Abstract
Orthohantavirus hantanense (HTNV) is a zoonotic pathogen transmitted by rodents and the causative agent of hemorrhagic fever with renal syndrome (HFRS) in East Asia. Long-term reservoir population ecology studies have enhanced our understanding of hantavirus infection patterns and support disease risk assessments critical for military and civilian populations in HTNV high-risk areas. Here, we evaluated fluctuations in the population dynamics of Apodemus agrarius, the primary reservoir of HTNV, assessed hantavirus seroprevalence, and conducted a descriptive analysis of HFRS disease risks in the Republic of Korea (ROK). From 2000 to 2019, a total of 12,476 small mammals representing 15 species were captured across locations spanning northern to southern regions. A. agrarius was the most frequently collected species in the ROK, with juvenile populations peaking during the late fall/winter seasons. A high proportion of A. agrarius captured at military installations/training sites near the Demilitarized Zone tested seropositive for immunoglobulin G antibodies against HTNV, whereas none of those trapped in Jeollanam Province were seropositive. Risk assessment identified high-risk HFRS zones in northern Gyeonggi and Gangwon Provinces. This study provides important insights for forecasting heightened viral transmission risks to humans and implementing targeted mitigation strategies in HFRS high-risk regions of the ROK.
Similar content being viewed by others
Introduction
The emergence of zoonotic diseases caused by Orthohantaviruses, a genus within the family Hantaviridae in the order Bunyavirales, demands urgent attention. Orthohantavirus hantanense (HTNV) and O. seoulense (SEOV) in Asia, as well as O. puumalaense (PUUV) and O. dobravaense in Europe, are causative viral agents of hemorrhagic fever with renal syndrome (HFRS) in humans1. Human exposure to Orthohantaviruses primarily occurs through inhalation of aerosolized urine, feces, or saliva and, less frequently, through bite or direct contact with infected rodents2. Clinical symptoms of HFRS vary widely depending on the viral agent, ranging from mild fever and headache to severe complications, including acute encephalomyelitis, acute respiratory distress syndrome, and kidney failure in infected individuals3. The global incidence of HFRS is a significant public health concern, with the Republic of Korea (ROK) reporting 400–500 cases annually, underscoring the critical need for comprehensive hantavirus risk assessments in the region4.
Hantaviruses remain restricted to specific geographical zones due to their strict association with distinct reservoir hosts, primarily rodents5. In the ROK, HTNV is linked to the striped field mouse, Apodemus agrarius, and accounts for the majority of HFRS cases, including the most severe manifestations6,7,8,9. SEOV, a globally distributed, rat-borne hantavirus, is responsible for approximately 20% of HFRS cases in the ROK with a mortality rate of < 1%10[,11[,12. Thottimvirus imjinense, a soricomorpha-borne hantavirus, has been identified in the Ussuri white-toothed shrew (Crocidura lasiura), but no human cases associated with this virus have been documented13,14. The incidence of HFRS follows seasonal trends and predominantly affects rural civilians residing or working in rodent-infested areas, as well as military personnel conducting training in HTNV endemic environments15,16,17. Thus, surveillance of reservoir populations remains essential for elucidating the epidemiology of HFRS and informing effective disease management strategies.
Rodent-to-human transmission of HFRS pathogens are influenced by factors related to reservoir host rodent populations and reproduction dynamics, environmental conditions, and anthropological activities6,18. Long-term monitoring of reservoir hosts is crucial for understanding the spatial and temporal patterns of HFRS infections and their ecological drivers in relation to its occurrence in associated human populations19,20,21,22. Population dynamic studies of reservoir populations identified ecological factors linked to SEOV and O. prospectense outbreaks in the United States23. Similarly, HFRS cases caused by PUUV in Scandinavia have been associated with cyclical changes in the density of bank vole (Clethrionomys glareolus) populations24. Seasonal environmental variations influenced by vegetation growth and food availability impact A. agrarius and Rattus norvegicus populations differently, shaping HFRS risks in urban and rural environments25,26. Rodent surveillance in the ROK has revealed habitat-based differences in hantaviral prevalence among reservoir populations, suggesting that hantavirus transmission risks varies across ecological landscapes27,28,29,30,31. However, comprehensive ecological studies on HTNV reservoirs in the ROK remain limited.
This study aims to identify HTNV endemic areas based on the geographic distribution of reservoir hosts and the prevalence of HTNV infections among different subpopulations. Temporal patterns of HTNV infections in reservoir hosts were analyzed using epidemiological data from seasonal trapping conducted over two decades in the ROK. Furthermore, a qualitative disease risk assessment was performed to evaluate HFRS disease risks at multiple trapping sites. Serological detection of anti-HTNV immunoglobulin G (IgG) antibodies was interpreted as evidence of prior infections and used as a proxy for past transmission dynamics within rodent populations. As field investigations were conducted within or near military training areas in the ROK, the inferences drawn from this study are primarily applicable to such settings. These findings provide a foundation for reducing human disease incidence through habitat modification, mitigation of environmental factors contributing to human infections, and reservoir population control.
Results
Small mammal capture during 2000–2019
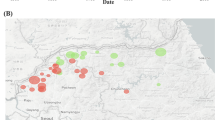
A total of 12,476 small mammals representing 15 species were live captured across four regions: Gyeonggi, Gangwon, Jeollabuk, and Jeollanam Provinces, yielding an overall trap success rate of 21.9% (Fig. 1; Table 1). A. agrarius (10,993; 88.1%) was the most frequently collected species, followed by C. lasiura (576; 4.6%), Microtus fortis (345; 2.8%), Mus musculus (225; 1.8%), Craseomys regulus (119; 1.0%), Micromys minutus (114; 0.9%), R. norvegicus (42; 0.3%), and Tscherskia triton (39, 0.3%). The remaining seven species accounted for only 23 individuals (0.2%) of all small mammals captured. Trap rates of A. agrarius varied across trapping sites, with higher rates generally observed for areas near the Demilitarized Zone (DMZ) in the ROK (Fig. 2, Supplementary Table 1).
Location of trapping sites for small mammal surveillance in the Republic of Korea (ROK) between 2000 and 2019. A geographic map of the ROK illustrating small mammal capture sites in (A) northern Gyeonggi and Gangwon Provinces near the Demilitarized Zone, (B) southern Gyeonggi Province, (C) Jeollabuk Province, and (D) Jeollanam Province. Black circles indicate 20 training areas, live-fire complexes, and command posts operated by the US and ROK military. Squares represent US Air Bases, upward-pointing triangles denote non-military sites, and inverted triangles indicate US military installations. The base map provided by Natural Earth (https://www.naturalearthdata.com) is freely available for use in any type of project, with no permission required. The figure was created from base layer of the map using a Quantum Geographical Information System (QGIS) 3.10 for Mac and modified using Adobe Illustrator CC 2024 (Adobe Systems Incorporated, California, USA). TA, training areas; FP, firing points; LTA, local training areas.
Trap rate of Apodemus agrarius at 32 collection sites in the Republic of Korea. A total of 56,810 trap nights were conducted at all collections sites between 2000 and 2019. In the figure, the X-axis represents the trap rate for A. agrarius (Trap rate (%) = [Number of A. agrarius collected/Number of trap nights] × 100), while the Y-axis indicates the names of the trapping sites. Orange bars indicate trap rates at sites located in northern Gangwon and Gyeonggi Provinces near the Demilitarized Zone, green bars correspond to sites in southern Gyeonggi Province, blue bars represent sites in Jeollabuk Province, and red bars denote sites in Jeollanam Province. TA, training areas; FP, firing points; LTA, local training areas.
Monthly capture and gravid rates of A. agrarius
Monthly variations in A. agrarius trap rates were observed, with the lowest trap rates recorded in February (13.9%) and the highest in July (25.0%) (Table 2). Overall, the trap rate for A. agrarius was 19.3%, with a nearly equal distribution of males (49.8%) and females (50.2%). In January, the majority of A. agrarius consisted of juveniles weighing < 20 g (Fig. 3). However, as their proportion gradually declined over the subsequent months, reaching its lowest level in August, adults and older individuals became relatively more prevalent. The proportion of juveniles began to increase in late summer and continued to rise throughout autumn, peaking in November.
Monthly proportion of Apodemus agrarius, by weight, collected at multiple trapping sites in the Republic of Korea. The trapped rodents were characterised on the basis of their weight as < 20 g comprising juvenile and young rodents, 20–30 g comprising adult rodents and > 30 g comprising old rodents. The percentage of total A. agrarius trapped in a month and categorised on basis of their weight are plotted on the Y-axis. Error bars indicate 95% confidence intervals.
Monthly variations in body weight correlated with changes in reproductive activity among females (Fig. 4). In January and February, only 0.7% (1/131) and 0% (0/171) females examined were gravid. The proportion of gravid females increased in March (10.3%), followed by a minor peak in April (19.4%) and a subsequent decline through June (6.9%). A secondary increase in the proportion of gravid females was observed in July (9.3%), with major peaks occurring in August (56.6%) and September (49.6%). Thereafter, the proportion declined to 15.8% in October and reached its lowest level in December (2.6%).
Percentage of gravid female Apodemus agrarius collected monthly from 2000–2019 in the Republic of Korea. The black solid circular dots represent the percentage of gravid females for each month labelled on the X-axis. In January and February, the proportion of gravid females was minimal, with only 0.7% (1/131) in January and none (0/171) in February. The prevalence of gravid females increased in March (10.3%), followed by a minor peak in April (19.4%) before declining through June (6.9%). A secondary increase was observed in July (9.3%), with pronounced peaks in August (56.6%) and September (49.6%). Thereafter, the proportion gradually declined, reaching 15.8% in October and the lowest level in December (2.6%). Error bars indicate 95% confidence intervals.
Spatial and seasonal variation of HTNV-infected A. agrarius
Serum samples from trapped rodents were analyzed for antibodies (Abs) reactive to HTNV (Fig. 5). Seropositivity rates were highest at military training areas (TAs) near the DMZ (range: 0-37.5%, mean: 16.2%). Monthly seroprevalence was lowest in July (2.7%) and peaked in September (19.0%), with an annual mean of 13.4% (Table 3). Adults and old rodents exhibited significantly higher Ab prevalence rates than juveniles (χ2 = 615.2, df = 2, p < 0.0001; Supplementary Fig. 1).
Seropositivity rate for Orthohantavirus hantanense (HTNV) in Apodemus agrarius between 2000 and 2019. Serum samples collected from A. agrarius trapped at multiple sites were analyzed for the presence of anti-HTNV immunoglobulin G antibodies. The horizontal bars indicate the percentage of A. agrarius exhibiting seropositivity. Orange bars represent rodents trapped at sites located near the Demilitarized Zone in northern Gangwon and Gyeonggi Provinces, green bars correspond to collection sites in southern Gyeonggi Province, and blue bars denote collection sites in Jeollabuk Province. No A. agrarius collected from the Haenam-si dairy farm, Gwangju Air Base, K-16 Air Base, Camp Stanley, KC-39 Telecommunication Tower, Watkins Range, or LTA 36 tested seropositive for HTNV. Error bars indicate 95% confidence intervals. TA, training areas; FP, firing points; LTA, local training areas.
Risk assessment of hantavirus infection
A qualitative risk assessment framework was developed to classify hantavirus infection risks at each military trapping site. The assessment incorporated criteria related to HTNV Ab-positive rodent densities, environmental features influencing dust generation, potential for human–rodent interface, and historical HFRS case occurrence (Supplementary Table 2). Among the 30 military trapping locations, seven were identified as HTNV high-risk areas based on a comprehensive assessment of both the estimated number of HTNV Ab-positive rodents and/or environmental conditions that were conducive to potential risks of virus-contaminated dusts (Supplementary Table 3). Sites with moderate densities of HTNV Ab-positive rodents, environmental conditions that were not conducive to high rates of potentially contaminated dusts, and low incidence of HFRS cases since 1995 were classified as moderate risk. Improved multipurpose TAs and military installations with heavily graveled and paved roads that reduced dusts, cut vegetation that reduced rodent habitat, low densities of seropositive rodents, and few or no reported HFRS cases were considered low-risk. The primary HFRS high-risk areas were in northern Gyeonggi and Gangwon Provinces, as the proportion of HTNV seropositive A. agrarius decreased at survey sites south of Seoul.
Discussion
The present study highlights the importance of longitudinal studies to better understand the dynamics of HTNV transmission and infection within reservoir populations. Long-term data facilitate the analysis of infection patterns across species sub-populations, as well as variations based on sex and relative age. Such epidemiological insights are particularly valuable for understanding zoonotic diseases, as they help anticipate potential risks and inform strategies to mitigate human infections32. In this study, A. agrarius accounted for more than 80% of all small mammals captured at military installations, TAs, and firing ranges in the ROK from 2000 to 2019 are consistent with previous findings28,33,34,35,36,37. High population densities of A. agrarius, particularly among military TAs, was associated with uncut grasses and herbaceous and scrub vegetation in agricultural lands that provide ground cover. High population densities, widespread distribution, and high HTNV seropositivity rates underscore the role of A. agrarius as the primary reservoir host of HTNV in the region and its significance in identifying areas and periods of elevated disease risks to human health.
Fluctuations in HFRS incidence are shaped by multiple ecological and epidemiological factors. While SEOV-related HFRS cases may remain stable throughout the year, most HTNV-associated HFRS cases occur in the fall, coinciding with an influx of young, naïve rodents. According to monthly statistics from the Korea Disease Control and Prevention Agency, peak HFRS cases occur from October to early December, with a minor peak from May to June4. This correlates with the breeding season of A. agrarius that is bimodal, with a minor peak extending from late March to May (spring) and a major peak from August to September/early October.
Seasonal pattern of HFRS may be driven by (1) increased movement as young rodents mature, along with greater human activity, including agricultural harvesting and military operations under dry and dusty conditions, and (2) reduced vegetation cover and senescence, which may intensify rodent-to-rodent contact, potentially including aggressive interactions related to territory establishment and resource competition. During the spring reproductive peak, emerging vegetation provides abundant food and ground cover, which may reduce competition and aggressive interactions among rodents. As winter progresses, the dieback of annual vegetation limits food availability and ground cover, constraining suitable rodent habitats. These ecological pressures may contribute to increased intra-specific interactions, some of which could involve increased aggression and wounding, thereby creating potential opportunities for virus transmission. These behavioral dynamics—particularly aggression and wounding—have been proposed as potential mechanisms facilitating horizontal transmission of SEOV among wild rat populations38. While such behavioral pathways have not been directly observed in A. agrarius, similar mechanisms may plausibly contribute to HTNV transmission, particularly during periods of increased competition and reduced resource availability.
Evidence of rodent-to-human transmission of HTNV indicates that infections occur through the inhalation of dust contaminated with virus-laden excreta, including feces, urine, and saliva2. Military personnel are at particularly high-risk during field training activities that generate large amounts of dust in areas with high densities of infected rodents, especially near the DMZ, where capture rates often range from 20 to 40%, and HTNV Ab-positive rates frequently exceed 15–20%. HFRS cases are not evenly distributed throughout the ROK4. In southern regions, reduced HFRS incidence among US military personnel has been attributed to the modernization of training facilities, including the construction of hard-surfaced roads and improved infrastructure. The development of large-scale live fire complexes (LFCs) often involves clearing tall grasses and herbaceous vegetation and replacing dirt roads with gravel or pavement. These efforts can reduce rodent habitats and limit the generation of virus-contaminated dusts, thereby lowering the risk of human exposure. However, while such habitat modification may offer short-term benefits in specific contexts, we acknowledge that it may also lead to unintended ecological consequences. The removal of vegetation and alteration of landscape features can disrupt rodent populations, potentially causing shifts in their movement patterns and encouraging the establishment of new habitats closer to human activity. These changes may increase the risk of zoonotic transmission in previously unaffected areas and could affect natural selection dynamics within rodent populations. Given these considerations, infrastructure-based interventions should be implemented with caution and, where possible, integrated into broader, ecologically informed disease control strategies. These may include targeted rodent surveillance, environmental risk mapping, seasonal adjustment of training activities, and enhanced education on HFRS prevention among personnel operating in high-risk areas. A multifaceted approach that balances immediate disease risk reduction with long-term ecological sustainability is essential for the effective prevention of HFRS transmission.
This study has several limitations that should be considered when interpreting the findings. (1) Although standardized trapping protocols were applied over the 20-year period, field conditions—such as military training schedules, seasonal access restrictions, and adverse weather—occasionally limited consistent sampling across all sites and seasons. These logistical constraints may have introduced regional or temporal sampling biases. Moreover, although multiple small mammal species were captured, the analysis focused solely on A. agrarius, the primary reservoir of HTNV in the ROK. As such, the findings may not fully reflect transmission dynamics involving other potential host species. (2) The study was designed around serological testing for anti-HTNV IgG antibodies as a practical and scalable approach to long-term surveillance. This strategy was adopted in consideration of evolving national biosafety regulations requiring HTNV-related laboratory procedures to be conducted under animal biosafety level 3 (ABSL-3) conditions, as well as the logistical and financial challenges associated with conducting large-scale molecular diagnostics in field-collected samples. While this serology-based design enabled sustained monitoring over two decades, the absence of PCR-based molecular testing limited the ability to assess current infection status and patterns of viral shedding. Future studies integrating both serological and molecular approaches will help advance understanding of hantavirus circulation in natural reservoirs.
In conclusion, understanding the ecology and epidemiology of A. agrarius is essential for assessing hantaviral disease risks in the ROK. A minor increase in HFRS cases in the spring, following elevated rodent reproduction rates, and a major rise in the fall and early winter, driven by increased gravid rates from August to September, suggest that the influx of young mice contributes, at least partially, to heightened HTNV transmission and the subsequent surge in HFRS cases. To better understand the role of A. agrarius reproductive behavior in HTNV transmission, further studies are needed to investigate rodent behavior and bionomics, seasonal and annual population dynamics, primary transmission pathways, and environmental factors, such as land modification due to agricultural practices and military training activities. Additionally, other ecological and epidemiological factors influencing focal transmission patterns should be explored.
Methods
Ethics statement
Small mammal trapping and handling procedures were conducted in full compliance with relevant institutional and national guidelines and regulations. Specifically, the experimental protocols involving animals were reviewed and approved by the Korea University Institutional Animal Care and Use Committee (KUIACUC) under the following protocol numbers: #2010–212, #2016–49, and #2019–4. Field collection of wild rodents was carried out by Force Health Protection and Preventive Medicine, 65th Medical Brigade, 8th US Army, US Forces Korea (USFK), in accordance with USFK Regulation 40–1, which governs the “Prevention, Surveillance, and Treatment of Hemorrhagic Fever with Renal Syndrome” at US military installations and US/ROK-operated military training sites. Standardized protocols were followed to minimize risks associated with potentially infected animals. All laboratory personnel handling rodents were vaccinated with the Korean-authorized Hantavax vaccine. Small mammals were euthanized via cardiac puncture under isoflurane anesthesia in accordance with approved IACUC protocols. All procedures adhered to the ARRIVE guidelines (https://arriveguidelines.org) and complied with all applicable ethical standards for animal research. Every effort was made to minimize animal suffering throughout the study.
Habitat description
Small mammal surveillance was conducted at 32 sites, including US military installations/air bases, TAs, LFCs, and a communications post from November 2000 through December 2019 (Supplementary Table 4). Additionally, a civilian dairy farm in Singye-ri, Haenam-si, Jeollanam Province, was surveyed in this study. The topography and ecology of the training sites varied, influenced in part by military activities and designations. These areas ranged from flat, low-lying regions to mountainous, rocky multipurpose range complexes, as well as narrow and expanded river valleys with unmanaged grasses and forested hillsides. Over the survey period, multipurpose TAs/LFCs were modernized and characterized by hardened, heavily graveled roads and short-cut grasses, with limited unmanaged tall grasses, herbaceous and scrub vegetation, or forested hillsides. Some areas, such as the Dagmar North TA in Jangjwa-ri, consisted entirely of unmanaged lands. Military operations at these sites included command and control exercises, weapons firing, and limited maneuvers at smaller training sites. Larger multipurpose TAs and LFCs hosted personnel and mechanized infantry maneuvers, artillery and rocket impact zones, and air assault, helicopter, and aircraft training activities. Agricultural activities surrounding the TAs included land cultivation beginning in March, irrigation, planting, and harvesting of rice, fruit, and vegetable crops, as well as tree removal and occasional burning and cutting of tall grasses and trees along the military perimeters. Civilian encroachment onto training sites also occurred but has been limited as perimeter fences have been installed at many areas.
Sampling
Sherman live capture folding traps (7.5 × 8.75 × 22.5 cm; H.B. Sherman, Tallahassee, FL, USA), baited with peanut butter placed between two saltine crackers, were set during daylight hours (12:00–17:00) and collected the following morning as previously described39. Global positioning system coordinates and elevations were recorded at both the beginning and end of each trap line (20–60 traps set at 4–5 m intervals). Field collection records, including photographs of the collection site, were entered into an electronic database (Microsoft Excel, Microsoft Corp., Redmond, WA, USA) to document each trap line along with vegetation density, diversity, and composition at the time of the survey. Traps positive for small mammals were numbered sequentially and placed in secured containers for transport to an ABSL-3 laboratory, Korea University, Seoul, ROK. In the laboratory, each small mammal was assigned a unique identification number, euthanized, identified to species, sexed, weighed, and examined for gravid status (2002–2019). Spleen, lung, and kidney samples were placed in separate cryovials, and all blood and tissue samples were stored at −70 °C until processed for diagnostic testing.
Serological screening
Indirect immunofluorescence Ab testing was performed to detect IgG Abs against HTNV in rodent sera. Briefly, Vero E6 cells (ATCC, DR-L2785) infected with HTNV were fixed on multi-well slides using cold acetone for 10 min. A total of 25 µL of 1:32 diluted rodent sera in phosphate-buffered saline (PBS) was added to each well, followed by incubation at 37 °C for 30 min. The antigen slides were washed twice with PBS, and 25 µL of fluorescein isothiocyanate-conjugated anti-mouse IgG (ICN Pharmaceuticals, Laval, Canada) was added to each well. After incubation and washing, the slides were mounted with glycine-buffered glycerol under cover slips and examined under a fluorescence microscope (Axio Scope; Zeiss, Berlin, Germany).
Statistical analysis
Chi-square (χ2) analyses were performed using GraphPad Prism 10.4.1 for Windows (GraphPad Software, Boston, MA, USA; www.graphpad.com).
Risk assessment for hantavirus infection
To evaluate HFRS risk across multiple rodent survey sites, several parameters were considered, including HTNV prevalence in rodent populations, disease severity, environmental factors (e.g., dirt and gravel roads, unmanaged lands), rodent bionomics (capture rates, peak reproductive periods, distribution, and habitat characteristics), HTNV Ab-seropositivity rates, rodent density (estimated number of HTNV Ab-positive rodents per 100 traps), transmission dynamics, potential for human exposure, human activities, and available protective measures. Risk levels were classified as high, moderate, or low.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kim, W. K. et al. Genomic epidemiology and active surveillance to investigate outbreaks of hantaviruses. Front. Cell. Infect. Microbiol. 10, 532388. https://doi.org/10.3389/fcimb.2020.532388 (2020).
Forbes, K. M., Sironen, T. & Plyusnin, A. Hantavirus maintenance and transmission in reservoir host populations. Curr. Opin. Virol. 28, 1–6 (2018).
Noh, J. Y., Jung, J. & Song, J. W. Hemorrhagic fever with renal syndrome. Infect. Chemother. 51, 405–413 (2019).
Infectious Disease Portal. Korea Disease Control and Prevention Agency (KDCA) (2025). https://dportal.kdca.go.kr/pot/index.do
Bennett, S. N., Gu, S. H., Kang, H. J., Arai, S. & Yanagihara, R. Reconstructing the evolutionary origins and phylogeography of hantaviruses. Trends Microbiol. 22, 473–482 (2014).
Kim, W. K. et al. Active targeted surveillance to identify sites of emergence of hantavirus. Clin. Infect. Dis. 70, 464–473 (2020).
Lee, G. Y. et al. Clinical and immunological predictors of hemorrhagic fever with renal syndrome outcome during the early phase. Viruses 14, 595. https://doi.org/10.3390/v14030595 (2022).
Kim, W. K. et al. Phylogeographic analysis of hemorrhagic fever with renal syndrome patients using multiplex PCR-based next generation sequencing. Sci. Rep. 6, 26017. https://doi.org/10.1038/srep26017 (2016).
Prayitno, S. P. et al. Etiological agent and clinical characteristics of haemorrhagic fever with renal syndrome in the Southern Republic of korea: a genomic surveillance study. Clin. Microbiol. Infect. 30, 795–802 (2024).
Kim, W. K. et al. Multiplex PCR-based next-generation sequencing and global diversity of Seoul virus in humans and rats. Emerg. Infect. Dis. 24, 249–257 (2018).
Kim, Y. S. et al. Hemorrhagic fever with renal syndrome caused by the Seoul virus. Nephron 71, 419–427 (1995).
Park, K. et al. A portable diagnostic assay, genetic diversity, and isolation of Seoul virus from Rattus norvegicus collected in Gangwon province, Republic of Korea. Pathogens 11, 1047. https://doi.org/10.3390/pathogens11091047 (2022).
Song, J. W. et al. Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura). J. Virol. 83, 6184–6191 (2009).
Lee, S. H. et al. Dynamic circulation and genetic exchange of a shrew-borne hantavirus, Imjin virus, in the Republic of Korea. Sci. Rep. 7, 44369. https://doi.org/10.1038/srep44369 (2017).
Song, J. W. et al. Hemorrhagic fever with renal syndrome in 4 US soldiers, South korea, 2005. Emerg. Infect. Dis. 15, 1833–1836 (2009).
Lee, S. H., Chung, B. H., Lee, W. C. & Choi, I. S. Epidemiology of hemorrhagic fever with renal syndrome in korea, 2001–2010. J. Korean Med. Sci. 28, 1552–1554 (2013).
Cho, S. et al. Urinary genome detection and tracking of Hantaan virus from hemorrhagic fever with renal syndrome patients using multiplex PCR-based next-generation sequencing. PLoS Negl. Trop. Dis. 15, e0009707. https://doi.org/10.1371/journal.pntd.0009707 (2021).
Wang, Y. et al. Spatiotemporal trends of hemorrhagic fever with renal syndrome (HFRS) in China under climate variation. Proc. Natl. Acad. Sci. U S A https://doi.org/10.1073/pnas.2312556121 (2024).
Fang, L. Q. et al. Spatiotemporal dynamics of hemorrhagic fever with renal syndrome, beijing, people’s Republic of China. Emerg. Infect. Dis. 15, 2043–2045 (2009).
Lee, G. Y. et al. Phylogeographic diversity and hybrid zone of Hantaan orthohantavirus collected in Gangwon province, Republic of Korea. PLoS Negl. Trop. Dis. 14, e0008714. https://doi.org/10.1371/journal.pntd.0008714 (2020).
Park, K. et al. A novel genotype of Hantaan orthohantavirus harbored by Apodemus agrarius chejuensis as a potential etiologic agent of hemorrhagic fever with renal syndrome in Republic of Korea. PLoS Negl. Trop. Dis. 15, e0009400. https://doi.org/10.1371/journal.pntd.0009400 (2021).
Park, K. et al. Epidemiological surveillance and genomic characterization of Soochong virus from Apodemus species using multiplex PCR-based next-generation sequencing, Republic of Korea. J. Med. Virol. 96, e70077. https://doi.org/10.1002/jmv.70077 (2024).
Childs, J. E., Glass, G. E., Korch, G. W. & LeDuc, J. W. Prospective seroepidemiology of hantaviruses and population dynamics of small mammal communities of baltimore, Maryland. Am. J. Trop. Med. Hyg. 37, 648–662 (1987).
Niklasson, B., Hornfeldt, B., Lundkvist, A., Bjorsten, S. & Leduc, J. Temporal dynamics of puumala virus antibody prevalence in voles and of nephropathia epidemica incidence in humans. Am. J. Trop. Med. Hyg. 53, 134–140 (1995).
Xiao, H. et al. Ecology and geography of hemorrhagic fever with renal syndrome in changsha, China. BMC Infect. Dis. 13, 305 (2013).
Xiao, H. et al. Spatial heterogeneity of hemorrhagic fever with renal syndrome is driven by environmental factors and rodent community composition. PLoS Negl. Trop. Dis. 12, e0006881. https://doi.org/10.1371/journal.pntd.0006881 (2018).
Klein, T. A. et al. Hantaan virus surveillance in small mammals at firing points 10 and 60, yeoncheon, Gyeonggi province, Republic of Korea. Vector Borne Zoonotic Dis. 12, 674–682 (2012).
Klein, T. A. et al. Hantaan virus surveillance targeting small mammals at Dagmar North training area, Gyeonggi province, Republic of korea, 2001–2005. J. Vector Ecol. 36, 373–381 (2011).
Kim, J. et al. High-resolution phylogeographical surveillance of Hantaan orthohantavirus using rapid amplicon-based flongle sequencing, Republic of Korea. J. Med. Virol. 96, e29346. https://doi.org/10.1002/jmv.29346 (2024).
Kim, H. C. et al. Urban rodent surveillance, Climatic association, and genomic characterization of Seoul virus collected at U.S. Army garrison, seoul, Republic of korea, 2006–2010. Am. J. Trop. Med. Hyg. 99, 470–476 (2018).
Park, K. et al. Multiplex PCR-based nanopore sequencing and epidemiological surveillance of Hantaan orthohantavirus in Apodemus agrarius, Republic of Korea. Viruses 13, 847. https://doi.org/10.3390/v13050847 (2021).
Mills, J. N., Ksiazek, T. G., Peters, C. J. & Childs, J. E. Long-term studies of hantavirus reservoir populations in the Southwestern united states: a synthesis. Emerg. Infect. Dis. 5, 135–142 (1999).
Sames, W. J. et al. Ecology of Hantaan virus at twin bridges training area, Gyeonggi province, Republic of korea, 2005–2007. J. Vector Ecol. 34, 225–231 (2009).
Kim, J. A. et al. Genetic diversity and reassortment of Hantaan virus tripartite RNA genomes in nature, the Republic of Korea. PLoS Negl. Trop. Dis. 10, e0004650. https://doi.org/10.1371/journal.pntd.0004650 (2016).
Klein, T. A. et al. Hantaan virus surveillance targeting small mammals at nightmare range, a high elevation military training area, Gyeonggi province, Republic of Korea. PLoS One. 10, e0118483. https://doi.org/10.1371/journal.pone.0118483 (2015).
Park, K. et al. Epidemiological surveillance and phylogenetic diversity of Orthohantavirus hantanense using high-fidelity nanopore sequencing, Republic of Korea. PLoS Negl. Trop. Dis. 19, e0012859. https://doi.org/10.1371/journal.pntd.0012859 (2025).
Kim, H. C. et al. Hantavirus surveillance and genetic diversity targeting small mammals at camp humphreys, a US military installation and new expansion site, Republic of Korea. PLoS One. 12, e0176514. https://doi.org/10.1371/journal.pone.0176514 (2017).
Hinson, E. R., Shone, S. M., Zink, M. C., Glass, G. E. & Klein, S. L. Wounding: the primary mode of Seoul virus transmission among male Norway rats. Am. J. Trop. Med. Hyg. 70, 310–317 (2004).
O’Guinn, M. L. et al. Ecological surveillance of small mammals at firing points 10 and 60, Gyeonggi province, Republic of korea, 2001–2005. J. Vector Ecol. 33, 370–384 (2008).
Acknowledgements
This funding was provided by the Armed Forces Health Surveillance Division, Global Emerging Infections Surveillance Branch (GEIS), ProMIS ID P0131_20_ME_03, Silver Spring, MD, and the National Center for Medical Intelligence, Fort Detrick, MD. In addition, this study was partially funded by the Agency for Defense Development (UE242006TD and 411FF5-912A01201), the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2023R1A2C2006105), the Basic Research Program through the National Research Foundation of Korea (NRF) by the Ministry of Education (NRF-2021R1I1A2049607), and Korea University. We thank the commanders and many soldiers of the 5th, 38th, and 154th Medical Detachments, 168th Multifunctional Medical Battalion and Force Health Protection and Preventive Medicine, 65th Medical Brigade/US Army MEDDAC-Korea, who supported and/or conducted rodent surveillance. We especially thank the graduate students, Korea University, for their support for bionomic data collection for small mammals. We sincerely thank Korea Disease Control and Prevention Agency for their support and reports. We are grateful to the 18th Medical Command Commanders, COL(Ret) Phillip Volpe and COL Brian Allgood (deceased), 65th Medical Brigade Commanders, Wendy L. Harter, BG, Derek C. Cooper, COL, and Charles D. Zimmerman, COL, and Preventive Medicine Consultants who provided support and encouragement throughout the study.
Author information
Authors and Affiliations
Contributions
T.A.K., W.-K.K., and J.-W.S. designed the study. T.A.K., K.P., J.K., S.-H.L., S.H.G., W.J.S., M.L.O., M.J.T., and Y-J.K. captured animals. T.A.K., K.P., S.R., and H.C.K analyzed and interpreted data, and wrote the manuscript. K.P., J.K., S.-H.L., and S.H.G. performed experiments. J.K. and J.P. analyzed data and provided scientific discussion. W.-K.K. and J.-W.S. supervised the study and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Klein, T.A., Park, K., Rajoriya, S. et al. Longitudinal study of Orthohantavirus hantanense in Apodemus agrarius and disease risk assessment in the Republic of Korea during 2000–2019. Sci Rep 15, 30872 (2025). https://doi.org/10.1038/s41598-025-16897-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16897-3