Abstract
This study investigates how melatonin (Mel) supplementation mitigates Lead (Pb) toxicity in rice by evaluating plant growth, physiology, and molecular responses. Pb stress markedly reduced root and shoot lengths by 63% and 33%, respectively, compared to control plants; however, Mel supplementation effectively mitigated this inhibition, enhancing root and shoot lengths by 100% and 47%, respectively, relative to Pb-stressed plants after 10 days. Furthermore, prolonged Mel application under Pb stress sustained growth improvement, increasing root and shoot lengths by 36% and 35%, respectively, compared to Pb-stressed plants. We observed a 19.5% increase in plant height with Mel treatment, along with improvements in yield-related traits such as panicle length and seed weight. Beyond morphological traits, Mel reduced Pb-induced oxidative stress by decreasing \({\text{O}}_{2}^{ \cdot - }\), H2O2, and MDA levels by 36%, 26%, and 46%, respectively. Moreover, Mel modulated antioxidant enzyme activities in Pb + Mel-treated plants by decreasing ascorbate peroxidase (APX) activity and enhancing catalase (CAT) activity. Additionally, Mel regulated ion homeostasis, with K+ and Ca2+ contents increasing by 74% and 89%, respectively. At molecular level, Mel reduced OsMTP1 levels by 45% and increased OsPCS1 by up to 193%. Overall, Mel significantly alleviates Pb toxicity by enhancing growth, physiological traits, and stress resilience in rice plants, highlighting its potential as a sustainable strategy for improving crop performance under heavy metal stress and offering promising directions for future agricultural research.
Similar content being viewed by others
Introduction
The increasing global concern about environmental degradation and ecosystem decline caused by both organic and inorganic pollutants has become a significant issue. In recent decades, heavy metals have emerged as a prominent environmental problem, particularly affecting the sustainability of agro-ecosystems, especially in industrial areas1,2. Heavy metals, due to their persistent and toxic characteristics, represent a primary source of environmental toxicity among inorganic pollutants. Industries, mining activities, automobile emissions, inorganic fertilizers, and other agrochemicals are the main contributors to heavy metal pollution3,4. Trace metals such as copper (Cu), cadmium (Cd), lead (Pb), and mercury (Hg) are recognized as soil contaminants due to their potential to disrupt plant metabolic processes and reduce crop productivity5. Pb is identified as one of the most hazardous heavy metals, posing severe threats to both animals and plants6. Its detrimental effects on plant growth and development are profound, impacting various aspects of plant metabolism, ranking second only to arsenic in terms of hazard level7,8,9. Pb toxicity significantly interferes with seed germination, seedling growth, cell division, cell membrane permeability, and photosynthesis10,11,12. The degree of Pb toxicity varies depending on factors such as exposure duration, concentration, the capacity of plants to uptake Pb, and the growth stage of the plant13.
Heavy metal exposure triggers complex responses in plants, including the activation of enzymatic and non-enzymatic antioxidant systems. These mechanisms are integral to the plant’s adaptive strategy, orchestrating the regulation of reactive oxygen species (ROS) accumulation to bolster tolerance against stressors14. Nevertheless, the unchecked accumulation of ROS can precipitate oxidative stress, culminating in cellular damage and programmed cell death (PCD). Concurrently, heightened concentrations of heavy metals, notably lead (Pb), prompt ROS generation, exacerbating oxidative stress and instigating detrimental effects on cellular membranes, as well as disrupting cellular metabolism and physiological processes. These perturbations extend to alterations in crucial cellular constituents, including nucleic acids, soluble sugars, and chloroplast pigments15,16,17.
In response, plants deploy sophisticated mechanisms for metal uptake, storage, transportation, detoxification, elimination, and compartmentalization, mirroring their adaptive strategies to diverse environmental stressors18. Substantial evidence underscores the variability among plant species or varieties in their proficiency to uptake, translocate, and accumulate Pb, underscoring the intricate interplay between plant physiology and metal exposure19. Moreover, extensive investigations have delved into the ramifications of Pb contamination in soils, elucidating its intricate dynamics in plant uptake, translocation, and accumulation, particularly within grain crops, and its consequential impacts on human health20,21,22.
Additionally, at lower concentrations, plants respond to Pb uptake by initiating the biosynthesis of phytochelatins (PCs) within their roots. These PCs subsequently form complexes with Pb, effectively reducing its mobility to the aerial parts of the plant23,24. However, when Pb accumulation exceeds a critical threshold, detrimental effects ensue, leading to diminished uptake of water and nutrients, impaired respiration, transcription, photosynthesis, and nitrogen assimilation in plants25,26. Furthermore, the generation of reactive oxygen species (ROS) within plant cells exacerbates the situation, causing damage to cell membranes and vital cellular components such as proteins, lipids, and nucleic acids, ultimately resulting in cellular death27,28. Heavy metal stress is linked to the synthesis of phytochelatins, and it has been reported that plants lacking functional phytochelatins, such as phytochelatin-mutant plants, exhibit increased susceptibility to heavy metals29. Two genes related to phytochelatin synthesis, OsPCS1 and OsPCS2, are induced in rice plants under Pb stress30. Additionally, plants experiencing heavy metal stress undergo transcriptional regulation of CDF protein family members like OsMTP1 and OsMTP5, which are critical for maintaining cation homeostasis, chelation, sequestration, or expelling excess heavy metals31. The expression of OsMTP1 increases during Cd stress exposure; studies involving overexpression and gene silencing have confirmed its role in Cd transport32.
To mitigate the oxidative stress induced by the generation of reactive oxygen species (ROS) during stress conditions, including Pb contamination, a sensible approach involves employing a bioregulator to improve plant tolerance to harmful stressors. Among the various bioregulators, melatonin is a prominent bioactive compound naturally available in plants. Researchers have focused on melatonin’s function against several stresses, either through exogenous application or by enhancing its endogenous levels33,34. For instance, melatonin has been found to induce tolerance to cold stress in carrots and peaches, and to reduce oxidative stress in Bermuda grass35,36,37. Melatonin enhances antioxidant activity by inducing the synthesis of antioxidant enzymes and scavenging ROS when plants are exposed to stress conditions38. Melatonin acts directly as a free radical scavenger and protects plants from water and soil contamination39.
Melatonin alleviates the negative effects of Pb stress on maize growth by boosting chlorophyll content, photosynthetic efficiency (Fv/Fm), and key minerals such as Ca2+ and K+40,41. As a universal antioxidant, melatonin can easily pass through the plasma membrane into subcellular compartments, where it helps mitigate Pb stress by reducing H2O2 and MDA levels, and decreasing electrolyte leakage. The regulation of antioxidant enzyme activity, such as CAT, superoxide dismutase (SOD), and peroxidase (POD), as well as antioxidant assays like ABTS and DPPH, is a crucial part of the plant’s defense system against stress conditions42. Melatonin enhances maize tolerance to Pb stress by stimulating the activities of CAT, POD, and SOD41.
This study aims to investigate the role of melatonin as a bioregulator in enhancing rice plant tolerance to Pb toxicity. We focus on assessing its impact on plant growth, oxidative stress alleviation, and the regulation of stress-responsive genes. The ultimate objective is to explore melatonin’s potential as a sustainable strategy for mitigating heavy metal stress in crops.
Materials and methods
Experimental set up
Rice seeds, obtained from the National Rice Research Institute (NRRI), Hangzhou, China, were used as the experimental material. The experiment was conducted in two setups: pot culture and petri dish-based germination, each designed to evaluate the effect of Pb stress and melatonin treatment.
Seed preparation
To break dormancy, rice seeds were soaked in distilled water for three days. After germination, healthy and uniform seedlings were selected for further treatments.
Pot experiment
Germinated seedlings were transplanted into pots filled with soil and grown under controlled greenhouse conditions (30 °C, 60% relative humidity, and 6/8 h light/dark photoperiod). After 3 weeks of growth: Melatonin was applied at concentration of 100 µM (dissolved in water) following the method of Jan et al.43. After 24 h of melatonin treatment, 1.2 mM of pB was applied in the form of Pb(NO3)2, as described in Ashraf et al.44. The pot experiment included four treatment groups such as: Control (no treatment), Pb (treated with 1.2 mM Pb), Mel (treated with 100 µM melatonin), and Pb + Mel (treated with both melatonin and Pb, melatonin applied 24 h prior to Pb). Root and shoot lengths were measured 5 weeks after the initiation of treatments. Plant height, panicle length, number of seeds per panicle, and 100-grain weight were recorded at the final stage of plant maturity (when the plants naturally reached full maturity under the given growth conditions), which was not recorded in exact days or weeks.
Petri dish experiment
In parallel, a petri dish experiment was conducted to observe early seedling growth under Pb stress. Healthy rice seeds were placed on filter paper in petri dishes and subjected to the same treatment groups as the pot experiment. Melatonin (100 µM) was applied 24 h before Pb (1.2 mM) treatment. Seedling were grown for 10 days, and root and shoot lengths were measured thereafter.
ROS analysis and lipid peroxidation
To quantify H2O2 levels, 0.2 g of fresh leaf tissue was collected after 24 h of Pb and melatonin treatment. Samples were accurately weighed, powdered in liquid nitrogen, and homogenized in 5 mL of 0.1% trichloroacetic acid (TCA). The homogenization process ensures that the plant tissues are thoroughly disrupted, facilitating the release of H2O2 from the cells. The homogenate was then subjected to centrifugation at 12,000 × g for 15 min at 4 °C to separate the supernatant from the cell debris. This high-speed centrifugation ensures the removal of particulate matter and results in a clear supernatant containing the soluble H2O2. After centrifugation, 0.5 mL of the clear supernatant was carefully collected using a micropipette. This aliquot of the supernatant was mixed with 1 mL of a 1 M potassium iodide (KI) solution. The KI acts as a reagent that reacts with H2O2 to produce iodine (I2), which subsequently forms a yellow-colored complex. Additionally, 0.5 mL of a 10 mM phosphate buffer at pH 7.0 was added to maintain a stable pH environment, which is crucial for the accuracy and consistency of the reaction. The reaction mixture, now containing the supernatant, KI, and phosphate buffer, was thoroughly mixed and allowed to react for 10 min at room temperature. This incubation period ensures complete reaction between the H2O2 and KI. The absorbance of the resulting mixture was then measured at 390 nm using a spectrophotometer.
Superoxide anion (\({\text{O}}_{2}^{ \cdot - }\)) production was quantified using 0.2 g of fresh plant tissue homogenized in liquid nitrogen to ensure complete cell disruption. The homogenate was then mixed with 100 mM sodium–phosphate buffer (pH 7.8) containing 1 mM diethyl dithiocarbamate (DTC), an inhibitor of superoxide dismutase that helps in stabilizing the \({\text{O}}_{2}^{ \cdot - }\). After homogenization, the mixture was centrifuged at 12,000 × g for 15 min at 4 °C to remove debris, yielding a clear supernatant. The supernatant was used to measure \({\text{O}}_{2}^{ \cdot - }\) levels. This was done by evaluating its capacity to reduce nitro blue tetrazolium (NBT). For this, an aliquot of the supernatant was incubated with a reaction mixture containing 0.5 mM NBT in 50 mM sodium–phosphate buffer (pH 7.8). The NBT reduction by \({\text{O}}_{2}^{ \cdot - }\) results in the formation of a blue formazan compound, which is quantitatively measured by its absorbance at 540 nm using a spectrophotometer.
Lipid peroxidation was determined by quantifying MDA contents by using lipid peroxidation (MDA) kit from sigma (detail protocol is published in Jan et al.45).
ABTS radical scavenging activity
The ABTS radical scavenging activity of rice plant was assessed after 24 h of Pb and melatonin treatment. The protocol was used as described by Lee et al.46. Stock solutions of 7 mM ABTS and 2.4 mM potassium persulfate were prepared in double distilled water. To generate the ABTS cation radical, equal volumes of these stock solutions were combined and left to react in the dark for 12 to 16 h. This mixture, known as the working solution, was then diluted by adding 1 mL of the solution to 20 mL of double distilled water, adjusting the absorbance to approximately 0.7. For the assay, 20 µL of the leaf extract was mixed with 180 µL of the diluted working solution. The absorbance was measured at 734 nm using a Multiskan GO microplate spectrophotometer (Thermo Fisher Scientific, Vantaa, Finland).
The ABTS radical scavenging activity of the extracts was calculated using the following formula:
where Ac represents the absorbance of the ABTS radical cation alone, and AS represents the absorbance of the ABTS radical solution mixed with the sample extract. Each experiment was conducted in triplicate to ensure accuracy.
DPPH radical scavenging activity
The DPPH free radical scavenging activity of rice plant was assessed after 24 h of Pb and melatonin treatment. The protocol was used as described by Xu et al.47. A 0.1% DPPH solution in absolute methanol was freshly prepared. Equal volumes (100 µL each) of this DPPH solution and the sample extracts were mixed in microplates and incubated in the dark at room temperature (22–25 °C) for 30 min. A control was prepared by mixing 100 µL each of DPPH and methanol. Absorbance was measured at 517 nm using a Multiskan GO microplate spectrophotometer (Thermo Fisher Scientific, Vantaa, Finland).
The DPPH radical scavenging activity (%) was calculated using the formula:
where A is the absorbance of DPPH and sample, A0 is the absorbance of methanol and sample, B is the absorbance of DPPH and methanol, and B0 is the absorbance of methanol. Experiments were conducted in triplicate.
APX and CAT measurement
To measure APX activity, method described by Imran et al.48 was followed. Briefly, fresh leaves of rice plant was collected after 24 h of Pb and melatonin treatment. A 100 mg sample of plant tissue was extracted using 1 mL of 50 mM phosphate buffer (pH 7.0) containing 1 mM ascorbic acid and 1 mM EDTA. The homogenized sample was then centrifuged at 5000 × g for 15 min at 4 °C to obtain the supernatant. The resulting supernatant was mixed with a reaction buffer consisting of 50 mM phosphate buffer (pH 7.0), 15 mM ascorbic acid, and 0.3 mM H2O2. The enzymatic reaction was initiated by adding the hydrogen peroxide, and the decrease in absorbance at 290 nm was monitored using a spectrophotometer. This decrease in absorbance corresponds to the oxidation of ascorbic acid, which is catalyzed by APX, thus providing a measure of the enzyme’s activity.
To measure CAT activity, we adhered to the protocol optimized by Johansson and Borg49. The detailed procedure is as follows: Fresh leaf tissue were collected after 24 h of Pb and melatonin treatment and then 200 mg was accurately weighed and immediately frozen in liquid nitrogen to prevent enzymatic degradation. The frozen leaf tissue was then ground into a fine powder using a mortar and pestle cooled with liquid nitrogen. The powdered tissue was homogenized with 200 µL of methanol to extract the CAT enzyme. This homogenate contained crude CAT extract. Next, 0.5 mL of 0.2 mM phosphate buffer was prepared. This buffer consisted of H2O2 and a KH2PO4-NaOH solution, adjusted to a pH of 7.0. The crude CAT extract was mixed with the phosphate buffer to initiate the reaction. The activity of the CAT enzyme was determined by measuring the decrease in absorbance of H2O2 at a wavelength of 240 nm using a spectrophotometer. The reduction in absorbance indicated the breakdown of H2O2 by CAT. One unit of CAT was defined as the amount of H2O2 decomposed per minute per milligram of protein in the sample.
Chlorophyll contents, relative water contents, and electrolyte leakage measurement
Chlorophyll content was measured 1 month after Pb stress using a portable chlorophyll meter (SPAD-502, Konica Minolta, Japan). The second-to-last fully mature leaf was selected for chlorophyll measurement. Readings were taken from the leaf base, middle, and near the tip at the same time. Five leaves were measured from each treatment group, and the measurements were averaged to obtain the SPAD value.
To determine the relative water content (RWC), fully mature leaves were randomly collected 1 week after Pb and melatonin treatment. The fresh weight (FW) of these leaves was measured immediately. Subsequently, the leaves were submerged in distilled water in petri dishes for 3 h to reach full turgidity, and the turgid weight (TW) was measured. The same leaves were then dried at 70 °C for 48 h, and the dried weight (DW) was recorded. The relative water content was calculated using the formula:
To evaluate electrolyte leakage, fresh leaves were collected after 1 week of Pb and melatonin treatment. A 100 mg of fresh leaf tissue was cut into small pieces, approximately 5 mm in size. These pieces were placed into test tubes containing 10 mL of distilled deionized water. The test tubes were sealed to prevent evaporation and contamination. The samples were then incubated at 32 °C for 2 h to allow the initial release of electrolytes from the plant cells into the water. Following the incubation, the initial electrical conductivity (EC1) of the solution was measured using a conductivity meter (model CM-115, Kyoto Electronics, Kyoto, Japan). This measurement reflects the amount of electrolytes that have leaked from the leaf tissues due to any damage or membrane permeability changes. To ensure complete release of all electrolytes, the leaf samples were then subjected to autoclaving at 121 °C for 20 min. This process causes the cell walls and membranes to break down completely, releasing the remaining intracellular electrolytes into the solution. After autoclaving, the samples were allowed to cool to room temperature, specifically 25 °C, to ensure that the temperature did not influence the conductivity measurements.
Melatonin measurement in plant tissue
Melatonin from roots and leaves was extracted using a melatonin ELISA kit (Colorimetric, catalog No. NBP2-62160) by Novus Biologicals, USA. The extraction process is described below in detail: First, 0.5 g of root and leaf samples were collected and homogenized with 125 µL of 1X stabilizer solution provided in the kit. After homogenization, approximately 750 µL of ethyl acetate was added to the homogenate. The mixture was then vortexed vigorously to ensure thorough mixing of the components. Following vortexing, the samples were incubated on ice for 5 min to facilitate phase separation. After incubation, the samples were centrifuged at 1000 × g for 10 min to further separate the organic and aqueous phases. The organic layer, which contains the melatonin, was carefully removed and transferred to a new tube. The organic extract was then dried, typically under a stream of nitrogen gas or in a vacuum concentrator, to remove the ethyl acetate. The dried residue was re-suspended in 125 µL of 1X stabilizer solution to prepare it for the ELISA. The quantification of melatonin was carried out according to the manufacturer’s instructions provided in the ELISA kit manual. This involved adding the re-suspended samples to the wells of the ELISA plate, along with standards and controls. The plate was then processed as per the kit instructions, which included incubation steps and the addition of detection reagents. Finally, the optical density of each well was measured at 450 nm using a spectrophotometer (Multiskan GO, Thermo Fisher Scientific, Vantaa, Finland). The optical density readings were used to determine the concentration of melatonin in the root and leaf samples by comparing them to the standard curve generated during the assay.
Quantification of Pb contents in plant tissue
The concentration of lead (Pb) in root and shoot of the rice plants was determined following the method described by Ashraf et al.44. The detailed procedure is outlined below: Initially, the rice root and shoot were oven-dried to remove any moisture content. The dried plant material was then finely ground into a powder in liquid nitrogen. For the digestion process, a mixture of nitric acid (HNO3) and perchloric acid (HClO4) in a 4:1 volume/volume (v/v) ratio was used. Each powdered plant sample was digested with this acid mixture to break down the organic matter and release the Pb into the solution. After digestion, the resultant solutions were diluted to a final volume of 50 mL with deionized water. This dilution ensured that the concentrations of the analytes were within the detectable range of the instrumentation. The diluted samples were then filtered to remove any remaining particulates. The Pb concentrations in the filtrate were measured using an Agilent® 720ES inductively coupled plasma optical emission spectrometer (ICP-OES). This technique allowed for precise and accurate quantification of Pb in the plant samples.
RNA extraction and qRT-PCR analysis
Total RNA was extracted from rice plant after 6 h of Pb and melatonin treatment, using the RNeasy® Plant Mini Kit (Qiagen, Valencia, CA, USA), following the manufacturer’s protocol to ensure high-quality RNA suitable for further analysis. For cDNA synthesis, 2 µg of the extracted RNA was utilized. The cDNA was synthesized using the qPCR-Bio cDNA Synthesis Kit, according to the instructions provided by the manufacturer. This process involved the reverse transcription of RNA into complementary DNA (cDNA), which is necessary for quantitative PCR (qPCR) analysis. Specific primers for each target gene were used. The relative gene expression levels were measured using the StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific, Seoul, Korea). The reaction mixture consisted of 10 µL of 2X Real-time PCR Master Mix, which includes SYBR® Green I, from BIOFACT (Daejeon, Korea), 1 µL of each forward and reverse primer at a concentration of 20 pmol/µL, and 100 ng of synthesized cDNA. The final volume of the mixture was adjusted to 20 µL with nuclease-free water. The qPCR reaction was carried out under the following thermal cycling conditions: an initial polymerase activation step at 95 °C for 10 min to activate the DNA polymerase, followed by a denaturation step at 95 °C for 15 s to separate the DNA strands. The annealing and extension steps were conducted at 60 °C for 1 min to allow the primers to bind to the target sequences and facilitate DNA synthesis. For normalization of the gene expression data, OsActin was used as the internal control or housekeeping gene. The consistent expression of OsActin across different samples provided a reliable reference point for comparing the relative expression levels of the target genes.
Tissue sectioning and microscopy
Root samples were collected approximately 5 cm from the tip of mature roots, leaf samples were taken from the middle portion of fully expanded leaves, and stem samples were obtained from a position 5 cm above the root-shoot junction. All samples were fixed in FAA solution (containing 90% ethanol, 5% formaldehyde, and 5% glacial acetic acid) for 24 h. Following fixation, the samples were dehydrated in a graded ethanol series (70%, 85%, 95%, and 100%), with each step lasting 24 h. The dehydrated tissues were then embedded in Leica Historesin (Leica, Nussloch, Germany). Transverse sections were manually prepared using a sharp scalpel, stained with toluidine blue, rinsed with autoclaved water, and examined under a bright-field microscope (Nikon, Tokyo, Japan).
Ca 2+ , K + , and amino acid analysis
Ca2+ (calcium) and K+ (potassium) in rice plant after Pb and melatonin treatment were determined by following the methodology outlined by50. Initially, leaf samples were washed with water and then dried at 65 °C for two days to remove moisture content. Once dried, the plant tissues were ground into a fine powder using liquid nitrogen to maintain sample integrity. Subsequently, 100 mg of the powdered tissue samples were extracted using 10 mL of 0.1 N nitric acid (HNO3) for a period of 30 min. This extraction process facilitated the dissolution of ions from the plant material into the acid solution. After extraction, the samples were filtered to remove any particulate matter, ensuring clarity of the solution for subsequent analysis. The concentrations of Ca2+ and K+ present in the samples were determined using an Inductively Coupled Plasma (ICP) Spectrometer (Optima 7300DV and Avio500 by PerkinElmer). These are the instruments commonly used for elemental analysis in plant samples. These spectrometers utilize optical emission or mass spectrometry techniques to precisely quantify the concentrations of various ions in the extracted solutions.
Free amino acids were quantified 1 week after exposure to Pb and melatonin treatment. Approximately 500 mg of fresh leaf tissue was collected, flash-frozen in liquid nitrogen, and subsequently ground into a fine powder. This powdered sample was then homogenized in 10 mL of 70% methanol to extract the amino acids. The homogenate was shaken vigorously at room temperature for 24 h to ensure thorough extraction. Following homogenization, the free amino acid content was measured using the EZ amino acid analysis kit (Phenomenex, Santa Clara, CA, USA), which simplifies and accelerates the preparation and analysis of amino acids. The procedure was conducted according to the manufacturer’s instructions, ensuring accurate quantification. For further analysis, the extracted amino acids were analyzed by Gas Chromatography-Mass Spectrometry (GC–MS) using a Hewlett-Packard 6890N/5975 instrument (Agilent Technologies, Torrance, CA, USA). The separation was achieved on a ZB-AAA amino acid analysis column (10 m × 0.25 mm), which is designed specifically for amino acid analysis. The GC–MS system operated with a constant carrier gas flow, and the oven temperature was programmed according to a protocol previously described by Pavlik et al.51. This setup enabled the precise identification and quantification of individual amino acids in the leaf samples.
Statistical analysis
Statistical analysis was conducted on the entire dataset using GraphPad Prism software (version 5.01; GraphPad, San Diego, CA, USA). A one-way analysis of variance (ANOVA) was employed. The analysis included three independent biological replicates, and the means were compared using Bonferroni post hoc tests. The relationships among the various parameters were analyzed using Pearson’s correlation coefficient. The analysis was performed using GraphPad Prism 8 and Microsoft Excel to assess the strength and direction of associations between different parameters.
Results
Melatonin supplementation enhance rice plant growth under lead toxicity
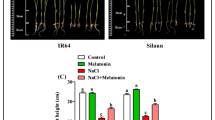
To investigate the effects of Pb, melatonin, and their combined application on rice seedling growth, we conducted an experiment using petri dishes. The results after 10 days of treatment indicated that Pb stress significantly reduced root and shoot lengths by 63% and 33%, respectively, compared to control plants (Fig. 1A, B). In contrast, melatonin treatment significantly increased both root and shoot lengths compared to control plants. Specifically, the combined Pb + Mel treatment improved root and shoot lengths by 100% and 47%, respectively, compared to Pb-only treated plants after 10 days of exposure to both Pb and melatonin. To further substantiate the impact of Pb and melatonin on plant growth, we evaluated root and shoot lengths after 5 weeks of treatment. The data revealed that prolonged Pb stress significantly reduced both root and shoot lengths, whereas melatonin treatment significantly enhanced shoot length (after 5 week) (Fig. 1C, D). Moreover, plants treated with the Pb + Mel combination exhibited 36% and 35% increases in root and shoot lengths, respectively, compared to Pb-only treated plants (after 5 week). At the final growth stage, plant height was markedly reduced under Pb stress but increased with melatonin supplementation compared to control plants (Fig. 1E). Notably, the Pb + Mel treatment resulted in a 19.5% increase in plant height compared to Pb-only treated plants. Additionally, panicle length, the number of seeds per panicle, and the seed weight per 100 grains were all significantly reduced by Pb treatment but were enhanced by melatonin treatment compared to control plants (Fig. 1F–H). Under Pb stress, melatonin supplementation significantly improved these yield attributes compared to Pb-only treated plants. Overall, these results demonstrate that Pb exposure negatively impacts rice plant growth and yield attributes, while melatonin supplementation mitigates these adverse effects and enhances plant growth and productivity.
Exogenous application of melatonin promotes growth parameters in rice plants under Pb stress. (A, B) Display root and shoot lengths of rice plants grown in petri dishes after 10 days of Pb and melatonin treatment, respectively. (C, D) Illustrate root and shoot lengths after 5 weeks of exposure to Pb and melatonin, plants grown in pots. (E) Shows plant height at the final stage of development. (F, G, H) Present panicle length, number of seeds per panicle, and seed weight of 100 grains, respectively. Data represented in the graphs are means of three independent biological replicates ± SD. Asterisks on the bars indicate significant differences using Bonferroni post hoc tests.
Melatonin reduces lead-induced oxidative stress
Melatonin is widely recognized for its role in mitigating oxidative stress across various biological systems, including plants. We conducted a comprehensive study to assess how melatonin influences rice seedling growth under Pb toxicity. Our findings revealed that Pb-treated plants exhibited significantly elevated levels of \({\text{O}}_{2}^{ \cdot - }\), H2O2, and MDA compared to control plants (Fig. 2A–C). Specifically, melatonin supplementation in Pb-stressed plants (Pb + Mel) resulted in a 36% reduction in \({\text{O}}_{2}^{ \cdot - }\), a 26% reduction in H2O2, and a 46% reduction in MDA contents compared to Pb-only treated plants. To further understand melatonin’s role in Pb stress mitigation, we assessed vegetative indices using a hyperspectral camera. These indices included the Normalized Difference Vegetative Index (NDVI), Photochemical Reflectance Index (PRI), and Anthocyanin Reflectance Index (ARI). Hyperspectral spectral images and their data were analyzed using the Environment for Visualizing Images (ENVI) software (Supplementary Fig. 1A–F). The analysis indicated that Pb stress significantly decreased the mean values of NDVI and PRI, while it increased ARI compared to control plants. In contrast, Pb + Mel-treated plants showed a 97% increase in NDVI, a 92% increase in PRI, and a 104% decrease in ARI compared to Pb-stressed plants. These results demonstrate that melatonin effectively alleviates Pb-induced oxidative stress in rice plants. The mitigation is achieved through the regulation of photosynthetic performance and the protection of chlorophyll from oxidative degradation. Consequently, melatonin treatment enhances overall plant growth and resilience under Pb stress conditions.
Melatonin application mitigates Pb-induced oxidative stress by modulating reactive oxygen species and lipid peroxidation. (A) Illustrates superoxide accumulation in plants treated with Pb and melatonin, (B) Depicts hydrogen peroxide accumulation, and (C) shows malondialdehyde content accumulation. Data represented in the graphs are means of three independent biological replicates ± SD. Asterisks on the bars indicate significant differences as determined by Bonferroni post hoc tests.
Melatonin regulate antioxidant defense system in response to Pb stress in rice
We evaluated the enzymatic antioxidant activity in Pb-stressed rice plants treated with melatonin to understand how exogenous melatonin mitigates Pb stress.Our findings revealed that the radical scavenging activities of ABTS and DPPH were elevated in Pb-stressed plants compared to control and melatonin-treated plants (Fig. 3A, B). Specifically, ABTS and DPPH activities were 11% and 8.8% higher, respectively, in Pb + Mel plants compared to Pb-only treated plants. Interestingly, the activities of the antioxidant enzymes APX and CAT were also altered by Pb stress (Fig. 3C, D). Pb stress alone enhanced the activities of both APX and CAT compared to control plants. In Pb + Mel plants, APX activity decreased by 14%, while CAT activity increased by 4%, both relative to Pb-only treated plants. This apparent contradiction, where APX activity decreases and CAT activity increases in Pb + Mel plants, can be explained by the overlapping roles of these enzymes in H2O2 detoxification. It is possible that melatonin modulates the activity of these enzymes such that only one predominates at a time to efficiently manage oxidative stress. Overall, these results demonstrate that melatonin enhances the antioxidant defense system in rice plants during Pb stress by differentially modulating the activities of key antioxidant enzymes. This modulation likely contributes to the improved stress resilience and growth observed in melatonin-supplemented plants under Pb stress conditions.
Melatonin enhances the antioxidant defense system in rice plants under Pb stress. (A, B) Illustrate ABTS and DPPH activities, respectively, in plants treated with Pb and melatonin. (C, D) Display APX and CAT activities, respectively under the same treatments. Data represented in the graphs are means of three independent biological replicates ± SD. Asterisks on the bars indicate significant differences as determined by Bonferroni post hoc tests.
Melatonin and Pb accumulation in rice tissues and gene expression analysis
To validate the uptake of melatonin and Pb, we performed a quantitative analysis of these compounds in rice roots and shoots (Fig. 4). Our results demonstrated that melatonin levels were highest in melatonin-treated plants (Mel), followed by Pb + Mel treatments in both roots and shoots, when compared to control plants (Fig. 4A, B). Specifically, melatonin levels were significantly reduced by 63% in the roots of Pb-treated plants relative to control plants (Fig. 4A). Furthermore, Pb concentrations were significantly reduced in Pb + Mel treated plants. Compared to Pb-only treated plants, Pb levels decreased by 23% in roots and by 36% in shoots (Fig. 4C). Notably, the concentration of Pb was higher in roots than the shoot. These findings confirm that melatonin supplementation reduces Pb uptake and alleviates Pb-induced oxidative stress.
Melatonin and Pb accumulation in plant tissues and transcript expression of OsMTP1 and OsPCS1. (A, B) Show melatonin accumulation in rice roots and shoots, respectively, while (C) depicts Pb accumulation in roots and shoots of Pb-only treated plants. (D, E) illustrate the expression levels of OsMTP1 and OsPCS1 in rice plants respectively, under Pb and melatonin treatment at three different time points. Data represented in the graphs are means of three independent biological replicates ± SD. Asterisks on the bars indicate significant differences as determined by Bonferroni post hoc tests.
Furthermore, we assessed the transcript levels of the OsMTP1 and OsPCS1 genes under Pb stress and Pb stress supplemented with melatonin (Pb + Mel). Both genes are crucial for plant responses to heavy metal stress. OsMTP1, a component of the Metal Tolerance Protein (MTP) family known as the cation diffusion facilitator (CDF), plays a vital role in heavy metal stress response. In contrast, the OsPCS1 gene encodes the phytochelatin synthase enzyme, which binds to heavy metals and facilitates their detoxification. Our findings revealed that the transcript level of OsMTP1 was significantly elevated in both Pb and Pb + Mel treated plants after 12 h of exposure compared to control plants (Fig. 4D). However, OsMTP1 expression was reduced by 45% in Pb + Mel treated plants compared to those treated only with Pb. These results indicate that melatonin supplementation decreases OsMTP1 gene expression. Conversely, the transcript level of OsPCS1 was significantly modulated in Pb, Mel, and Pb + Mel treated plants compared to control plants (Fig. 4E). Notably, OsPCS1 expression increased by 193% and 44% in Pb + Mel treated plants compared to those treated only with Pb after 6 h and 12 h respectivelly. These results suggest that melatonin supplementation enhances OsPCS1 gene expression under Pb stress, thereby promoting the detoxification of lead.
Furthermore, we visualized the uptake and distribution of Pb in various parts of rice plants (Fig. 5A–C). Pb accumulation was predominantly observed in the roots, specifically in the exodermis and cortex regions. In the stem, Pb was found mainly in the vascular tissues and parenchyma. In the leaf midrib, Pb accumulation was primarily located within the vascular tissues. These observations indicate that Pb can be transported through the plant’s vascular system to the aerial parts, highlighting its mobility within the plant.
Visualization of Pb accumulation in different parts of the rice plant. (A) Presents root cross-sections, with the upper image displaying a control plant root cross-section and the lower image showing a Pb-treated plant root cross-section. Arrows highlight Pb deposition in the root tissues. (B) Shows stem cross-sections, with the upper image representing a control plant and the lower image depicting a Pb-treated plant. Arrows indicate the presence of Pb traces in the stem tissues. (C) Illustrates cross-sections of the leaf midrib region, where the upper image shows a control plant leaf midrib and the lower image reveals the leaf midrib under Pb stress. Arrows point to Pb accumulation in these sections.
Melatonin regulate K+, Ca2+ ions, and free amino acids under lead stress
Ca2+ and K+ ions contribute significantly to the mitigation of Pb stress in plants, ensuring better growth, development, and productivity. To further investigate the dynamics of Ca2+ and K+ ions under Pb stress and the influence of melatonin supplementation, we analyzed their trends in Pb-treated plants. Our results revealed that Pb stress alone significantly reduced the accumulation of both Ca2+ and K+ ions compared to control plants (Fig. 6A, B). However, melatonin treatment (Mel) and the combined treatment of Pb and melatonin (Pb + Mel) led to a significant increase in the accumulation of these ions. Specifically, K+ content in Pb + Mel plants increased by 74% compared to Pb-treated plants, while Ca2+ content in Pb + Mel plants increased by 89% compared to Pb-treated plants. These findings suggest that melatonin supplementation enhances the accumulation of K+ and Ca2+ ions during Pb stress.
Regulation of potassium, calcium, and free amino acids by melatonin application under Pb stress. (A, B) Show K+ and Ca2+ accumulation, respectively, in rice plants subjected to Pb and melatonin treatments. (C–F) Depict the accumulation of aspartic acid, proline, glutamic acid, and alanine, respectively, under the same conditions. Data in the graphs are presented as means of three independent biological replicates ± SD. Asterisks on the bars denote significant differences as determined by Bonferroni post hoc tests.
In addition to ion accumulation, we observed similar trends in the accumulation of free amino acids, such as aspartic acid, proline, glutamic acid, and alanine. Pb stress alone significantly reduced the levels of these amino acids in plants (Fig. 6C–F). However, melatonin-treated plants and Pb + Mel plants exhibited enhanced accumulation of these amino acids compared to control plants. Notably, Pb + Mel plants showed increases of 53%, 116%, 49%, and 49% in aspartic acid, proline, glutamic acid, and alanine, respectively, compared to Pb-treated plants. These results indicate that melatonin supplementation mitigates Pb stress by facilitating the accumulation of essential ions (K+ and Ca2+) and free amino acids. The enhanced accumulation of these ions and amino acids under melatonin treatment suggests improved ion homeostasis and stress resilience, contributing to the overall better growth and development of plants under Pb stress. Therefore, melatonin plays a crucial role in reducing Pb toxicity and promoting plant health by enhancing both ion balance and the accumulation of stress-responsive amino acids.
Melatonin modulates physiological traits in plants under Pb stress
Rice plants subjected to lead (Pb) stress exhibit significant physiological impairments, including a reduction in leaf width, chlorophyll content, and relative water content, alongside a notable increase in electrolyte leakage compared to control plants (Fig. 7A–D). Specifically, Pb stress leads to narrower leaves, diminished chlorophyll levels, reduced water retention, and heightened membrane damage, indicating severe stress effects on the plants’ physiological status. In contrast, the application of melatonin demonstrates a protective effect on these physiological parameters. Melatonin treatment enhances leaf width, chlorophyll content, and relative water content, while significantly reducing electrolyte leakage compared to both control and Pb-stressed plants. Under Pb stress conditions, melatonin supplementation significantly improves physiological parameters. Specifically, leaf width increased by 23%, chlorophyll content by 12%, relative water content by 27%, and electrolyte leakage decreased by 26% compared to plants subjected to Pb stress alone.These findings suggest that melatonin mitigates the detrimental effects of Pb stress by promoting leaf expansion, enhancing photosynthetic pigment levels, improving water retention, and maintaining cellular membrane integrity. Consequently, melatonin not only alleviates the stress-induced physiological damage but also supports the overall growth and health of rice plants under Pb contamination.
Effect of melatonin on leaf area, chlorophyll content, relative water content, and electrolyte leakage under Pb stress. (A) Illustrates leaf width, (B) depicts leaf chlorophyll content, (C) shows leaf relative water content, and (D) displays leaf electrolyte leakage. Data in the graphs are presented as means of three independent biological replicates ± SD. Asterisks on the bars denote significant differences as determined by Bonferroni post hoc tests.
Discussion
In this study, we examined the effects of melatonin supplementation on rice plants under Pb stress. The results indicated that melatonin significantly enhances the resilience of rice plants to lead toxicity. A schematic representation of melatonin-induced mitigation of Pb stress in rice plant is illustrated in Fig. 8. Melatonin treatment improved plant growth, as evidenced by increased shoot and root length and overall biomass. It also reduced oxidative stress, as indicated by decreased levels of reactive oxygen species (ROS) like H2O2 and \({\text{O}}_{2}^{ \cdot - }\). Additionally, melatonin enhanced the activity of antioxidant enzymes such as APX and CAT, which are crucial for mitigating oxidative damage. The ion balance was also improved, with reduced lead accumulation and increased uptake of essential ions like potassium and calcium. Furthermore, melatonin boosted amino acid levels, including proline, which is vital for stress responses. Overall, melatonin supplementation offers a promising strategy to enhance the growth and stress tolerance of rice plants under lead stress. The relationships among 29 distinct parameters were visualized through a heat map of Pearson correlation coefficients (Fig. 9). The red squares represent positive correlations, while green squares indicate negative correlations, and dark squares signify no significant correlation. Strong positive correlations were observed among root and shoot length, panicle number, seeds per panicle, seed weight, K+, Ca2+, free amino acids, leaf width, chlorophyll content, and relative water content. Conversely, negative correlations were found between reactive oxygen species (ROS) markers such as \({\text{O}}_{2}^{ \cdot - }\), H2O2, MDA, and antioxidant activities (ABTS, DPPH, APX, and CAT), as well as the expression levels of OsMTP1 and OsPCS, and electrolyte leakage, highlighting the interconnectedness of oxidative stress and antioxidant defense mechanisms in Pb-stressed plants.Our study demonstrated that Pb stress significantly impairs the growth and development of rice at both the seedling and final reproductive stages. This was evidenced by substantial reductions in root and shoot length, plant height, panicle length, the number of seeds per panicle, and the seed weight per 100 grains (Fig. 1). These reductions can be attributed to Pb-induced oxidative stress, which impairs cellular division, disrupt membrane integrity, and interferes with nutrients uptake and hormonal imbalance. However, melatonin supplementation markedly counteracted these adverse effects. The enhancement in root and shoot lengths, particularly in Pb + Mel-treated plants, underscores melatonin’s role in promoting growth even under toxic conditions. This growth promotion likely arises from melatonin’s known antioxidant properties, which reduce ROS levels, protect membrane structures, and preserve cellular function under stress. Moreover, possibly melatonin may help restore endogenous auxin gradients and stimulate cell division and expansion, facilitating root and shoot elongation. The observed improvements in plant height, panicle length, seed count, and seed weight indicate that melatonin not only aids in vegetative growth but also supports reproductive development, thereby improving yield attributes under Pb stress. Previous research has consistently shown that heavy metals, specifically lead, adversely affect plant growth52,53,54. Our results are align with the findings reported by Namdjoyan et al., which demonstrated that Pb significantly reduced safflower biomass, whereas melatonin application increased biomass under Pb stress55. The improved growth and biomass in Pb-stressed rice plants following melatonin application can be attributed to melatonin’s role in mitigating Pb uptake and reducing subsequent root-to-shoot translocation. We observed that higher concentrations of Pb were accumulated in the roots compared to the shoots in Pb + Mel-treated plants (Fig. 4C). This differential accumulation suggests that melatonin restricts Pb movement to aerial parts, likely by modulating gene expression associated with metal transport and detoxification. Further investigation revealed that melatonin supplementation under Pb stress reduced the transcript levels of OsMTP1 and increased the transcript levels of OsPCS1 compared to Pb-only treated plants (Fig. 4D, E). OsMTP1 is known to promote the transport of metals, while OsPCS1 is involved in the chelation of metals and other toxic compounds. Our results suggest that the induction of OsPCS1 enhances the chelation of Pb, thereby reducing its toxicity. Concurrently, the downregulation of OsMTP1 limits the translocation of Pb from root to shoot, mitigating the overall Pb burden in the aerial parts of the plant. These findings are consistent with those reported by Namdjoyan et al., who found that melatonin promoted the levels of phytochelatins (PCs) and reduced root-to-shoot translocation of Pb55. This reinforces the notion that melatonin enhances the capacity of plants to manage and tolerate heavy metal stress by modulating metal uptake, transport, and detoxification pathways.
Schematic representation of melatonin-induced mitigation of Pb stress in rice plants. Green upward arrows indicate parameters that increased, while red downward arrows indicate those that decreased in response to melatonin treatment. R.W.C, Relative water content; E.L, Electrolyte leakage; Chl, Chlorophyll content.
Heavy metal toxicity is a significant issue that affects plants both biochemically and physiologically, necessitating various approaches to mitigate its toxicity. Excessive accumulation of heavy metals in soil leads to their uptake by plants, which is associated with growth inhibition, elevated oxidative stress, and a significant reduction in crop productivity56,57. Melatonin, an important biostimulator, protects plants against heavy metal stress by scavenging free radicals39,58. Its amphiphilic nature allows it to easily cross lipid membranes, localize within subcellular compartments, and interact with both aqueous and lipid-phase ROS. Numerous studies have examined the effects of melatonin on plants exposed to Pb stress, and increasing evidence suggests that melatonin plays a crucial role in plant responses to environmental stress59,60. In the current study, plants exposed to only Pb stress exhibited a considerable elevation in \({\text{O}}_{2}^{ \cdot - }\), H2O2, and MDA contents compared to control plants (Fig. 2). This indicates a high level of oxidative stress, as MDA is a key marker of lipid peroxidation and ROS-mediated membrane damage. However, melatonin supplementation to Pb-stressed plants significantly reduced the levels of \({\text{O}}_{2}^{ \cdot - }\), H2O2, and MDA compared to plants treated only with Pb. This suggests that melatonin not only acts as a direct ROS scavenger but also modulates endogenous antioxidant defense pathways. Specifically, melatonin may interact with ROS signaling to upregulate the expression and capacity of key antioxidant enzymes, thereby accelerating the detoxification of \({\text{O}}_{2}^{ \cdot - }\) and H2O2. Several other studies have reported similar findings, indicating that Pb induces reactive oxygen species (ROS) and impairs membrane integrity61,62,63. The exogenous application of melatonin mitigates oxidative stress induced by Pb by lowering H2O2 and MDA content and reducing the electrolyte leakage (EL) rate41.
It is well-documented that melatonin is a universal antioxidant capable of easily passing through plasma membranes and moving into subcellular sections. Therefore, it can neutralize ROS in both the cytoplasm and organelles such as chloroplasts and mitochondria, which are major sites of ROS generation. Thus, it is reasonable to conclude that melatonin acts as an antioxidant in plants under stress conditions64. Furthermore, our study found that melatonin application enhanced the antioxidant activity of ABTS, DPPH, and CAT in rice plants under Pb stress compared to those treated only with Pb (Fig. 3). The increase in ABTS and DPPH scavenging activities reflects a higher non-enzymatic antioxidant capacity, while elevated CAT activity indicates improved enzymatic detoxification of H2O2. This multi-tiered antioxidant response helps maintain redox homeostasis and reduces oxidative injury under Pb stress. These results align with previous reports that melatonin enhances CAT activity against Pb stress and ABTS and DPPH activity against arsenic stress41,43. As a universal antioxidant, melatonin scavenges ROS either directly or by enhancing the activity of antioxidant enzymes, resulting in reduced oxidative stress and lipid peroxidation. A recent study also suggested that melatonin enhances the bioaccumulation of phytochelatins (such as anthocyanins), which scavenge ROS, chelate heavy metals, and sequester them into vacuoles, thereby reducing oxidative stress43. Overall, these results suggest that the application of melatonin under lead stress induces ROS scavenging, enhances the antioxidant defense system, and reduces lipid peroxidation. Consequently, this reduces oxidative stress and promotes plant growth and development under lead stress.
The improvement of plant growth parameters through melatonin application under Pb stress is often attributed to the enhancement of mineral elements such as Ca2+ and K+, as well as photosynthetic pigments and amino acids. Our results confirmed that Pb stress reduced these parameters, whereas melatonin application enhanced them under Pb stress (Figs. 6, 7). Mustafa Okant and Cengiz Kaya reported that melatonin application mitigated Pb toxicity in maize plants by increasing chlorophyll, Ca2+, and K+ contents41. Consistent with this, our study demonstrated that melatonin alleviated Pb stress in rice plants, as also suggested by Ni et al.40. A primary cause of chlorophyll reduction under stress conditions is the overaccumulation of H2O2 in plant tissues. Our findings showed that Pb-stressed rice plants had higher levels of H2O2 and \({\text{O}}_{2}^{ \cdot - }\), along with reduced chlorophyll content. In contrast, melatonin treatment reduced these ROS levels and enhanced chlorophyll content under Pb stress. These results suggest that melatonin mitigates the detrimental effects of Pb stress on chlorophyll, possibly by counteracting H2O2 accumulation. Furthermore, our investigation revealed that melatonin increased the accumulation of amino acids such as aspartic acid, proline, glutamic acid, and alanine, as well as relative water content, while reducing electrolyte leakage under Pb stress compared to plants treated only with Pb (Figs. 6, 7). Under heavy metal stress, plants synthesize a diverse array of metabolites, including amino acids such as proline and histidine, as well as glutathione, spermidine, and spermine65. Proline, in particular, is a crucial amino acid that functions as an osmolyte, free radical scavenger, and stabilizer of macromolecules66. The enhanced level of proline correlates with the enhanced heavy metal stress. Our study assumed that melatonin enhanced proline against Pb stress, which binds to heavy metal ions and reduces their availability and toxicity within the plant cell. Elevated levels of proline are correlated with increased tolerance to heavy metal stress. Our study hypothesizes that melatonin enhances proline accumulation in plants exposed to Pb stress. This increase in proline not only helps in osmoprotection and antioxidant defense but also binds to heavy metal ions, thereby reducing their availability and toxicity within plant cells. By chelating heavy metals, proline mitigates their harmful effects and contributes to the overall resilience of the plant under stress conditions. This dual role of proline in both detoxification and protection underscores its importance in the plant’s adaptive response to heavy metal stress.
Conclusion
This study highlighted the detrimental effects of Pb stress on rice plant growth and physiology. Plants exposed to only Pb stress significantly impairs growth, induces oxidative stress, disrupts ion homeostasis, and effect essential physiological traits. However, melatonin supplementation effectively mitigates these adverse effect, enhancing growth, reducing oxidative stress, modulating antioxidant enzyme activities, and improving ion balance and amino acid accumulation. These protective effects of melatonin are likely mediated through antioxidative properties, regulation of stress-responsive genes, and maintenance of cellular homeostasis. Consequently, melatonin emerges as a promising agent for enhancing plant resilience and productivity under heavy metal stress conditions, offering potential application in sustainable agriculture and environmental remediation.
Data availability
The data that support the findings of this study are available in the supplementary material of this article.
References
Anjum, S. A. et al. Chromium and aluminum phytotoxicity in maize: Morpho-physiological responses and metal uptake. CLEAN Soil Air Water 44, 1075–1084 (2016).
Wang, X. et al. Biotransfer of Cd along a soil-plant-mealybug-ladybird food chain: A comparison with host plants. Chemosphere 168, 699–706 (2017).
Hu, J. et al. Effects of Pb 2+ on the active oxygen-scavenging enzyme activities and ultrastructure in Potamogeton crispus leaves. Russ. J. Plant Physiol. 54, 414–419 (2007).
Yu, L. et al. Heavy metal contamination and source in arid agricultural soil in central Gansu Province, China. J. Environ. Sci. 20, 607–612 (2008).
Ghazaryan, K. et al. Soil pollution: an agricultural and environmental problem with nanotechnological remediation opportunities and challenges. Discov. Sustain. 5, 1–33 (2024).
Rahman, Z. & Singh, V. P. The relative impact of toxic heavy metals (THMs)(arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 191, 1–21 (2019).
Islam, E. et al. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 147, 806–816 (2007).
Gaya, U. & Ikechukwu, S. Heavy metal contamination of selected spices obtained from Nigeria. J. Appl. Sci. Environ. Manag. 20, 681–688 (2016).
Uzu, G., Sobanska, S., Aliouane, Y., Pradere, P. & Dumat, C. Study of lead phytoavailability for atmospheric industrial micronic and sub-micronic particles in relation with lead speciation. Environ. Pollut. 157, 1178–1185 (2009).
Dogan, M., Saygideger, S. D. & Colak, U. Effect of lead toxicity on aquatic macrophyte Elodea canadensis Michx. Bull. Environ. Contam. Toxicol. 83, 249–254 (2009).
Gupta, D. et al. Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J. Hazard. Mater. 172, 479–484 (2009).
Maestri, E., Marmiroli, M., Visioli, G. & Marmiroli, N. Metal tolerance and hyperaccumulation: Costs and trade-offs between traits and environment. Environ. Exp. Bot. 68, 1–13 (2010).
Ashraf, U. et al. Lead toxicity in rice: Effects, mechanisms, and mitigation strategies—A mini review. Environ. Sci. Pollut. Res. 22, 18318–18332 (2015).
Verma, S. & Dubey, R. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 164, 645–655 (2003).
Clemens, S. Evolution and function of phytochelatin synthases. J. Plant Physiol. 163, 319–332 (2006).
Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410 (2002).
Ali, B. et al. Promotive role of 5-aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Ind. Crops Prod. 52, 617–626 (2014).
Jiang, W. & Liu, D. Pb-induced cellular defense system in the root meristematic cells of Allium sativum L. BMC Plant Biol. 10, 1–8 (2010).
Cheng, W.-D., Zhang, G.-P., Yao, H.-G., Wu, W. & Xu, M. Genotypic and environmental variation in cadmium, chromium, arsenic, nickel, and lead concentrations in rice grains. J. Zhejiang Univ. Sci. B 7, 565–571 (2006).
Davis, J. M., Elias, R. W. & Grant, L. Current issues in human lead exposure and regulation of lead. Neurotoxicology 14, 15–27 (1993).
Feng, J. et al. Source attributions of heavy metals in rice plant along highway in Eastern China. J. Environ. Sci. 23, 1158–1164 (2011).
Liu, J., Ma, X., Wang, M. & Sun, X. Genotypic differences among rice cultivars in lead accumulation and translocation and the relation with grain Pb levels. Ecotoxicol. Environ. Saf. 90, 35–40 (2013).
Salavati, J., Fallah, H., Niknejad, Y. & Barari Tari, D. Methyl jasmonate ameliorates lead toxicity in Oryza sativa by modulating chlorophyll metabolism, antioxidative capacity and metal translocation. Physiol. Mol. Biol. Plants 27, 1089–1104 (2021).
Saini, S., Kaur, N. & Pati, P. K. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol. Environ. Saf. 223, 112578 (2021).
Naeem, I., Masood, N., Turan, V. & Iqbal, M. Prospective usage of magnesium potassium phosphate cement combined with Bougainvillea alba derived biochar to reduce Pb bioavailability in soil and its uptake by Spinacia oleracea L. Ecotoxicol. Environ. Saf. 208, 111723 (2021).
Lamhamdi, M. et al. Effect of lead stress on mineral content and growth of wheat (Triticum aestivum) and spinach (Spinacia oleracea) seedlings. Saudi J. Biol. Sci. 20, 29–36 (2013).
Gupta, D. K., Chatterjee, S. & Walther, C. Lead in Plants and the Environment. (2020).
Turan, V. Potential of pistachio shell biochar and dicalcium phosphate combination to reduce Pb speciation in spinach, improved soil enzymatic activities, plant nutritional quality, and antioxidant defense system. Chemosphere 245, 125611 (2020).
Andresen, E. et al. Different strategies of cadmium detoxification in the submerged macrophyte Ceratophyllum demersum L. Metallomics 5, 1377–1386 (2013).
Rahim, W. et al. Exogenously applied sodium nitroprusside mitigates lead toxicity in rice by regulating antioxidants and metal stress-related transcripts. Int. J. Mol. Sci. 23, 9729 (2022).
Jan, R. et al. Metal resistant endophytic bacteria reduces cadmium, nickel toxicity, and enhances expression of metal stress related genes with improved growth of Oryza sativa, via regulating its antioxidant machinery and endogenous hormones. Plants 8, 363 (2019).
Yuan, L., Yang, S., Liu, B., Zhang, M. & Wu, K. Molecular characterization of a rice metal tolerance protein, OsMTP1. Plant Cell Rep. 31, 67–79 (2012).
Byeon, Y., Lee, H. Y. & Back, K. Chloroplastic and cytoplasmic overexpression of sheep serotonin N-acetyltransferase in transgenic rice plants is associated with low melatonin production despite high enzyme activity. J. Pineal Res. 58, 461–469 (2015).
Arnao, M. B. & Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 121, 195–207 (2018).
Lei, X. Y., Zhu, R. Y., Zhang, G. Y. & Dai, Y. R. Attenuation of cold-induced apoptosis by exogenous melatonin in carrot suspension cells: the possible involvement of polyamines. J. Pineal Res. 36, 126–131 (2004).
Cao, S. et al. Exogenous melatonin treatment increases chilling tolerance and induces defense response in harvested peach fruit during cold storage. J. Agric. Food Chem. 64, 5215–5222 (2016).
Shi, H. et al. Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. J. Pineal Res. 59, 102–108 (2015).
Martinez, V. et al. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules 23, 535 (2018).
Reiter, R. J., Tan, D. X. & Galano, A. Melatonin: Exceeding expectations. Physiology (2014).
Ni, J. et al. Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 23, 799 (2018).
Okant, M. & Kaya, C. The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ. Sci. Pollut. Res. 26, 11864–11874 (2019).
Li, X. et al. Physiological and proteomics analyses reveal the mechanism of Eichhornia crassipes tolerance to high-concentration cadmium stress compared with Pistia stratiotes. PLoS ONE 10, e0124304 (2015).
Jan, R. et al. Melatonin alleviates arsenic (As) toxicity in rice plants via modulating antioxidant defense system and secondary metabolites and reducing oxidative stress. Environ. Pollut. 318, 120868 (2023).
Ashraf, U. et al. Alterations in growth, oxidative damage, and metal uptake of five aromatic rice cultivars under lead toxicity. Plant Physiol. Biochem. 115, 461–471 (2017).
Jan, R. et al. Flavonone 3-hydroxylase relieves bacterial leaf blight stress in rice via overaccumulation of antioxidant flavonoids and induction of defense genes and hormones. Int. J. Mol. Sci. 22, 6152 (2021).
Lee, M.-Y. et al. Melanin synthesis inhibition and radical scavenging activities of compounds isolated from the aerial part of Lespedeza cyrtobotrya. J. Microbiol. Biotechnol. 20, 988–994 (2010).
Xu, B. J. & Chang, S. K. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 72, S159–S166 (2007).
Imran, M. et al. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants 13, plab026 (2021).
Johansson, L. H. & Borg, L. H. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 174, 331–336 (1988).
Farooq, M., Park, J.-R., Jang, Y.-H., Kim, E.-G. & Kim, K.-M. Rice cultivars under salt stress Show differential expression of genes related to the regulation of Na+/K+ balance. Front. Plant Sci. 12, 680131 (2021).
Pavlík, M. et al. Trace elements present in airborne particulate matter—Stressors of plant metabolism. Ecotoxicol. Environ. Saf. 79, 101–107 (2012).
Hasanuzzaman, M. & Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22, 584–596 (2013).
Hasan, M. K. et al. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 6, 601 (2015).
Namdjoyan, S., Kermanian, H., Abolhasani Soorki, A., Modarres Tabatabaei, S. & Elyasi, N. Interactive effects of salicylic acid and nitric oxide in alleviating zinc toxicity of Safflower (Carthamus tinctorius L). Ecotoxicology 26, 752–761 (2017).
Namdjoyan, S., Soorki, A. A., Elyasi, N., Kazemi, N. & Simaei, M. Melatonin alleviates lead-induced oxidative damage in safflower (Carthamus tinctorius L.) seedlings. Ecotoxicology 29, 108–118 (2020).
Azarin, K. et al. Impact nano-and micro-form of CdO on barley growth and oxidative stress response. J. King Saud Univ. Sci. 36, 103493 (2024).
Vardumyan, H. et al. Additive-mediated phytoextraction of copper-contaminated soils using Medicago lupulina L. Egypt. J. Soil Sci. 64, 599–618 (2024).
Reiter, R. J., Tan, D.-X., Manchester, L. C. & Qi, W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem. Biophys. 34, 237–256 (2001).
Park, S. et al. Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J. Pineal Res. 54, 258–263 (2013).
Lee, H. Y., Byeon, Y. & Back, K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res. 57, 262–268 (2014).
Reddy, A. M., Kumar, S. G., Jyothsnakumari, G., Thimmanaik, S. & Sudhakar, C. Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.). Chemosphere 60, 97–104 (2005).
Liu, N. et al. Lead and cadmium induced alterations of cellular functions in leaves of Alocasia macrorrhiza L. Schott. Ecotoxicol. Environ. Saf. 73, 1238–1245 (2010).
Shahid, M., Pinelli, E. & Dumat, C. Review of Pb availability and toxicity to plants in relation with metal speciation; Role of synthetic and natural organic ligands. J. Hazard. Mater. 219, 1–12 (2012).
Marta, B., Szafrańska, K. & Posmyk, M. M. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 7, 575 (2016).
Sharma, S. S. & Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 57, 711–726 (2006).
Matysik, J., Alia, Bhalu, B. & Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 82, 525–532 (2002).
Acknowledgements
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. RS-2025-02214096)” Rural Development Administration, Republic of Korea. The authors extend their appreciation to the Northern Border University, Arar, KSA for funding this research “work through the Project Number (NBU-CRP-2025-249)”.
Author information
Authors and Affiliations
Contributions
JK, EE, and RJ, conceptualized the study; JK and EE, conducted experiments; JK, and RJ, conducted data analysis; JK, RJ, and EE, wrote the draft. RJ, YF, and KMK supervise the study, YF, KMK, and EE, review and edited the draft, YF and KMK support the financial acquisition. All the authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Khan, J., Elsharkawy, E., Fu, Y. et al. Melatonin alleviates lead-induced stress in rice through physiological regulation and molecular defense mechanisms. Sci Rep 15, 34788 (2025). https://doi.org/10.1038/s41598-025-18514-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18514-9