Abstract
Anesthesia is essential, but not selective, in resting-state functional magnetic resonance imaging (fMRI) for pre-clinical studies. To mitigate stress and minimize head-movement artifacts, animals should be anesthetized during resting-state fMRI. Although the type, dosage, and timing of anesthesia can influence fMRI outcomes, responses to stimulation, and functional connectivity, the appropriate dosage of anesthesia is among the most important considerations. However, little is known about the effects of anesthetic dosage on innate fear responses induced by electrical stimulation. Therefore, we aimed to investigate the effects of medetomidine dosage on electrical stimulation and functional connectivity in fear-related regions. We conducted a graph-based network analysis of functional connectivity before and after electrical stimulation, based on different medetomidine dosages. We observed increased functional connectivity post-stimulation in the high-dose condition, but not in the low-dose condition. The high-dose condition showed increased global network properties post-stimulation compared to those observed pre-stimulation. In contrast, the low-dose condition showed no significant difference in global network properties between pre- and post-stimulation. The results suggest that high-dose medetomidine suppresses functional connectivity in fear-related regions in the brain; however, this suppressed functional connectivity can be recovered by electrical stimulation.
Similar content being viewed by others
Introduction
The use of fMRI is becoming increasingly important in animal studies because of its noninvasive imaging without sacrificing animals. During fMRI scanning, animals should be anesthetized to mitigate stress and minimize head-movement artifacts that can distort the images1,2,3,4. However, the choice of anesthesia can impact the results due to its varied effects on cerebral blood flow and brain function5,6,7. Numerous studies have indicated that the type, dosage, and timing of anesthesia can influence fMRI outcomes, such as baseline blood oxygen level-dependent (BOLD) signals, responses to stimulation, and functional connectivity8,9,10,11,12,13,14,15.
Various anesthetic agents have been used, including isoflurane, medetomidine, α-chloralose, urethane, ketamine, and thiobutabarbital, in rodents. Moreover, medetomidine, either alone or in conjunction with isoflurane, has been repeatedly and successfully used for evoked mouse fMRI studies10,16,17,18. Medetomidine is a selective alpha-2 adrenergic agonist that is commonly used as an anesthetic adjunct. Unlike many other sedatives and anesthetics, which deeply suppress central nervous system activity, medetomidine has been shown to preserve neural activity and maintain functional connectivity networks more closely resembling wakefulness16,19,20,21. In addition, it provides a stable sedative state characterized by a state of “arousable sedation” where patients are easily rousable and responsive to stimuli. The effects of medetomidine dosage on fMRI outcomes have been systematically evaluated9,10,22. For example, a gradual rise in the BOLD response with higher stimulation frequency serves as an indicator of sedation depth and may be adjusted by the dosage of medetomidine infusion10. To ensure a stable sedation state over an extended period using medetomidine, a high dosage is required, but it is associated with side effects that disrupt the synchrony in the brain22. In general, numerous studies have adopted the continuous medetomidine infusion of less than 0.2 mg/kg/h12,17,23.
While anesthesia generally suppresses brain activity24,25,26certain parts of the brainstem and limbic system, such as the amygdala, hippocampus, and anterior cingulate cortex, demonstrate less suppression than other brain areas in specific situations27,28. This is reasonable because the brainstem governs critical activities, such as heart rate and respiration, while the limbic system may contribute to the modulation of emotional responses and memory formation by remaining less suppressed during anesthesia. Moreover, the anterior cingulate cortex is involved in various functions, such as cognitive control, emotional regulation, pain processing, decision-making, and innate fear responses.
Notably, innate fear can be triggered instantaneously, even without conscious awareness, due to its crucial role in survival. The innate fear response is controlled by the basolateral amygdala and the anterior cingulate cortex29with the orbitofrontal cortex and insula also involved in controlling fear memory to predatory threats30,31,32,33,34,35. Since the anterior cingulate cortex receives information from the orbitofrontal cortex, it is expected that the anterior cingulate cortex and orbitofrontal cortex may remain less suppressed during the anesthetized state, ensuring the maintenance of vital functions. However, little is known about the specific effects of anesthesia agents on these areas and how anesthesia concentration influences emotional and cognitive processes. We focused on the fear-related regions derived from the electrical stimulation of the mouse forepaw, which is commonly used to test neural activation in the somatosensory cortex, as electrical shocks can activate fear-processing brain regions, such as the anterior cingulate cortex29.
Therefore, this study aimed to use functional connectivity to examined whether the anterior cingulate cortex and orbitofrontal cortex may be affected by the use of medetomidine doses. We examined functional connectivity changes in the fear-related regions composed of the anterior cingulate cortex and orbitofrontal cortex before and after electrical stimulation that can induce pain and lead to fear response. Utilizing structural and functional MRI data36 and Allen Mouse Brain Atlas37we constructed the fear-related regions. We then explored the functional connectivity changes in the fear-related regions between pre- and post-stimulations according to medetomidine dosages. To further test the functional connectivity changes at the network level, we conducted a graph-based network analysis and compared global network properties in the fear-related regions. We predicted the dosage of medetomidine affects functional pathways of fear processing.
Methods
Data acquisition
We obtained structural MRI and resting-state fMRI of the mice under different anesthesia dosages and electrical stimulation conditions from the data repository at the University of Queensland (https://doi.org/10.48610/3b35b94)38. All experimental protocols were approved by the Institutional Animal Ethics Committee at the University of Queensland (IACUC number: QBI/SCMB/089/16/QBI). All experiments were prepared in accordance with ARRIVE guidelines39and carried out following the Australian code of practice for the care and use of animals for scientific purposes40. All methods were performed in accordance with the relevant guidelines and regulations of the Korea Brain Research Institute.
Electrical stimulation was applied to the forepaw using sub-dermal electrodes inserted near the second and fourth digits of the left dorsal forepaw. To deliver consistent and mild stimulation, a current source (Isostim A320, World Precision Instrument, USA) was used with the following parameters: 6 Hz pulse frequency, 0.3 ms pulse width, and 0.2 mA current. These parameters were chosen to ensure non-noxious stimulation.
The obtained data were composed of low-dose (0.1 mg/kg/h, n = 9) and high-dose (0.3 mg/kg/h, n = 9) medetomidine conditions that medetomidine does not typically induce epileptic seizures in mice41. We excluded one mouse due To no acquisition of structural MRI images, and 8 mice were used for the analysis. A sample size of 8 mice is within the typical range for mouse fMRI studies, where sample sizes of n = 6–13 are commonly employed18,42,43,44,45. This range is often based on both practical constraints and ethical considerations, which limit the number of animals that can be used in such experiments.
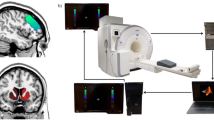
Notably, no animal was exposed to any other anesthesia before the first session. All animals were exposed to repeated anesthesia, as this was vital to the experimental design, allowing for a paired t-test statistical analysis design. The effects and potential bias of prior exposures on the conclusion and analysis of the differential effects of different doses of anesthesia were controlled by randomizing the dose administered to each animal in the first session. In the second session, which was conducted at least a week later, the alternate dose was used. A brief experimental procedure is shown in Fig. 1a and detailed information on MRI acquisition is available in To and Nasrallah’s study36,38. Briefly, medetomidine anesthesia was initiated with an intraperitoneal bolus injection (0.5 mg/kg for the low-dose condition; 0.15 mg/kg for the high-dose condition), followed by continuous infusion at the designated dose (0.1 or 0.3 mg/kg/h). Isoflurane was tapered off gradually and maintained at ~ 0.5% during imaging. Functional scans were initiated approximately 55 min after medetomidine onset to ensure a stable anesthetic state. Throughout scanning, physiological parameters including respiration rate (90–180 breaths/min) and rectal temperature (36.5 ± 0.5 °C) were continuously monitored. No animals exhibited signs of arousal or distress during stimulation, and no imaging sessions required exclusion due To motion artifacts. Structural and functional MRI data were acquired using a 9.4T MRI scanner (Bruker Biospin, Germany) with a cryogenically cooled transmit and receive coil. The MRI parameters for the structural T2-weighted image were as follows: Turbo Rapid Acquisition with Refocused Echoes (TurboRARE), acquisition Matrix of 192× 192, field of view of 19.2 mm, 52 contiguous slices, voxel size of 0.1 × 0.1 × 0.3 mm³, repetition time (TR) of 7200 ms, and echo time (TE) of 39 ms, averages = 2, RARE factor = 8. The functional MRI parameters were as follows: a 2D gradient-echo echo-planar-imaging (GE-EPI), acquisition Matrix of 64× 64, field of view of 19.2 mm, 36 interleaved slices, voxel size of 0.3 × 0.3 × 0.6 mm³, TR for 1000 ms, TE of 14 ms, flip angle of 70°, and 600 volumes (total scan time = 10 min)36.
fMRI data preprocessing
Resting-state fMRI data were preprocessed using statistical parametric mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm)46 and FMIRB Software Library (https://fsl.fmrib.ox.ac.uk/fsl/)47. As used in our previous study48the fMRI data underwent seven preprocessing steps, orientation correction, voxel scaling (×10)49,50slice timing correction, head motion correction, distortion correction, co-registration, and spatial normalization to the template space Allen Mouse Brain Common Coordinate Framework (CCFv3)37, using non-linear transformation. The normalized data were interpolated to 0.8 × 0.8 × 0.8 mm3 voxels, which is equivalent to 0.08 mm with respect to the actual size of the mouse brain. Spatial smoothing was not conducted to avoid spill-over effects between voxels51. For the preprocessing of the fMRI time series, six rigid motion parameters and their derivatives, three principal components of the white Matter, and cerebrospinal fluid masks, Linear and quadratic regressors were regressed out. Band-pass filtering from 0.01 to 0.3 Hz was applied. We calculated Pearson correlation coefficients (r-values) between the filtered time series of two reference regions. Functional connectivity was defined by converting the r-values to Fisher z scores.
Construction of fear-related and sensory-motor-related brain regions
To investigate the topological characteristics of functional connectivity by electrical stimulation, we constructed the fear-related and sensory-motor-related regions of the mouse (Fig. 1). According to previous studies30,31,32,33,52,53we defined the fear-related regions with the anterior cingulate area (ACA), orbital area (ORB), and dentate gyrus (DG) of hippocampus of the Allen Mouse Brain Atlas (Supplementary Fig. 1). We excluded basolateral amygdala because of signal loss in the ventral brain areas around the amygdala54. We also defined the sensory-motor-related regions with the somatosensory areas (SS) and somatomotor areas (MO).
Overview of experimental procedure and subnetworks for functional connectivity analysis. (A) A mice fMRI study under different anesthesia dosage and stimulation conditions was conducted in To and Nasrallah’s work36. Some images were used with permission from https://biorender.com/z75s689 under a CC BY license. (B) The fear-related and sensory-motor-related regions were constructed with the following brain regions: ACAd1: Anterior cingulate area, dorsal part, layer 1; ACAv1: Anterior cingulate area, ventral part, layer 1; ORBm2: Orbital area, medial part, layer 2; DG/mo: Dentate gyrus, molecular layer; MOs1: Secondary motor area, layer 1; MOp1: Primary motor area, layer 1; SSp-ul1: Primary somatosensory area, upper Limb, layer 1; SSp-ul4: Primary somatosensory area, upper Limb, layer 4; SSp-ul6a: Primary somatosensory area, upper Limb, layer 6a; SSp-un1: Primary somatosensory area, unassigned, layer 1; SSp-m1: Primary somatosensory area, mouth, layer 1; SSp-n1: Primary somatosensory area, nose, layer 1; SSp-bfd1: Primary somatosensory area, barrel field, layer 1; SSs1: Supplemental somatosensory area, layer 1; SSs4: Supplemental somatosensory area, layer 4, SSs6a: Supplemental somatosensory area, layer 6a.
Network analysis of fear-related and sensory-motor-related brain regions
To test whether functional connectivity changes can be observed at the network level, we analyzed functional connectivity with graph theoretical methods using the Brain Connectivity Toolbox (BCT, brain-connectivity-toolbox.net)55. The BCT toolbox, a MATLAB toolbox for complex brain-network analysis, can measure functional segregation and integration, quantify the importance of individual brain regions, and test the optimization of brain networks. Using the BCT toolbox, we calculated three global network properties: (i) global node degree (to test how a node is important), (ii) global node strength (to test how a node is strongly connected to other nodes), and (iii) global efficiency (to test how the optimization of functional network).
In the functional connectivity matrix (\(\:G\)), node degree (\(\:d\)) is calculated based on the number of edges connected between a node (\(\:i\)) and other nodes (\(\:j\)), and global node degree (\(\:D\)) is calculated by dividing the sum of node degrees with the total number (\(\:N\)) of nodes.
Node strength (\(\:s\)) is calculated based on the sum of the weights of edges connected between a node (\(\:i\)) and other nodes (\(\:ii\)). Global node strength (\(\:S\)) is calculated by dividing the sum of node strengths by the total number (\(\:N\)).
where \(\:w\) indicates the weight of edges between nodes \(\:i\) and \(\:j\).
Node efficiency is calculated by the mean of inverse shortest-path distance from a node to other nodes and global efficiency is defined by averaging all node efficiencies56. Global efficiency (\(\:E\)) is calculated as below:
where \(\:gd\) is the geodesic distance between nodes \(\:i\) and \(\:j\).
Results
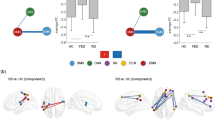
Figure 2 displays the functional connectivity of the fear-related regions before and after electrical stimulation according to low- and high-dose conditions. The functional connections averaged across the group are shown in Figs. 2a and b. In the high-dose condition, functional connections were increased after electrical stimulation. Statistical differences in node strengths of functional connectivity between pre- and post-stimulation were found using a paired t-test and a false discovery rate (FDR) < 0.01 (Fig. 2b; Table 1). Significantly increased edges (FDR-adjusted p = 0.0018) were found between the ventral part of ACA, the lateral part of ORB, and DG (molecular layer) (Fig. 2c; Table 2).
Functional connectivity in the fear network and its difference between pre- and post-stimulation in low- and high-dose conditions. (A) Group-average functional connectivity matrices, (B) group-average functional networks, stimulation difference in (C) node strength, and (D) edge strength. ACA: Anterior cingulate area, ORB: Orbital area, DG: Dentate gyrus.
Our principal findings were that functional connectivity of the fear-related regions was significantly increased post-stimulation in high-dose condition, but not in low-dose condition. Based on these findings, we further questioned whether the increase in functional connectivity after post-stimulation is specific to the fear-related regions or not. To answer this question, we investigated global network properties in the sensory-motor-related regions that are engaged in the feeling of being afraid.
Figure 3 shows the global network properties (global node degree, global node strength, and global efficiency) in the fear and sensory-motor-related regions. In the fear-related regions, the high-dose condition showed significantly increased global network properties in post-stimulation compared to those in pre-stimulation. However, in the low-dose condition, there was no significant difference in global network properties between pre- and post-stimulations (Fig. 3a; Table 3).
To test whether the neuromodulation effect is significantly larger in high-dose conditions than in low-dose conditions, we conducted a two-way 2 (Stimulation: Pre-stim, Post-stim) × 2 (Dosage: Low-dose, High-dose) repeated measures analysis of variance (ANOVA). As a post-hoc analysis, paired t-tests were applied to assess the differences in global network properties between pre- and post-stimulation.
In the ANOVA analysis, global node degree, global node strength, and global efficiency showed significant interaction between Stimulation and Dosage (global node degree: F1,14 = 11.92, p = 0.0039; global node strength: F1,14 = 5.75, p = 0.0310; global efficiency: F1,14 = 6.66, p = 0.0218) as well as significant main effects of Stimulation (global node degree: F1,14 = 7.39, p = 0.0167; global node strength: F1,14 = 9.37, p = 0.0085; global efficiency: F1,14 = 13.73, p = 0.0024). However, there were no significant main effects of Dosage in the global network properties (global node degree: F1,14 = 0.40, p = 0.5352; global node strength: F1,14 = 0.45, p = 0.5121; global efficiency: F1,14 = 0.40, p = 0.5369). In the post-hoc analysis, significantly higher global network properties post-stimulation were observed in the high-dose condition compared to those pre-stimulation (Table 3). However, there were no significant differences in global network properties between pre- and post-stimulation in the low-dose condition.
In contrast, regardless of concentrations, global network properties of the sensory-motor-related regions did not show any significant differences between pre- and post-stimulations (Fig. 3b; Table 1). In addition, the two-way 2 × 2 ANOVA analysis revealed non-significant interaction effects for Stimulation × Dosage in the global network properties (global node degree: F1,14 = 0.82, p = 0.3803; global node strength: F1,14 = 1.75, p = 0.2065; global efficiency: F1,14 = 1.61, p = 0.2248) as well as no significant main effects for Stimulation (global node degree: F1,14 = 1.37, p = 0.2618; global node strength: F1,14 = 0.59, p = 0.4558; global efficiency: F1,14 = 0.53, p = 0.4791). However, there were significant main effects for Dosage in global node strength (F1,14 = 6.2, p = 0.0259) and global efficiency (F1,14 = 7.8, p = 0.0144) but not in global degree (F1,14 = 1.37, p = 0.2618). These results indicate a significant neuromodulation effect in the high-dose condition of the fear-related regions, not the sensory-motor-related regions.
Global network properties of functional connectivity in the fear-related and sensory-motor-related regions. Significant interactions between Stimulation (Pre-stim, Post-stim) × Dosage (Low-dose, High-dose) of the global network properties were observed in (A) the fear-related regions, not in (B) the sensory-motor-related regions. In the fear-related regions, global node degree, global node strength, and global efficiency were increased in the high-dose condition but not in the low-dose condition after electrical stimulation. However, in the sensory-motor-related regions, there were no significant increases after electrical stimulation in both high- and low-dose conditions.
Discussion
In the current study, we tested how functional connectivity in the fear-related regions changes after electrical stimulation according to the medetomidine doses. This is important for animal fMRI studies, as brain functions may be differently represented in cortical and subcortical regions according to the dosage of anesthesia. In fact, the use of low-dose medetomidine has several advantages for sensory study such as sedation57preservation of sensory processing18,58less impairment of motor function12,59reduced cardiovascular and respiratory effects60and longitudinal study facilitation61. In contrast, the use of high-dose medetomidine can maintain sedation and physiological parameters10 and block somatosensory-evoked potentials62shift frequency power spectrum toward the higher frequency63,64and cause loss of consciousness65. Therefore, while higher-order cognitive networks may be disrupted with loss of consciousness, specific brain networks related to basic physiological functions may still be active in anesthesia. In this respect, we focused on innate fears because functional connectivity changes in the fear-related regions could explain the effects of medetomidine doses.
It is generally expected that the use of low-dose medetomidine may be advantageous in representing cognitive function, whereas the use of high-dose medetomidine may be difficult in representing cognitive function due to the loss of consciousness. This is likely because a lower medetomidine dose is better, considering the stable sedation of the functional network before and after stimulation, which has often been used in previous studies66,67. In the current study, we observed change (increase) in functional connectivity after electrical stimulation in the fear-related regions. In particular, the difference was larger for the high-dose condition than for the low-dose condition (Fig. 2a). Similarly, increased node strengths and edge strengths of the fear-related regions following electrical stimulation were only observed in the high-dose condition (Figs. 2b and c). Our findings are consistent with those of previous studies demonstrating neural activation in the fear-related brain regions by electrical stimulation33.
In the network analysis of functional connectivity, we also found that when high-dose medetomidine was used, global node degree, global node strength, and global efficiency after electrical stimulation were increased in the fear-related regions but not in the sensory-motor-related regions (Fig. 3). Previous studies on rodent resting-state fMRI have repeatedly observed that lower dose of medetomidine is consistently associated with higher BOLD signal and functional connectivity strength in cortical and subcortical regions9,12,63. Similarly, we observed significant main effects for the dosage of medetomidine in global node strength (F1,14 = 6.2, p = 0.0259) and global efficiency (F1,14 = 7.8, p = 0.0144) in global network properties of the sensory-motor-related regions. However, irrespective of medetomidine concentrations, no significant differences were observed in the global network properties of the sensory-motor-related regions before and after stimulations. Our findings are in line with those of previous studies reporting that under different levels of medetomidine, stimulation-induced neural activity in the somatosensory areas has no changes22and neurovascular coupling in the somatosensory cortex is not affected19. Interestingly, medetomidine dose-dependent anesthesia did not affect the changes in functional connectivity in the sensory-motor-related regions, but the fear-related regions remained functionally dependent on the anesthesia state. These findings show that changes in functional connectivity in the high-dose condition may be specific to individual networks.
However, upon comparing global network properties between low- and high-dose conditions, we found that high-dose medetomidine suppressed the functional connectivity in both the fear-related regions (two-sample t-tests, global node degree: low-dose = 19.16 ± 1.32, high-dose = 14.51 ± 1.26, p = 0.0232; global node strength: low-dose = 9.76 ± 0.77, high-dose = 7.36 ± 0.52, p = 0.0243; global efficiency: low-dose = 0.41 ± 0.02, high-dose = 0.35 ± 0.02, p = 0.0286) and sensory-motor-related regions (global node strength: low-dose = 13.79 ± 0.42, high-dose = 10.94 ± 0.66, p = 0.0025; global efficiency: low-dose = 0.36 ± 0.01, high-dose = 0.31 ± 0.01, p = 0.0013). These results are consistent with a previous finding that a higher dosage of medetomidine reduces functional connectivity strength43. Our findings also indicate that high dosage may disrupt functional synchrony in the brain with strong sedation.
Our main findings were that the functional network properties of the fear-related regions with high-dose medetomidine were increased after stimulation. These increases seem to represent the normalization of functional connectivity by neuromodulation. To clarify this, we additionally compared global network properties post-stimulation in low- and high-dose conditions in the fear-related and the sensory-motor-related regions. As a result, we observed no significant differences in global network properties between low- and high-dose medetomidine in both the fear-related regions (two-sample t-tests, global node degree: p = 0.3359; global node strength: p = 0.6087; global efficiency: p = 0.6657) and the somatosensory network (global node degree: p = 0.6416; global node strength: p = 0.3089; global efficiency: p = 0.2250). Furthermore, upon comparing global network properties in the pre-stimulus phase for the low- and high-dose conditions, we observed significant differences in global node degree (p = 0.0232), global node strength (p = 0.0243), and global efficiency (p = 0.0286) in the fear-related regions (Supplementary Fig. 2). Considering the baseline differences, the changes observed between pre- and post-stimulation are likely due to electrical stimulation.
These results indicate that the fear-related and sensory-motor-related brain regions are stable because of no changes in the functional network properties before and after stimulation in the low-dose condition. Considering these results, we conclude that the lower dosage of medetomidine is preferable for various fMRI studies, promoting the consistent maintenance of brain networks, as supported by previous research66,67. Conversely, our results suggest that a higher dosage increases the network’s responsiveness to external stimuli, similar to electrical stimulation in this study, facilitating the identification of brain regions responsive to such stimuli.
The hippocampus plays important roles in fear processing, as do the anterior cingulate cortex29,68,69,70,71 and orbitofrontal cortex32,72. In particular, the anterior cingulate cortex is connected to the basolateral amygdala29periaqueductal gray73nucleus accumbens74bed nucleus of the stria terminalis75and superior colliculus motor region76 to form a circuit or work cooperatively to control fear, pain-related defensive behavior, or pain avoidance response. It seems to be that neural activity in the anterior cingulate cortex and orbitofrontal cortex was less suppressed during the anesthetized state, allowing it to respond actively to stimuli at high-dose anesthesia conditions in line with a previous study77.
This study had some Limitations that should be considered for future studies. First, no fMRI data were available at 90 min in the high-dose medetomidine condition, which would have allowed to examine changes in functional connectivity over time. Second, actual fear response could not be measured from the mouse even though it is expected to respond similarly to humans.
In conclusion, this study is concerned with functional connectivity changes focusing on the fear-related regions that have not been previously addressed36,38. Our findings demonstrate the effects of medetomidine dosage on electrical stimulation and how it affects functional connectivity in the fear-related regions. Awake rodent fMRI scanning has many advantages when suitably performed78,79,80. However, when the use of awake fMRI is precluded, our findings remain important for planning animal fMRI studies that require anesthesia. Importantly, functional connectivity can be increased or decreased according to the agent and dosage of anesthesia used.
Data availability
There were no experimental datasets obtained in this study. The raw mouse MRI datasets are available from the data repository at the University of Queensland (https://doi.org/10.48610/3b35b94). Full access to the data used in this study is available from the corresponding author upon reasonable request.
References
Adamczak, J. M., Farr, T. D., Seehafer, J. U., Kalthoff, D. & Hoehn, M. High field BOLD response to forepaw stimulation in the mouse. Neuroimage 51, 704–712. https://doi.org/10.1016/j.neuroimage.2010.02.083 (2010).
Becker, K. Animal welfare aspects in planning and conducting experiments on rodent models of subarachnoid hemorrhage. Cell. Mol. Neurobiol. 43, 3965–3981. https://doi.org/10.1007/s10571-023-01418-5 (2023).
Birn, R. M. The role of physiological noise in resting-state functional connectivity. Neuroimage 62, 864–870. https://doi.org/10.1016/j.neuroimage.2012.01.016 (2012).
Ueki, M., Mies, G. & Hossmann, K. A. Effect of alpha-chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta Anaesthesiol. Scand. 36, 318–322. https://doi.org/10.1111/j.1399-6576.1992.tb03474.x (1992).
Crosby, G., Crane, A. M., Jehle, J. & Sokoloff, L. The local metabolic effects of somatosensory stimulation in the central nervous system of rats given pentobarbital or nitrous oxide. Anesthesiology 58, 38–43. https://doi.org/10.1097/00000542-198301000-00007 (1983).
Lindauer, U., Villringer, A. & Dirnagl, U. Characterization of CBF response to somatosensory stimulation: model and influence of anesthetics. Am. J. Physiol. 264, H1223–1228. https://doi.org/10.1152/ajpheart.1993.264.4.H1223 (1993).
Masamoto, K., Kim, T., Fukuda, M., Wang, P. & Kim, S. G. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb. Cortex. 17, 942–950. https://doi.org/10.1093/cercor/bhl005 (2007).
Gass, N. et al. Sub-anesthetic ketamine modulates intrinsic BOLD connectivity within the hippocampal-prefrontal circuit in the rat. Neuropsychopharmacology 39, 895–906. https://doi.org/10.1038/npp.2013.290 (2014).
Nasrallah, F. A., Tay, H. C. & Chuang, K. H. Detection of functional connectivity in the resting mouse brain. Neuroimage 86, 417–424. https://doi.org/10.1016/j.neuroimage.2013.10.025 (2014).
Pawela, C. P. et al. A protocol for use of Medetomidine anesthesia in rats for extended studies using task-induced BOLD contrast and resting-state functional connectivity. Neuroimage 46, 1137–1147. https://doi.org/10.1016/j.neuroimage.2009.03.004 (2009).
Peltier, S. J. et al. Functional connectivity changes with concentration of Sevoflurane anesthesia. Neuroreport 16, 285–288. https://doi.org/10.1097/00001756-200502280-00017 (2005).
Pradier, B. et al. Combined resting state-fMRI and calcium recordings show stable brain States for task-induced fMRI in mice under combined ISO/MED anesthesia. Neuroimage 245, 118626. https://doi.org/10.1016/j.neuroimage.2021.118626 (2021).
Schlegel, F., Schroeter, A. & Rudin, M. The hemodynamic response to somatosensory stimulation in mice depends on the anesthetic used: implications on analysis of mouse fMRI data. Neuroimage 116, 40–49. https://doi.org/10.1016/j.neuroimage.2015.05.013 (2015).
Schrouff, J. et al. Brain functional integration decreases during propofol-induced loss of consciousness. Neuroimage 57, 198–205. https://doi.org/10.1016/j.neuroimage.2011.04.020 (2011).
Wu, T. et al. Altered regional connectivity reflecting effects of different anaesthesia protocols in the mouse brain. Neuroimage 149, 190–199. https://doi.org/10.1016/j.neuroimage.2017.01.074 (2017).
Magnuson, M. E., Thompson, G. J., Pan, W. J. & Keilholz, S. D. Time-dependent effects of isoflurane and Dexmedetomidine on functional connectivity, spectral characteristics, and Spatial distribution of spontaneous BOLD fluctuations. NMR Biomed. 27, 291–303. https://doi.org/10.1002/nbm.3062 (2014).
Bukhari, Q., Schroeter, A., Cole, D. M. & Rudin, M. Resting state fMRI in mice reveals anesthesia specific signatures of brain functional networks and their interactions. Front. Neural Circuits. 11, 5. https://doi.org/10.3389/fncir.2017.00005 (2017).
You, T., Im, G. H. & Kim, S. G. Characterization of brain-wide somatosensory BOLD fMRI in mice under dexmedetomidine/isoflurane and ketamine/xylazine. Sci. Rep. 11, 13110. https://doi.org/10.1038/s41598-021-92582-5 (2021).
Nasrallah, F. A., Lew, S. K., Low, A. S. & Chuang, K. H. Neural correlate of resting-state functional connectivity under alpha2 adrenergic receptor agonist, Medetomidine. Neuroimage 84, 27–34. https://doi.org/10.1016/j.neuroimage.2013.08.004 (2014).
Steiner, A. R., Rousseau-Blass, F., Schroeter, A., Hartnack, S. & Bettschart-Wolfensberger, R. Systematic Review: Anesthetic Protocols and Management as Confounders in Rodent Blood Oxygen Level Dependent Functional Magnetic Resonance Imaging (BOLD fMRI)—Part B: Effects of Anesthetic Agents, Doses and Timing. Animals 11 (2021).
Zhao, F., Zhao, T., Zhou, L., Wu, Q. & Hu, X. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. Neuroimage 39, 248–260. https://doi.org/10.1016/j.neuroimage.2007.07.063 (2008).
Nasrallah, F. A., Tan, J. & Chuang, K. H. Pharmacological modulation of functional connectivity: alpha2-adrenergic receptor agonist alters synchrony but not neural activation. Neuroimage 60, 436–446. https://doi.org/10.1016/j.neuroimage.2011.12.026 (2012).
Sirmpilatze, N., Baudewig, J. & Boretius, S. Temporal stability of fMRI in medetomidine-anesthetized rats. Sci. Rep. 9, 16673. https://doi.org/10.1038/s41598-019-53144-y (2019).
Jonckers, E. et al. Different anesthesia regimes modulate the functional connectivity outcome in mice. Magn. Reson. Med. 72, 1103–1112. https://doi.org/10.1002/mrm.24990 (2014).
Yoshida, K. et al. Physiological effects of a habituation procedure for functional MRI in awake mice using a cryogenic radiofrequency probe. J. Neurosci. Methods. 274, 38–48. https://doi.org/10.1016/j.jneumeth.2016.09.013 (2016).
Ma, Y., Hamilton, C. & Zhang, N. Dynamic connectivity patterns in conscious and unconscious brain. Brain Connect. 7, 1–12. https://doi.org/10.1089/brain.2016.0464 (2017).
Brown, E. N., Purdon, P. L. & Van Dort, C. J. General anesthesia and altered States of arousal: a systems neuroscience analysis. Annu. Rev. Neurosci. 34, 601–628. https://doi.org/10.1146/annurev-neuro-060909-153200 (2011).
Mashour, G. A. Cognitive unbinding: a neuroscientific paradigm of general anesthesia and related States of unconsciousness. Neurosci. Biobehav Rev. 37, 2751–2759. https://doi.org/10.1016/j.neubiorev.2013.09.009 (2013).
Jhang, J. et al. Anterior cingulate cortex and its input to the basolateral amygdala control innate fear response. Nat. Commun. 9, 2744. https://doi.org/10.1038/s41467-018-05090-y (2018).
de Lima, M. A. X., Baldo, M. V. C., Oliveira, F. A. & Canteras, N. S. The anterior cingulate cortex and its role in controlling contextual fear memory to predatory threats. Elife 11 https://doi.org/10.7554/eLife.67007 (2022).
Heany, S. J. et al. Effects of testosterone administration on threat and escape anticipation in the orbitofrontal cortex. Psychoneuroendocrinology 96, 42–51. https://doi.org/10.1016/j.psyneuen.2018.05.038 (2018).
Hsieh, H. T. & Chang, C. H. Activation of medial orbitofrontal cortex abolishes fear extinction and interferes with fear expression in rats. Neurobiol. Learn. Mem. 169, 107170. https://doi.org/10.1016/j.nlm.2020.107170 (2020).
Lee, J. Y. et al. Role of anterior cingulate cortex inputs to periaqueductal gray for pain avoidance. Curr Biol 32, 2834–2847 e2835, (2022). https://doi.org/10.1016/j.cub.2022.04.090
Shih, C. W. & Chang, C. H. Medial or lateral orbitofrontal cortex activation during fear extinction differentially regulates fear renewal. Behav. Brain Res. 412, 113412. https://doi.org/10.1016/j.bbr.2021.113412 (2021).
Tao, D., He, Z., Lin, Y., Liu, C. & Tao, Q. Where does fear originate in the brain? A coordinate-based meta-analysis of explicit and implicit fear processing. Neuroimage 227, 117686. https://doi.org/10.1016/j.neuroimage.2020.117686 (2021).
To, X. V. & Nasrallah, F. A. A mice resting-state functional magnetic resonance imaging dataset on the effects of Medetomidine dosages and prior-stimulation on functional connectivity. Data Brief. 42, 108279 (2022).
Wang, Q. et al. The Allen Mouse Brain Common Coordinate Framework: A 3D Reference Atlas. Cell 181, 936–953 e920, (2020). https://doi.org/10.1016/j.cell.2020.04.007
To, X. V., Vegh, V. & Nasrallah, F. A. Towards data-driven group inferences of resting-state fMRI data in rodents: comparison of group ICA, GIG-ICA, and IVA-GL. J. Neurosci. Methods. 366, 109411 (2022).
Percie du Sert. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 18, e3000411. https://doi.org/10.1371/journal.pbio.3000411 (2020).
Health, N. & Council, M. R. Australian code of practice for the care and use of animals for scientific purposes. 8th ed. Australian Government, National Health and Medical Research Council, Canberra. https://doi.org/10.1111/j.1751-0813.1998.tb10161.x (2013)
Bortel, A., Pilgram, R., Yao, Z. S. & Shmuel, A. Dexmedetomidine - Commonly used in functional imaging Studies - Increases susceptibility to seizures in rats but not in wild type mice. Front. Neurosci. 14, 832. https://doi.org/10.3389/fnins.2020.00832 (2020).
Li, Q. et al. Resting-state functional MRI reveals altered brain connectivity and its correlation with motor dysfunction in a mouse model of huntington’s disease. Sci. Rep. 7, 16742. https://doi.org/10.1038/s41598-017-17026-5 (2017).
Ferrier, J., Tiran, E., Deffieux, T., Tanter, M. & Lenkei, Z. Functional imaging evidence for task-induced deactivation and Disconnection of a major default mode network hub in the mouse brain. Proc. Natl. Acad. Sci. U S A. 117, 15270–15280. https://doi.org/10.1073/pnas.1920475117 (2020).
Grandjean, J. et al. Common functional networks in the mouse brain revealed by multi-centre resting-state fMRI analysis. Neuroimage 205, 116278. https://doi.org/10.1016/j.neuroimage.2019.116278 (2020).
Whitesell, J. D. et al. Regional, Layer, and Cell-Type-Specific Connectivity of the Mouse Default Mode Network. Neuron 109, 545–559 e548, (2021). https://doi.org/10.1016/j.neuron.2020.11.011
Friston, K. J. et al. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain. Mapp. 2, 189–210 (1994).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 (Suppl 1), 208–219. https://doi.org/10.1016/j.neuroimage.2004.07.051 (2004).
Lee, D., Lee, Y., Lee, Y. & Kim, K. Functional connectivity in the mouse brainstem represents signs of recovery from concussion. J. Neurotrauma. 40, 240–249 (2023).
Niranjan, A., Christie, I. N., Solomon, S. G., Wells, J. A. & Lythgoe, M. F. fMRI mapping of the visual system in the mouse brain with interleaved snapshot GE-EPI. Neuroimage 139, 337–345. https://doi.org/10.1016/j.neuroimage.2016.06.015 (2016).
Grimm, C., Wenderoth, N. & Zerbi, V. An optimized protocol for assessing changes in mouse whole-brain activity using opto-fMRI. STAR. Protoc. 3, 101761. https://doi.org/10.1016/j.xpro.2022.101761 (2022).
van den Heuvel, M. P., Stam, C. J., Boersma, M. & Hulshoff Pol, H. E. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. Neuroimage 43, 528–539. https://doi.org/10.1016/j.neuroimage.2008.08.010 (2008).
Bernier, B. E. et al. Dentate gyrus contributes to retrieval as well as encoding: evidence from context fear conditioning, recall, and extinction. J. Neurosci. 37, 6359–6371. https://doi.org/10.1523/JNEUROSCI.3029-16.2017 (2017).
Lee, J. et al. Phospholipase C beta 1 in the dentate gyrus gates fear memory formation through regulation of neuronal excitability. Sci. Adv. 10, eadj4433. https://doi.org/10.1126/sciadv.adj4433 (2024).
Imamura, A. et al. Zero-echo time imaging achieves whole brain activity mapping without ventral signal loss in mice. Neuroimage 307, 121024. https://doi.org/10.1016/j.neuroimage.2025.121024 (2025).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069. https://doi.org/10.1016/j.neuroimage.2009.10.003 (2010).
Latora, V. & Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 87, 198701. https://doi.org/10.1103/PhysRevLett.87.198701 (2001).
Pertovaara, A., Kauppila, T., Jyvasjarvi, E. & Kalso, E. Involvement of supraspinal and spinal segmental alpha-2-adrenergic mechanisms in the medetomidine-induced antinociception. Neuroscience 44, 705–714. https://doi.org/10.1016/0306-4522(91)90089-7 (1991).
Shim, H. J. et al. Protocol for mouse optogenetic fMRI at ultrahigh magnetic fields. STAR. Protoc. 3, 101846. https://doi.org/10.1016/j.xpro.2022.101846 (2022).
Chen, H. F. et al. Generation of a whole-brain hemodynamic response function and sex-specific differences in cerebral processing of mechano-sensation in mice detected by BOLD fMRI. Front. Neurosci. 17, 1187328. https://doi.org/10.3389/fnins.2023.1187328 (2023).
Sinclair, M. D. A review of the physiological effects of alpha2-agonists related to the clinical use of Medetomidine in small animal practice. Can. Vet. J. 44, 885–897 (2003).
Weber, R., Ramos-Cabrer, P., Wiedermann, D., van Camp, N. & Hoehn, M. A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage 29, 1303–1310. https://doi.org/10.1016/j.neuroimage.2005.08.028 (2006).
Li, B. H., Lohmann, J. S., Schuler, H. G. & Cronin, A. J. Preservation of the cortical somatosensory-evoked potential during Dexmedetomidine infusion in rats. Anesth. Analg. 96, 1155–1160. https://doi.org/10.1213/01.ANE.0000053239.62623.32 (2003).
Grandjean, J., Schroeter, A., Batata, I. & Rudin, M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage 102 Pt. 2, 838–847. https://doi.org/10.1016/j.neuroimage.2014.08.043 (2014).
To, X. V., Vegh, V. & Nasrallah, F. A. Towards data-driven group inferences of resting-state fMRI data in rodents: comparison of group ICA, GIG-ICA, and IVA-GL. J. Neurosci. Methods. 366, 109411. https://doi.org/10.1016/j.jneumeth.2021.109411 (2022).
Flecknell, P. Laboratory Animal Anaesthesia (Academic, 2015).
To, X. V. & Nasrallah, F. A. A roadmap of brain recovery in a mouse model of concussion: insights from neuroimaging. Acta Neuropathol. Commun. 9 https://doi.org/10.1186/s40478-020-01098-y (2021).
Leinenga, G. et al. Scanning ultrasound-mediated memory and functional improvements do not require amyloid-beta reduction. Mol. Psychiatry. https://doi.org/10.1038/s41380-024-02509-5 (2024).
Frankland, P. W., Bontempi, B., Talton, L. E., Kaczmarek, L. & Silva, A. J. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304, 881–883. https://doi.org/10.1126/science.1094804 (2004).
Carrillo, M. et al. Emotional Mirror Neurons in the Rat’s Anterior Cingulate Cortex. Curr Biol 29, 1301–1312 e1306, (2019). https://doi.org/10.1016/j.cub.2019.03.024
Ortiz, S. et al. Anterior cingulate cortex and ventral hippocampal inputs to the basolateral amygdala selectively control generalized fear. J. Neurosci. 39, 6526–6539. https://doi.org/10.1523/JNEUROSCI.0810-19.2019 (2019).
Wu, K. et al. Distinct circuits in anterior cingulate cortex encode safety assessment and mediate flexibility of fear reactions. Neuron 111, 3650–3667 e3656, (2023). https://doi.org/10.1016/j.neuron.2023.08.008
Rolls, E. T. Emotion, motivation, decision-making, the orbitofrontal cortex, anterior cingulate cortex, and the amygdala. Brain Struct. Funct. 228, 1201–1257. https://doi.org/10.1007/s00429-023-02644-9 (2023).
Mobbs, D. et al. From threat to fear: the neural organization of defensive fear systems in humans. J. Neurosci. 29, 12236–12243. https://doi.org/10.1523/JNEUROSCI.2378-09.2009 (2009).
Smith, M. L., Asada, N. & Malenka, R. C. Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science 371, 153–159. https://doi.org/10.1126/science.abe3040 (2021).
Deyama, S., Nakagawa, T., Kaneko, S., Uehara, T. & Minami, M. Involvement of the bed nucleus of the stria terminalis in the negative affective component of visceral and somatic pain in rats. Behav. Brain Res. 176, 367–371. https://doi.org/10.1016/j.bbr.2006.10.021 (2007).
Lischinsky, J. E. & Lin, D. Looming danger: unraveling the circuitry for predator threats. Trends Neurosci. 42, 841–842. https://doi.org/10.1016/j.tins.2019.10.004 (2019).
Tang, C. Y. & Ramani, R. Functional connectivity and anesthesia. Int. Anesthesiol Clin. 54, 143–155. https://doi.org/10.1097/AIA.0000000000000083 (2016).
Chen, X. et al. Sensory evoked fMRI paradigms in awake mice. Neuroimage 204, 116242. https://doi.org/10.1016/j.neuroimage.2019.116242 (2020).
Tsurugizawa, T. et al. Awake functional MRI detects neural circuit dysfunction in a mouse model of autism. Sci. Adv. 6, eaav4520. https://doi.org/10.1126/sciadv.aav4520 (2020).
Gutierrez-Barragan, D. et al. Unique spatiotemporal fMRI dynamics in the awake mouse brain. Curr Biol 32, 631–644 e636, (2022). https://doi.org/10.1016/j.cub.2021.12.015
Funding
This research was supported by the KBRI basic research program through the Korea Brain Research Institute funded by the Ministry of Science and ICT (25-BR-03-01, 25-BR-03-02, 25-BR-08-01) and the Electronics and Telecommunications Research Institute (ETRI) grant funded by the Korean government (25YB1210, 25ZB1330).
Author information
Authors and Affiliations
Contributions
D.L. contributed to the conceptualization, formal analysis, methodology, funding acquisition, supervision, writing - original draft, writing - review & editing. D.Y.K. contributed to the investigation, writing - original draft, writing - review & editing. X.V.T. contributed to data curation, writing - review & editing. F.A.N. contributed to data curation, writing - review & editing. H-K.L. contributed to conceptualization, investigation, funding acquisition, supervision, writing - original draft, writing - review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, D., Kim, D.Y., To, X.V. et al. High-dose medetomidine increases functional connectivity in the fear-related regions after electrical stimulation. Sci Rep 15, 33264 (2025). https://doi.org/10.1038/s41598-025-18556-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18556-z