2024.118 (2024).
Maleki, B., Abdulhasan, A. G., Khlaif, T. H. & Mansouri, M. Synthesis and photocatalytic properties of zinc-copper bimetallic oxide nanoparticles for removal of reactive blue 19 dye in aqueous suspension. Int. J. Environ. Anal. Chem. 1–24. https://doi.org/10.1080/03067319.2024.2337222 (2024).
Liu, Y. et al. Highly effective adsorption of cationic and anionic dyes on magnetic fe/ni nanoparticles doped bimodal mesoporous carbon. J. Colloid Interface Sci. 448, 451–459. https://doi.org/10.1016/j.jcis.2015.02.037 (2015).
Balta, Z. & Simsek, E. B. Understanding the structural and photocatalytic effects of incorporation of hexagonal boron nitride whiskers into ferrite-type perovskites (BiFeO3, MnFeO3) for effective removal of pharmaceuticals from real wastewater. J. Alloy. Comp. 898, 162897. https://doi.org/10.1016/j.jallcom.2021.162897 (2022).
Xu, T., Wang, G., Liu, Z., Deng, N. & Zhang, X. Construction of magnetically separable S-scheme BiFeO3/Cl-g-C3N4 heterostructure photocatalyst for simultaneous removal of Cr(VI) and Tetracycline in aqueous solution: performance and mechanism insight. J. Catal. 440, 115821. https://doi.org/10.1016/j.jcat.2024.11582 (2024).
Nguyen, T. H. et al. Novel biogenic silver and gold nanoparticles for multifunctional applications: green synthesis, catalytic and antibacterial activity, and colorimetric detection of Fe(III) ions. Chemosphere 287, 132271. https://doi.org/10.1016/j.chemosphere.2021.132271 (2022).
Si, Y. et al. Photocatalytic performance of a novel MOF/BiFeO3 composite. Material 10, 1161. https://doi.org/10.3390/ma10101161 (2017).
Bhoi, Y. P. & Mishra, B. G. Photocatalytic degradation of alachlor using Type-II CuS/BiFeO3 heterojunctions as novel photocatalyst under visible light irradiation. Chem. Eng. J. 344, 391–401. https://doi.org/10.1016/j.cej.2018.03.094 (2018).
Subramanian, Y. et al. Investigations on the enhanced dye degradation activity of heterogeneous BiFeO3–GdFeO3 nanocomposite photocatalyst. Heliyon 5, e01831. https://doi.org/10.1016/j.heliyon.2019.e01831 (2019).
Mohanty, L., Pattanayak, D. S., Singhal, R., Pradhan, D. & Dash, S. K. Enhanced photocatalytic degradation of Rhodamine B and malachite green employing BiFeO3/G-C3N4 nanocomposites: an efficient visible-light photocatalyst. Inorg. Chem. Commun. 138, 109286. https://doi.org/10.1016/j.inoche.2022.109286 (2022).
Funding
M. M. thanks the University of Ilam, for the award research fellowship.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by F.A., M.M., and M.T. All authors contributed to the study conception and design. M.M. was the senior supervisor of the study. All authors are fully aware of this manuscript and have permission to submit the manuscript for possible publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Abbasi, F., Mansouri, M. & Tanzifi, M. RSM optimization and morphological investigation of bismuth ferrite for photodegradation of organic pollutant under visible light. Sci Rep 15, 30920 (2025). https://doi.org/10.1038/s41598-025-16568-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16568-3