Abstract
In normally sighted adult volunteers, applying a monocular patch for a few hours produces a short-term shift of ocular dominance in favor of the deprived eye—a phenomenon often interpreted as a form of homeostatic plasticity. We recently showed that the same effect can be elicited without eye-patching, by delaying the image in one eye (by 333 ms) over a 1 h period, during which participants engaged in a visuomotor coordination task; at the end of this period, ocular dominance shifted in favor of the delayed eye. Here we extended these findings, showing that passive exposure to the dichoptic replay of the same video with the same monocular delay did not affect ocular dominance. Moreover, we showed that the ocular dominance shift elicited by monocular delay during goal-directed actions had the same size as the effect of monocular deprivation, achieved by replacing the delayed image with a homogeneous gray screen, and that the two effects were correlated across participants. These results suggest that homeostatic plasticity is gated by a mismatch between vision in one eye and its multimodal context, and it is not necessarily linked with visual deprivation.
Similar content being viewed by others
Introduction
Monocular deprivation has been extensively used to study neuroplasticity in the visual system1. In animal models, depriving one eye of visual stimulation for days/weeks early in life leads to a permanent shift of ocular dominance in favor of the non-deprived eye2,3,4. This reorganization is linked with Hebbian plasticity, and it is characteristic of a “critical period” during development1. In humans, a much shorter period of monocular deprivation (minutes/hours) produces a paradoxically opposite and transient shift of ocular dominance in favor of the deprived eye5,6—as reported in a large set of studies, which we recently meta-analyzed7. This form of plasticity has been reliably observed across a large age range: between 68 and 81 years of age9,10. Since it dynamically up-regulates neural excitability in response to reduced sensory input11,12,13 it is often termed homeostatic plasticity14.
However, we recently demonstrated that reducing the sensory input to one eye is not necessary to induce the short-term ocular dominance shift15. We used an altered-reality setup to delay the visual input in one eye by 333 ms while participants continuously performed visually-guided goal-directed actions (building towers with wooden blocks) for 1 h. At the end of this period of monocular delay, ocular dominance shifted towards the delayed eye, qualitatively reproducing the shift of ocular dominance towards the deprived eye elicited by monocular deprivation.
This result aligns with previous evidence that a short-term ocular dominance shift can be elicited by manipulating the input in one eye (without attenuating it): through temporal reversal of a visual sequence16,17,18, application of an inverting prism19, phase scrambling20,21,22,23 or application of a kaleidoscopic lens24. In all cases, ocular dominance shifted in favor of the manipulated eye. These manipulations share a key feature: they make the input in the two eyes incompatible, inevitably discouraging binocular fusion and likely promoting perceptual suppression of the distorted/inverted eye. It is possible that such inter-ocular suppression mimicked the effects of the physical removal of input to the same eye16,17,18,25, hence reproducing the post-deprivation homeostatic boost. Our approach differed from these paradigms in two important ways. First, our monocular delay only introduced sparse and local mismatches between the eyes: only at the spatiotemporal coordinates of moving objects did dichoptic vision become incongruent, while all static elements in the scene remained congruent and promoted binocular fusion. Second, monocular delay alone did not promote any ocular dominance shift, indicating that our manipulation of the visual input was not responsible for the effect. The ocular dominance shift was only observed when monocular delay was paired with active engagement in a visually-guided goal-directed series of actions; it was not observed when participants passively viewed (with the same monocular delay) someone else performing the task. This result is indicative of the importance of visually-guided goal-directed actions (with the associated motor, perceptual and cognitive effects) for the generation of the ocular dominance shift.
There are multiple classically described mechanisms through which actions could modulate visual perception. One is through the feedback signals generated by action execution, e.g. through integration of proprioceptive signals generated by the action26. Another is through a “corollary discharge” or “efference copy” signal, which is generated in-synch with the motor command and sent to the sensory systems to predict the consequences of the action and eliminate them from awareness (e.g. the retinal motion generated during a saccadic eye movement27,28,29). This view implies that actions may be encoded as complex representations, which include their consequences on the external world and the agents themselves30. Action planning and execution are also inextricably linked with shifts of covert attention, which focus processing resources at the spatio-temporal coordinates that are most relevant for the action31. That plasticity was selectively elicited when monocular delay was combined with goal-directed actions, not in passive viewing conditions, suggests that any of these mechanisms (or all of them) contributes to gating the short-term ocular dominance shift.
One important limitation of our previous study15 consists in the imperfect control of the passive viewing conditions. The visual input during the passive control was not the same as during visually-guided actions, because actions in the two conditions were performed by different agents at different times. This difference could in principle be responsible for the different results in the active vs. passive conditions. The first aim of the present study was to exclude this possibility, by ensuring that visual stimulation was identical across the two conditions. For this, we recorded each participant’s binocular view while they performed the tower-building task with the 333 ms monocular delay15 and then replayed the same binocular visual input to the same participant, who passively watched the scene in first-person perspective (promoting sense of agency32). We asked whether the ocular dominance shift induced by monocular delay would still be larger during goal-directed actions than in passive viewing, even though visual stimulation was identical across conditions.
With the present study, we also pursued a second aim, to evaluate the magnitude of the ocular dominance shift induced by monocular delay during goal-directed actions, compared with the effect induced by monocular deprivation. To measure both effects in comparable conditions, we presented the same participants with another replay of their own actions, but this time we only showed the image in the non-delayed eye, while the other was replaced with a static homogeneous gray screen. This allowed us to directly compare the effects of monocular deprivation (nulling contrast in one eye) and those of monocular delay, with these two conditions applied for the same 1 h period and with the same visual stimulation of the non-manipulated eye—two of the main factors affecting the monocular deprivation effect-size (duration33,34; visual experience during the experimental condition15,19,35), as reviewed in7.
Results
We used a custom-built altered-reality set-up to control binocular visual stimulation. In the main experiment, participants (N = 19) performed goal-directed actions under visual guidance (they used wooden blocks to build towers), while the altered-reality set-up delayed the input to their dominant eye by 333 ms (Fig. 1). We consistently applied monocular delay to each participant’s dominant eye, like in our previous study15 and following a common practice in short-term monocular deprivation studies7. The tower-building task lasted 1 h, during which the visual input to the two eyes was recorded. We then replayed the video to two subsets of the same participants (both N = 15), who watched it passively in two comparison conditions (approximately 2 weeks apart): in the first, the two monocular images were delivered to the corresponding eyes, reproducing the same visual stimulation as in the main experiment including the monocular delay; in the second condition, the delayed image was replaced with a homogeneous gray screen, inducing a form of monocular deprivation.
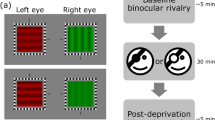
Experimental set-up for the three monocular conditions. (A) Monocular delay during visually-guided goal-directed actions, during which participants built towers with wooden blocks, monitoring their actions through an altered-reality set-up (stereo-camera mounted above an Oculus set); the image in their dominant eye was delayed by 333 ms. (B) Monocular delay in passive viewing, where participants passively watched a replay of the images recorded in A, including the delayed image in their dominant eye. (C) Monocular deprivation: participants passively watched a replay of the images recorded in A except the image in their dominant eye was replaced with a homogeneous gray screen. Each condition lasted 1 h, preceded and followed by binocular rivalry tests that measured ocular dominance. The active condition was always tested first; its recordings were used in the other two conditions, the order of which was counterbalanced across participants.
Immediately before and after each 1 h experiment, ocular dominance was assessed with binocular rivalry. Ocular dominance may be quantified with two binocular rivalry parameters: dominance proportions and mean dominance phase durations.
Dominance proportion is defined as the total time during which the image in the dominant eye was perceived, divided by the total dominance time (i.e. the total testing time minus the short intervals during which perception was a mixture of the stimuli in the two eyes) and expressed as percentage. Figure 2 shows the dominance difference (ΔOD%) after vs. before each 1 h experiment. A one-way ANOVA considering the 14 participants tested in all three conditions revealed a significant effect of conditions (F(2, 26) = 5.45, p = 0.011, ηp2 = 0.29).
Shifts of ocular dominance (ΔOD%, defined as the dominance proportion of the manipulated eye, measured after each 1 h experiment, minus the dominance measured before the experiment) elicited in the three monocular conditions. The dashed line at y = 0 indicates no effect. The box plots show the median, interquartile and range of the three distributions (same color coding as in Fig. 1) for all participants tested in each condition (19, 15 and 15 respectively); symbols above each distribution report the significance of each distribution (***p < 0.001, ns = not significant). The ANOVA and the post-hoc tests (symbols above horizontal lines, *p < 0.05, **p < 0.01, ns = not significant, Bonferroni corrected) were run on the subset of N = 14 participants with data in all conditions.
Follow-up post-hoc t-tests (Bonferroni corrected) compared the three conditions, showing a significant difference between the monocular delay during visually-guided goal-directed actions and the monocular delay during passive viewing conditions (post-hoc ttest, t(26) = 2.59, p = 0.046, logBF = 0.42), as well as between the monocular delay during passive viewing and the monocular deprivation conditions (post-hoc t-test, t(26) = 3.06, p = 0.015, logBF = 0.45). In contrast, there was no significant difference between the monocular delay during visually-guided goal-directed actions and monocular deprivation (post-hoc t-test, t(26) = 0.47, p = 1, logBF = −0.48). We also performed one-sample t-tests on the three ΔOD% values (considering all participants tested in each condition), which showed significant effects in both the monocular delay during visually-guided goal-directed actions (t(18) = 4.19, p < 0.001, logBF = 1.79) and the monocular deprivation conditions (t(14) = 5.21, p < 0.001, logBF = 2.35), and no significant effect of monocular delay during passive viewing (t(14) = 1.17, p = 0.258, logBF = −0.32).
Figure 3 shows an alternative metric based on mean phase durations, which are commonly used to characterize rivalry dynamics36 and can provide an indirect index of ocular dominance, with longer mean phase durations associated with the dominant eye vs. the non-dominant eye. Dominance phases are time-intervals during which perception is dominated by the image presented in one eye, before switching to the image presented in the other eye (or to a mixture of the two). We studied mean phase durations across time-points (before vs. after the 1 h experiment) and eyes (dominant vs. non-dominant) for the three conditions of our experiment (monocular delay during visually-guided goal-directed actions, monocular delay during passive viewing and monocular deprivation); we entered these values in a three-way ANOVA for repeated measures after log-transforming the values. This revealed a significant main effect of eye (dominant vs. non dominant: F(2, 26) = 25.98, p < 0.001, ηp² = 0.66) meaning that, as expected, dominance phases lasted longer for the dominant eye than for the non-dominant eye. This dominance was modulated over time (significant eye × time interaction: F(2, 26) = 13.62, p = 0.003, ηp2 = 0.51), suggesting that ocular dominance shifted between the pre- and post-experiment phases. With one exception, the other main effects or interactions were far from the significance threshold (condition: F(2, 26) = 2.20, p = 0.13, ηp2 = 0.14; time: F(2, 26) = 1.80, p = 0.20, ηp2 = 0.12; condition × time: F(2, 26) = 0.68, p = 0.51, ηp2 = 0.05; condition × eye: F(2, 26) = 0.53, p = 0.59, ηp2 = 0.04; see also Supplementary Materials for two-way ANOVAs on data from each condition). The exception was the three-way interaction, which revealed a non-significant trend for ocular dominance shifts to vary across conditions (eye × time × condition: F(2, 26) = 2.78, p = 0.08, ηp2 = 0.17). This trend is qualitatively consistent with the effect revealed by analyzing the proportion-based ocular dominance index in Fig. 2; its non-significance is likely related to the lower reliability of ocular dominance estimates based on mean phase durations, compared with estimates based on dominance proportions37.
Mean dominance phase durations for the two eyes, before and after each 1 h experimental condition: continuous lines represent the manipulated eye (delayed or deprived), while dashed lines represent the non-manipulated eye. Error bars are standard errors across participants. Text insets report the significance of the interaction term of two-way ANOVAs with factors time (pre vs. post) and eye (manipulated vs. non-manipulated) run separately in each condition—complete statistics are reported in the Supplementary Material; *p < 0.05; **p < 0.01; ***p < 0.001; ns non-significant.
For completeness, we also checked the proportion of binocular rivalry mixed percepts, another important parameter of rivalry dynamics that represents the time during which neither eye completely dominates perception. We found no change in mixed percepts in either condition (see Supplementary Fig. S1 and related text for statistics).
Using the more reliable proportion-based ocular dominance index (reported in Fig. 2), we proceeded to analyze the relations between the ocular dominance shifts observed in the three conditions. Figure 4 shows individual participants’ ΔOD% values, correlating the results for monocular delay during action (x-axes) with the results for the other two conditions. There was no correlation between the non-significant ocular dominance shifts induced by monocular delay during passive viewing and the significant shifts induced either by monocular delay during actions (robust correlation38: r(15) = 0.15, p = 0.608, logBF = −0.65, 1 outlier; Fig. 4A) or by monocular deprivation (r(14) = −0.04, p = 0.898, logBF = −0.65, no outliers, not shown). In contrast, the effects of monocular delay during visually-guided goal-directed actions were significantly correlated with those of monocular deprivation (Fig. 4B: r(15) = 0.61, p = 0.015, logBF = 0.56, no outliers); the fact that the bisection line lies within the confidence interval of the robust linear fit confirms that the effects of the two conditions were statistically indistinguishable.
Correlation between the ocular dominance shifts (ΔOD %, defined as the dominance of the manipulated eye measured after each 1 h experiment, minus the same value before the experiment) observed across the three experiments: (A) Monocular delay during visually-guided goal-directed actions vs. passive viewing and (B) Monocular delay during visually-guided goal-directed actions vs. monocular deprivation. Text insets report Pearson’s correlation coefficients (r) and corresponding p values, computed after automatic removal of the outliers with the robust regression toolbox38 (*p < 0.05, **p < 0.01, ns = not significant). Dashed black lines indicate the identity and zero functions. The continuous black lines show the best linear fit with confidence intervals.
Our experimental design required that the active condition was always tested first, recording the videos to be replayed in the other two conditions; however, there are several indications that this did not confound our results. First, our experimental sessions were widely spaced (on average every 2 weeks), making it very unlikely that the short-lived ocular dominance shifts could have accumulated across sessions39,40. Second, we tested for variations in baseline ocular dominance across the three experiments and found no evidence for any habituation or familiarization effects (one-way ANOVA on preOD%: F(2, 26) = 1.03, p = 0.36, ηp2 = 0.07).
Discussion
Using an altered-reality system, we induced a temporal discrepancy between the visual input in the two eyes, while participants performed visually-guided goal-directed actions, or while they passively viewed a replay of their own actions with the same monocular delay. After 1 h, ocular dominance shifted in favor of the delayed eye, but only if participants had actively engaged in the task, not when they had passively viewed the same visual scene. These results confirm and extend our previous observations15 showing that monocular delay is sufficient to elicit short-term ocular dominance plasticity and that the effect is selectively observed during active task performance, not in passive viewing.
In passive viewing, monocular delay implies that the two eyes provide inconsistent but equally valid descriptions of the visual scene. Lacking strong temporal cues that adjudicate which eye is “online”, perception may sample from both eyes (perhaps in an alternating fashion), predicting no impact on ocular balance. In contrast, active task performance unambiguously flags the delayed eye as the one providing an invalid description of the visual scene. We suggest that this mismatch lies at the origin of the ocular dominance plasticity effect, promoting a boost of the mismatched eye representation. Multiple sources of action-related signals could be involved, including motor plans leading up to the actions, multisensory feedback from their execution and the accompanying attentional shifts.
Motor planning can influence perception through a corollary discharge mechanism27,28,29. In this model, the preparation of an action is characterized by the generation of two signals: a motor command, and a copy or corollary signal that is sent to the sensory systems to predict the consequences of the action. In this model, the corollary discharge signal serves to alert the sensory systems of the impending changes that will result from action execution, usually suppressing them from awareness41. In our paradigm, the corollary discharge can only predict the signals from the non-delayed eye, while the delayed eye could escape its suppressing effects. As a result, these mismatched signals may be transiently boosted42 explaining the ocular dominance shift in favor of the delayed eye.
A similar mismatch occurs between signals from the delayed eye and the multisensory feedback from the actions. The execution of our tower-building task produced proprioceptive and tactile feedback signals linked with the manipulation of the wooden blocks, as well as auditory feedback signals, e.g. the sound of wooden blocks moved by the experimenter or falling on the desk. All these were out-of-synch with the visual changes in the delayed eye. In the present study, auditory cues were not included in the passive viewing condition, implying that we cannot evaluate their effect. However, in our previous study this mismatch was preserved in the passive condition, where participants watched and heard another person performing the tower-building task15. Nevertheless, no ocular dominance plasticity was observed. This suggests that audiovisual correspondence alone is not sufficient to flag the delayed eye as inconsistent. It remains an open question whether this is due to insufficient strength of the auditory cues or of multisensory integration43. An alternative possibility would be that motor signals related to the planning of goal-directed actions are a necessary component of the short-term plasticity effect, which cannot be reproduced by manipulating multisensory congruence alone. Follow-up studies are required to adjudicate between these competing interpretations.
There is evidence that watching a replay of one’s own actions in first-person perspective reactivates sensorimotor signals associated with the action plan44,45. According to an influential theory30 the same network is activated when performing or imagining/observing an action, which may predict similar effects in our active and passive conditions (as long as observation triggered an internal simulation of the action). This could have predicted that passive viewing in first-person perspective elicited a similar ocular dominance shift as during actions, in contrast with the lack of effect with passive viewing in third-person perspective15. Instead, we find that the first- or third-person perspective produced comparable results: in both cases, passive viewing elicited no ocular dominance shift (note that first- and third-person perspective results are not directly comparable, as they were tested in different participants and under slightly different conditions, with auditory cues available in the third- not the first-person perspective case). This suggests that reactivating one’s action schemes does not suffice to disambiguate which eye is mismatched and that plasticity is selectively elicited when monocular delay is combined with the execution of visually-guided goal-directed actions.
Actions are also tightly linked with the allocation of attention46. During goal-directed actions, attention tends to shift toward the action targets47 and withdrawn from other locations46. As a result, attentional resources may be focused on visual changes seen by the online eye and withdrawn from those in the delayed eye. Can attention withdrawal explain the enhancement of the delayed eye after restoring normal vision? A recent series of studies16,17 supported this view showing that directing attention to the movie-frames in one eye eventually shifted ocular dominance in favor of the unattended eye (which saw the same movie frames in reverse order). However, in those experiments the images in the two eyes were incompatible, disrupting binocular fusion like in most other monocular manipulations (monocular image distortion20,21,22,24, inversion19 or continuous suppression25,48). Under such conditions, directing attention to the image in one eye can promote interocular suppression of the other eye, essentially reproducing the effects of attenuating its input (deprivation), hence providing a possible explanation for its rebound boost. Contrary to most other monocular manipulations, ours (monocular delay) preserves the congruency between the two eyes at most visual locations, promoting binocular fusion. Mismatched signals primarily come from the hands and motor targets (only about 20% of the image); it is possible that allocation of action-linked attention to these targets is sufficient to trigger inter-ocular suppression and the rebound ocular dominance shift.
The effect of monocular delay during action had the same magnitude as the effect of monocular deprivation across our participants. This implies that mismatching visual signals at sparse locations in space/time is sufficient to reproduce the effect of eliminating monocular visual information across most of the visual field. Thus, we conclude that short-term ocular dominance plasticity does not require manipulating the strength of visual input in the two eyes, or preventing binocular fusion, at least under conditions requiring visually-guided goal-directed actions.
One apparent difference between monocular delay and monocular deprivation is their relation to action execution. While monocular delay selectively affected ocular dominance when combined with actions, monocular deprivation shifted ocular dominance even in our passive viewing condition. We suggest that this may be interpreted based on the different mismatch that monocular deprivation and monocular delay introduce relative to the multimodal context. While passive viewing eliminates the disadvantage of the delayed eye (both eyes provide an equally valid description of the visual field), the deprived eye may be flagged as inconsistent by its inability to provide any visual information. On this view, coherence with multimodal context could be seen as one of the mechanisms gating ocular dominance plasticity. This is in line with evidence suggesting that this form of plasticity engages multimodal factors50,51; it is also consistent with evidence that its effects extend beyond the primary visual cortex, where ocular dominance is set, encompassing a large-scale network reorganization52.
Conclusion
We conclude that a small mismatch between monocular vision and the multimodal context is sufficient to elicit a short-term ocular dominance shift, reproducing the homeostatic plasticity elicited by monocular deprivation. Our results strengthen the evidence that performing visually-guided goal-directed actions gates this form of plasticity. This implies that multimodal signals (action plans and/or multisensory feedback and/or the accompanying attentional allocation) participate in setting the plasticity of sensory processing, even in adult individuals and even for features encoded at the earliest levels of the visual system – where the balance between monocular signals is set, likely in the primary visual cortex.
Methods
Human participants
Experimental procedures were approved by the regional ethics committee [Comitato Etico Pediatrico Regionale—Azienda Ospedaliero-Universitaria Meyer—Firenze (FI); protocol “Plasticita’ del Sistema visivo”] and are in line with the principles of the declaration of Helsinki. Participants gave written informed consent before participating in the studies. We estimated the necessary sample size based on our previous study15; we took the difference between ΔOD% (ocular dominance shift measured with proportions, see Eq. 1 below) in the condition where participants actively engaged in the tower building task vs. passively viewed another person performing the task. The Cohen’s d of this difference indicated a large effect size (d = 0.9); we computed the number of participants required to replicate such effect with 80% power and alpha 0.05, yielding 12 participants.
We recruited 21 participants, all with normal or corrected-to-normal vision, with no history of amblyopia, strabismus, or eye surgery. Two participants were excluded due to difficulty in following the instructions (they failed to maintain continuous activity on the tower-building tasks, frequently pausing the task despite being urged not to). Nineteen participants took part in the active monocular delay condition; a subset of these also took part in the monocular deprivation (N = 15) or the passive monocular delay conditions (N = 15). Fourteen participants completed all three conditions.
In 14 participants, we verified the quality of stereovision with the Firsby Stereotest™; they had a mean stereoacuity of 107 (s.e.m. = 3) seconds of arc. For all participants the dominant eye was assessed by binocular rivalry (see below, “Binocular Rivalry” section).
Altered-reality set-up
Participants sat at a desk with a 37 × 29 cm area containing wooden bricks that they could manipulate; the working area was comfortably within their arm range (20 to 60 cm from the participant’s head). Participants viewed the desk area and their hands through an Oculus VR headset (Oculus Rift, DK2, fit to the headrest) displaying the images captured with a stereo camera (Zed) mounted upon the Oculus frame (Fig. 1). Participants’ head and the Oculus set were stabilized with a chin and headrest, minimizing the sense of discomfort and nausea that is typically reported when the Oculus set was used head-free.
The combined stereo camera and Oculus VR system had a visual field of 80 × 60 deg with a resolution of 960 × 720 pixels per eye. Camera vergence was adjusted for each participant to account for small differences in interpupillary distance. The Zed camera and Oculus system had refresh rates of 60 Hz and 75 Hz respectively, and a minimum delay of 35 ms. In the main experiment, participants engaged in a visuomotor task, using wooden bricks (average size: 2 cm) to build block-towers continuously for 1 h, with visual information conveyed through the altered-reality set-up. They were instructed to construct as many and as tall towers as possible, rebuilding them when they collapsed. We recorded the images streamed on the altered-reality set-up, capturing participants’ activities with a first-person perspective; the recording was achieved through the same integrated software that operated the altered-reality system. Sounds were not recorded.
In two participants, we additionally acquired an image of the eye, to ensure that participants kept them open throughout the experiment. We quantified the percentage of time during which one eye was closed (e.g. due to blinks); this was less than 5% on average, stable over the course of the 1 h experiment.
Experimental procedure
Participants were tested in three sessions, each lasting 1 h and conducted on separate days, with binocular rivalry assessed at the beginning and the end of each session.
In the first condition, participants engaged in a visuomotor task while their dominant eye vision was delayed by 333 ms (ocular dominance was determined with binocular rivalry, see below). The delay produced large interocular differences for any moving object including their hands. Participants reported seeing the moving object twice or seeing a moving object with a tail or shadow trailing behind it. However, this was selective for the visual field regions containing visual motion. For all other regions (> 80% of pixels), the two binocular images were fused.
In the other two conditions (monocular delay in passive viewing and monocular deprivation), participants watched a replay of their own recorded activity through the same altered-reality set-up with the dominant eye delayed by 333 ms or substituted with a homogeneous gray screen. In both conditions, participants passively re-viewed the scene without engaging in any task (Fig. 1); in both cases, only the video was replayed, without sounds.
Binocular rivalry
Before and after the 1 h exposure to monocular delay or monocular deprivation participants quickly removed the Oculus VR set and turned to an adjacent apparatus for measuring binocular rivalry and estimating ocular dominance. We used two different set-ups to assess binocular rivalry, one using Ferro-Magnetic shutter goggles, the other using a mirror stereoscope. For the first set-up, a ViSaGe (Cambridge Research Systems) housed in a PC (Asus) controlled by MATLAB (MATLAB r2010a, The MathWorks) generated and displayed the stimuli on a linearized monitor (Barco CDCT, 800 × 600 pixels) driven at 100 Hz, synchronized with the Ferro-Magnetic shutter goggles (Cambridge Research Systems). For the mirror stereoscope set-up, stimuli were generated with the Psycho-Toolbox routines53,54 for MATLAB (MATLAB r2010a, The MathWorks) housed in Mac PC and displayed on a linearized monitor (Display ++, CRS, Cambridge Research Systems, 1920 × 1080 pixels) driven a 120 Hz. In both set-ups, participants viewed the display at a distance of 57 cm with a chinrest stabilizing head position. Binocular rivalry stimuli were two orthogonal sinusoidal gratings (orientation: ±45°, spatial frequency: 2 cycles per degree), Gaussian-vignetted within a s = 2.5 deg window. Gratings were presented against a mean luminance background (5.85 cd/m2); they were overlayed with a central black fixation point and framed by a black square, both presented binocularly to facilitate fusion.
The stimulus was delivered in periods of 180 s, during which participants reported perceptual oscillations by holding down one of three keys to indicate: dominance of the clockwise grating, dominance of the counterclockwise grating, or mixed perception (fusion or piecemeal combination of the two gratings). Four periods of stimulation (about 12 min) were tested both before and after the 1 h exposure to monocular delay; the assignment of the two grating orientations to the left and right eye was swapped across periods. 12 min represents a compromise between collecting enough data to reliably assess the ocular dominance shift and measuring the effect before it exponentially decays back to baseline55.
For the analysis, we averaged across the second, third and fourth periods; we excluded the first period to ensure data homogeneity, as it typically exhibits stronger but also more variable effects across participants. Due to a failure in data storage, one participant only had two periods of stimulation for the baseline measurements of one session, and we averaged across the two available periods.
We quantified binocular rivalry dynamics by analyzing the duration of dominance phases (periods of time where either eye dominated perception) and by computing the percentage of dominance time spent seeing through one eye (Eq. 1).
where DE stands for Dominant Eye and NDE stands for Non-dominant Eye. We marked one eye as dominant (preOD > 50%) with a preliminary binocular rivalry session. We quantified the effect of each experimental condition by taking the the difference between ocular dominance indices calculated before and after each experiment (ΔOD% = postOD%−preOD%).
For completeness, we also computed the percentage of total testing time spent seeing mixed percepts (i.e. incomplete dominance of either eye), and compared before and after each experiment.
Statistical analyses
Statistical analyses were performed in Matlab56 and in JASP57. A robust correlation toolbox38 was used to detect and exclude outliers using the “boxplot” option.
Data availability
The experimental data that support the findings of this study are available on Zenodo at the following link: https:/doi.org/10.5281/zenodo.16419149 .
References
Espinosa, J. S. & Stryker, M. P. Development and plasticity of the primary visual cortex. Neuron 75, 230–249. https://doi.org/10.1016/j.neuron.2012.06.009 (2012).
Hubel, D. H. & Wiesel, T. N. Effects of monocular deprivation in kittens. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 248, 492–497. https://doi.org/10.1007/BF00348878 (1964).
Wiesel, T. N. & Hubel, D. H. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J. Neurophysiol. 26, 978–993. https://doi.org/10.1152/jn.1963.26.6.978 (1963).
Wiesel, T. N. & Hubel, D. H. Single-Cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 26, 1003–1017. https://doi.org/10.1152/jn.1963.26.6.1003 (1963).
Lunghi, C., Burr, D. C. & Morrone, C. Brief periods of monocular deprivation disrupt ocular balance in human adult visual cortex. Curr. Biol. 21, R538–539. https://doi.org/10.1016/j.cub.2011.06.004 (2011).
Zhou, J., Clavagnier, S. & Hess, R. F. Short-term monocular deprivation strengthens the patched eye’s contribution to binocular combination. J. Vis. 13 https://doi.org/10.1167/13.5.12 (2013).
Steinwurzel, C., Pennella, G. & Binda, P. Short-term monocular deprivation in adult humans: A meta-analysis and new perspectives. BioRxiv https://doi.org/10.1101/2025.02.20.639298 (2025).
Lunghi, C., Morrone, M. C., Secci, J. & Caputo, R. Binocular rivalry measured 2 h after occlusion therapy predicts the recovery rate of the amblyopic eye in anisometropic children. Invest. Ophthalmol. Vis. Sci. 57, 1537–1546. https://doi.org/10.1167/iovs.15-18419 (2016).
Nguyen, B. N., Malavita, M., Carter, O. L. & McKendrick, A. M. Neuroplasticity in older adults revealed by temporary occlusion of one eye. Cortex 143, 1–11. https://doi.org/10.1016/j.cortex.2021.07.004 (2021).
Nguyen, B. N., Srinivasan, R. & McKendrick, A. M. Short-term homeostatic visual neuroplasticity in adolescents after 2 h of monocular deprivation. IBRO Neurosci. Rep. 14, 419–427. https://doi.org/10.1016/j.ibneur.2023.04.003 (2023).
Turrigiano, G. G. & Nelson, S. B. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 5, 97–107. https://doi.org/10.1038/nrn1327 (2004).
Fagiolini, M., Pizzorusso, T., Berardi, N., Domenici, L. & Maffei, L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: Dark rearing and monocular deprivation. Vis. Res. 34, 709–720. https://doi.org/10.1016/0042-6989(94)90210-0 (1994).
Kaneko, M. & Stryker, M. P. Homeostatic plasticity mechanisms in mouse V1. Philos. Trans. R Soc. Lond. B Biol. Sci. 372 https://doi.org/10.1098/rstb.2016.0504 (2017).
Lunghi, C., Emir, U. E., Morrone, M. C. & Bridge, H. Short-term monocular deprivation alters GABA in the adult human visual cortex. Curr. Biol. 25, 1496–1501. https://doi.org/10.1016/j.cub.2015.04.021 (2015).
Steinwurzel, C., Morrone, M. C., Sandini, G. & Binda, P. Active vision gates ocular dominance plasticity in human adults. Curr. Biol. 33, R1038–R1040. https://doi.org/10.1016/j.cub.2023.08.062 (2023).
Song, F., Lyu, L., Zhao, J. & Bao, M. The role of eye-specific attention in ocular dominance plasticity. Cereb. Cortex. 33, 983–996. https://doi.org/10.1093/cercor/bhac116 (2023).
Song, F., Lyu, L. & Bao, M. Adaptation of ocular opponency neurons mediates Attention-Induced ocular dominance plasticity. Neurosci. Bull. 40, 339–349. https://doi.org/10.1007/s12264-023-01103-z (2024).
Song, F. et al. Causal role of the frontal eye field in attention-induced ocular dominance plasticity. Elife 12 https://doi.org/10.7554/eLife.93213 (2024).
Wang, M., McGraw, P. & Ledgeway, T. Attentional eye selection modulates sensory eye dominance. Vis. Res. 188, 10–25. https://doi.org/10.1016/j.visres.2021.06.006 (2021).
Bai, J., Dong, X., He, S. & Bao, M. Monocular deprivation of fourier phase information boosts the deprived eye’s dominance during interocular competition but not interocular phase combination. Neuroscience 352, 122–130. https://doi.org/10.1016/j.neuroscience.2017.03.053 (2017).
Lyu, L., He, S., Jiang, Y., Engel, S. A. & Bao, M. Natural-scene-based steady-state visual evoked potentials reveal effects of short-term monocular deprivation. Neuroscience 435, 10–21. https://doi.org/10.1016/j.neuroscience.2020.03.039 (2020).
Wang, J., Song, F., He, X. & Bao, M. Negligible contribution of adaptation of ocular opponency neurons to the effect of short-term monocular deprivation. Front. Psychol. 14, 1282113. https://doi.org/10.3389/fpsyg.2023.1282113 (2023).
Zhou, J., Reynaud, A. & Hess, R. F. Real-time modulation of perceptual eye dominance in humans. Proc. Biol. Sci. https://doi.org/10.1098/rspb.2014.1717 (2014).
Ramamurthy, M. & Blaser, E. Assessing the kaleidoscope of monocular deprivation effects. J. Vis. 18, 14. https://doi.org/10.1167/18.13.14 (2018).
Kim, H. W., Kim, C. Y. & Blake, R. Monocular perceptual deprivation from interocular suppression temporarily imbalances ocular dominance. Curr. Biol. 27, 884–889. https://doi.org/10.1016/j.cub.2017.01.063 (2017).
Sherrington, C. Observations on the sensual role of the proprioceptive nerve-supply of the extrinsic ocular muscles. Brain. 41, 332–343 (1918).
von Helmholtz, H. & Southall, J. P. C. Helmholtz’s Treatise on Physiological Optics (Dover Publication, 1962).
Sperry, R. W. Neural basis of the spontaneous optokinetic response produced by visual inversion. J. Comp. Physiol. Psychol. 43, 482–489. https://doi.org/10.1037/h0055479 (1950).
von Holst, E. & Mittelsttaedt, H. The reafference principle: Interaction between the central nervous system and the periphery. Die Naturwiss. 37, 464–476 (1950).
Jeannerod, M. Neural simulation of action: A unifying mechanism for motor cognition. Neuroimage. 14, 103–109. https://doi.org/10.1006/nimg.2001.0832 (2001).
Rizzolatti, G., Riggio, L., Dascola, I. & Umilta, C. Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia. 25, 31–40. https://doi.org/10.1016/0028-3932(87)90041-8 (1987).
Haggard, P. Sense of agency in the human brain. Nat. Rev. Neurosci. 18, 196–207. https://doi.org/10.1038/nrn.2017.14 (2017).
Min, S. H. et al. Issues revisited: Shifts in binocular balance depend on the deprivation duration in normal and amblyopic adults. Ophthalmol. Ther. 11, 2027–2044. https://doi.org/10.1007/s40123-022-00560-5 (2022).
Prosper, A., Pasqualetti, M., Morrone, M. C. & Lunghi, C. The duration effect of short-term monocular deprivation measured by binocular rivalry and binocular combination. Vis. Res. 211, 108278. https://doi.org/10.1016/j.visres.2023.108278 (2023).
Chen, X. et al. Action video gaming does not influence short-term ocular dominance plasticity in visually normal adults. eNeuro. https://doi.org/10.1523/ENEURO.0006-20.2020 (2020).
Brascamp, J. W., Klink, P. C. & Levelt, W. J. The ‘laws’ of binocular rivalry: 50 years of levelt’s propositions. Vis. Res. 109, 20–37. https://doi.org/10.1016/j.visres.2015.02.019 (2015).
Acquafredda, M., Sari, I. D., Steinwurzel, C., Lunghi, C. & Binda, P. Measuring the reliability of binocular rivalry. J. Vis. 23, 5. https://doi.org/10.1167/jov.23.10.5 (2023).
Pernet, C. R., Wilcox, R. & Rousselet, G. A. Robust correlation analyses: False positive and power validation using a new open source matlab toolbox. Front. Psychol. 3, 606. https://doi.org/10.3389/fpsyg.2012.00606 (2012).
Min, S. H., Baldwin, A. S. & Hess, R. F. Ocular dominance plasticity: A binocular combination task finds no cumulative effect with repeated patching. Vis. Res. 161, 36–42. https://doi.org/10.1016/j.visres.2019.05.007 (2019).
Zou, L., Zhou, C., Hess, R. F., Zhou, J. & Min, S. H. Daily dose-response from short-term monocular deprivation in adult humans. Ophthalmic Physiol. Opt. 44, 564–575. https://doi.org/10.1111/opo.13282 (2024).
Binda, P. & Morrone, M. C. Vision during saccadic eye movements. Annu. Rev. Vis. Sci. 4, 193–213. https://doi.org/10.1146/annurev-vision-091517-034317 (2018).
Rao, R. P. & Ballard, D. H. Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 2, 79–87. https://doi.org/10.1038/4580 (1999).
Deneve, S. & Pouget, A. Bayesian multisensory integration and cross-modal spatial links. J. Physiol. Paris. 98, 249–258. https://doi.org/10.1016/j.jphysparis.2004.03.011 (2004).
Campanella, F., Sandini, G. & Morrone, M. C. Visual information gleaned by observing grasping movement in allocentric and egocentric perspectives. Proc. Biol. Sci. 278, 2142–2149. https://doi.org/10.1098/rspb.2010.2270 (2011).
Rizzolatti, G. & Craighero, L. The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192. https://doi.org/10.1146/annurev.neuro.27.070203.144230 (2004).
Kreyenmeier, P., Deubel, H. & Hanning, N. M. Theory of visual attention (TVA) in action: Assessing premotor attention in simultaneous eye-hand movements. Cortex 133, 133–148. https://doi.org/10.1016/j.cortex.2020.09.020 (2020).
Deubel, H. & Schneider, W. X. Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vis. Res. 36, 1827–1837. https://doi.org/10.1016/0042-6989(95)00294-4 (1996).
Xu, J. P., He, Z. J. & Ooi, T. L. Effectively reducing sensory eye dominance with a push-pull perceptual learning protocol. Curr. Biol. 20, 1864–1868. https://doi.org/10.1016/j.cub.2010.09.043 (2010).
Lunghi, C. & Sale, A. A cycling lane for brain rewiring. Curr. Biol. 25, R1122–1123. https://doi.org/10.1016/j.cub.2015.10.026 (2015).
Sari, I. D. & Lunghi, C. Different forms of plasticity interact in adult humans. eNeuro. https://doi.org/10.1523/ENEURO.0204-22.2023 (2023).
Federici, A. et al. Crossmodal plasticity following short-term monocular deprivation. Neuroimage. 274, 120141. https://doi.org/10.1016/j.neuroimage.2023.120141 (2023).
Acquafredda, M. K., Biagi, J. W., Tosetti, L., Morrone, M. & Binda, M. C. The pulvinar regulates plasticity in human visual cortex. BioRxiv https://doi.org/10.1101/2025.02.24.639829 (2025).
Brainard, D. H. The psychophysics toolbox. Spat. Vis. 10, 433–436 (1997).
Pelli, D. G. The videotoolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis. 10, 437–442 (1997).
Lunghi, C., Burr, D. C. & Morrone, M. C. Long-term effects of monocular deprivation revealed with binocular rivalry gratings modulated in luminance and in color. J. Vis. https://doi.org/10.1167/13.6.1 (2013).
MATLAB v. version 24.1.0 (R2024a) (2024a).
JASP v. Version 0.19.3 (2024).
Acknowledgements
This research was funded by: the European Union (ERC, PREDACTIVE, 101170249); the European Union—Next Generation EU, in the context of The National Recovery and Resilience Plan, Investment 1.5 Ecosystems of Innovation, Project Tuscany Health Ecosystem (THE, CUP I53C22000780001); the European Union—Next Generation EU, grant PRIN 2022 (Project ‘RIGHTSTRESS—Tuning arousal for optimal perception’, Grant no. 2022CCPJ3J, CUP I53D23003960006); the Italian Ministry of University and Research under the program FARE-2 (grant SMILY). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council. Neither the European Union nor the granting authority can be held responsible for them.
Author information
Authors and Affiliations
Contributions
G.S.: Methodology and Resources; C.S, G.P.: Investigation; P.B., C.S, G.P.: Formal Analysis and Resources; P.B., C.S.: Writing – first draft; P.B., C.S., M.C.M., G.P.: Conceptualization; All authors: Writing – Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Steinwurzel, C., Pennella, G., Morrone, M.C. et al. Monocular delay during visually guided actions is as effective as monocular deprivation in driving ocular dominance plasticity. Sci Rep 15, 34743 (2025). https://doi.org/10.1038/s41598-025-18464-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18464-2