Abstract
Prelingual sensorineural hearing loss (SNHL) represents about 80% of genetic SNHL, with at least 90 causative genes identified. In order to identify the genetic diagnosis of prelingual SNHL, we performed a prospective study by systematic history-taking and phenotyping, followed by whole-exome sequencing (WES) with target gene analysis in 100 Thai patients. We found an overall diagnostic yield of 46%, 58.3% for familial cases, and 39.0% for sporadic cases. These included 41 cases with nonsyndromic SNHL(nsSNHL) and 5 cases with syndromic SNHL (sSNHL). We identified 41 P/LP and 4 VUS variants of 15 genes. Of those sSNHL, the causative genes were PAX3, SOX10, MITF (Waardenburg and Teitz syndromes), and SLC26A4 (Pendred syndrome). The genetic defects identified among those with nsSNHL were GJB2 and SLC26A4 as the most prevalent causes, followed by MYO15A, MYO7A, POU3F4, OTOF, PCDH15, GSDME, PTEN, ACTG1, TMPRSS3, MITF, and MPZL2. The inheritance of these nsSNHL genes involved X-linked recessive (n = 3), autosomal dominant (n = 3), and autosomal recessive in the remainder (n = 36). Patients with positive mutations underwent surveillance for associated symptoms like goiter and retinitis pigmentosa. In conclusion, most prelingual SNHL was nsSNHL with autosomal recessive inheritance. Identifying the causative gene benefits patients for specific management and genetic counseling.
Similar content being viewed by others
Introduction
Hearing loss (HL) is defined as when a person does not hear at the normal hearing threshold of 15 dB in the better ear1 The incidence of hearing loss (HL) is about 1–2 in 1,000 infants2,3,4,5,6. Sensorineural HL (SNHL) refers to any cause of hearing loss due to a pathology of the cochlea, auditory nerve, or central nervous system4,7. It is noted that > 95% of permanent HL represents sensorineural HL (SNHL). The severity of HL is classified into mild (26–40 dB), moderate (41–70 dB), severe (71–90 dB), and profound (> 90 dB)8.
The etiology of prelingual SNHL is divided into 3 groups, including acquired causes (TORCH infection, prematurity, severe hyperbilirubinemia, ototoxic drug use), genetic causes, and unknown etiology4,5,6. Genetic HL is classified into syndromic (sSNHL) and non-syndromic (nsSNHL) based on the presence or absence of associated abnormalities of other organs, respectively. It can also be divided into prelingual and postlingual SNHL according to the onset before or after the development of verbal speech, usually at 2 years of age. Prelingual SNHL represents ∼80% of genetic HL, with at least 90 genes identified as the cause, creating difficulty in genetic diagnosis4,5,9. With the advance of next-generation sequencing (NGS), specifically whole exome sequencing (WES) and gene panel testing, the diagnostic yield of HL is approaching 40–60% in some studies3,10,11,12,13,14 as compared to 29% or less in the pre-NGS era4,15.
Common sSNHL include Waardenburg syndrome (blue iris, heterochromia, telecanthus, and Hirschsprung disease), Pendred syndrome (cochlear hypoplasia or 1 ½ turn-cochlear, and adolescent onset goiter), Usher syndrome (retinitis pigmentosa, vestibular abnormality in some patients), Alport syndrome (microhematuria, later onset renal failure)9,16. Other rare sSNHL were such as Tietz albinism (light-colored hair and skin, blue/grey iris) and Jervell and Lange-Nielsen syndrome (syncope, prolonged QT-interval, and ventricular arrhythmia)4,9. Nonsyndromic SNHL(nsSNHL), on the other hand, has no clinically relevant associated symptoms.
Variants of the gap junction beta 2, GJB2, gene are the most common cause of hereditary HL worldwide, followed by other genes, depending on the studies and ethnicity of the populations investigated4,12,17,18,19,20.
The American College of Medical Genetics (ACMG) and the American Academy of Pediatrics have recommended stepwise investigations, including clinical and audiological data, to exclude acquired causes, followed by single gene testing of GJB2 as the first-tier genetic test if a negative acquired cause. Patients with a GJB2-negative test are recommended to undergo an HL gene panel or WES sequencing as the second-tier test8.
In order to identify the genetic diagnosis of prelingual SNHL, we performed a prospective study by systematic history-taking and phenotyping, followed by WES with target gene analysis in 100 Thai patients.
Results
Demographic data
A total of 116 eligible individuals agreed to participate in the study; 16 were further excluded due to a clinical history suggesting acquired causes, delayed onset hearing loss, and mild SNHL. Of the 100 participants from 75 families (67 from the hospital and 33 from the school), there were 85 children and 15 adults, comprising 48 males and 52 females, and 36 familial and 64 sporadic cases. The 36 familial cases were from 21 families, including 14 siblings from 7 unrelated families, 12 affected trios (child-father-mother) from 4 families, and 10 children whose affected relatives were not enrolled in the study (Table 1 and Supplementary data: Table S1-S2). Only 4 patients had a history of parental consanguinity. The severity of HL was severe/severe-to-profound/profound in 70% and moderate/moderate-to-severe in 30%, respectively. Among 38 patients with available data on newborn hearing screening, all were tested by transient evoked otoacoustic emissions (TEOAEs or, in short, OAEs), and 7 patients were found to “Pass”, (Supplementary data, Table S2).
Clinical findings, syndromic and non-syndromic SNHL
Twenty-nine patients were found to have a history of motor delay, non-audiological symptoms, and/or abnormal inner ears (Table 2). These included a history of mild gross motor delay (n = 7), abnormal gait (n = 3), epilepsy (n = 1), hypopigmentation defects (n = 5), hyperpigmented spot (n = 2), goiter (n = 1), intellectual disability (n = 1), attention deficit (n = 1), preauricular skin tags (n = 2), and nonspecific facial dysmorphism (n = 1, Patient 100), abnormal cochlear (n = 3), enlarged vestibular aqueduct (n = 1), and abnormal internal acoustic canal with semicircular canal abnormality (n = 1). Only 6 out of 28 patients had recognizable sSNHL based on clinical characteristic features, including Waardenburg syndrome (n = 3), Tietz albinism syndrome (n = 2), and Pendred syndrome (n = 1). Clinical findings in the other patients were nonspecific for a recognized hearing loss syndrome. In sum, there were 94 patients with likely nsSNHL prior to the WES analysis.
Diagnostic yield and causative genes
In total, clinically relevant or positive variants were identified in 46 out of 100 patients. These included 41 P/LP (25P and 16 LP) and 4 VUS variants of 15 genes. The diagnostic yield of familial cases was 58.3% (21/36 cases), and 39.0% (25/64) for sporadic cases.
Of those with a prior diagnosis of sSNHL, the LP/P variants were detected in PAX3 and SOX10, each in a single patient with Waardenburg syndrome; an LP variant of MITF in two patients with Tietz albinism syndrome (Patients 40 and 67, a daughter and the mother); and SLC26A4 variants in a patient with Pendred syndrome (Patient 65; Tables 2 and 3). Another patient with Waardenburg syndrome was negative by WES and single gene testing (PCR-Sanger sequencing of PAX3) but was found to have a PAX3 deletion of exons 5–8 by a commercial gene panel study (Invitae®); therefore, this individual was counted as WES-negative.
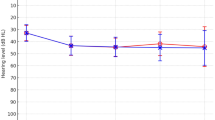
Among nsSNHL, genetic defects were identified in 41 out of 94 patients, or 43.6%, involving 39 P/LP (22P and 13 LP) and 4 VUS of 12 genes. The leading causative genes identified were GJB2 and SLC26A4, followed by MYO15A, MYO7A, POU3F4, OTOF, PCDH15, and then each in TMPRSS3, MITF, PTEN, ACTG1, MPZL2, and GSDME (Table 4and Fig. 1 ). As for Patient 40, who inherited Tietz-albinism syndrome-related MITF variant from her affected mother, and an autosomal dominant nsSNHL-related GSDME variant from her affected father, only the MITF variant was counted for diagnostic yield; this was to avoid complex calculations. Of those having autosomal recessive SNHL, 13 patients were homozygous and 28 were compound heterozygotes (Table 3 and Supplementary Table S3).
Of the 7 patients who “passed” OAEs, their WES was found positive in four patients: two for OTOF (Patients 35 and 49), one for SLC26A4 (Patient 13), and the other for MYO7A (Patient 3).
The inheritance of these nsSNHL genes involved X-linked recessive (POU3F4) in 3 patients, autosomal dominant (ACTG1, GSDME, and PTEN) in 3 patients, and autosomal recessive in the remainder. Compound heterozygosity was consistently confirmed through segregation analysis, including the Sanger sequencing result of the patient and at least one parent.
Identified variants
The details of each genetic variant and its frequency are shown in Tables 3 and 4. Eleven of the 45 positive variants detected were novel findings. The most common GJB2 variants were autosomal recessive alleles of c.109G > A, followed by c.235 delC (Table 4). Of those homozygous c.109G > A, two patients had severe-to-profound HL (Patients 83 and 100) while the other three had moderate-to-severe HL (Patients 54, 58, and 106; Table 3 and Table S3). Patients with the other variants had severe-to-profound HL.
The common SLC26A4 variants detected were c.290T > A, c.706 C > G, c.1229 C > T, and c.2162 C > T, c.1547dup, c.1971 C > A (Table 4). The severity of HL in these individuals was mostly severe-to-profound (n = 6), and the remainder was moderate-to-severe (n = 1) and moderate (n = 2). None of the affected children exhibited goiter, but one of the affected mothers did (Patient 65). There were two children with possible Pendred syndrome prior to WES as follows: the child (Patient 37) of a mother with Pendred syndrome, and the other child (Patient 79) due to the presence of 1 ½ turned cochlear noted in the temporal bone CT scan. However, we listed these children in the nsSNHL prior to the WES study because of the genetic heterogeneity of SNHL and cochlear abnormalities. The remaining patients with SLC26A4 either have enlarged vestibular aqueduct (EVA; Patient 15), normal cochlear (Patient 13), or unavailable data (n = 7).
As for the variants of MYO15A, MYO7A, and PCDH15 (a known gene for Usher syndrome type 1 F), POU3F4, OTOF, TMPRSS3, MITF, and MPZL2, the detected variants appear to be rather dispersed, and no common variants were identified (Tables 3 and 4). The MYO7A- and PCDH15-positive patients, whose ages ranged from 1 to 8 years, denied vision problems at night, and their ophthalmological examination is pending.
There were three autosomal dominant genes/variants detected in nsSNHL of this cohort. The GSDME c.781 C > T variant was found in a father and a child with severe-to-profound SNHL. The ACTG1 c.547 C > T variant was found in a sporadic case with moderate-to-severe SNHL (Patient 44). This ACTG1 variant was inherited from the father, who reported normal hearing but never had an audiological evaluation. A de novo PTEN variant, c.19G > T, was observed in another patient with profound SNHL and additional features of blue pigment macules at the left buttock, flat foot, questionable wide-base gait, and mild developmental delay (Patient 59; Table 2 and Supplementary Table S3).
Among six patients with apparently nsSNHL and inner ear abnormalities: EVA (Patients 15 and 28), hypoplastic/aplastic cochlear (Patients 47 and 85), 1 ½ turned cochlear (Patient 79), and atretic semicircular canal and enlarged vestibule (Patient 100), only three patients were found positive in SLC26A4 (Patients 15 and 79) and GJB2 (Patient 100).
Discussion
We identified an overall genetic diagnostic yield of 46% among the prelingual SNHL in the present study, 58.3% for familial cases, and 39.0% for sporadic cases. The majority, 89.1% (41/46), were nsSNHL and the remainder, 10.9% (5/46), represented sSNHL. The data revealed the frequency of different inheritance of HL as follows: autosomal recessive − 76.1% (36/46), autosomal dominant − 17.4% (8/46), and X-linked recessive − 6.5% (3/46). The leading causative genes were GJB2 and SLC26A4 in equivalent frequency, followed by MYO7A.
The high diagnostic yield in the present study is equivalent to 40 to 65% reported from other populations3,10,11,12,13,14.
Interestingly, the frequency of parental consanguinity was low at 4% in this cohort, but a family history of HL was frequent at 36%, suggesting that it could be attributed to the result of assortative marriage and high prevalence of HL gene/variants among the Thai population, specifically GJB2: c.109G > A and c.235delC alleles, and several SLC26A4 variants. A previous studies including a systematic review have demonstrated that c.109G > A was the most common GJB2 pathogenic allele detected among the combined (control + hearing loss) populations from Southeast Asia, including Thailand (9.3%), Taiwan (11.7%), Vietnam (13.2%), Malaysia (4.9%), China (6.8%), with the overall frequency at 6.6%, followed by c.235delC at 1.1%21,22,23,24. However, the most common GJB2 pathogenic allele found in the Asian HL population was the c.235delC (9.7%) followed by the c.109G > A (4.1%), whereas the leading causative variant among Thai HL populations in this study was c.109G > A. This has raised an interesting question of whether or not there is a modifier gene/variant potentiating the severity of HL among Thai patients with homozygous c.109G > A, which remains to be studied. Although the c.109G > A has been functionally confirmed to be a pathogenic recessive variant with incomplete penetrance and is typically associated with mild-to-moderate SNHL21 most of our patients had moderate-to-severe or profound SNHL. The c.35delG is extremely rare in Southeast Asian and East Asian populations, while it is the prevalent variant found among Caucasian and Arab descendants22,23,24,25,26.
Notably, there was high allelic heterogeneity of the LP/P SLC26A4 variants identified in the present study, with several variants being present in similar frequencies (Table 4). The presence of a high rate of compound heterozygosity also supports the assumption of assortative marriage among the deaf, which may lead to a positive family history of hearing loss, but not due to consanguinity. The finding of several LP/P variants among those positive for SLC26A4 was also observed among the Vietnamese HL population27. In contrast, there is evidence of a common SLC26A4 allele found among some HL populations, such as c.706 C > G (p.Leu236Val) in the Philippines28,29.
To our knowledge, this is the largest cohort of SNHL studied by NGS on the Thai population. Our data demonstrated high genetic heterogeneity of SNHL in Thai patients, with 45 variants detected in 15 genes of 46 patients, which is consistent with those found in other populations but in contrast to lower genetic heterogeneity of populations with high consanguinity26,30 Additionally, this is supported by the presence of a high proportion of compound heterozygous over homozygous individuals (27 vs. 13) among those with positive autosomal recessive SNHL, and around one-fifth of the positive variants being novel findings (Table 3).
Of those with inner ear anomalies in the present cohort, the genetic diagnosis was positive in 50.0% (3/6), in agreement with 54.5–73.9% from earlier reports31,32.
SLC26A4 is the only known gene responsible for Pendred syndrome, the most common autosomal recessive sSNHL. SLC26A4 also causes autosomal recessive nsSNHL with or without an enlarged vestibular aqueduct (EVA). SLC26A4 has been observed and the second or third common causative gene, next to GJB2, MYO15A, or STRC for nsSNHL in several populations12,17,18,19. SLC26A4 can be present in digenic with other recessive genes, namely, FOXI1 and KCNJ10, in SNHL; however, it is not detected here.
MYO15A variants found in 4 affected individuals were compound heterozygous, again supporting the notion that it is not a result of parental consanguinity. Interestingly, an individual (Patient 63) inherited two distinct variants, each from the homozygous parents.
MYO7A is known to cause autosomal dominant postlingual nsSNHL as well as autosomal recessive congenital nsSNHL and Usher syndrome type 1B4,9. However, only recessive MYO7A variants were found in this cohort, likely due to the inclusion of only prelingual SNHL in the study.
The presence of two autosomal dominant causative genes/variants, GSDME c.781 C > T and MITF: c.971G > A, in an affected individual (Patient 40) is again consistent with the assortative marriage among the deaf population. This also addresses the extreme genetic heterogeneity and complexity of genetic HL, as well as the necessity of extended gene panel testing or WES to capture HL-related genes as many as possible to avoid misdiagnosis due to the limited number of HL genes provided in the panel, as previously observed33.
Moreover, some genes can cause both autosomal dominant and autosomal recessive hearing loss, both sSNHL and nsSNHL, depending on the type of the variants. Variants in GJB2 can result in autosomal dominant sSNHL with ichthyosis/keratitis or palmoplantar hyperkeratosis, and autosomal recessive nsSNHL4,9,21. MITF variants may cause autosomal dominant Waardenburg and Tietz syndromes and autosomal recessive nsSNHL as seen in this and earlier studies33. MYO7A mutated alleles could lead to autosomal dominant postlingual HL with slow progression, and autosomal recessive prelingual nsSNHL and Usher syndrome type 1B9. Therefore, patients with MYO7A recessive variants require regular monitoring for retinitis pigmentosa and vestibular dysfunction, as these symptoms may not develop until the 2nd -3rd decades of life.
As for ACTG1, there were only 53 LP/P variants reported previously, and all of them were missense changes (https://www.ncbi.nlm.nih.gov/clinvar). ACTG1-related HL is often progressive in nature, first evident at high frequency, and is clearly observed by the twenties34,35. The ACTG1 variant, c.547 C > T, found in our patient (Patient 44) was listed as LP in ClinVar (VCV000808333.34), and there could be phenotypic variability; therefore, we counted this variant as a positive, although the heterozygous father reported normal hearing. We advised the father to have an audiological evaluation and monitoring. It should be noted that another amino acid substitution at this codon, c.548G > A or p.Arg183Gln (VCV000505063.7), was found as a de novo occurrence in HL patient, and confirmed to be likely pathogenic based on the protein modeling and molecular dynamics simulations35. PTEN is a known cause of macrocephaly/autism and cancer-predisposition disorders, Bannayan-Riley-Ruvalcaba, and Cowden syndrome, of which SNHL is a part of the conditions. Patient 59 could, in fact, represent a PTEN-related disorder.
For cases with VUS in an autosomal recessive gene, some are potentially pathogenic because of their presence in trans with another VUS or LP/P variant, along with the segregation with the hearing loss phenotype, such as the VUS variants of MYO7A detected in Patients 10 and 11, and that of the TMPRSS3 gene in Patient 33. Functional analysis and/or the recurrence of these variants in trans, either with another VUS or an LP/P variant only in the affected individuals, could support its pathogenicity. Additionally, periodic review and reanalysis every 1–2 years could lead to a reclassification, owing to new phenotypes and new variants of the genes being described.
In Thailand, universal newborn hearing screening has been around since 2003 and recommended by professional societies; however, it was not until recently, in 2022, that the screening became the public health policy (https://eng.nhso.go.th). This may explain the low percentage of patients with available records of OAEs in the present study.
Among patients with positive genes/variants, despite ‘passing” OAEs in this cohort, the OTOF-positive individual is likely that OAEs generally fail to detect OTOF-related SNHL because of the nature of auditory neuropathy of this disorder4,36. Only automated auditory brainstem evoked response (AABR) hearing screening can detect SNHL caused by auditory neuropathy36,37. The cases of positive SLC26A4 and MYO7A variants cannot be explained by the pathogenic mechanism of HL related to these two genes but possibly be supported by imperfect sensitivity and negative predictive value of OAEs that range around 66.7–67.7% and 45.6–99.7%, respectively38,39.
By integrating the result of WES-target gene analysis and systematic phenotyping, it is possible that the percentage of sSNHL of the present cohort could be up to 22% (6 Patients with Waardenburg and Tietz syndrome, 10 patients with SLC26A4, 3 patients with MYO7A, 2 patients with PCDH15, and one patient with PTEN positive alleles. We cannot conclude for sure at the present time because some features of Pendred syndrome (goiter) and Usher syndrome (retinitis pigmentosa) may not appear until later in life, and there is no available data on the CT temporal bone of these patients. These patients deserve a close follow-up for possible associated symptoms accordingly.
Data from the present study could benefit the patients and families in providing an accurate diagnosis and precise gene-guided management, such as long-term surveillance for retinal changes and vision loss in patients with Usher-related genes of which symptom may not be detected in early childhood, and genetic counseling and reproductive options, including in vitro fertilization (IVF) and pre-gestational testing. The pre-designed data collection form can be used as an assisting tool for physicians in collecting data systematically and more efficiently in excluding acquired causes and recruiting cases suitable for genetic analysis. This data could be useful in developing a clinical guideline for genetic testing following a positive newborn hearing screening and for newly confirmed patients. Moreover, the genetic epidemiological data would provide strong supporting information for the development of a new universal coverage benefit package of exome sequencing for prelingual hearing loss in Thai patients. The data could be useful for preconceptional carrier screening for common variants/causative genes of SNHL in the Thai general population.
Limitations of this study are that it is a single-center study, not a very large cohort, as well as the inability to detect structural variants, deep intronic mutations, or copy number variants, which WES might have missed.
In conclusion, WES with targeted gene analysis resulted in a high diagnostic yield among Thai patients with prelingual SNHL, both sporadic and familial cases, with high genetic heterogeneity. Identification of genetic defects plus systematic phenotyping has led to more precise clinical diagnosis and medical management, genetic counseling for patients and families, as well as broader aspects for national health policy related to early-onset hearing loss. This data also expands the phenotypes and genotypes of genetic HL.
Materials and methods
Patients
The study was a cross-sectional prospective study conducted from July 2023 to July 2024. Eligible participants were children aged < 18 years and adults aged ≥ 18 years with a history of prelingual-onset and moderate-to-profound SNHL and no known acquired causes, who were followed up at Ramathibodi Hospital and/or from a school for the deaf in Bangkok.
Exclusion criteria included postlingual and mild HL and acquired hearing loss, as detailed in Supplementary Table S4). Demographic data were collected, including age, sex, prenatal/perinatal/postnatal history, family history of hearing loss, and developmental disorder. A three-generation pedigree was also constructed for each family. Systematic phenotyping of the participants and first-degree relatives was obtained, including clinical history and physical examination (performed by the researchers, TD and DW). We used the screening questions and examination targeted at a medical history and phenotypes suggesting common syndromic HL as follows: clinical history of renal disease, goiter, night blindness, vertigo, white forelock, premature grey hair, blue iris, arrhythmia and/or sudden cardiac death in the young (before age 45 years), and developmental history; and systematic physical examination to identify possible dysmorphic features and signs of HL syndromes, namely growth parameters, coarse facial features, head size and shape, preauricular skin tag/pits, size and shape of the ear pinna, ear canal, pigmentation defects of skin (e.g., skin color, café au lait spots), white forelock and hypopigmented hair, telecanthus, periorbital minor anomalies (e.g., up-slanted eye, macro/micro cornea, coloboma, blue iris/heterochromia), cleft lip/palate, sinus tract/cyst along the anterior border of the sternocleidomastoid muscle, thyromegaly, heart sounds, extremities (e.g., tapered fingers, extra/missing digits, syndactyly, nail hypoplasia, stiff joints), signs of skeletal dysplasia and/or disproportionate short stature, and gait ataxia or abnormal movement, according to the case record form (CRF) designed for systematic data collection. (Table 5). Patients with positive mutations underwent surveillance for associated symptoms, such as goiter and retinitis pigmentosa, as clinically indicated.
The severity of hearing loss, as measured by auditory brainstem response (ABR), auditory steady-state response (ASSR), and/or audiometry, was retrospectively reviewed from the medical records or from the personal health records of the patients. The definition of degree of hearing loss was as follows: 41–70 dB = moderate; 71–90 dB = severe; >90 dB or having a cochlear implant = profound. As for those unavailable audiological reports, moderate-to-severe was assigned for patients who could hear some sound with/without some words, severe-to-profound for those who did not hear any sound and had no words.
Whole exome sequencing and targeted gene analysis
Peripheral blood was obtained from each participant and their parents. DNA was extracted following standard protocols, then subjected to WES using Illumina NovaSeq 6000 (Macrogen, South Korea) with Agilent’s SureSelect (V5 and 6G) for target enrichment and 150 bp Pair End mode (∼average read depth 125x), with exome capture ∼97% of the target regions. The exome data were quality assessed by using the FastQC package and read alignment against a reference genome (hg19 from UCSC genome browser database) by using Burrows-Wheeler aligner (BWA, version 0.5.9); SAMTOOLS for variant identification; ANNOVAR for variant annotation, filtering, and prioritizing the potential variants called for further analysis, following Broad Institute’s best practice guidelines for GATK v3.4 (https://www.broadinstitute.org/) and the previous established protocols40.
The VCF files were then analyzed using the Human Phenotype Ontology of hearing impairment (HP0000365: 1589 genes) and variants having minor allele frequencies (MAF) > 0.03 in the 1000 Genomes Project (November 2010 and October 2011 releases) were filtered out, except for the GJB2 gene variants that MAF > 0.05 was applied (owing to the high frequency of GJB2:p.Val37Ile variant among the Thai affected population24. The pathogenicity of the variants detected was initially classified into pathogenic (P), likely pathogenic (LP), variants of uncertain significance (VUS), likely benign (LB), and benign (B), according to ACMG/AMP 2015 general guidelines for interpretation of the sequence variants41. Those of VUS were further classified based on the 2018 modified criteria according to the expert specification for genetic HL42. The frequency of the variants identified was checked against the Genome Aggregation Database (gnomAD, available at https://gnomad.broadinstitute.org/) and Thai Genome Reference Database (ThaiGeR, available at https://thaiger.genomicsthailand.com).
Sanger sequencing was done to confirm the presence of the variants in the participants and parents to determine the inheritance pattern (cis, trans, or de novo) and disease segregation.
Clinically relevant variants were defined as P/LP variants, VUS in compound with P/LP variants, bi-allelic VUS in trans, or de novo VUS variants of autosomal dominant or X-linked genes. The relevant variants were counted as positive and calculated for diagnostic yield.
Research ethics
The research protocol was approved by the Ramathibodi Hospital Human Ethics Research Committee (MURA2023/528 and MURA2021/440 Ref.2024/502). All research was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants and/or their legal guardians.
Data availability
The datasets generated and/or analyzed during the current study are available in the ClinVar repository (https://www.ncbi.nlm.nih.gov/clinvar), with accession numbers as follows: SCV005901549, SCV005882697, SCV005882690, SCV005882692, SCV005882693, SCV005882694, SCV005882696, SCV005882695, SCV005894845, and SCV005894844.
References
Shearer, A. E. et al. H. in GeneReviews(®) (eds M. P. Adam University of Washington, Seattle Copyright © 1993–2024, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved., (1993).
Jariengpraser, C., Lertsukprasert, K., Kasemsuwan, L. & Nannareumitra, P. Newborn hearing screening using otoacoustic emission (OAEs): a 1-year study at Ramathibodi hospital. Thai J. Otolaryngol. Head Neck Surg. 4, 27–41 (2003).
Shadab, M. et al. Autosomal recessive non-syndromic hearing loss genes in Pakistan during the previous three decades. J. Cell. Mol. Med. 28, e18119. https://doi.org/10.1111/jcmm.18119 (2024).
Shearer, A. E. Genetic testing for pediatric sensorineural hearing loss in the era of gene therapy. Curr. Opin. Otolaryngol. Head Neck Surg. 32, 352–356. https://doi.org/10.1097/MOO.0000000000001005 (2024).
Shearer, A. E. et al. in GeneReviews((R)) (eds M. P. Adam (1993).
Smith, R. J., Bale, J. F. Jr. & White, K. R. Sensorineural hearing loss in children. Lancet 365, 879–890. https://doi.org/10.1016/S0140-6736(05)71047-3 (2005).
Tanna, R. J., Lin, J. W. & De Jesus, O. in StatPearls (2025).
Alford, R. L. et al. American college of medical genetics and genomics guideline for the clinical evaluation and etiologic diagnosis of hearing loss. Genet. Med. 16, 347–355. https://doi.org/10.1038/gim.2014.2 (2014).
Walls, W. D., Azaiez, H. & Smith, R. J. H. Hereditary Hearing Loss Homepage, https://hereditaryhearingloss.org (2024).
Elander, J. et al. Extended genetic diagnostics for children with profound sensorineural hearing loss by implementing massive parallel sequencing. Diagnostic outcome, family experience and clinical implementation. Int. J. Pediatr. Otorhinolaryngol. 159, 111218. https://doi.org/10.1016/j.ijporl.2022.111218 (2022).
Mazzola, S. & Schreiber, A. Genetics evaluation outcomes of patients with pediatric hearing loss: 2008–2022 retrospective study. Am. J. Otolaryngol. 45, 104196. https://doi.org/10.1016/j.amjoto.2023.104196 (2024).
Van Heurck, R. et al. Benefits of exome sequencing in children with suspected isolated hearing loss. Genes (Basel). 12 https://doi.org/10.3390/genes12081277 (2021).
Xiang, J. et al. Comprehensive genetic testing improves the clinical diagnosis and medical management of pediatric patients with isolated hearing loss. BMC Med. Genomics. 15, 142. https://doi.org/10.1186/s12920-022-01293-x (2022).
Yamamoto, N. et al. Comprehensive gene panel testing for hearing loss in children: Understanding factors influencing diagnostic yield. J. Pediatr. 262, 113620. https://doi.org/10.1016/j.jpeds.2023.113620 (2023).
Morzaria, S., Westerberg, B. D. & Kozak, F. K. Systematic review of the etiology of bilateral sensorineural hearing loss in children. Int. J. Pediatr. Otorhinolaryngol. 68, 1193–1198. https://doi.org/10.1016/j.ijporl.2004.04.013 (2004).
Koffler, T., Ushakov, K. & Avraham, K. B. Genetics of hearing loss: syndromic. Otolaryngol. Clin. North. Am. 48, 1041–1061. https://doi.org/10.1016/j.otc.2015.07.007 (2015).
Downie, L. et al. Exome sequencing in infants with congenital hearing impairment: a population-based cohort study. Eur. J. Hum. Genet. 28, 587–596. https://doi.org/10.1038/s41431-019-0553-8 (2020).
Likar, T. et al. Diagnostic outcomes of exome sequencing in patients with syndromic or non-syndromic hearing loss. PLoS One. 13, e0188578. https://doi.org/10.1371/journal.pone.0188578 (2018).
Ma, H. et al. Genetic and phenotypic analysis of 225 Chinese children with developmental delay and/or intellectual disability using whole-exome sequencing. BMC Genom. 25, 391. https://doi.org/10.1186/s12864-024-10279-1 (2024).
Shearer, A. E. & Smith, R. J. Massively parallel sequencing for genetic diagnosis of hearing loss: the new standard of care. Otolaryngol. Head Neck Surg. 153, 175–182. https://doi.org/10.1177/0194599815591156 (2015).
Posukh, O. L., Maslova, E. A., Danilchenko, V. Y., Zytsar, M. V. & Orishchenko, K. E. Functional consequences of pathogenic variants of the GJB2 gene (Cx26) localized in different Cx26 domains. Biomolecules 13 https://doi.org/10.3390/biom13101521 (2023).
Feng, Y., Hu, S., Zhao, S. & Chen, M. Recent advances in genetic etiology of non-syndromic deafness in children. Front. Neurosci. 17, 1282663. https://doi.org/10.3389/fnins.2023.1282663 (2023).
Ozgur, Z. et al. Systematic review and meta-analysis of pathogenic GJB2 variants in the Asian population. Int. J. Pediatr. Otorhinolaryngol. 189, 112233. https://doi.org/10.1016/j.ijporl.2025.112233 (2025).
Wattanasirichaigoon, D. et al. High prevalence of V37I genetic variant in the connexin-26 (GJB2) gene among non-syndromic hearing-impaired and control Thai individuals. Clin. Genet. 66, 452–460. https://doi.org/10.1111/j.1399-0004.2004.00325.x (2004).
Zainal, S. A., Md Daud, M. K., Abd Rahman, N., Zainuddin, Z. & Alwi, Z. Mutation detection in GJB2 gene among Malays with non-syndromic hearing loss. Int. J. Pediatr. Otorhinolaryngol. 76, 1175–1179. https://doi.org/10.1016/j.ijporl.2012.04.027 (2012).
Alkhidir, S. et al. The genetic basis and the diagnostic yield of genetic testing related to nonsyndromic hearing loss in Qatar. Sci. Rep. 14, 4202. https://doi.org/10.1038/s41598-024-52784-z (2024).
Han, J. J. et al. Elucidation of the unique mutation spectrum of severe hearing loss in a Vietnamese pediatric population. Sci. Rep. 9, 1604. https://doi.org/10.1038/s41598-018-38245-4 (2019).
Chiong, C. M. et al. The SLC26A4 c.706C > G (p.Leu236Val) variant is a frequent cause of hearing impairment in Filipino cochlear implantees. Otol Neurotol. 39, e726–e730. https://doi.org/10.1097/MAO.0000000000001893 (2018).
Truong, B. T. et al. Exome sequencing reveals novel variants and unique allelic spectrum for hearing impairment in Filipino cochlear implantees. Clin. Genet. 95, 634–636. https://doi.org/10.1111/cge.13515 (2019).
Reis, C. S., Quental, S., Fernandes, S., Castedo, S. & Moura, C. P. Whole-Exome sequencing targeting a gene panel for sensorineural hearing loss: the first Portuguese cohort study. Cytogenet. Genome Res. 162, 1–9. https://doi.org/10.1159/000523840 (2022).
Baldyga, N. et al. The genetic background of hearing loss in patients with EVA and cochlear malformation. Genes (Basel). 14. https://doi.org/10.3390/genes14020335 (2023).
Yilmaz, U. et al. Identifying DNA variants in a Turkish cohort with inner ear anomalies. Ear Nose Throat J. 103, 32S–36S. https://doi.org/10.1177/01455613241287290 (2024).
Thongpradit, S. et al. MITF variants cause nonsyndromic sensorineural hearing loss with autosomal recessive inheritance. Sci. Rep. 10, 12712. https://doi.org/10.1038/s41598-020-69633-4 (2020).
Morell, R. J. et al. A new locus for late-onset, progressive, hereditary hearing loss DFNA20 maps to 17q25. Genomics 63, 1–6. https://doi.org/10.1006/geno.1999.6058 (2000).
Morgan, A. et al. Genomic studies in a large cohort of hearing impaired Italian patients revealed several new alleles, a rare case of uniparental disomy (UPD) and the importance to search for copy number variations. Front. Genet. 9, 681. https://doi.org/10.3389/fgene.2018.00681 (2018).
Azaiez, H. et al. in GeneReviews((R)) (eds M. P. Adam (1993).
Iwasa, Y. et al. OTOF mutation screening in Japanese severe to profound recessive hearing loss patients. BMC Med. Genet. 14, 95. https://doi.org/10.1186/1471-2350-14-95 (2013).
Kalambe, S., Gaurkar, S., Jain, S. & Deshmukh, P. Comparison of otoacoustic emission (OAE) and brainstem evoked response audiometry (BERA) in high risk infants and children under 5 years of age for hearing assessment in Western india: A modification in screening protocol. Indian J. Otolaryngol. Head Neck Surg. 74, 4239–4253. https://doi.org/10.1007/s12070-021-02876-3 (2022).
Yousefi, J., Ajalloueyan, M., Amirsalari, S. & Hassanali Fard, M. The specificity and sensitivity of transient otoacustic emission in neonatal hearing screening compared with diagnostic test of auditory brain stem response in Tehran hospitals. Iran. J. Pediatr. 23, 199–204 (2013).
Van der Auwera, G. A. et al. From FastQ data to high confidence variant calls: the genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinf. 43 (10 33), 11–11. https://doi.org/10.1002/0471250953.bi1110s43 (2013).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet. Med. 17, 405–424. https://doi.org/10.1038/gim.2015.30 (2015).
Oza, A. M. et al. Expert specification of the ACMG/AMP variant interpretation guidelines for genetic hearing loss. Hum. Mutat. 39, 1593–1613. https://doi.org/10.1002/humu.23630 (2018).
Acknowledgements
We thank the patients and their families for their participation in this study, as well as the teachers and staff at Setsatien School for the Deaf for their kind support in distributing the project brochure to the student’s families, the Ramathibodi Foundation for financial support for genetic analysis. We are thankful to the Faculty of Medicine Ramathibodi Hospital for giving the Research Career Development Awards to DW, TT, and PW.
Funding
Rare Disease Fund, Ramathibodi Hospital Foundation.
Author information
Authors and Affiliations
Contributions
TD and DW conceptualized and designed the study. TD and DW collected clinal data, performed a physical examination, and prepared the manuscript draft. TD, DW, RT, NK, SK, TT, and PW recruited participants and provided patient care. TD, AT, and SN performed and/or analyzed the molecular data. DW acquired funding, supervised the study, and performed critical editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Damrongchietanon, T., Wattanasirichaigoon, D., Khongkraparn, A. et al. Diagnostic yield of whole exome sequencing with targeted gene analysis in prelingual sensorineural hearing loss in Thailand. Sci Rep 15, 32784 (2025). https://doi.org/10.1038/s41598-025-18038-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18038-2