Abstract
Accessibility to outcome measures that reliably assess disease pathology in preclinical studies are vital, especially for rare disorders such as Duchenne muscular dystrophy (DMD). The non-invasive technique of electrical impedance myography (EIM) has shown potential as an outcome measure in patients and mouse models of neuromuscular disorders. However, these preclinical observations have been limited to a single site. Here, we performed a longitudinal, parallel two-site study to assess the potential of EIM as an outcome measure by using two DMD mouse models – mdx mice on a C57BL/10ScSn/J or DBA/2J genetic background – differing in disease severity, and being hypertrophic and atrophic, respectively, over a ten-month period, in which mice were evaluated monthly. We found that longitudinally acquired phase values were the most reliable, with relatively minor differences between study sites. Histopathology, assessed at 8, 12, 28, or 52 weeks of age correlated well with EIM phase values in both models. Our findings highlight the applicability of EIM as a robust, preclinical outcome measure for DMD.
Similar content being viewed by others
Introduction
Duchenne muscular dystrophy (DMD) is the most common inherited dystrophy of childhood, affecting approximately 1:5000 newborn males worldwide1. DMD is an X-linked recessive disorder caused by mutations in the DMD gene resulting in a lack of dystrophin protein in myofibers1,2. Dystrophin and its binding partners form the dystrophin-associated glycoprotein complex (DGC), which is crucial to ensure sarcolemmal stability and proper contractile activity3. The absence of dystrophin and consequential loss of the DGC result in a continuous pathological environment, ultimately leading to inefficient regeneration and myofiber death, and replacement of muscle tissue with fibro-adipose tissue1,2,3. DMD patients experience progressive muscle weakness and wasting, affecting skeletal, respiratory and cardiac function. Most patients become wheelchair-bound by their early teens and median life expectancy is approximately 35 years, with cardiorespiratory failure as the leading cause of death1.
Despite the extensive efforts and progress achieved toward optimizing the standards of care and developing new therapies, DMD remains a severe disorder representing a major unmet clinical need and a huge burden for affected boys and their families2,4,5. Game-changing therapies demand high-quality translational research which incorporates effective models and reliable endpoints, helping to provide a key step towards bridging the gap between basic science and their clinical application6.
The classic C57BL/10ScSn-Dmdmdx (BL10-mdx) mouse is the most extensively used and best characterized DMD model that represents an invaluable tool for providing insights into DMD pathobiology and testing therapeutic interventions. BL10-mdx mice lack dystrophin because of a spontaneous point mutation in exon 23 of the Dmd gene and consequently develop a dystrophic phenotype characterized by muscle hypertrophy. Starting at 3–4 weeks of age, muscles undergo extensive cycles of degeneration and regeneration leading to inflammation, fibrosis and central nucleation in myofibers7,8,9. However, when compared to humans, BL10-mdx mice are only mildly affected both at muscle tissue and function levels, which complicates preclinical studies8,9. More recently, a new DMD mouse model was generated by crossing BL10-mdx mice on a DBA/2J genetic background10,11. Compared to BL10-mdx mice, D2.B10-Dmdmdx/J (D2-mdx) mice are atrophic and have a more severe dystrophic phenotype with an earlier onset. The D2-mdx model exhibits greater muscle damage with decreased muscle mass and myofiber size, impaired regeneration, and exacerbated intramuscular fibrosis11,12,13. These features are mainly attributed to the presence of variants in genetic modifiers of muscular dystrophy, i.e., Ltbp4, implicated in pro-fibrotic TGF-β1 activation and negatively impacting disease progression and severity14,15, and Anxa6, involved in membrane repair and muscle regeneration16. Lastly, D2-mdx mice carry the dyscalc1 locus involving Abcc6 or Emp3 genes¸ which have been associated with calcifications seen in skeletal muscle and heart – a feature not observed in DMD patients, thus possibly representing an intrinsic limitation of the model17. Although the number of studies aimed at characterizing various aspects of the D2-mdx phenotype11,12,13 or using this model to evaluate the efficacy of treatments18,19 is increasing, a complete natural history of mdx mice on a DBA/2J genetic background is not yet available20. Importantly, despite this model being proposed as more severely affected and therefore a better alternative for preclinical studies, D2-mdx mice also display fluctuations in disease severity over time, which may call for caution in data interpretation21.
Regardless of which mouse model is studied, identifying validated tools to monitor disease status and potential response to therapy is of major importance. A tool that can be applied and interpreted analogously in both preclinical and clinical settings would be ideal for this purpose 6,8. One convenient potential technology is electrical impedance myography (EIM)22. In EIM, a very low intensity, painless, high-frequency current is passed across a muscle or muscle group of interest via four linearly placed surface electrodes. The resulting voltage patterns, related to tissue properties, are measured, including resistance (R, i.e., how easily the current passes through the tissue), reactance (X, i.e., how easily the tissue can store an electric charge), and phase angle, or simply phase, ((θ), calculated as θ = arctan (X/R), providing a summative measure, which is less affected than resistance or reactance by tissue volume or electrode spacing or size22. Changes in these parameters can provide information on muscle status and composition in health and disease and the impact of therapy22,23.
Although some aspects of this technique are still in development24, studies in animals and humans support the usefulness of EIM as a primary diagnostic and tracking tool, with its outputs being potential biomarkers for longitudinal assessment of disease course and therapy response, in various conditions affecting muscle and nerves22,23,24,25,26, including muscular dystrophies27. Importantly, EIM has several practical advantages over some other tools (such as magnetic resonance imaging): it is portable and non-invasive, has low-costs, and allows for rapid acquisition time with minimal subject cooperation22. While EIM post-processing can be more challenging, this technique does not require image generation or interpretation and offers the possibility of muscle-specific assessments across different disease stages22,24. Potential EIM applications are especially promising in certain clinical populations, often excluded from functional testing and clinical trials, such as early-symptomatic children or advanced, non-ambulatory patients.
Indeed, this method has already demonstrated sensitivity to detect human disease progression in children under age 7 and the beneficial effects of steroid therapy in DMD patients22,27,28. Moreover, initial studies in BL10-mdx mice revealed that EIM values are altered when compared to wild type (WT) animals, with significant correlations to histological changes29. EIM could also detect the effects of therapeutic interventions (e.g., myostatin inhibition and cell-penetrating peptide conjugated phosphorodiamidate morpholino oligomers (PPMO)) in this model30,31. More recently, explorative studies in D2-mdx mice showed a correlation between EIM and functional outcomes; also, D2-mdx EIM data combined with a predictive algorithm have been proposed as a surrogate measure to estimate muscle pathology32,33. However, to date, these last findings have been obtained at only a single site and in animals belonging to limited age ranges.
Hence, as part of a broader multi-stakeholder research initiative called ‘Of Mice and Measures’ (OMAM) aimed at obtaining comparative data on natural history of DMD murine models20, we further explored EIM’s potential as a translatable tool in dystrophic settings. This was achieved by means of an independent, two-site, longitudinal study conducted in D2-mdx and BL10-mdx mice (8–52 weeks of age), with WT animals from each genetic background as control groups. Moreover, since both study sites had no previous experience with EIM, it would also provide insight into its implementation as an outcome measure in new settings. The ultimate goal of this collaboration was to help identify and advance opportunities for expanded EIM applications in preclinical studies on DMD, further contributing to the definition of the best Standard Operating Procedures (SOPs) for use in selected mouse models.
Results
Phase values in longitudinal configuration are the most reliable and accurate across study sites
Surface EIM measurements were performed monthly at both study sites on the gastrocnemius muscle of male BL10-mdx and D2-mdx mice, compared with WT mice of each strain. In total, 2.65% of the data from the UniBa dataset and 26.88% from the LUMC dataset were excluded due to poor data quality, primarily caused by acquisition errors early in data collection (Supplementary Table 1). More specifically, the number of observations excluded from the LUMC dataset considerably exceeded that from the UniBa dataset, since data acquisition started much earlier at the LUMC, when both teams were still less experienced in identifying low-frequency artifacts, which significantly improved after remote guidance from Dr. Rutkove’s team.
To assess the strength of agreement between the two sites, we calculated the Spearman correlation coefficients for phase, reactance, and resistance obtained from all datapoints (n = 1204) derived from all mice strains at each age analyzed, for both longitudinal and transverse directions (BL10-WT n = 306, BL10-mdx n = 302, D2-WT n = 292 and D2-mdx n = 304). Specifically, we focused on the comparison of EIM data recorded at 105 kHz, which is considered a standard, reliable frequency for surface EIM measurements.
The correlations (Table 1; Fig. 1) between sites were moderate-to-good for phase and reactance (e.g., longitudinal phase: 0.79, P < 0.001, and longitudinal reactance: 0.62, P < 0.01), but worse for resistance: (e.g., longitudinal resistance 0.38, NS). Neither mouse strain nor age cohort seemed to affect the correlation values. Generally, data obtained in the longitudinal configuration were more consistent across sites than those from the transverse direction. Thus, in the following analyses, we focused on data obtained in the longitudinal direction only.
Comparison of 105 kHz EIM data of phase, reactance, and resistance obtained at UniBa and LUMC, being split into cohorts and strains, obtained with current flow both longitudinal and transverse to the major fiber direction. Correlations between observations of the study sites for phase (A), reactance (B) and resistance (C). The colors represent the different mouse cohorts (blue 8-week-old, green 12-week-old, red 28-week-old, purple 52-week-old). The symbols represent the different mouse strains (circle D2-mdx, triangle D2-WT, square BL10-mdx and diamond BL10-WT). Variation in the LUMC group is represented by the vertical darker SD-bars, while that of UniBa by the horizontal lighter SD-bars.
To further investigate the agreement between study sites, we assessed bias and precision, standard readouts obtained as part of the Bland-Altman analysis (Table 1). A small positive bias was observed for the phase, indicating that LUMC values exceeded those of UniBa. Conversely, a large negative bias was found for the reactance and resistance, again, indicating that UniBa values were higher. The bias for the resistance was greater than that of reactance in both directionalities. By far, the best agreement was found for phase with relatively small bias and high precision. Based on these observations, subsequent analyses focused on phase; however, for the sake of completeness and transparency, data on reactance and resistance at this same frequency are also reported herein.
Phase values obtained longitudinally over time are affected by mouse strain and age
Given the consistency in observations for phase between both study sites, we combined the datasets from LUMC and UniBa and assessed whether EIM outcomes were affected by mouse strain and/or disease state (Fig. 2, Supplementary Table 2). In BL10-mdx mice, phase values were consistently higher and stable over time compared to those of BL10-WT mice, with significant differences (P < 0.05) between mdx and WT animals at most of the time points (Fig. 2A, left panel). The reactance values for these mice almost overlapped with those of healthy controls throughout the entire study protocol, except for significant differences observed in 8-week-old mice (Fig. 2B, left panel). Conversely, resistance data resulted in a trend towards lower values, in BL10-mdx vs. WT mice throughout the study (Fig. 2C, left panel).
105 kHz EIM data of the phase, reactance, and resistance obtained monthly for the longitudinal orientation for all animals. Combined EIM data obtained from UniBa and the LUMC for the phase (A), reactance (B), and resistance (C). Data is represented as mean ± SEM. Asterisks indicate a P < 0.05, comparing mdx and WT animals at each time point.
Focusing on D2 mice, phase values had more variability, which exhibited an increase in the age range between 8 and 24 weeks, followed by a plateau in D2-mdx mice only (Fig. 2A, right panel). Moreover, opposite to what was observed in BL10-mdx mice, phase values in D2-mdx mice were consistently lower than those of D2-WTs from 24 weeks of age onwards, with significant differences (P < 0.05) at most time points. This was paralleled by a similar trend observed for reactance data (Fig. 2B, right panel), while no remarkable differences were found between the groups for resistance, except for the 16-week and 40-week time points (Fig. 2C, right panel).
Figure 2 shows the complete data set obtained collectively in all animals at each time point. However, mice were euthanized at 8, 12, and 28 weeks, thus over time, there was a gradually decreasing set of animals being evaluated and week-to-week comparison can be misleading given this gradual loss. Therefore, Fig. 3 shows the longitudinal phase values for only the 10 animals followed out to 52 weeks for each group, both combined across sites and also separately for each site (Supplementary Fig. 1 shows the analogous resistance and reactance data). As is obvious here, there is an offset in values as already identified in the Bland-Altman analysis.
105 kHz EIM data of the phase of only those 10 animals in each group that were carried through to 52 weeks. These results are provided collectively across both sites, as well as separately at each individual site. See Supplemental Fig. 1 for analogous presentation of reactance and resistance values.
When looking separately at the complete data sets obtained at each site (Supplementary Fig. 2), these were consistently in line with those shown in Figs. 2 and 3. Phase data obtained by LUMC generally exceeded those of UniBa, regardless of the strain or age (Supplementary Fig. 2A – B), as well as for reactance values (Supplementary Fig. 2C – D), while resistance values were higher at UniBa (Supplementary Fig. 2E – F).
Histopathological outcomes and correlation to EIM measures
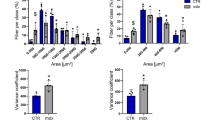
Gastrocnemius muscle sections were acquired at the key endpoints (i.e., 8, 12, 28, and 52 weeks of age) and were stained to assess overall pathology, fibrosis, and calcifications. In both dystrophic strains, the percentage of unhealthy tissue (comprised of fibrosis, necrosis, inflammatory infiltrates, recently regenerated fibers, and calcifications) peaked in the window of 8 − 12 weeks of age (i.e., the acute and active phase of pathology) and were followed by a relative stabilization thereafter8,9,12 Supplementary Fig. 3). Differently, the amount of fibrosis, assessed as collagen deposits in gastrocnemius muscle sections, increased with age (Supplementary Fig. 4), while intramuscular calcifications were almost exclusively observed in D2-mdx mice, especially at younger ages, with levels decreasing in the older cohorts (Supplementary Fig. 5).
Significant relationships between histopathological features and EIM measures were obtained and are summarized in Fig. 4. Significant positive correlations were found between phase values and the percentage of unhealthy tissue and fibrosis in BL10-mdx mice aged 28 and 52 weeks (Fig. 4A, C, respectively; P ≤ 0.04). Conversely, significant negative correlations were observed between phase and the percentage of unhealthy tissue and fibrosis in the D2-mdx strain from 12 weeks of age onwards (Fig. 4B, D; P ≤ 0.01). The more severe histopathology of D2-mdx mice is reflected by the stronger and significant correlations found, with an earlier onset. Notably, despite the ongoing cycles of muscle degeneration and regeneration displayed by dystrophic mice of both strains at the age of 8 weeks, this did not translate into significant correlations with phase values, nor with reactance or resistance values in these cohorts. Importantly, gastrocnemius muscles of D2-mdx mice were characterized by the presence of calcifications, especially in young adult mice, not found in the BL10-mdx strain (Fig. 4E–F). These calcifications were strongly inversely correlated with phase values irrespective of age (P ≤ 0.01). For the reactance and resistance, correlations reached significance at only a few ages especially for D2-mdx mice (Supplementary Figs. 6 and 7).
Spearman correlations between histopathological features and phase values acquired in the longitudinal direction at 105 kHz per strain and age cohort. (A) Overall pathology, consisting of inflammation, fibrosis, necrosis, regeneration in BL10-mdx and BL10-WT mice. (B) Overall pathology in D2-mdx and D2-WT mice. (C) Fibrosis in BL10-mdx and BL10-WT mice. (D) Fibrosis in D2-mdx and D2-WT mice. (E) Calcification in BL10-mdx and BL10-WT mice. (F) Calcification in D2-mdx and D2-WT mice.
Discussion
In this study, we assessed EIM features over 10 months in two different mouse models of DMD: the long-established, classic BL10-mdx mouse, characterized by a very mild disease and typical muscle hypertrophy, and the emerging D2-mdx mouse, which exhibits a more severe phenotype with muscle atrophy, also associated with atypical features including intramuscular calcifications. Unsurprisingly, given the dissimilarity in the character of these two models, EIM profiles for the gastrocnemius muscle were also very different between the two strains. Specifically, when focusing on phase recorded at the frequency of 105 kHz – a simple and reliable feature commonly employed in both humans or animal models22,27,34,35 – we found that gastrocnemius muscles from BL10-mdx mice had consistently and significantly higher phase values vs. BL10-WT controls throughout the study. This phase difference was mainly due to the lower resistance values but similar reactance values in BL10-mdx animals as compared to BL10-WT. Conversely to classic mdx, dystrophic mice on the D2 background, although starting similarly to D2-WT mice, quickly diverged over time, with phase values being markedly and consistently reduced compared to their WT counterparts. This directly resulted from a marked reduction in reactance and a smaller relative reduction in resistance in D2-mdx animals. It is worth noting that the persistent decrease in reactance found in D2-mdx mice is consistent with the dramatically reduced myofiber size typical of this model, this being the primary histological feature exerting the major influence on this value. The absence of a reduction in reactance in BL10-mdx mice is in line with the smaller proportion of small-caliber muscle fibers in these mice. Indeed, in a previous study12, it has been shown via immunofluorescence staining for laminin that 33.8% of the fibers in gastrocnemius muscles of D2-mdx mice have a small size (i.e. with a cross-sectional area between 100 and 500 µm2), compared to the 19.6% found in BL10-mdx mice. However, it is the early and persistently reduced resistance in BL10-mdx mice that caused the higher phase. The explanation for this finding, however, is not immediately evident. While muscle size could provide a sufficient cause (e.g., the gastrocnemius in the BL10-mdx is abnormally hypertrophied compared to WT, with 15% of the fibers > 3000 μm in BL10-mdx mice vs. 2.7% in BL10-WT mice12), if overall muscle hypertrophy had been the sole reason, we would have expected a concomitant decrease in reactance as well. Thus, it is possible that the reduced resistance observed in BL10-mdx mice was due to other factors, such as persistent interstitial edema, or more likely, the presence of fibrosis, which acts as a low-resistance path through the tissue and is known to gradually increase in this mouse model. Importantly, this finding in BL10-mdx was different from that of an earlier study29, which included some of the same authors, as well as a second work by an independent group31. In those earlier studies, both reactance and resistance were reduced in BL10-mdx mice compared to WT counterparts, resulting in a lower phase. Critically, there were some major methodological differences, including the fact that the first work included only two cohorts of mice, one at 2 months and one at 18 months of age, subjected to one-time evaluations. In addition, that study reported data for 50 kHz (and not 105 kHz), which may further justify the differences in the two experimental settings. However, when analyzing the data obtained at 50 kHz in the current work, we confirmed the increase in phase in BL10-mdx vs. BL10-WT (data not shown), also at this frequency. Moreover, while the mdx strains between these two older studies and the current one, were identical, the actual WT animals differed in one29, with that earlier study employing the C57BL/6J as healthy controls and this study and the other earlier study31, more appropriately, using the C57BL/10ScSn/J. However, these WT strains exhibited very similar phase values between the studies, while BL10-mdx values measured here were considerably higher. To summarize, no clear explanation is apparent for these differences, apart from potential issues relating to chow composition, accommodation, or other unmeasurable characteristics and is perhaps worthy of further study. In contrast to the earlier work in BL10-mdx mice, D2-mdx results are consistent with earlier analysis of D2-mdx mice32, which also showed marked reductions in reactance driving the observed reductions in phase.
Moving beyond these comparisons, we were also interested in evaluating the relationship between histological features and EIM parameters. Once again, the picture was very different between the dystrophic mice of the two strains. Indeed, globally, D2-mdx mice showed the expected alterations, with a strong negative correlation observed between unhealthy tissue, fibrosis, and calcifications. These are all features of disease progression, with a decrease generally observed over time in neuromuscular diseases11,12. Intriguingly, the relationship between EIM results and histopathology features in BL10-mdx muscles appears less straightforward. Firstly, given the minimal calcification observed in this model, it is unsurprising that there is no strong correlation between these values. As fibrosis increases, however, we observed a direct relationship with phase. This is consistent with the fact that a higher amount of fibrosis causes a reduction in resistance and is likely to explain the direct relationship with phase since the overall health of the myofibers is relatively preserved in this model29. Given the lack of clear pathophysiological signatures across DMD models, strain-specific validation should be performed before EIM is being used as a biomarker for muscular dystrophies in mice.
One of the underlying reasons for initiating this study was to determine whether two different sites could obtain consistent data using the EIM methodology to evaluate dystrophic mouse models. Investigators from both LUMC and UniBa were rigorously trained onsite at BIDMC by Dr. Rutkove and his team and then provided with EIM devices. Nevertheless, while both EIM devices were intended to be identical, since both were prototypes, there were inevitably small differences between them, including slight variations in electrode length and inter-electrode distance, which could impact the raw resistance and reactance values themselves. Such differences could have resulted in the observed discrepancies in reactance and resistance values, which, to some extent, would be mitigated in the calculation of the phase and may help explain why that parameter showed the highest consistency. It could also explain the consistent deviations observed in Fig. 1, for both correlation and Bland-Altman plots. Device variability is an important aspect to acknowledge, particularly in multi-site studies, since differences in device geometry may introduce site-specific bias. As EIM continues to develop toward broader preclinical and clinical applications, future efforts should aim to standardize devices and to establish cross-platform calibration procedures to minimize site-specific bias and ensure reproducibility across studies. It is worth mentioning that the on-site training took place before the start of the COVID-19 pandemic, but the actual study began 11 months later during the pandemic, which considerably slowed down animal studies and data collection at both institutions. Data collection started at the LUMC, and their experiences and results were discussed with the BIDMC and UniBa teams after the first cohorts were included. Several acquisition errors were identified by Dr. Rutkove which led to revision of the protocol and data quality checks, which were implemented directly at the start of the data acquisition at UniBa. Consequently, the LUMC dataset suffers from higher exclusion rates for the 8- and 12-week timepoints. This intervention significantly improved data quality, with both groups pursuing data collection separately with greater independence. Despite this, the two laboratories demonstrated moderate to good concordance for phase values in both longitudinal and transverse configurations, with longitudinal assessments performing better overall. Another reason for these discrepancies is likely attributable to the smaller hind limb size of D2 mice, especially dystrophic ones, which makes the proper placement of the surface electrode array over the entire gastrocnemius muscle quite challenging24. Globally, the consistency of results produced at the two sites supports that longitudinal, parallel validation can be performed successfully at independent laboratories, though it is recommended to focus on longitudinal orientation measurements on gastrocnemius muscle in this specific model. Clearly, the study of additional mouse and perhaps rat models would also be warranted. Recent advances in surface EIM technology have enabled the generation of smaller-sized electrodes applicable for assessments in other species such as zebrafish36. These next-generation electrodes could further enhance specificity and data reproducibility in mice.
In addition to these broader points, there are other limitations to this study. First, we did not attempt a week-by-week comparison between the two sites, in part because the animal numbers were simply too small and would likely lead to false conclusions and excessive number of analyses. Second, in the correlations with histology (Fig. 4), we pooled the data from both sites. Given the variation in the raw values in both sites, performing these analyses separately may have yielded higher correlation values, but doing so would also substantially reduce our sample sizes. In the end, these correlations can be interpreted as more conservative assessments. Third, our histological assessments of collagen at the two sites were completed using different staining techniques (Sirius Red and Masson’s trichrome), reflecting previous experience and expertise. This could lead to additional errors, but since both techniques are essentially comparable, we believe any new variation introduced by this difference to be modest.
In conclusion, our study demonstrated distinct, yet generally consistent, impedance signatures over 10 months in two selected DMD mouse models. Also, we confirmed that EIM results correlate with histological features in these mice, with stronger associations observed in the more severe D2-mdx model33. This, together with the recently explored correlations between EIM parameters and in vivo functional outcomes in D2-mdx mice32, will contribute to further validating EIM as biomarker for disease severity and therapeutic response. Importantly, our results indicate that, with proper training, the EIM technique can be implemented at different research sites. To allow application of EIM in preclinical studies for DMD, including evaluation of therapeutic interventions, future efforts should focus on the standardization of the EIM probes and cross-platform calibrations to further increase reproducibility in multi-site studies.
Finally, while the current work focused on the BL10-mdx and D2-mdx models, in line with the overall objectives of the OMAM initiative, future studies may extend the use of EIM to additional DMD models carrying patient-relevant mutations, as well as large animal models, as has already been done in the canine form of DMD40. This will further enhance its translational relevance to human studies, especially considering EIM’s non-invasiveness, portability, and ease of use across different muscles and disease stages, features that have already shown promise in clinical studies involving patients with DMD and other neuromuscular disorders.
Materials and methods
Experimental animals
Study data collection was performed at two sites: the University of Bari (UniBa) in the group of Prof., Dr. De Luca, and the Leiden University Medical Center (LUMC) in the group of Prof., Dr. Aartsma-Rus/Dr. van Putten. Initial training of these two groups in the EIM technique and data analysis in the main persons of Dr. van Putten and Dr. Mantuano, was completed with the assistance of Dr. Rutkove’s group onsite at the reference site Beth Israel Deaconess Medical Center/Harvard Medical School in Boston (BIDMC).
At UniBa, 34 males of each of the following strains: C57BL/10ScSn/J wild types (BL10-WT), C57BL/10ScSn-Dmdmdx/J (BL10-mdx), DBA/2J (D2-WT) and D2.B10-Dmdmdx/J (D2-mdx) (a total of 136 animals) were purchased from The Jackson Laboratory (USA, distributed by Charles River, Calco, Italy). Mice were housed in cages (2–5 mice per cage) filled with sawdust and bedding (LIGNOCEL 3–4 S, Charles River Laboratories) at 22–24 °C, with a humidity of 50–60%, and 12 h light/12 h dark cycles. Each mouse was provided with water ad libitum and with a daily portion of chow (RM3, Special Diets Services (SDS), Essex, United Kingdom) amounting to 5 g per mouse. This predetermined quantity, which significantly surpassed the typical daily intake of ~ 3 g per mouse, enabled the experimenter to effectively monitor for any noticeable differences in food consumption between the groups. Notably, no such deviations were observed. The study was performed in conformity with the Italian law for Guidelines for Care and Use of Laboratory Animals (D.L. 116/92), and European Directive (2010/63/UE), while the experimental protocol was approved by the Italian Ministry of Health (Authorization n. 1119/2020-PR).
At the LUMC, 34 males of each of the four strains were generated with breeding couples obtained from The Jackson Laboratory. Two to five mice were group housed in individually ventilated cages (Makrolon type II) filled with sawdust and enriched with nesting (Bed-r’Nest BRN8SR) and bedding (LIGNOCEL BK-8-15-00433) materials and a cardboard tunnel (GLP fun tunnels mini-1022006). Ad libitum access to water and standard RM3 chow (SDS) was provided to the mice. The study was approved by the Animal Ethics Committee of the LUMC (AVD 1160020171407, PE.17.246.037) and executed conform with the Directive 2010/63/EU of the European Parliament. The laboratories complied with the ARRIVE guidelines and all methods were carried out in accordance with relevant guidelines and regulations.
Study setup
At each study site, mice were randomly assigned to various cohorts that underwent identical parallel EIM evaluations, with the primary distinction being the time selected for euthanasia. Surface EIM measurements of the gastrocnemius were performed once a month from the age of 8 weeks onwards until the day of sacrifice (except for the 48-week time point, due to temporary unavailability of operators). The mice were euthanized within a week of the EIM recording at either 8 weeks (n = 8 per strain), 12 weeks (n = 8 per strain), 28 weeks (n = 8 per strain), or 52 weeks (n = 10 per strain). To allow direct correlations with muscle histology, the gastrocnemius was isolated and processed to assess different aspects of histopathology. This identical cross-sectional study design of both study sites allowed us to analyze a total of n = 68 males per strain (across the two sites) at the age of 8 weeks, dropping to n = 20 mice per strain at the age of 52 weeks (Supplementary Table 1).
Electrical impedance myography
To assess muscle conductivity, surface EIM experiments were performed at both study sites in an identical manner, utilizing the bioimpedance spectroscopy device EIM 1103 system obtained from Myolex Inc22. The surface electrode consisted of four metal electrodes with a distance of 2.16 mm between them. The two outer plates were 4.27 mm long, while the two inner ones were 3.23 mm long. Mice were anesthetized with isoflurane (4% induction, 2% maintenance and 1.5% O2 l/min), and the fur covering the posterior hind limb was removed with depilatory cream (Nair™), applied twice, each for 1 min maximum. The cream was delicately, but completely, removed with moist saline gauze, after which the leg, with the gastrocnemius muscle facing upwards, was taped to the measuring surface at a 45⁰ angle to the body, away from the head. After calibration with saline, resistance and reactance data were obtained during three to four individual repeated tests, from which the phase was calculated. Impedance was assessed in the transverse and longitudinal configurations. For the transverse direction, the electrode array was oriented along the myofibers, allowing the current to run across the myofibers. For the longitudinal direction, the array was oriented perpendicularly to the fiber direction, allowing the current to run parallel to the fibers. As myofibers run from tendon to tendon, currents can run more easily along the fibers than across them. The impedance data were collected across multiple frequencies (ranging from 8 kHz to 8.4 MHz) per timepoint per mouse. During the experimental session, the surface area was continuously kept moist with saline gauze.
Histology
At the ages of 8, 12, 28, or 52 weeks, mice were euthanized by cervical dislocation, and the gastrocnemius muscle was dissected, mounted on cork, and snap-frozen in liquid nitrogen-cooled isopentane. At UniBa, frozen gastrocnemius muscles were transversally sliced in a cryostat microtome set at − 20 °C (HM 525 NX, Thermo Fisher Scientific, Waltham, MA, USA), to obtain 10 μm-thick cross-sections, placed on Superfrost™ Plus slides (Thermo Fisher Scientific)37. Similarly, at LUMC, sections of 8 μm thickness were generated with a CM3050 S Cryostat (Leica Biosystems, Buffalo Grove, IL, USA) and placed on Superfrost™ Plus Adhesion microscope slides (Epredia, Braunschweig, Germany). Samples at both study sites were stored at − 80 °C until further processing.
Overall muscle pathology staining
The Hematoxylin and Eosin (H&E) staining was performed at both sites to assess overall pathology in dystrophic mice muscles – namely, fibrosis, necrosis, inflammatory infiltrates, recently regenerated fibers, and calcifications – herein altogether defined as “unhealthy tissue”.
At UniBa, slides were thawed at room temperature for approximately 20 min and then stained with filtered Carazzi’s hematoxylin (Bio-Optica, Milan, Italy) for 10 min. Thereafter, the slides were rinsed twice with tap water. Destaining was performed in acid ethanol, after which the sections were rinsed in tap water for 3 min. Sections were then stained with eosin (Bio-Optica) for 3 min, followed by quick immersion in distilled water. After this, they were placed in 70%, 80%, and 100% ethanol for 1 min each. The slides were incubated in xylene overnight and then mounted with Eukitt® mounting medium (Bio-Optica), which was used to place the cover slip on the slides. Images covering the entire section of the muscle were acquired at 4× magnification by using a brightfield microscope (CL-I Eclipse Nikon) and then stitched via the Photomerge feature in Adobe Photoshop v.20.0.637.
At LUMC, slides were thawed at room temperature for 30 min, fixed with ice-cold acetone for 5 min, and air-dried for 30 min. Thereafter, the slides were rinsed with water and stained for 3 min with Mayer’s hematoxylin (Agilent, Santa Clara, CA, USA). The stain was developed with running tap water for 5 min. Destaining was performed in acid ethanol after which the sections were rinsed twice in tap water for one min and once in deionized water for 2 min. Sections were then stained with eosin (Sigma-Aldrich, Saint Louis, MO, USA) for 30–45 s after which they were placed in 80%, 90%, and 100% ethanol for 1 min each, followed by two times for 5 min in xylene. Slides were mounted with PERTEX® mounting medium (HistoLab, Askim, Sweden) and images covering the entire slide were captured with the 3DHISTECH PANNORAMIC® 250 Flash III slide scanner at a 20× magnification. Thereafter, a whole muscle cross-section representing the middle part of the muscle was selected, saved as tiff image and used for subsequent analyses.
At both sites, the percentage of unhealthy tissue was quantified in a blinded manner by two to three experimenters via a semi-automated process using the ImageJ® software version 1.53a (https://imagej.net/ij/) colour deconvolution plugin (https://imagej.net/Deconvolution), which separates the haematoxylin component (displaying the nuclei) from the eosin component (displaying the intact muscle fibers, but excluding the unhealthy parts of the muscle). The eosin channel was used to measure the percentage of healthy tissue, which was subtracted from the total area to calculate the percentage of unhealthy tissue38.
Fibrosis staining
The Masson’s trichrome staining and the Sirius Red staining were used for the visualization of collagen, as a marker for fibrosis. Both dyes detect most collagen types and tend to reveal similar trends in fibrosis. However, the use of different staining methods could have added variability.
At UniBa, the Masson’s trichrome staining (all reagents from Bio-Optica), was used. To this end, sections were thawed at room temperature for approximately 20 min, and then stained with filtered Carazzi’s hematoxylin for 10 min. Thereafter, the slides were rinsed with running tap water for about 3 min and then stained with picric acid solution for 4 min, followed by quick immersion in distilled water. After this, slides were stained with Ponceau B solution for 3 min. Slides were then rinsed with distilled water and stained with phosphomolybdic acid solution for 8 min. Without rinsing, the slides were stained with aniline blue solution for 4 min. Slides were rinsed with distilled water and dehydrated (1 min in 70%, 80%, and 100% ethanol, followed by two times 5 min in xylene) and then mounted with Eukitt®. Images covering the entire section of the muscle were acquired at 4× magnification by using a brightfield microscope (CL-I Eclipse Nikon) and then stitched via Photomerge feature in Adobe Photoshop v.20.0.6. Positive (blue) areas were quantified by using ImageJ® by two blinded experimenters and normalized to the total area37.
At the LUMC, fibrosis was assessed with the Sirius Red staining. Sections were kept at room temperature for 30 min. They were fixed in 4% paraformaldehyde for 10 min and in 100% ethanol for 5 min, after which they were air dried for 30 min. Slides, rinsed with deionized water were then stained with Sirius Red solution for 45 min and washed twice with Acetic Acid water for 5 min. Slides were rinsed with deionized water and dehydrated (1 min in 80%, 90% and 100% ethanol, followed by two times 5 min in xylene). Thereafter, slides were mounted with PERTEX® mounting medium. Images were taken with the 3DHISTECH PANNORAMIC® 250 Flash III slide scanner at a 20× magnification covering the entire slide. Thereafter, a whole muscle cross-section representing the middle part of the muscle was selected, saved as tiff image and used for subsequent analyses. The percentage of fibrosis, excluding the perimysium, but including fibrosis in inflammatory regions in the section, was assessed with ImageJ (version 1.53a, https://imagej.net/ij/) by two to three blinded experimenters.
Muscle calcification staining
Muscle calcification was assessed with Alizarin Red staining at both sites. Again, frozen sections were left at room temperature for 30 min before they were fixed with ice-cold acetone for 10 min. After being left to dry for 30 min, the slides were stained with Alizarin Red S solution for 1 min and rinsed in acetone for 30 s and in acetone/xylene (1:1) for 15 s. Slides were then placed in xylene for 1.5 h in total, while refreshing the solution once. After mounting the slides with mounting medium (Eukitt® for UniBa, PERTEX® for LUMC), these were dried. At UniBa, a whole muscle section was imaged with the CL-I Eclipse Nikon brightfield microscope at 4× magnification; single images acquired were stitched via Photomerge feature in Adobe Photoshop v.20.0.6. At LUMC, the whole glass slide was imaged with the 3DHISTECH PANNORAMIC® 250 Flash III slide scanner at 20× magnification, and the muscle cross-sectional area representing the middle part of the muscle was selected and used for analyses. The percentage of calcification was assessed with ImageJ (version 1.53a, https://imagej.net/ij/) by two blinded experimenters.
Statistical analysis
EIM surface data for both study sites were reviewed for quality. Data affected by artifacts due to poor electrode contact or erratic multifrequency data were excluded. The outlier removal process was carried out in two phases for each of the impedance data collected across multiple frequencies per timepoint per mouse. First, the low-frequency range, which included data up to 105 kHz, was checked for outliers, considering the noise effect on the low frequencies, and when these were identified, the entire measurement (spanning all frequencies), was excluded from further analysis. Secondly, as some outliers were not detectable using statistical methods alone, the entire frequency range was checked for additional outliers and excluded if they demonstrated atypical artifacts, including dramatic frequency-to-frequency variation or non-physiological patterns. Following outlier removal, repeated measurements (ranging from one to four per mouse) were averaged, to obtain a single measurement, set per mouse. We selected the data extracted from the 105 kHz frequency for further analyses given strong reliability at approximately this frequency on previous studies in animal models and humans22,27,34,35. All statistical analyses were performed using Python (v3.12.4) with the following libraries: pandas (v2.2.3), matplotlib (v3.8.4), scipy (v1.13.1), and stats models (v0.14.2).
Spearman correlation coefficients were calculated to compare measurements between the two study sites. This approach was chosen so as to quantify the average consistency of measurements across each group relative to one another. Bland-Altman analyses were also performed, including calculation of precision and bias38.
To compare the four mouse strains across multiple time points (8, 12, 16, 20, 24, 28, 32, 36, 40, 44 and 52 weeks of age), one-way ANOVA with Tukey post-hoc testing was performed at each time point. Correlation analyses between histological parameters and EIM measurements across cohorts (8, 12, 28, and 52 weeks of age) were conducted using Spearman’s rank correlation coefficient due to its robustness in handling non-parametric data and its suitability for identifying monotonic relationships. Distributions of measurements were summarized as mean and standard error of the mean (SEM). Data were considered significant with P < 0.05.
Data availability
Data is provided within the manuscript and supplementary information files.
References
Duan, D., Goemans, N., Takeda, S. & Mercuri, E. Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers. 7, 13 (2021).
Gatto, F., Benemei, S., Piluso, G. & Bello, L. The complex landscape of DMD mutations: moving towards personalized medicine. Front. Genet. 15, 1360224 (2024).
Gao, Q. Q. & McNally, E. M. The dystrophin complex: structure, function, and implications for therapy. Compr. Physiol. 5, 1223–1239 (2015).
Birnkrant, D. J. et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and Gastrointestinal and nutritional management. Lancet Neurol. 17, 251–267 (2018).
Dang, U. J. et al. Efficacy and safety of Vamorolone over 48 weeks in boys with Duchenne muscular dystrophy: A randomized controlled trial. Neurology 102, e208112 (2024).
Willmann, R. et al. Improving translatability of preclinical studies for neuromuscular disorders: lessons from the TREAT-NMD advisory committee for therapeutics (TACT). Dis. Model. Mech. 13, dmm042903 (2020).
Sicinski, P. et al. The molecular basis of muscular dystrophy in the Mdx mouse: a point mutation. Science 244, 1578–1580 (1989).
Grounds, M. D., Radley, H. G., Lynch, G. S., Nagaraju, K. & De Luca, A. Towards developing standard operating procedures for pre-clinical testing in the Mdx mouse model of Duchenne muscular dystrophy. Neurobiol. Dis. 31, 1–19 (2008).
Massopust, R. T. et al. Lifetime analysis of Mdx skeletal muscle reveals a progressive pathology that leads to myofiber loss. Sci. Rep. 10 (1), 17248 (2020).
Fukada, S. et al. Genetic background affects properties of satellite cells and Mdx phenotypes. Am. J. Pathol. 176, 2414–2424 (2010).
Coley, W. D. et al. Effect of genetic background on the dystrophic phenotype in Mdx mice. Hum. Mol. Genet. 25, 130–145 (2016).
van Putten, M. et al. Natural disease history of the D2-mdx mouse model for Duchenne muscular dystrophy. FASEB J. 33, 8110–8124 (2019).
Hammers, D. W. et al. The D2.mdx mouse as a preclinical model of the skeletal muscle pathology associated with Duchenne muscular dystrophy. Sci. Rep. 10, 14070 (2020).
Heydemann, A. et al. Latent TGF-beta-binding protein 4 modifies muscular dystrophy in mice. J. Clin. Invest. 119, 3703–3712 (2009).
Flanigan, K. M. et al. LTBP4 genotype predicts age of ambulatory loss in Duchenne muscular dystrophy. Ann. Neurol. 73, 481–488 (2013).
Swaggart, K. A. et al. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proc. Natl. Acad. Sci. U S A. 111, 6004–6009 (2014).
Korff, S. et al. Fine mapping of Dyscalc1, the major genetic determinant of dystrophic cardiac calcification in mice. Physiol. Genomics. 25, 387–392 (2006).
Kennedy, T. L. et al. Micro-utrophin improves cardiac and skeletal muscle function of severely affected D2/mdx mice. Mol. Ther. Methods Clin. Dev. 11, 92–105 (2018).
Bellissimo, C. A. et al. The slow-release adiponectin analog ALY688-SR modifies early-stage disease development in the D2.mdx mouse model of Duchenne muscular dystrophy. Am. J. Physiol. Cell. Physiol. 326, C1011–C1026 (2024).
Gordish-Dressman, H. et al. Of mice and measures: A project to improve how we advance Duchenne muscular dystrophy therapies to the clinic. J. Neuromuscul. Dis. 5, 407–417 (2018).
Aartsma-Rus, A., van Putten, M., Mantuano, P. & De Luca, A. On the use of D2.B10-Dmdmdx/J (D2.mdx) versus C57BL/10ScSn-Dmdmdx/J (mdx) mouse models for preclinical studies on Duchenne muscular dystrophy: A cautionary note from members of the TREAT-NMD advisory committee on therapeutics. J. Neuromuscul. Dis. 10, 155–158 (2023).
Rutkove, S. B. & Sanchez, B. Electrical impedance methods in neuromuscular assessment: an overview. Cold Spring Harb Perspect. Med. 9, a034405 (2019).
Mortreux, M. et al. Performing in vivo and ex vivo electrical impedance myography in rodents. J. Vis. Exp. 84, 10379163513 (2022).
Sanchez, B., Rutkove, S. B. & Present Uses Future applications, and technical underpinnings of electrical impedance myography. Curr. Neurol. Neurosci. Rep. 17, 86 (2017).
Rutkove, S. B. et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotroph. Lateral Scler. 13, 439–445 (2012).
Rutkove, S. B., Gregas, M. C. & Darras, B. T. Electrical impedance myography in spinal muscular atrophy: a longitudinal study. Muscle Nerve. 45, 642–647 (2012).
Rutkove, S. B. et al. Electrical impedance myography for assessment of Duchenne muscular dystrophy. Ann. Neurol. 81, 622–632 (2017).
Leitner, M. L. et al. Electrical impedance myography for reducing sample size in Duchenne muscular dystrophy trials. Ann. Clin. Transl Neurol. 7, 4–14 (2020).
Li, J. et al. Electrical impedance myography for the in vivo and ex vivo assessment of muscular dystrophy (mdx) mouse muscle. Muscle Nerve. 49, 829–835 (2014).
Sanchez, B. et al. Evaluation of electrical impedance as a biomarker of myostatin Inhibition in wild type and muscular dystrophy mice. PLoS One. 10, e0140521 (2015).
Hiyoshi, T. et al. Electrical impedance myography detects dystrophin-related muscle changes in Mdx mice. Skelet. Muscle. 13, 19 (2023).
Chrzanowski, S. M., Nagy, J. A., Pandeya, S. & Rutkove, S. B. Electrical impedance myography correlates with functional measures of disease progression in D2-mdx mice and boys with Duchenne muscular dystrophy. J. Neuromuscul. Dis. 10, 81–90 (2023).
Pandeya, S. R. et al. Estimating myofiber cross-sectional area and connective tissue deposition with electrical impedance myography: A study in D2-mdx mice. Muscle Nerve. 63, 941–950 (2021).
Verga, S. A. et al. Electrical impedance myography in healthy dogs: normative values, repeatability, and the impact of age. Front. Vet. Sci. 9, 1025528 (2022).
Rutkove, S. B. et al. Loss of electrical anisotropy is an unrecognized feature of dystrophic muscle that May serve as a convenient index of disease status. Clin. Neurophysiol. 127, 3546–3551 (2016).
Rutkove, S. B. et al. Surface electrical impedance myography detects skeletal muscle atrophy in aged wildtype zebrafish and aged gpr27 knockout zebrafish. Biomedicines 11, 1938 (2023).
Boccanegra, B. et al. A. Growth hormone secretagogues modulate inflammation and fibrosis in Mdx mouse model of Duchenne muscular dystrophy. Front. Immunol. 14, 1119888 (2023).
van Putten, M. et al. A 3 months mild functional test regime does not affect disease parameters in young Mdx mice. Neuromuscul. Disord. 20, 273–280 (2010).
Bland, J. M. & Altman, D. G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 8, 135–160 (1999).
Hakim, C. H. et al. Non-invasive evaluation of muscle disease in the canine model of Duchenne muscular dystrophy by electrical impedance myography. PloS One, 12(3), e0173557 (2017).
Acknowledgements
This study has been funded by Charley’s Fund and Duchenne UK, in the framework of the OMAM “Of Mice And Measures” project, with funds granted to Annemieke Aartsma-Rus and Annamaria De Luca, that are both kindly acknowledged for reading the manuscript and for support. Special thanks to TUBITAK (2219-International Postdoctoral Research Fellowship Program for Turkish Citizens) for providing the scholarship for postdoctoral studies of Buket Sonbas Cobb.
Author information
Authors and Affiliations
Contributions
PM; conceptualization, data collection, data analysis, writing manuscript, supervision. MvP; conceptualization, data collection, data analysis, writing manuscript, supervision. BSC; data analysis. KP; data collectio. nBB; data collection. CTdW; data collection. LT; data collection. AS; data collection. OC; data collection. DvdV; data collection. SE; data collection. SP; data collection. JN; data analysis. SR; training, data analysis, writing manuscript, supervision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Rutkove has equity in and serves as a consultant and scientific advisor to Myolex Inc., a company that designs impedance devices for clinical and research use and used in this study; he is also a member of Myolex’s Board of Directors. The company also has an option to license patented impedance technology of which Dr. Rutkove is named as an inventor. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mantuano, P., van Putten, M., Cobb, B.S. et al. A two-site collaborative study of electrical impedance myography for evaluation of disease progression in murine Duchenne muscular dystrophy models. Sci Rep 15, 33210 (2025). https://doi.org/10.1038/s41598-025-17137-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17137-4