Abstract
Geriatric syndromes are posing an increasing health threat in an aging population. The reliable indicator of geriatric syndromes is of great clinical value for early diagnosis and intervention. To investigate the potential application of slow gait speed as a signal for identifying common geriatric syndromes in the elderly. A total number of 985 elderly outpatients (457 men and 528 women) were recruited in the study. The subjects were classified into two groups according to the gait speed cut-off (1.0 m/s), with the individuals being assigned as normal speed group (NSG) when the gait speed ≥ 1.0 m/s and the slow speed group (SSG) was defined as the gait speed < 1.0 m/s. CGA management system Simply Edition (CGA-SE) software was implemented to collect data, compare the demographic variations and assess the prevalence of functional decline in the two groups. Compared to the NSG, SSG subjects were significantly older, shorter in height, lighter in weight and consumed more medicine. SSG subjects also showed a higher score in Edmonton symptom assessment, Self-Rating Depression Scale (SDS), Self-rating Anxiety Scale (SAS), and Mini Nutritional Assessment (MNA), and had a lower score in Barthel index of Activities of Daily Living (BADL) assessment and Mini-Mental State Examination (MMSE). There was a significantly higher prevalence of frailty, disability, depression, and dementia in SSG when compared to NSG. In addition, gait speed was an independent predictive factor associated with a higher risk of frailty, disability, dementia, and swallowing dysfunction. Slow gait speed could be used as an indicator for several common geriatric syndromes in elderly outpatients. We recommended the 6 m walk test as a routine examination for the elderly in the primary hospitals.
Similar content being viewed by others
Introduction
Global aging has arrived, in China the pace of population aging is dramatically fast and would reach its peak of approximately 385 million by 20581. To fill the foreseen gap between inadequate geriatricians and the growing elder population, the government has required half of the primary hospitals should establish geriatric departments before 20252. So there is an urgent demand for expanding the training of physicians with comprehensive geriatric assessment (CGA) and management of geriatric syndromes (GS). This is especially necessary for the clinician of primary care, and hospital-based community health services, who will be on the front line in the care of the growing population of the elderly3.
The core components of the CGA are functional status, including cognition, mobility, and comorbidities with a focus on geriatric syndromes, polypharmacy, mood, nutrition, and so on4. It involves the application of functional scales to quantify the manifestations of complicated symptoms and behavioral performance related to daily life functions, and helps geriatricians to find risky domains and body disorders5. The use of CGA has been widely accepted in geriatric therapy with multiple benefits being reported6, but it also suffers from obvious limitations. Firstly, it is difficult for some elder people with impaired hearing, sight and poor understanding or movement to answer abundant questions or perform specific motions. Secondly, CGA mainly replies on the physician’s judgment. If the physician lacks training about those functional scales, the diagnosis might be unreliable. In addition, the implementation of CGA is time-consuming even for the experienced geriatrician. So it is impractical for a general physicians to screen geriatric syndromes based on lots of unfamiliar scales in a limited time.
Some of the prevalent manifestation indicators may be used for the preliminary screening of geriatric syndromes in the elderly. Former studies have shown that the prevalence of sarcopenia was about 13.1–14.9% in elderly men and 11.4% in elderly women in Korea7; the incidence of frailty was estimated to be 18% in the elderly in the Europe8; the rate of mild cognitive impairment among Chinese elderly was about 15.4%9; Activities of daily life (ADL) impairment was significantly more pervasive in old-old participants (12.7%) than in young-old ones (3.0%) in Korea10, and the disability of the elder in Palestinian population was at high incidence (31.2%)11. Sarcopenia or other geriatric syndromes usually leads to falls, morbidity and the undermined quality of life, which incurs a high level of financial burden on the health and social systems12. The identification of reliable manifestation indicator for geriatric syndromes is conducive to early diagnosis and intervention.
Sarcopenia is defined as age-related skeletal muscle atrophy, the reduced muscle strength and/or impaired physical performance13, which is closely associated with with frailty14, cognitive impairment15, and disability16. According to Asian Working Group for Sarcopenia (AWGS) 2019 consensus17, sarcopenia diagnosis is based on the evaluation of skeletal muscle mass, grip strength, and balance ability. Bioelectrical impedance analysis (BIA) and Dual-energy X-ray absorptiometry (DXA) have been widely used to evaluate skeletal muscle mass; pinch meter equipment is designed to evaluate the grip strength, and 6 m walk test has been implemented to assess the walking performance. However, many primary hospitals in underdeveloped regions or countries are not equipped with BIA, DXA, even pinch meter equipment for routine examinations. So the gait speed test (over a 6-m course) becomes an economic way for sarcopenia screening without special instruments and expensive cost.
Actually, European Working Group on Sarcopenia in Older People (EWGSOP) 2010 had recommended the gait speed test18 as a practical evaluation of sarcopenia in ten years earlier. Although the 5-times chair stand test or Short-Physical Performance Battery (SPPB) score have been formulated in the recent AWGS consensus 201917, gait speed test still stands a place due to its easy implementation. Since sarcopenia has a wide interaction with several geriatric syndromes, we hypothesized that a slower gait speed implies a higher risk of geriatric syndromes. Therefore we recruited elder outpatients and classified the subjects into two groups: the normal speed group (NSG) and slow speed group (SSG) according to the gait speed cut-off (1.0 m/s). Through comprehensive analysis of the demographic variations and functional cognitive performance in the two groups, we concluded that slow gait speed could be used as an indicator for several common geriatric syndromes in elderly outpatients. 6 m walk test is recommended as a routine examination for the elderly in the primary hospitals.
Methods
Study design and participants
This study was a retrospective study conducted in Yunnan province located in southwestern China. An independently developed CGA management system was used as the database for the recruited subjects. The participants were obtained from different regions in Yunnan, such as the cities of Kunming, Qujing, Kaiyuan, Chuxiong, and Wenshan, from December 2021 to December 2024. The inclusion criteria were as follows: (1) geriatric patient over 60 years; (2) complete CGA ; (3) the ability to walk for 6 m independently or with assistive devices or with a companion. The exclusion criteria were as follows: (1) incomplete assessment owing to physical dysfunction such as severe cardiac insufficiency, respiratory failure, acute cerebrovascular diseases, cognitive impairment, and disability or disagree with assessment; (2) communication defects due to the loss of hearing or sight. Finally, 985 cases were included. Each case recording healthy information including height, weight, age, gender, BMI, gait speed, the status of chronic pain, comorbidity, polypharmacy, fall, functional scales of frailty, mood, cognition, nutrition, and dysphagia. This study protocol was approved by the Research Ethics Committee of the First People’s Hospital of Yunnan province (Ethics approval number: KHLL2021-KY038), and all the personal sensitive information was removed from the data and had no information that could lead to the identification of a participant. All methods were performed in accordance with the relevant guidelines and regulations.
CGA management system
This management system was established by the geriatric department of the First People’s Hospital of Yunnan Province. It consisted of generally used assessment scales: Edmonton Frail Scale (EFS)19, Barthel index of Activities of Daily Living (BADL) assessment20, Self-Rating Depression Scale (SDS)21 and Self-rating Anxiety Scale (SAS)22 for mood, Mini-Mental State Examination (MMSE) for cognition performance23, Mini Nutritional Assessment (MNA) for nutrition24, water swallow test (WST) for swallow function25. The database stored the assessment scale score, diagnosis of dysfunction and severity, also stored details of each scales. Participants experiencing continuous pain which seriously undermined the daily life quality are classified as chronic pain. Those who had more than two kinds of chronic diseases and required long-term treatment26 were classified as comorbidity. Cases taking more than five medications at a time were classified as polypharmacy. We only counted the regular medications that patients take daily for chronic diseases, excluding vitamins, dietary supplements, as well as short-term prescriptions and over-the-counter (OTC) medications. Cases had falls in recent one year were classified as high risk of falls. In this study, the gait speed test was performed in accordance with the standardized protocol for the 6-meter walk test as specified by AWGS (2019). The participants could complete 6 m straight-walking test by themselves or with assistance of a companion or facilities. According to AWGS 2019 consensus17, the elderly walked with a gait speed over 1.0 m/s was classified as good performance of movement, otherwise classified as the performance of sarcopenia.
To eliminate bias, every CGA evaluators among this study has homogeneous training.
Statistical analyses
To compare the height, weight, age, BMI, and scores of EFS, BADL, SDS, SAS, MMSE, and MNA, a two-tailed unpaired Student’s t-test was employed for statistical analysis and the values were presented as mean ± standard deviation. With categorical variables like sex, age group, BMI degree, chronic pain, comorbidity, polypharmacy, fall, frailty degree, disability degree, depression, anxiety degree, education, malnutrition degree, and dysphagia degree, a chi-square test was conducted. Bonferroni’s adjustment was used for the correction of multiple comparisons. All statistical analyses were performed using GraphPad Prism, version 9.4. P values < 0.05 were considered to be statistically significant.
Results
According to the AWGS 2019 recommendation, in Asian gait speed cut-off was 1.0 m/s to indicate physical function decline. The enrolled subjects were classified into two groups, the normal speed group (NSG, gait speed ≥ 1.0 m/s) which comprised 11.88% of total cases (men, 52.14%; women, 47.86%), and the slow speed group (SSG, gait speed < 1.0 m/s) which comprised 88.12% of total cases (men 45.62%, women 54.38%). There was no sex-related difference in the proportions between these two groups(X2 = 1.759, p = 0.200) (Table 1).
Characteristics of participants
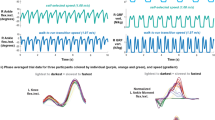
The result of the non-parametric test for continuous variables was expressed by median and quartile. Compared to NSG, SSG showed significant differences (p < 0.0001) in several characteristics. SSG subjects were much older in age (age: 77 vs. 72 years), shorter in height (height: 159 vs. 162 cm), lighter in weight (weight: 55 vs.59 kg), relied on more types of drugs (number: 4 vs. 3), displayed a higher score in Edmonton (score: 8 vs. 4, frailty cutoff ≥ 6), SDS (T score: 52.5 vs. 42.5,depress cutoff ≥ 50), SAS (T score: 37.5 vs. 31.25,anxiety cutoff ≥ 50) and MNA (score: 16 vs.14,malnutrition cutoff ≤ 24). In contrast, there were significantly lower scores of BADL (score: 95 vs. 100, disability cutoff ≤ 90) and MMSE (score: 22 vs. 28, according to the educational level, in normal education dementia cutoff ≤ 27 ) in SSG group. Although participants in SSG were shorter and lighter than those of NSG, the body weight index (BMI) between the two groups showed no significant difference (p = 0.325). The number of diagnosed disorders were also similar between NSG and SSG (p = 0.752). (see Table 2; Figs. 1 and 2).
Characteristic of participant in two group. The individuals was assigned as normal speed group (NSG) when the gait speed ≥ 1.0 m/s and the slow speed group (SSG) was defined as the gait speed < 1.0 m/s. Individuals in SSG had significant difference compared to NSG in age, height, weight, BADL score, SDS T score and SAS T score, which marked as “*“( p < 0.05).
Characteristic of participant in two group(continue). The individuals was assigned as normal speed group (NSG) when the gait speed ≥ 1.0 m/s and the slow speed group (SSG) was defined as the gait speed < 1.0 m/s. Except of numbers of diagnosis and BMI, participant in SSG had significant difference compared to NSG in numbers of drugs, Edmonton score, MNA score, MMSE score which marked as “*“( p < 0.05).
Prevalence of geriatric syndrome
In terms of the prevalence of geriatric syndromes, most of the results were consistent with the characteristics described in Table 3. Subjects in SSG had a higher incidence in frailty (68.7 vs. 29.91%), disability (48.6 vs. 15.4%), depression (60.0 vs. 30.8%), and dementia (38.7 vs. 11.1%) than NSG. The rate of the illiterate in SSG subjects were also higher than that of NSG (14.45 vs. 3.4%) (p < 0.001). But analysis of chronic pain (49.55 vs. 48.7%), comorbidity (59.9 vs. 60.7%), polypharmacy (47.7 vs. 41.0%), fall (13.65 vs. 9.4%), anxiety (16.1 vs. 9.4%), malnutrition (96.9 vs. 96.6%) and dysphagia (6.6 vs. 5.1%) showed no significant difference between SSG and NSG (p > 0.05). Although SSG subjects used more kinds of drugs and had higher SAS T scores than NSG, the prevalence of polypharmacy and anxiety were not significantly different between the two groups (p = 0.175, 0.068) (Table 3). Overall, these data showed that the subjects with low gait speed (< 1.0 m/s) had a higher risk of developing frailty, disability, depression, and dementia (Fig. 3).
The prevalence of GS between NSG and SSG. The individuals was assigned as normal speed group (NSG) when the gait speed ≥ 1.0 m/s and the slow speed group (SSG) was defined as the gait speed < 1.0 m/s. According to Mann-Whitney U test, SSG had higher prevalence in frailty, disbility, depress, dementia (p < 0.05,expressed as *). Because of MMSE classified cogonition depends on education, we also analysised illiterate in both group.
The predictive value of gait speed as an indicator of geriatric syndromes
Finally, we assessed the predictive value of gait speed as an indicator of geriatric syndromes. All the demographic and clinical assessment information including height, weight, sex, age, BMI, gait speed, slow gait speed(< 1.0 m/s), chronic pain, numbers of diagnoses and drugs, comorbidity, polypharmacy, and fall history in the recent one year was used to construct the logistic regression model. The results of binary logistic regression showed that gait speed could serve as an independent protective factor associated with frailty, disability, dementia, and swallow dysfunction (Fig. 4). Slow gait speed was an independently risk factor associated with frailty, depression, and dementia. But in swallow dysfunction analysis, low gait speed was a protective factor. Gait speed showed no clear association with the anxiety level. Age was found to be an independent risk factor in frailty, disability and dementia, but a protective factor in anxiety. Comorbidity was an independent risk factor in disability, depression, anxiety, dementia, and the number of diagnoses was a risk factor in frailty and swallowing dysfunction. The history of falls was also found to be an independent risk factor in frailty, depression, and anxiety. The status of malnutrition showed no clear association with the above factors. Overall, subjects with normal or above gait speed had a lower risk of frailty, disability, dementia, and swallowing dysfunction. Contrarily, the subjects with a slow gait speed (< 1.0 m/s) suffered from a higher risk of frailty, depression, dementia, and swallowing dysfunction (Fig. 4). These data suggest that slow gait speed was a potential predictive factor associated with frailty, disability, depression, dementia, and swallowing dysfunction.
The impact of walking speed in geriatric syndromes. Analyzed of the demographic and clinical assessment information in the GS logistic regression model. It showed that gait speed could serve as an independent protective factor associated with frailty, disability, dementia, and swallow dysfunction; Slow gait speed was an independently risk factor associated with frailty, depression, and dementia.
Discussion
In this study, we examined the hypothesis that gait speed test could be a fast and reliable screening method not only for sarcopenia, but also for other common geriatric syndromes. In our study, SSG subjects showed higher scores in Edmonton symptom assessment, SDS, SAS, and MNA, while lower scores in BADL and MMSE. It suggests that slow gait speed was associated with decline in cognition and frailty. Compared to NSG, it has a significantly higher prevalence of frailty, disability, depression, and dementia in SSG. The result was similar to existing researches27,28 Importantly, we demonstrated that gait speed could be employed as an independent predictive factor for frailty, disability, dementia, and swallowing dysfunction. Overall, our study provided novel insights into the clinical application of slow gait speed as an indicator of common geriatric syndromes, indicating the feasibility of the 6 m walking test as a routine examination for fast screening of geriatric syndromes in elder outpatients.
Although some former studies regarded gait speed as an objective result of one specific disease or geriatric syndromes, the routine examination of a large cohort of elderly outpatients may reveal a different perspective of slow gait speed as comorbidity. In our study, the difference in the prevalences of comorbidity between NSG and SSG was about 60%. From the multiple linear regression analysis, age (over the 60s), sex, BMI or bodily form, chronic pain, fall, the scores of SDS, SAS, and swallow function did not affect gait speed. In contrast, the comorbidity status, polypharmacy, and education level seem to have significant influences on the gait speed. The underlying reasons for these observations need to be further clarified in the future studies.
Many previous studies reported that gait speed could be used as an identified characteristic of frailty29, daily life function30, dementia31 or cognition impairment32, malnutrition33, depression34, and chronic pain35. Most of these studies considered the reduced gait speed to be a functional decline as a result of other syndromes, which did not classify the subjects based on the gait speed cut-off. At variance with the previous studies involving elder patients diagnosed with certain diseases or syndromes, we included the elder outpatients in an unbiased manner without considering basic diseases or dysfunctions. Our study showed that slow gait speed (< 1.0 m/s) was an independent risk factor for frailty, depression, dementia, and swallowing dysfunction. Contrarily, subjects with faster gait speed were associated with reduced level of frailty, improved daily life function, cognition, and swallow function. Meanwhile, gait speed had no relativity with malnutrition and chronic pain from our regression analysis.
Gait speed test has been proposed as a key examination for sarcopenia, and sarcopenia is closely related to several geriatric syndromes. Thus, the slow gait speed may also have relationship with other geriatric syndromes. Since 6 m walk test need nothing about site and equipment, all the primary hospital could carry out it easily. This is very important for the construction of grass-roots hospitals that carry out geriatric medicine diagnosis and treatment. In contrast, the 6-minute walk test focuses more on evaluating cardiac and respiratory function. It is not only more time-consuming but also requires a larger space to administer. Similarly, the 30-meter walk test imposes greater demands in terms of both time and space compared to the 6-meter walk test .
Notably, a walking test for gait speed assessment could not replace the CGA as a comprehensive assessment scheme for geriatric syndromes, since slow gait speed could not inform all spectrum of geriatric syndromes. Nevertheless, the assessment of gait speed may be employed as a preliminary screening approach to signify the potential tendency of the development of geriatric syndromes before the time-consuming CGA assessment is implemented.
Limitations
The subjects in this study were recruited from outpatients in the geriatric department. The characteristics of outpatients in other departments or inpatient and long-term care center may differ. Meanwhile, some of the confounding factors such as race, economic condition, smoking or drinking, sports habit, pain intensity, and sleeping quality were not included in our analyses, which may also influence the result interpretation. Although in this study, cases with acute musculoskeletal conditions such as gout, arthritis flares, or trauma were in the minority, and while these diseases reduce gait speed only temporarily, they still need to be excluded to enhance the accuracy of the analysis. In addition, in this study, we allowed the elderly walk with assistance or facilities, but when these cases walk without help, the gait speed would be different. So further research on these cases may yield additional findings in the future.
Furthermore, after intervention with strength training whether the gait speed test can still serve as a good indicator for the development of other geriatric syndromes is unclear, which warrants further follow-up study in the future.
Conclusion
Taken together, we compared the clinical characteristics and geriatric syndromes in elderly outpatients with the normal gait speed and the slow gait speed (cutoff < 1.0 m/s). Slow gait speed could be a signal for some geriatric syndromes in elderly outpatients. Elder outpatients with a gait speed less than 1.0 m/s has a higher risk of frailty, disability, depression, and dementia. In addition, outpatients with higher scores in BADL, MMSE and lower scores in Edmonton and MNA displayed a better gait speed. It is recommended to include 6 m walk test as a routine assessment for the primary hospitals to early warning of the presence of geriatric syndromes.
Data availability
The datasets generated and analysed during the current study are not publicly available due to material involved personal relative information, but are available from the corresponding author on reasonable request.
References
Wu, L., Huang, Z. & Pan, Z. The spatiality and driving forces of population ageing in China. PLoS ONE 1, e0243559 (2021).
China tNHCotPsRo. Circular of the National Health Commission on the Issuance of the Guiding Principles for the Planning of Medical Institutions (2021–2025) (No. 3 of the National Health Commission of the People’s Republic of China (2022). Bull. Natl. Health Commiss. People’s Rep. China 1, 12–21 (2022).
Gonzalez, M. R., Miller, R. K. & Michener, A. R. Overview of High Yield Geriatrics Assessment for Clinic and Hospital. Med. Clin. North Am. 5, 777–789 (2020).
Kaur, P., Rowland, J. & Whiting, E. The ABCD of the comprehensive geriatric assessment. Med. J. Aust. 5, 206–7(e1) (2021).
Hindley, E. Comprehensive Geriatric Assessment. Br. J. Hosp. Med. 6, 356 (2018).
Alberto, P. Three decades of comprehensive geriatric assessment: Evidence coming from different healthcare settings and specific clinical conditions. J. Am. Med. Dir. Assoc. 18(2), 192.e1-.e11 (2017).
Choo, Y.J. & Chang, M.C. Prevalence of sarcopenia among the elderly in Korea: A meta-analysis. J. Prevent. Med. Public Health (Yebang Uihakhoe Chi) 2, 96–102 (2021).
O'Caoimh, R., Galluzzo, L., Rodrguez-Laso, N. et al. Prevalence of frailty at population level in European ADVANTAGE Joint Action Member States: A systematic review and meta-analysis. Ann. dell’Ist. Superior. Sanita 3, 226–38 (2018).
Deng, Y. et al. The prevalence of mild cognitive impairment among Chinese people: A meta-analysis. Neuroepidemiology 2, 79–91 (2021).
Kim, S., Han, D. & Lee, J. Prevalence and correlates of impairments in activities of daily living in older koreans: Comparison of young-old and old-old. J. Men’s Health. 3, e1–e10 (2019).
Harsha, N., Llyc, Z. & Rrbe, G. Disability among Palestinian elderly in the occupied Palestinian territory (oPt): Prevalence and associated factors. BMC Public Health 1, 1–9 (2019).
Almada, M., Brochado, P., Portela, D. et al. Prevalence of falls and associated factors among community-dwelling older adults: A cross-sectional study J. Frailty Aging 1, 10–6 (2021).
Kato, T. et al. Differences in lower limb muscle strength and balance ability between sarcopenia stages depend on sex in community-dwelling older adults. Aging Clin. Exp. Res. 3, 527–534 (2022).
Kusunoki, H. & Shinchon, K. Prevention of frailty and cognitive impairment in elderly patients with heart failure. Nihon Ronen Igakkai Zasshi. Jpn. J. Geriatr. 2, 107–14 (2019).
Cho, J., Park, M., Moon, W.-J. et al. Sarcopenia in patients with dementia: Correlation of temporalis muscle thickness with appendicular muscle mass. Neurol. Sci. 5, 3089–95 (2022).
Xu, W., Chen, T., Cai, Y. et al. Sarcopenia in community-dwelling oldest old is associated with disability and poor physical function J. Nutr. Health Aging 3, 339–45 (2020).
Shan, J., Lin, K. & Xiaohong ,L. Interpretation of Asian Working Group for Sarcopenia Consensus update on sarcopenia diagnosis and treatment. Chin. J. Geriatr. 4, 373-6 (2020).
Cruz-Jentoft, A.J., Baeyens, J.P., Bauer, J.M. et al. Sarcopenia: European consensus on definition and diagnosis : Report of the European Working Group on Sarcopenia in Older People. Age Ageing 4, 412–23 (2010).
Hynes, C. & Galvin, R. 30 the diagnostic and predictive accuracy of the edmonton frail scale for screening frailty in older adults: A systematic review and meta-analysis. Age Ageing (2022).
Peng, W. et al. Cross-sectional association of residential greenness exposure with activities of daily living disability among urban elderly in Shanghai. Int. J. Hyg. Environ. Health 1, 113620 (2020).
Jokelainen, J., Timonen, M., Keinnen-Kiukaanniemi, S. et al. Validation of the Zung self-rating depression scale (SDS) in older adults. Scand. J. Prim. Health Care (2019).
MacPhail, M., Redshaw, M. & Martin, C.R. Here comes the Zung: Revisiting the Zung Self-rating Anxiety Scale (SAS) for application within maternal mental health contexts J. Reprod. Infant Psychol. 3, E18-E9 (2013).
Zhao, L., Han, C., Zheng, Z., Xiu, S.L. et.al. Risk of Mini-Mental State Examination (MMSE) decline in the elderly with type 2 diabetes: A Chinese community-based cohort study. BMC Endocr. Disord. 1, 129 (2020).
Zhigang, X., Wang, Y. & Yu, J. MON-LB324: Application of NRS-2002 and MNA to screen nutritional risk in elderly patients in a Chinese University Hospital. Clin. Nutr. 1, S299 (2017).
Nishida, T. et al. Utility of the Eating Assessment Tool-10 (EAT-10) in evaluating self-reported dysphagia associated with oral frailty in Japanese community-dwelling older people. J. Nutr. Health Aging 1, 3–8 (2020).
Stuhec, M. General principles of pharmacotherapy selection in elderly patients for different comorbidities. Eur. Psychiatry. 1, S45 (2022).
Liu, X. et al. Characteristics of decline in cognition and locomotion among the elderly in seven provinces of China. Aging Med. 4, 190–197 (2019).
Yano, Y., Kario, K. & Inokuchi, T. Walking speed is a useful marker of frailty in older persons–reply. JAMA Intern Med. 4, 325–326 (2013).
Shinohara, T., Miyata, K. & Usuda, S. Sections of the brief-balance evaluation systems test relevant for discriminating fast versus slow walking speeds in community-dwelling older women. J. Geriatr. Phys. Ther. 2022(1), E1–E7 (2001).
Felício, D. et al. Handgrip strength is associated with, but poorly predicts, disability in older women with acute low back pain: A 12-month follow-up study (article). Maturitas 2017, 19–23 (2017).
Montero-Odasso, M. et al. Motor and Cognitive Trajectories Before Dementia: Results from Gait and Brain Study. J. Am. Geriatr. Soc. 9, 1676–1683 (2018).
Peel, N.M., Jones, L.V. & Hubbard, R.E. The association between gait speed and cognitive status in community-dwelling older people: A systematic review and meta-analysis. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 6, 943–8 (2019).
Reis, J. M. S. et al. According to revised ewgsop sarcopenia consensus cut-off points, low physical function is associated with nutritional status and quality of life in maintenance hemodialysis patients. J. Renal Nutr. 4, 469–475 (2022).
Naharci, M.I., Veizi, B. & Tasci, I. Gait Speed is independently associated with depression symptoms in mild cognitive impairment neuropsychology, development, and cognition. Sect. B Aging Neuropsychol. Cognit. 4, 637–50 (2022).
Cruz-Almeida, Y., Rosso, A., Marcum, Z. et al. Associations of musculoskeletal pain with mobility in older adults: Potential cerebral mechanisms. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 9, 1270–6 (2017).
Funding
This work was supported by Zhang Cuntai Expert Workstation of Yunnan Province[202405AF140057], Yunnan Province Clinical Research Center for Geriatrics [202102AA310002], Open Project of Clinical Medical Research Center for Geriatric Diseases in Yunnan Province[2023YJZX-LN-14].
Author information
Authors and Affiliations
Contributions
[Xu. He]and[Yan. Li]wrote the main manuscript text; [Jun. Chen], [Li.Zhang]and [Ying. Zhou] prepared figures and tables; [Jin. Quan], [Sunrui. Lu], [Yan. Huang] and [Kehua. Wang] selected and analysed data .All authors reviewed the manuscript. All the authors endorsed the publication of this study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study protocol was approved by the Research Ethics Committee of the First People’s Hospital of Yunnan province (Ethics approval number: KHLL2021- KY038).
Informed consent
The content of the study was explained to the participants, and written informed consent was obtained from them.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, X., Li, Y., Chen, J. et al. The primary hospitals should take gait speed as a routine test for elderly patients. Sci Rep 15, 34512 (2025). https://doi.org/10.1038/s41598-025-17685-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17685-9