Abstract
The efficient valorization of lignocellulosic biomass (LCB) into high-value platform chemicals such as 5-hydroxymethylfurfural (5-HMF) and furfural offers a sustainable alternative to fossil-based resources. In this study, we report the design and application of efficiently recyclable, silica-supported imidazolium-based acidic ionic liquid (IL) heterogeneous catalysts for the conversion of real, extracted cellulose and hemicellulose derived from wheat straw, rice husk and bagasse. The synthesized catalysts were extensively characterized using FTIR, XRD, TGA, BET and their Brønsted acidity was quantified. Catalytic activity was optimized by evaluating key reaction parameters, including catalyst loading, time, and temperature. Under mild operational conditions (80 °C for cellulose and 120 °C for hemicellulose), high yields of 5-HMF (91%) and furfural (86%) were achieved. The catalysts also demonstrated excellent recyclability over five consecutive reaction cycles without significant loss in activity. This study offers a scalable and environmentally benign approach for biomass valorization, highlighting the potential for industrial implementation.
Similar content being viewed by others
Introduction
Biomass resources are a promising renewable alternative for the imperishable supply of liquid fuels, fine chemical intermediates, and their precursors. Concerns about global warming and the depletion of fossil fuel resources have garnered the attention of researchers worldwide to develop substitute sources of energy and chemicals1,2. Development of efficient methodologies for the conversion of biomass is important for initiating the replacement of fossil fuels, crude oil derivatives, and petro-based systems. Biomass mainly comprises three prime components, namely hemicellulose, lignin, and cellulose. Valorization of these carbohydrate constituents yields sugars that act as substrates to produce fuels via platform/fine chemicals3. Amongst various biomass-procured chemicals, furfural, and 5-hydroxymethylfurfural (5-HMF) are valuable platform chemicals, which can be produced via dehydration of six and five-carbon sugars, respectively4,5. 5-HMF can further be converted to useful chemical intermediates such as 2,5-dimethylfuran6 and 2,5-furandicarboxylic acid (2,5-FDCA)7. Similarly, furfural can be converted to intermediates such as furfuryl alcohol8 and 2-methyltetrahydrofuran9. These industrially relevant bio-based intermediates have shown great potential in replacing fossil-derived molecules in the synthesis of industrial polymers. However, for their economic production, it is vital to develop novel technologies that result in the conversion of biomass-derived sugars to furfurals in high yields and purity.
Various catalytic strategies have been implemented to produce 5-HMF usually from various 6-C monomeric, dimeric, and polymeric bio-based starting materials such as glucose, fructose, sucrose, cellulose10,11,12,13,14,15,16 and furfural from five-carbon bio-based components such as xylose, xylan, hemicellulose, corn cobs17,18,19,20,21,22 using Lewis and Bronsted acids23,24, metal based25,26, mineral acids27,28, zeolites29,30, ionic liquids31,32 and several other homogeneous/heterogeneous catalysts33,34,35,36.
Among all the catalytic systems reported in recent literature, ionic liquids have been used as environmentally benign reaction media and promising catalysts for the conversion of carbohydrate components to 5-HMF and furfural. Literature also reports the co-production of furfural (74%) and 5-HMF (32%) from untreated corncobs at 175 °C using polytriphenylamine-SO3H (SPTPA) solid acid catalyst in γ-valerolactone37; the production of 5-HMF and furfural in lower yields respectively from untreated straw and barley husk in a biphasic solvent system using sulphanilic acid as a recyclable bifunctional organocatalyst38. Al-incorporated SBA-15 catalyst has also been reported for synthesizing 5-HMF from cotton cellulose using the hydrothermal degradation method with 68.5% cellulose conversion and 62.1% selectivity of 5-HMF at 170 °C in 2 h reaction time39.
An in-situ methodology has been developed for the conversion of raw biomass using 1-methyl-3(3-sulfopropyl)-imidazolium hydrogen sulfate Bronsted acidic ILs (BAILs) as catalysts in a biphasic solvent system into furfural and C5-sugars40. The application of SO4H-functionalized acidic ionic liquids (SFILs) in xylan hydrolysis and dehydration of xylose to furfural has also been reported, wherein the super acid SFIL [Ch-SO4H] [CF3SO3] showed efficient performance for the process, superior to usual acidic ILs, mineral acids and resins41. Silica-supported boric acid catalyst has been used as an efficient catalyst for the conversion of carbohydrates to 5-HMF in a 1.5–7 h process time at 120 °C, using ionic liquids as reaction medium42.
Overall, the literature reports on approaches that utilize whole biomass carbohydrates for 5-HMF and furfural production is sparse, and the yields obtained are rather low to moderate. This warrants the development of new methodologies that allow further improvement in the yield of the platform chemicals obtained, which can be achieved by minimizing the formation of humins and other degradation products43. This is very similar to how controlled elemental doping strategies have been shown to significantly alter the crystal structure and functional performance of nanomaterials44, tailoring the catalyst composition can be a promising pathway to enhance selectivity and efficiency in biomass valorization.
In continuation of our previous work on the extraction of lignocellulosic biomass (LCB) components, namely cellulose, hemicellulose and lignin and their subsequent valorization45,46, we herein report the implementation of efficiently recyclable, heterogeneous, silica-supported acidic imidazolium-based ionic liquid catalysts47,48 for the conversion of extracted cellulose and hemicellulose to enhance the yield of 5-HMF and furfural. Additionally, the effects of various reaction parameters on the conversion of cellulose and hemicellulose into these value-added platform chemicals were systematically investigated to optimize the reaction conditions. Unlike previous studies that primarily focused on pure model sugars, the present work utilizes real lignocellulosic biomass-derived cellulose and hemicellulose for high-yield platform chemical production. Furthermore, this study emphasizes the development of a recyclable and reusable silica-supported imidazolium-based ionic liquid catalyst, ensuring both sustainability and cost-effectiveness for potential industrial-scale biomass valorization processes.
In addition to addressing the selectivity and recyclability challenges observed in conventional catalytic systems, the present work emphasizes the industrial feasibility of the developed approach. By employing real lignocellulosic biomass substrates and achieving high product yields under mild and scalable reaction conditions, this study demonstrates the potential of silica-supported ionic liquid catalysts for large-scale, sustainable biomass valorization processes.
Materials and methods
Cellulose and hemicellulose were extracted from three lignocellulosic agricultural residues: wheat straw, rice husk and sugarcane bagasse. These substrates were selected due to their high availability, cost-effectiveness, and relevance as agro-industrial waste in biomass valorization processes. The extraction and purification procedures for cellulose and hemicellulose were based on previously reported protocols 44, involving alkaline pre-treatment, bleaching and acid hydrolysis steps to ensure component separation. The extracted biopolymers were washed thoroughly with deionized water, dried at 60 °C under vacuum and stored in desiccators before use.
Imidazolyl-propyl functionalized silica gel and 1,3-propane sultone (used for quaternization) were purchased from Sigma-Aldrich and were used without further purification. Other chemicals, including [BMIM] Cl (1-butyl-3-methylimidazolium chloride, ionic liquid solvent), solvents (ethyl acetate, ethanol), reagents (KOH, HCl, phenolphthalein) and analytical standards for 5-HMF and furfural, were of analytical grade and purchased from standard suppliers (SRL, Merck).
Instrumentation and characterization
-
Fourier-transform infrared (FTIR) spectroscopy was performed using a PerkinElmer RXI-FTIR spectrometer in the range of 400–4000 cm-1 using KBr pellet technique to identify functional groups present in the catalysts and confirm sulfonation and anion exchange.
-
Powder X-ray diffraction (XRD) analysis was conducted using PANalytical X’Pert Pro diffractometer with Cu-Kα radiation (λ = 1.5406 Å), operated at 40 kV and 30 mA. XRD patterns were used to assess crystallinity and confirm the successful immobilisation of ionic liquid on the silica support.
-
Thermogravimetric analysis (TGA) was performed using a Mettler Toledo TGA/SDTA 851 system under a nitrogen atmosphere with a heating rate of 10 °C/min up to 800 °C to assess thermal stability and decomposition profiles of the catalysts.
-
1H-NMR and 13C-NMR spectra were recorded to confirm the chemical structure of the synthesised ionic liquids and products using Bruker Avance II 500 MHz and 100 MHz spectrometers, respectively, using CDCl3 as solvents and TMS as internal reference.
-
High-performance liquid chromatography (HPLC) was used to quantify 5-HMF and furfural yields. The setup consisted of a Waters 515 HPLC pump, Waters 2998 photodiode array (PDA) detector and manual single injection system. Separations were carried out on a Waters Spherisorb ODS2 column (4.6 × 250 mm, 5 μm particle size) at 30 °C, using a mobile phase of water: acetonitrile (80:20 v/v) at a flow rate of 1.0 mL/min. Calibration curves were constructed using external standards of known concentrations using Empower 2 software.
-
Mass spectrometry (MS) was used to confirm the molecular masses and structures of the synthesised platform chemicals. Analyses were carried out on Waters Micromass Q-TOF Micro-spectrometer and Finnigan Mat LCQ systems in electrospray ionisation (ESI) mode.
Acidity measurement of catalysts
The Brønsted acidity of the synthesised ionic liquid-functionalized silica catalysts was measured by a back-titration method. In this method, 500 mg of each catalyst was suspended in 20 mL of 0.1 N aqueous KOH solution and stirred at room temperature for 24 h. After filtration, the residual KOH in the supernatant was titrated against 0.1 N HCl solution using phenolphthalein as a visual indicator. The endpoint of the titration was marked by the colour change from pink to colourless. The total Brønsted acidity was calculated based on the difference in normality using the standard formula:
All measurements were conducted in triplicate, and the reported results are expressed as mean values with a standard deviation of less than ± 2%. The consistency in acidity measurements is critical for correlating the catalyst’s acid strength with its catalytic performance during biomass conversion reactions.
General procedure for conversion of Cellulose to 5-HMF
The valorization of cellulose was carried out using the synthesised catalyst(s) in the presence of an appropriate volume of [BMIM] Cl as the reaction medium. The reaction was conducted under conventional heating at 80 °C for 2 h, as illustrated in Scheme 1. Upon completion, the reaction mixture was allowed to cool to room temperature and was subsequently extracted with ethyl acetate. Product formation was monitored using thin-layer chromatography (TLC), after which the organic layer was evaporated. The resulting 5-hydroxymethylfurfural (5-HMF) was analysed by high-performance liquid chromatography (HPLC) equipped with a photodiode array (PDA) detector and a Waters Spherisorb 5 μm ODS2 column (4.6 × 250 mm), 1H-NMR, 13C-NMR and Mass spectroscopy. The yield of 5-HMF was calculated by weight %age using the following equation:
General procedure for the valorization of hemicellulose
The valorization of hemicellulose was carried out using the synthesised Si-Im-Pr-CF3SO3 catalyst in the presence of an appropriate volume of [BMIM]Cl as the reaction medium. The reaction was performed under conventional heating at 120 °C for 3 h, as illustrated in Scheme 2. Upon completion, the mixture was cooled to room temperature and extracted using ethyl acetate. The formation of furfural was monitored via thin-layer chromatography (TLC) and the organic layer was subsequently evaporated. The product was analysed by high-performance liquid chromatography (HPLC) equipped with a photodiode array (PDA) detector and a Waters Spherisorb 5 μm ODS2 column (4.6 × 250 mm), 1H-NMR, 13C-NMR and Mass spectroscopy. The yield of furfural was calculated by weight %age using the equation:
General procedure for synthesis of heterogeneous ionic liquid catalyst(s)
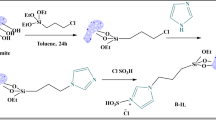
In a round bottomed flask 3-(imidazole)-1-yl propyl functionalized silica gel (500 mg, 0.5 mmol) dissolved in acetone (5 mL) and 1,3-propane sultone (0.61 g, 0.5 mmol) in acetone (10 mL) were mixed at 0 °C. The mixture was then stirred at ambient temperature for 5 days under nitrogen atmosphere. The solid formed was filtered, washed with diethyl ether and toluene to remove the unreacted starting material, and dried under vacuum to get desired silica-supported zwitterion in (0.987 g) 98% yield.
Subsequently, dropwise addition of trifluoromethanesulfonic acid (0.732 g, 19.27 mmol) was done to the solution of silica-supported zwitterionic solid (0.987 g, 19.27 mmol) in acetone (10 mL). The resulting mixture was then stirred for 2 h at 40 °C. The solid formed was filtered and dried to yield 3-propyl-1-(propyl-4-sulfonyl) imidazolium trifluoromethanesulfonate functionalized silica catalyst (1.66 g) in 97% yield. The prepared catalyst was labelled as Si-Im-Pr-CF3SO3.
Similar process and reactant ratio were used to synthesize the other two catalysts, 3-propyl-1-(propyl-4-sulfonyl) imidazolium sulfonate functionalized silica [Si-Im-Pr-HSO4] and 3-propyl-1-(propyl-4-sulfonyl) imidazolium phosphonate functionalized silica [Si-Im-Pr-H2PO4] using sulfuric acid and phosphoric acid in place of trifluoromethanesulfonic acid respectively as represented in Scheme 3.
Representative scheme for the valorization of cellulose
See Scheme 4
Representative scheme for the valorization of hemicellulose
See Scheme 5
Results and discussion
The valorization of lignocellulosic biomass (LCB) into high-value platform chemicals such as 5-hydroxymethylfurfural (5-HMF) and furfural involves a cascade of sequential reactions including hydrolysis, isomerization, and dehydration. Effective control over each of these steps is crucial to achieve high selectivity and product yield, especially when processing complex, real biomass-derived substrates like cellulose and hemicellulose. The present study focuses on the application of synthesised silica-supported imidazolium-based acidic ionic liquid (IL) heterogeneous catalysts for the conversion of cellulose and hemicellulose extracted from wheat straw, rice husk and sugarcane bagasse. The design of these catalysts addresses major limitations of existing homogeneous and heterogeneous acid catalysts, such as lack of recyclability, poor thermal stability, and dependence on pure model substrates.
Catalyst characterization
Comprehensive physicochemical characterization of the synthesised catalysts was performed using Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), thermogravimetric analysis (TGA), and Brunauer–Emmett–Teller (BET) surface area analysis.
The FTIR spectrum of the synthesized Si-Im-Pr-CF3SO3 catalyst (Fig. 1) confirmed successful immobilization of the sulfonic acid functionalized imidazolium ionic liquid on the silica support. A broad absorption band at 3465 cm⁻1 was assigned to O–H stretching vibrations from residual silanol groups on the silica surface. The peaks at 1657 cm⁻1 and 1530 cm⁻1 correspond to C = N stretching and aromatic ring vibrations of the imidazolium cation, respectively. Strong absorptions observed at 1444 cm⁻1 and 1096 cm⁻1 were attributed to asymmetric and symmetric S = O stretching of the sulfonate group, confirming the presence of the triflate anion. The band around 800 cm⁻1 was assigned to Si–O–Si bending modes of the silica framework. These characteristic vibrations collectively confirm both the chemical structure of the functionalized ionic liquid and its covalent anchoring to the silica support.
The amorphous nature of the silica framework in Si-Im-Pr-CF3SO3 was confirmed by XRD analysis, which revealed a single broad peak cantered at 2θ ≈ 22.8°, with no evidence of crystalline phases (Fig. 2). This observation indicates that the ionic liquid moieties were uniformly distributed on the silica support without causing long-range crystallinity.
The thermal stability of the catalyst was assessed by thermogravimetric analysis (TGA). The TGA curve displayed an initial weight loss below 150 °C, which can be attributed to the desorption of physically adsorbed moisture. A major weight loss occurred between 300 and 500 °C, corresponding to the decomposition of the organic ionic liquid species immobilized on the silica surface (Fig. 3). This thermal profile indicates that the catalyst can tolerate reaction temperatures up to 300 °C without significant degradation, making it suitable for biomass conversion applications typically performed below this temperature.
BET surface area measurements confirmed that the catalyst maintained a mesoporous structure after ionic liquid functionalization. With a specific surface area of 321.28 m2/g and an average pore volume in the range of 3–6 cm3/g, the nitrogen adsorption–desorption isotherms displayed typical Type IV behavior, showing mesoporosity and opening the door for free diffusion of the reactant and product. Such porosity is advantageous as it enhances substrate diffusion and increases the accessibility of active sites during catalysis (Fig. 4).
Optimization of reaction conditions for cellulose valorization to 5-HMF
The optimization of reaction conditions was done in terms of catalyst loading, time and temperature employing Si-Im-Pr-CF3SO3 as a model acid catalyst for the valorization of cellulose extracted from wheat straw to 5-HMF and the relevant observations are presented in Table 1.
Cellulose isolated from rice husk and bagasse was also converted to 5-HMF employing all the synthesized heterogeneous ILs (Si-Im-Pr-CF3SO3/Si-Im-Pr-HSO4/Si-Im-Pr-H2PO4) under optimized conditions. The results are tabulated in Table 2, wherein the best results were obtained using Si-Im-Pr-CF3SO3.
Optimization of reactions conditions for hemicellulose valorization to furfural
The reaction conditions were then optimized in terms of catalyst loading, time and temperature using Si-Im-Pr-CF3SO3 as model acid catalyst for conversion of hemicellulose extracted from wheat straw to furfural as summarized in Table 3. Firstly, the influence of catalyst loading with respect to hemicellulose conversion using Si-Im-Pr-CF3SO3 ranging from 1 to 7 mg was investigated and the yield of furfural kept on increasing with increase in the amount of catalyst used. It was observed that higher catalyst loading accelerated the formation of the product up to a certain limit and no further increase in the yield of furfural was observed beyond 6 mg catalyst loading.
Additionally, the reaction conditions were optimised in terms of time using Si-Im-Pr-CF3SO3 (6 mg), wherein a 3 h reaction time was found to be appropriate for the process to get the desired product in maximum yield. However, the yield of furfural dropped when the reaction time was extended over 3 h.
The hemicellulose valorization using Si-Im-Pr-CF3SO3 catalyst was also performed at different temperatures ranging from 60 to 160 ºC, wherein maximum yield of furfural (approx. 86%) was obtained at 120 ºC and further increase in temperature beyond 120 ºC led to a reduction in the yield. The optimized conditions for valorization of extracted hemicellulose to furfural were found to be 6 mg catalyst, 3 h reaction time and 120 °C temperature.
Transcending the range of reaction parameters beyond the reported optimized value probably results in further polymerization and product deterioration by charring of the reaction mixture, which forms humins on excessive heating, as evident from literature reports49.
Hemicellulose isolated from rice husk and bagasse was also converted to furfural employing all the synthesized heterogeneous (Si-Im-Pr-CF3SO3/Si-Im-Pr-HSO4/Si-Im-Pr-H2PO4) ILs under the optimized conditions, and the results are tabulated in Table 4, wherein better results in terms of furfural yield were obtained using Si-Im-Pr-CF3SO3 catalyst.
Product characterization (5-HMF and furfural)
The chemical identity and purity of the valorization products, 5-HMF and furfural, were confirmed through a combination of analytical techniques, including ^1H nuclear magnetic resonance (^1H-NMR) spectroscopy, high-performance liquid chromatography (HPLC), electrospray ionization mass spectrometry (ESI–MS), and elemental (CHN) analysis.
To ensure a concise yet informative presentation in the main text, only the HPLC chromatograms and elemental (CHN) analysis results are included herein, as these directly demonstrate product purity and retention characteristics. High-resolution structural confirmation was also carried out using ^1H-NMR spectroscopy and ESI–MS analysis; however, these spectra are provided in the Supporting Information (Figures S1–S4) to maintain clarity in the main manuscript while still allowing interested readers to access the complete spectral data.
The HPLC chromatogram of 5-HMF (Fig. 5) exhibited a single sharp peak with a retention time of 2.9 min, confirming its high purity. Similarly, the HPLC chromatogram of furfural (Fig. 6) displayed a single well-resolved peak at 3.2 min, further validating the isolation of a pure product. Elemental analysis results for both compounds were in close agreement with their theoretical molecular compositions (C6H6O3 for 5-HMF and C5H4O2 for furfural), providing additional confirmation of their chemical identities and purity.
Comparative assessment with reported catalytic systems
The catalytic valorization of lignocellulosic biomass into platform chemicals such as 5-HMF and furfural has been extensively studied using various homogeneous and heterogeneous acid catalysts. However, many of these reported systems rely on harsh reaction conditions, expensive or non-recyclable materials, or require the use of pure model substrates such as glucose, xylose, or commercial cellulose. In contrast, the present study employs real extracted cellulose and hemicellulose from agricultural waste (wheat straw, rice husk and bagasse), demonstrating the feasibility of converting complex biomass fractions without pre-purification or molecular simplification. This not only ensures alignment with practical biomass utilization scenarios but also addresses the limitations of substrate purity that affect scalability in earlier reports.
A comparison with recently reported catalytic systems (Table 5) reveals that the silica-supported imidazolium-based acidic ionic liquids, particularly Si-Im-Pr-CF3SO3, offer superior catalytic performance under significantly milder conditions. The valorization reactions were carried out at just 80 °C (for 5-HMF from cellulose) and 120 °C (for furfural from hemicellulose), which contrasts with the 150–220 °C temperature ranges typically required in hydrothermal or microwave-assisted systems. Moreover, the product yields achieved in this study, up to 91% for 5-HMF and 86% for furfural, are among the highest reported using heterogeneous catalysts with real biomass substrates and exceed many results obtained using pure model sugars under similar or harsher conditions.
An additional merit of the developed catalysts lies in their heterogeneous and recyclable nature, offering clear advantages over conventional mineral acids, deep eutectic solvents (DESs), or homogeneous ionic liquids that are difficult to recover or reuse. The Si-Im-Pr-CF3SO3 catalyst maintained its activity over five consecutive reaction cycles with negligible loss in performance, as evidenced by consistent product yields. This recyclability, combined with mild operational parameters and substrate flexibility, underscores the industrial and environmental sustainability of the presented catalytic system and positions it as a strong candidate for future biomass valorization technologies.
Catalyst recyclability and reusability
Catalyst recyclability is a critical parameter for assessing the industrial applicability of heterogeneous catalytic systems. The reusability of Si-Im-Pr-CF3SO3 was evaluated under optimized reaction conditions for both cellulose and hemicellulose valorization. After each catalytic run, the solid catalyst was separated by filtration, washed thoroughly with ethanol and water, dried and reused with fresh substrate under identical conditions.
The catalyst exhibited negligible loss in catalytic activity over five consecutive cycles, with product yields remaining consistently above 85% for both 5-HMF and furfural production (Fig. 7). This excellent reusability highlights the structural and functional stability of the catalyst under operational conditions, underlining its potential for large-scale biomass valorization processes.
Conclusion
This work demonstrates an eco-friendly, scalable method for biomass valorization with superior product yields under mild conditions. In this work, heterogeneous silica-supported imidazolium-based sulfonic acid ionic liquid catalysts have been synthesized and their efficacy as catalysts for valorization of the extracted cellulose or hemicellulose to platform chemicals was investigated. Among all the acidic IL catalysts, the Si-Im-Pr-CF3SO3 catalyst was found to be the most efficient for valorization of cellulose and hemicellulose. Using optimized reaction conditions, yields up to as high as 91% of 5-HMF and 86% of furfural were obtained in high purity, displaying appreciable catalytic performance. The design and utilization of silica-supported imidazolium-based IL catalysts provide a reliable strategy for improving the competence of the process, thus facilitating their potential applications in biomass valorization. The presented catalytic system offers promising potential for future scale-up in biorefinery applications targeting sustainable chemical production. Such advancements are in line with the broader push toward clean and renewable technologies, including emerging sustainable pathways such as fusion energy, which aim to meet future global energy demands50.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to confidentiality restrictions imposed by the laboratory where the analyses were conducted, but are available from the corresponding author on reasonable request.
References
Popp, J., Lakner, Z., Harangi-Rakos, M. & Fari, M. The effect of bioenergy expansion: Food, energy, and environment. Renew. Sustain. Energy Rev. 32, 559–578. https://doi.org/10.1016/j.rser.2014.01.056 (2014).
Ahorsu, R., Medina, F. & Constanti, M. Significance and challenges of biomass as a suitable feedstock for bioenergy and biochemical production: A review. Energies 11, 3366–3384. https://doi.org/10.3390/en11123366 (2018).
Busic, A. et al. Bioethanol production from renewable raw materials and its separation and purification: A review. Food Technol. Biotechnolo. 56, 289–311. https://doi.org/10.17113/ftb.56.03.18.5546 (2018).
Gallezot, P. Bio-renewable sources for synthesis of eco-friendly polyurethane adhesives–review. Chem. Soc. Rev. 41, 1538–1558. https://doi.org/10.1039/C1CS15147A (2012).
Isikgor, F. H. & Becer, C. R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 6, 4497–4559. https://doi.org/10.1039/C5PY00263J (2015).
Thananatthanachon, T. & Rauchfuss, T. B. Efficient production of the liquid fuel 2,5-dimethylfuran from fructose using formic acid as a reagent. Angew. Chem 122, 6766–6768. https://doi.org/10.1002/anie.201002267 (2010).
Motagamwala, A. H. et al. Toward biomass-derived renewable plastics: Production of 2,5-furandicarboxylic acid from fructose. Sci. Adv 4, 9722–9729. https://doi.org/10.1126/sciadv.aap9722 (2018).
Perez, R. F. & Fraga, M. A. Hemicellulose-derived chemicals: One-step production of furfuryl alcohol from xylose. Green Chem. 16, 3942–3950. https://doi.org/10.1039/C4GC00398E (2014).
Biswas, P., Lin, J. H., Kang, J. & Guliants, V. V. Vapor phase hydrogenation of 2-methylfuran over noble and base metal catalysts. Appl. Catal-A 475, 379–385. https://doi.org/10.1016/j.apcata.2014.01.054 (2014).
Zhou, C. et al. Conversion of glucose into 5-hydroxymethylfurfural in different solvents and catalysts: Reaction kinetics and mechanism. Egypt. J. Pet. 26, 477–487. https://doi.org/10.1016/j.ejpe.2016.07.005 (2017).
Rezayan, A., Zhang, Y., Li, B. & Xu, C. C. Catalytic conversion of cellulose to 5-hydroxymethylfurfural: Advancements in heterogeneous catalysts and cutting-edge hydrolysis strategies. ChemCatChem https://doi.org/10.1002/cctc.202300973 (2023).
Wang, C., Fu, L., Tong, X., Yang, Q. & Zhang, W. Efficient and selective conversion of sucrose to 5-hydroxymethylfurfural promoted by ammonium halides under mild conditions. Carbohyd. Res. 347, 182–185. https://doi.org/10.1016/j.carres.2011.11.013 (2012).
Rout, P., Nannaware, A., Prakash, O., Kalra, A. & Rajasekharan, R. Synthesis of hydroxymethylfurfural from cellulose using green processes: A promising biochemical and biofuel feedstock. Chem. Eng. Sci. 142, 318–346. https://doi.org/10.1016/j.ces.2015.12.002 (2016).
Tang, Z. & Su, S. Direct conversion of cellulose to 5-hydroxymethylfurfural (HMF) using an efficient and inexpensive boehmite catalyst. Carbohyd. Res. 481, 52–59. https://doi.org/10.1016/j.carres.2019.06.010 (2019).
Li, X., Peng, K., Xia, Q., Liu, X. & Wang, Y. Efficient conversion of cellulose into 5-hydroxymethylfurfural over niobia/carbon composites. Chem. Eng. J. 332, 528–536. https://doi.org/10.1016/j.cej.2017.06.105 (2018).
Yan, L. et al. Ruthenium trichloride catalyzed conversion of cellulose into 5-hydroxymethylfurfural in biphasic system. Biores. Technol. 279, 84–91. https://doi.org/10.1016/j.biortech.2019.01.120 (2019).
Liu, Y., Ma, C., Huang, C., Fu, Y. & Chang, J. Efficient conversion of xylose into furfural using sulfonic acid-functionalized metal-organic frameworks in a biphasic system. Ind. Eng. Chem. Res 57, 16628–16634. https://doi.org/10.1021/acs.iecr.8b04070 (2018).
Upare, P. P. et al. Direct chemical conversion of xylan into furfural over sulfonated graphene oxide. Catal. Today 324, 66–72. https://doi.org/10.1016/j.cattod.2018.07.002 (2019).
Luo, Y. et al. The production of furfural directly from hemicellulose in lignocellulosic biomass: A review. Catal. Today 319, 14–24. https://doi.org/10.1016/j.cattod.2018.06.042 (2019).
Zhong, C. et al. Selective hydrolysis of hemicellulose from wheat straw by a nanoscale solid acid catalyst. Carbohyd. Polym. 131, 384–391. https://doi.org/10.1016/j.carbpol.2015.05.070 (2015).
Zhang, S. et al. Efficient production of furfural from corncob by an integrated mineral-organic-Lewis acid catalytic process. BioResources 12, 2965–2981. https://doi.org/10.15376/biores.12.2.2965-2981 (2017).
Sun, D. et al. Efficient conversion of Xylan to furfural using niobium-modified sba-15 catalyst in biphasic solvents: Experiments and Simulations. Ind. Eng. Chem. Res. 64, 2069–2083. https://doi.org/10.1021/acs.iecr.4c04435 (2025).
Zhao, P. et al. Synergetic effect of Brønsted/Lewis acid sites and water on the catalytic dehydration of glucose to 5-hydroxymethylfurfural by heteropolyacid-based ionic hybrids. Chemistry Open 7, 824–832. https://doi.org/10.1002/open.201800138 (2018).
Choudhary, V., Sandler, S. I. & Vlachos, D. G. Conversion of xylose to furfural using Lewis and Brønsted acid catalysts in aqueous media. ACS Catal. 2, 2022–2028. https://doi.org/10.1021/cs300265d (2012).
Yang, L. et al. One-pot synthesis of 5-hydroxymethylfurfural from carbohydrates using an inexpensive FePO4 catalyst. RSC Adv. 5, 19900–19906. https://doi.org/10.1039/C4RA16145A (2015).
Luo, Y., Li, Z., Zuo, Y., Su, Z. & Hu, C. A simple two-step method for the selective conversion of hemicellulose in pubescens to furfural. ACS Sustain. Chem. Eng 5, 8137–8147. https://doi.org/10.1021/acssuschemeng.7b01766 (2017).
Cai, C. et al. Conversion of cellulose to 5-hydroxymethylfurfural using inorganic acidic catalysts in the presence of pressurized water steam. BioResources 12, 1201–1215. https://doi.org/10.15376/biores.12.1.1201-1215 (2017).
Lamminpaa, K., Ahola, J. & Tanskanen, J. Acid-catalysed xylose dehydration into furfural in the presence of kraft lignin. Biores. Technol. 177, 94–101. https://doi.org/10.1016/j.biortech.2014.11.074 (2015).
Sezgin, E., Esen Keçeci, M., Akmaz, S. & Koc, S. N. Heterogeneous Cr-zeolites (USY and Beta) for the conversion of glucose and cellulose to 5-hydroxymethylfurfural (HMF). Cellulose 26(17), 9035–9043 (2019).
Chen, H., Qin, L. & Yu, B. Furfural production from steam explosion liquor of rice straw by solid acid catalysts (HZSM-5). Biomass Bioenerg. 73, 77–83. https://doi.org/10.1016/j.biombioe.2014.12.013 (2015).
Sert, M., Aslanoglu, A. & Ballice, L. Conversion of sunflower stalk-based cellulose to the valuable products using choline chloride based deep eutectic solvents. Renew. Energy 118, 993–1000. https://doi.org/10.1016/j.renene.2017.10.083 (2018).
Carvalho, A. V., Lopesa, A. C. & Lukasik, R. B. Relevance of the acidic 1-butyl-3-methylimidazolium hydrogen sulphate ionic liquid in the selective catalysis of the biomass hemicellulose fraction. RSC Adv. 5, 47153–47164. https://doi.org/10.1039/C5RA07159C (2015).
Shuai, L. & Pan, X. Hydrolysis of cellulose by cellulase-mimetic solid catalyst. Energy Environ. Sci 5, 6889–6894. https://doi.org/10.1039/C2EE03373A (2012).
Fang, J., Zheng, W., Liu, K., Lia, H. & Li, C. Molecular design and experimental study on the synergistic catalysis of cellulose into 5-hydroxymethylfurfural with Brønsted-Lewis acidic ionic liquids. Chem. Eng. J. 385, 123796–123833. https://doi.org/10.1016/j.cej.2019.123796 (2020).
Deng, A. et al. A feasible process for furfural production from the pre-hydrolysis liquor of corncob via biochar catalysts in a new biphasic system. Biores. Technol. 216, 754–760. https://doi.org/10.1016/j.biortech.2016.06.002 (2016).
Dietz, C. H. J. T. et al. Sequential and in situ extraction of furfural from reaction mixture and effect of extracting agents on furfural degradation. Ind. Eng. Chem. Res. 58, 16116–16125. https://doi.org/10.1021/acs.iecr.9b00694 (2019).
Zhang, L., Xi, G., Zhang, J., Yu, H. & Wang, X. Efficient catalytic system for the direct transformation of lignocellulosic biomass to furfural and 5-hydroxymethylfurfural. Biores. Technol. 224, 656–661. https://doi.org/10.1016/j.biortech.2016.11.097 (2017).
Mirzaei, H. M. & Karimi, B. Sulphanilic acid as a recyclable bifunctional organocatalyst in the selective conversion of lignocellulosic biomass to 5-HMF. Green Chem. 18, 2282–2286. https://doi.org/10.1039/C5GC02440D (2016).
Pham, S. T. et al. Cellulose conversion to 5 Hydroxymethyl Furfural (5-HMF) using Al-incorporated SBA-15 as highly efficient catalyst. J. Chem. 2019, 1–8. https://doi.org/10.1155/2019/5785621 (2019).
Hua, D., Ding, H., Liu, Y., Li, J. & Han, B. Dehydration of xylose to furfural over imidazolium-based ionic liquid with phase separation. Catalysts 11, 1552. https://doi.org/10.3390/catal11121552 (2021).
Hui, W. et al. Efficient hydrolysis of hemicellulose to furfural by novel superacid SO4H-functionalized ionic liquids. Green Energy Environ. 4, 49–55. https://doi.org/10.1016/j.gee.2018.06.002 (2019).
Sidhpuria, K. B., Daniel-da-Silva, A. L., Trindade, T. & Countinho, J. A. P. Supported ionic liquid silica nanoparticles (SILnPs) as an efficient and recyclable heterogeneous catalyst for the dehydration of fructose to 5-hydroxymethylfurfural. Green Chem. 13, 340–350. https://doi.org/10.1039/C0GC00690D (2011).
Anchan, H. N. & Dutta, S. Recent advances in the production and value addition of selected hydrophobic analogs of biomass-derived 5-(hydroxymethyl)furfural. Biomass Convers. Biorefinery 13, 2571–2593. https://doi.org/10.1007/s13399-021-01315-1 (2021).
Katoch, G. et al. Impact of lanthanum doping on crystal structure and magnetic anisotropy of Mn–Zn soft nanoferrites. Sci. Rep. 15, 11663. https://doi.org/10.1038/s41598-025-91305-4 (2025).
Arora, S., Gupta, N. & Singh, V. Choline based basic ionic liquid (BIL)/Acidic DES mediated cellulose rich fractionation of agricultural waste biomass and valorization to 5-HMF. Waste Biomass Valorization 11, 3345–3354. https://doi.org/10.1007/s12649-019-00603-2 (2020).
Arora, S., Gupta, N. & Singh, V. Improved Pd/Ru metal supported graphene oxide nano-catalysts for hydrodeoxygenation (HDO) of vanillyl alcohol, vanillin and lignin. Green Chem. 22, 2018–2027. https://doi.org/10.1039/D0GC00052C (2020).
Kaur, A. & Singh, V. Silica bound sulphonic acid functionalized imidazolium ionic liquid as a recyclable and recoverable catalyst for N-B °C protection of amines. Curr. Cataly. 3, 316–322. https://doi.org/10.2174/2211544703666140812232332 (2014).
Shashni, S., Singh, V. & Toor, A. P. High-efficacy glycerol acetalization with silica gel immobilized Brønsted acid ionic liquid catalysts–preparation and comprehending the counter-anion effect on the catalytic activity. New J Chem 45, 21807–21823. https://doi.org/10.1039/D1NJ03508H (2021).
Chen, S., Wojcieszak, R., Dumeignil, F., Marceau, E. & Royer, S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem Rev 118, 11023–11117. https://doi.org/10.1021/acs.chemrev.8b00134 (2018).
Katoch, G. et al. Fusion energy: A sustainable pathway to meeting future global energy demands. Discov. Sustain. 6, 221. https://doi.org/10.1007/s43621-025-00906-6 (2025).
Acknowledgements
The authors are thankful to SAIF-CIL, Panjab University, Chandigarh, and TBRL-DRDO for spectroscopic analysis.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The contributions of all authors to this research work are duly acknowledged and specified as follows. Dr. Shalini Arora was primarily responsible for the conceptualization of the research idea, development of the experimental methodology, execution of laboratory experiments, data curation and preparation of the original draft of the manuscript. Dr. Sitanshu Kumar contributed significantly to the optimization of the catalytic reactions, experimental design support and assisted in the detailed analysis, interpretation of the data including FTIR, NMR, HPLC and Mass spectroscopy. Dr. Gaurav Katoch played a key role in the characterization of catalysts, including XRD, TGA and BET analysis, along with assisting in data validation, visualization and critical review and editing of the manuscript. Dr. Johnson Santhosh provided overall supervision, project administration, necessary resources and offered critical insights during the revision process. He also fulfilled the responsibilities of a corresponding author. All authors have read and approved the final version of the manuscript and take collective responsibility for the content presented in this research work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Arora, S., Sitanshu, Katoch, G. et al. Efficient biomass valorization using silica supported imidazolium based ionic liquid catalysts. Sci Rep 15, 32882 (2025). https://doi.org/10.1038/s41598-025-17712-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17712-9