Abstract
This prospective study aimed to investigate colonization of Ureaplasma spp. in preterm infants to evaluate the relationship between Ureaplasma spp. colonization and BPD including other neonatal morbidities. A cohort of 357 preterm infants was examined for Ureaplasma spp. detection. Samples as nasopharyngeal swabs and gastric aspirates (non-intubated infants) whereas tracheal aspirates (intubated infants), were collected within 24 h of birth from infants with < 37 weeks gestational age (GA). Each sample was tested with culture and real-time polymerase chain reaction (RT-PCR) to confirm Ureaplasma spp. colonization. A total of 12 (3.3%) preterm infants (GA, 30.5 ± 2.2 weeks, P = 0.001; BW, 1,529 ± 501 g, P = 0.005) were positive for Ureaplasma spp. colonization. 23 (6.8%) preterm infants (GA, 28.7 ± 1.1 weeks, P = 0.001; BW, 1,234 ± 441 g, P = 0.005) developed BPD. Ureaplasma spp. colonization and BPD development showed an inverse relation with GA and birth weight (BW), and were associated with one antenatal factor (premature rupture of membranes; PROM), two natal factors (smaller GA, lower BW), one postnatal factor (longer use of supplemental oxygen), and p-value was significant for all factors (P < 0.05). Incidence of BPD in Ureaplasma spp. positive group was higher than in Ureaplasma spp. negative group (n = 3, 25% vs. n = 20, 6%, P = 0.001). Multivariate logistic regression analysis revealed a common correlation of Ureaplasma spp. colonization and BPD development with smaller GA (P = 0.012 & 0.001; 95%CI: 0.590–0.937 & 0.181–0.627), independent correlation of Ureaplasma spp. colonization with PROM (P = 0.002; 95%CI: 2.226–35.547) whereas independent correlation of BPD development with Ureaplasma spp. colonization (P = 0.037; 95%CI: 1.167–123.809) and more ventilator days (P = 0.003; 95%CI: 1.197–2.486). Ureaplasma spp. colonization is associated with PROM, smaller GA, and higher incidence of BPD. All preterm infants delivered by mothers with PROM and/or with smaller GAs should be investigated for Ureaplasma spp. colonization to anticipate BPD incidence.
Similar content being viewed by others
Introduction
A mycoplasma species known as Ureaplasma spp. does not possess a cell wall, hydrolyzes urea to produce ATP, and is biosynthetically limited. This mycoplasma species adheres to the genitourinary tract in adults and to the respiratory tract in neonates1. It is estimated that 40 to 80 percent of these organisms can be detected in the vaginal flora of healthy women and are capable of causing infertility, early pregnancy loss, premature birth, stillbirth, and adverse neonatal outcomes in their hosts2. There is a decreased risk of respiratory colonization with the increase in gestational age (GA)3. Ureaplasma spp. is associated with adverse pregnancy outcomes4, and common neonatal morbidities including respiratory distress syndrome (RDS)5, necrotizing enterocolitis (NEC)6, intraventricular hemorrhage (IVH)7 and bronchopulmonary dysplasia (BPD)8.
Bronchopulmonary dysplasia, which is also known as chronic lung disease (CLD) of infancy was described for the first time by Northway et al. in 19679. More than 15 million babies are born prematurely (< 37 weeks gestational age) each year, representing more than 1 in 10 births in the world, and approximately 2.4 million of these are born before 32 weeks of postmenstrual age (PMA)10. There is a greater risk of developing BPD and chronic respiratory morbidity among infants with extremely low gestational ages11.
In terms of prevalence, the incidence of BPD ranges from 5 to 68 percent as a most common disease among extremely preterm infants (born before 32 weeks gestational age) with the incidence increasing steadily as gestational age increases12,13. GA is inversely related with respiratory colonization8, and was found the most likely & strongest risk factor for BPD development14. But some investigators have reported contradictory results. So, there is still need for further surveys on this issue.
The aim of present study was therefore to determine the colonization rate of Ureaplasma spp. in preterm infants to evaluate its relationship with BPD including common neonatal morbidities and to assess the best source for sample collection, choice of the optimum diagnostic tool, and identification of antenatal, natal and postnatal risk factors in association with Ureaplasma spp. colonization and BPD development.
Materials and methods
Eligibility criteria
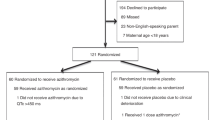
In this prospective study, a total of 357 preterm infants without any congenital anomaly except patent ductus arteriosus (PDA), below the GA of 37 weeks who were admitted to the neonatal intensive care unit no. 1 at The First Affiliated Hospital of Zhengzhou University between January 2022 and October 2023. All parents were informed about the study objectives and signed the respective consent forms. Informed consent was obtained from their legal guardian(s). Gastric aspirates and nasopharyngeal (NP) swabs (non-intubated infants) whereas tracheal aspirates (intubated infants) were obtained within 24 h of age, and all samples underwent culture as well as real-time polymerase chain reaction (RT-PCR) for the confirmation of Ureaplasma spp. colonization. Sterile saline (0.5 mL) was injected into the endotracheal tube and immediately aspirated back into the suction trap, and the procedure was repeated. Infants were placed into different groups on the basis of Ureaplasma spp. colonization and BPD development in order to make a comparison of characteristics and comorbid factors.
Diagnosis criteria for BPD
The need of supplemental oxygen or respiratory support at 28 postnatal days was the standard to diagnose BPD. Oxygen-dependence or respiratory support at 28 postnatal days is the definition to diagnose BPD and is proposed by 2018 National Institute of Child Health and Human Development (NICHD).
Culture and RT-PCR
Each specimen was collected in a specialized liquid transport system (growth medium for mycoplasmal organisms i.e. 10B broth), vortexed and 0.2 mL of each specimen was added to 1.8 mL of prepared 10B broth. Tubes were incubated at 37 °C. The inoculum was plated onto A8 agar if a color change occurred. A positive culture was defined as a positively colored broth (color change) confirmed by the presence of typical colony morphology on A8 agar.
A RT-PCR was conducted by diluting a fresh culture of Ureaplasma spp. in 10B broth, establishing the concentration of organisms in each dilution by measuring the colony-forming units and color-changing units (ccu) present. A parallel sample of each dilution was assayed by RT-PCR. Each dilution was centrifuged (12,000 g, 20 min, 4 °C) to pellet the cells, and the supernatant was removed. The cell pellet was then resuspended and processed. RT-PCR analysis was performed on 5 L of each dilution. As per standard procedures, all specimens were processed at The First Affiliated Hospital of Zhengzhou University.
Diagnosis criteria for clinical outcomes
Regarding comorbidities, the analysis encompassed respiratory distress syndrome (RDS) diagnosed through therapeutic surfactant treatment, patent ductus arteriosus (PDA), bronchopulmonary dysplasia (BPD) classified by the National Institute of Child Health and Human Development, necrotizing enterocolitis (NEC) identified by the modified Bell’s staging criteria, intraventricular hemorrhage (IVH) confirmed via brain ultrasonography or magnetic resonance imaging (MRI), and retinopathy of prematurity (ROP) classified according to the International Classification of Acute Retinopathy of Prematurity. Additional clinical variables being examined included the duration of ventilator and oxygen support, length of hospitalization, instances of preterm premature rupture of the membrane (defined as exceeding 18 h), utilization of antenatal steroids, pregnancy-induced hypertension (PIH), and various demographic variables.
Statistical methods
Statistical analyses were done with t test and Wilcoxon test for numerical data after checking the normality assumption with the Kolmogorov–Smirnov test. Categorical data was tested with Chi square using Yates correction and Fisher’s exact test as appropriate. Logistic Regression Analysis for the risk factors of UU colonization and BPD development. Analysis was done with SPSS version 16. A p value < 0.05 was considered statistically significant.
Results
Comparison of preterm infants’ characteristics and co-morbid factors between Ureaplasma spp. positive and Ureaplasma spp. negative groups
Twelve (3.3%) preterm infants (GA, 30.5 ± 2.2 wk, P = 0.001; BW, 1,529 ± 501 g, P = 0.005) were positive for Ureaplasma spp. infection, and 345 preterm infants (GA, 33.2 ± 2.4 wk; BW, 1,934 ± 601 g) were negative. There were significant differences of gestational ages (GA) and birth weights (BW) between the two groups. Ureaplasma spp. positive (UU+) infants in comparison with Ureaplasma spp. negative (UU–) infants were with smaller GA and lower BW (Table 1, Fig. 1A). Ureaplasma spp. positive (UU+) infants were under more days of oxygen than Ureaplasma spp. negative (UU–) infants (Table 1, Fig. 1A). The incidence of common neonatal illnesses such as RDS, PDA, NEC, ROP, and IVH were not significantly different between the two groups. BPD occurred more frequently in the (UU+) group than in the (UU–) group (Table 2, Fig. 1B). Ureaplasma spp. colonization was detected inversely related to the GA and BW. Antenatal factors like use of antenatal steroids, PROM and PIH were associated with Ureaplasma spp. colonization (Table 1, Fig. 1A). Multivariate logistic regression analysis revealed that risk factors related with Ureaplasma spp. colonization were smaller GA (P = 0.012; 95%CI: 0.590–0.937) and PROM (P = 0.002; 95%CI: 2.226–35.547) (Table 3).
Comparison of preterm infants’ characteristics and co-morbid factors between BPD positive and BPD negative groups
Regarding BPD development, out of 357 enrolled infants, 23 preterm infants (GA, 28.7 ± 1.1 wk, P = 0.001; BW, 1,234 ± 441 g, P = 0.005) developed BPD, and 334 preterm infants (GA, 33.4 ± 2.2 wk; BW, 1958 ± 578) didn’t develop BPD. Ureaplasma spp. colonization rate among BPD infants was higher than non-BPD infants. There were significant differences of gestational ages (GA) and birth weights (BW) between the two groups. BPD positive (BPD+) infants than BPD negative (BPD–) infants were with smaller GA and lower BW (Table 4, Fig. 1C). Infants in BPD+ group as compared to BPD− group showed significant differences in required number of supplemental oxygen days and number of ventilator days (Table 4, Fig. 1C). The incidence of common neonatal illnesses such as PDA and NEC were not significantly different between two study groups but occurrence rates of RDS, ROP and IVH were more frequent in the BPD (+) group than in the BPD (–) group (Table 5, Fig. 1D). BPD development was inversely related to GA and BW. Ureaplasma spp. colonization and antenatal factors like PROM and cesarean section (C-section) showed an association with BPD development (Table 4. Figure 1C). Logistic regression analysis revealed that risk factors related with BPD were smaller GA (P = 0.001; 95%CI: 0.181–0.627), Ureaplasma spp. Colonization (P = 0.037; 95%CI: 1.167–123.809), and ventilator days (P = 0.003; 95%CI: 1.197–2.486) (Table 6).
Discussion
The findings of this study point towards possible relationship between Ureaplasma spp. and BPD in preterm infants, this study emphasizes the importance of determining the best source for sample collection, choosing the most appropriate diagnostic tool, and identifying antenatal, natal, and postnatal risk factors in association with Ureaplasma spp. colonization and BPD development.
The present study confirms the previous observations15,16,17,18,19,20 that Ureaplasma spp. can be the pathogens to colonize the respiratory tract in preterm infants and can cause the development of bronchopulmonary dysplasia (BPD). Respiratory colonization rate and predominant role to cause BPD development by Ureaplasma spp. in preterm infants varies from study to study. Firstly, about colonization rate, different studies reported different incidence rate of Ureaplasma spp. colonization in newborn, 9 percent21, 14 percent22, 20 percent23, and 13 to 47 percent24. Gobec et al.25 reported that the average incidence of respiratory tract colonization in extremely low gestational age neonates (ELGANs) with Ureaplasma spp. was only 8.8% and suggested ELGANs should be screened soon after birth to reduce BPD incidence.
In present study, overall Ureaplasma spp. colonization rate was 3.3%, and colonization was associated with BPD development. Possibly, low colonization rates of only 3.3% and 8.8% are related to varied range of Ureaplasma species’ colonization rates in accordance with different areas geographically. Referring to predominance of Ureaplasma species to cause BPD development, few studies investigated about Ureaplasma species’ colonization in the respiratory tracts of preterm infants and observed that U. parvum was the predominant specie colonizing the respiratory tracts of preterm infants3,5,26. Another study from the University of Maryland confirmed that the U. parvum was the one most common specie detected in the samples not only from the tracheal aspirates but also from blood and CSF6. In comparison with these studies, Heggie et al.27 and Katz et al.26, found no differences between these two species, but in addition, Katz et al.26 found the higher rates of BPD for both species. Abele-Horne et al.28 found twofold higher rates for BPD in infants colonized with U. urealyticum than in the infants colonized with U. parvum and the same study also observed that U. urealyticum colonized infants were significantly less mature and had lower birth weight than the U. parvum colonized infants.
The samples used in the present study were gastric aspirates, NP swabs (non-intubated infants) and tracheal aspirates (intubated infants), and all samples underwent culture as well as RT-PCR for the confirmation of Ureaplasma spp. colonization. All the samples showing positive results for culture and/or RT-PCR were all NP swabs except for one positive sample, which was a gastric aspirate, and interestingly, this infant’s gastric aspirate was positive for Ureaplasma spp. colonization, whereas the NP swab was negative. Moreover, infants with confirmed Ureaplasma spp. colonization and BPD were positive for NP swabs but not for gastric aspirates and only one infant who was positive for gastric aspirate but not for NP swab developed necrotizing enterocolitis (NEC) rather than BPD. Nevertheless, this is only a finding in one infant, and even though it is not significant, it is still possible that the development of one organ system’s comorbid condition may be related to a colonization of that particular organ system without affecting any other organ system.
The diagnosis of infection with Ureaplasma spp. currently requires the use of culture, which is a costly procedure that requires special expertise and requires 2 to 5 days for results. To detect infections in newborns more rapidly, a more rapid diagnostic method is required. RT-PCR compared to culture showed better diagnostic potential to detect Ureapalsma spp.23,25,29,30,31, and infectious agents can be detected in < 24 h by specific amplification of genetic sequences by PCR32. So, faster detection of Ureaplasma spp. by PCR (< 24 h) compared to culture (2–5 days) will be particularly important in management of infants in whom this organism has been shown to be a significant cause of meningitis, respiratory disease, and death. It will also be useful to further establish the role of this organism in intra-amniotic infection and preterm delivery with this method of detection33. In present study, RT-PCR was found to be more sensitive than culture methods, and preterm infants with RT-PCR results from the NP source had negative cultures from the same source, confirming the enhanced sensitivity of RT-PCR in detecting Ureaplasma species. In order to minimize the risk of iatrogenic infection, it is therefore advised to use RT-PCR rather than culture to detect Ureaplasma species. Additionally, for the initial investigation, it is preferable to use a minimally invasive source (NP aspirate) rather than a highly invasive source (CSF, blood, tracheal, and gastric aspirates).
Several studies have shown that prenatal, natal and postnatal problems are associated with Ureaplasma spp. colonization34,35,36 and with the BPD development37,38,39,40. On one hand, in terms of Ureaplasma spp. colonization, present study and a study by Abele-Horne et al.28 found that there is significant association between Ureaplasma spp. colonization and less mature ages and lower birth weights of preterm infants. In a prospective study, it was observed that there is a decreased risk of Ureaplasma spp. respiratory colonization with the increase in gestational age (GA)3, and respiratory colonization rate is inversely proportional to the gestational age8, comparing these findings with the current study, Ureaplasma spp. colonization was more frequent in infants with smaller GA and lower BW. Furthermore, preterm infants born by mothers with premature rupture of membranes (PROM) were found having frequent respiratory colonization with Ureaplasma spp.23,41, and our study validates this observation.
On the other hand, the risk of BPD is inversely related with GA and BW13,42,43, and GA was found the most likely and strongest risk factor for BPD development28, present study also observed that BPD development showed an inverse association with smaller GA and lower BW, and GA was a risk factor for BPD in regression model. Moreover, present study and a study by Jo et al.44 reported similar results, suggesting that the infants delivered by C-section and with characteristics like smaller GA, lower BW are at risk to develop BPD and need more oxygen & ventilator days as postnatal problems. Few other studies have found that the rate of BPD is increasing due to the increased survival rate of preterm infants12,13,42,43,45, and it has also been clear that BPD survivors keep to suffer from the respiratory morbidities even in their later life46.
Common neonatal morbidities such as RDS, PDA, NEC, ROP and IVH were not associated with Ureaplasma spp. colonization by Eun et al.21, whereas RDS by Brown et al.47 and ROP by Zysman-Colman et al.48 were associated with BPD. In present study, Ureaplasma spp. colonization was associated only with BPD rather than any other neonatal morbidity, and RDS, ROP, IVH occurred more frequently in BPD+ group vs BPD− group.
This study is limited to a small sample size, single-centered study, a single-time point for Ureaplasma spp. colonization screening and limited generalizability of the findings.
Conclusions
-
1.
Ureaplasma spp. colonization is associated with a higher incidence of BPD than non-colonized infants, suggesting that this pathogen may contribute to BPD and needs to be addressed.
-
2.
Ureaplasma spp. colonization is linked with the use of antenatal steroid, PROM, PIH, smaller GA, and lower BW. Moreover, infants colonized with Ureaplasma spp. need longer use of supplemental oxygen and develop BPD more frequently.
-
3.
PROM, C-section, smaller GA, and lower BW are the factors associated with BPD. Infants with BPD require longer periods of supplemental oxygen and mechanical ventilation (MV). Furthermore, infants with BPD are at risk for RDS, ROP and IVH.
-
4.
There is a common correlation of Ureaplasma spp. colonization and BPD development with smaller GA, independent correlation of Ureaplasma spp. colonization with PROM whereas independent correlation of BPD development with Ureaplasma spp. colonization and more ventilator days.
-
5.
Finally, all preterm infants delivered by mothers with PROM and/or smaller GA should be investigated for Ureaplasma spp. colonization to anticipate the development of BPD. Despite the fact that these findings are quite logical and consistent with clinical practice, further studies are encouraged to gain a better understanding of these findings in relation to Ureaplasma spp. colonization and BPD.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- BPD:
-
Bronchopulmonary dysplasia
- BW:
-
Birth weight
- ELGANs:
-
Extremely low gestational age neonates
- GA:
-
Gestational age
- IVH:
-
Intraventricular hemorrhage
- MV:
-
Mechanical ventilation
- NIH:
-
National Institute of Health
- NEC:
-
Necrotizing enterocolitis
- NP:
-
Nasopharyngeal swab
- PDA:
-
Patent ductus arteriosus
- PMA:
-
Postmenstrual age
- PROM:
-
Premature rupture of membranes
- PIH:
-
Pregnancy-induced hypertension
- RDS:
-
Respiratory distress syndrome
- ROP:
-
Retinopathy of prematurity
- RT-PCR:
-
Real-time polymerase chain reaction
- Ureaplasma spp.:
-
Ureaplasma species
References
Waites, K. B. et al. Molecular methods for the detection of Mycoplasma and Ureaplasma infections in humans: A paper from the 2011 William Beaumont Hospital Symposium on molecular pathology. J. Mol. Diagn. 14, 437–450. https://doi.org/10.1016/j.jmoldx.2012.06.001 (2012).
Viscardi, R. M. Ureaplasma species: Role in diseases of prematurity. Clin. Perinatol. 37, 393–409. https://doi.org/10.1016/j.clp.2009.12.003 (2010).
Sung, T. J. et al. Frequency of Ureaplasma serovars in respiratory secretions of preterm infants at risk for bronchopulmonary dysplasia. Pediatr. Infect. Dis. J. 30, 379–383. https://doi.org/10.1097/INF.0b013e318202ac3a (2011).
Murtha, A. P. & Edwards, J. M. The role of Mycoplasma and Ureaplasma in adverse pregnancy outcomes. Obstet. Gynecol. Clin. 41, 615–627. https://doi.org/10.1016/j.ogc.2014.08.010 (2014).
Abele-Horn, M. et al. Vaginal Ureaplasma urealyticum colonization: Influence on pregnancy outcome and neonatal morbidity. Infection 25, 286–291. https://doi.org/10.1007/bf01720398 (1997).
Viscardi, R. M. et al. Incidence of invasive Ureaplasma in VLBW infants: Relationship to severe intraventricular hemorrhage. J. Perinatol. 28, 759–765. https://doi.org/10.1038/jp.2008.98 (2008).
Okogbule-Wonodi, A. C. et al. Necrotizing enterocolitis is associated with Ureaplasma colonization in preterm infants. Pediatr. Res. 69, 442–447. https://doi.org/10.1203/PDR.0b013e3182111827 (2011).
Lowe, J. et al. Association between pulmonary Ureaplasma colonization and bronchopulmonary dysplasia in preterm infants: Updated systematic review and meta-analysis. Pediatr. Infect. Dis. J. 33, 697–702. https://doi.org/10.1097/inf.0000000000000239 (2014).
Northway, W. H. Jr., Rosan, R. C. & Porter, D. Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 276, 357–368. https://doi.org/10.1056/nejm196702162760701 (1967).
World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth (World Health Organization, 2012).
Greenough, A. Long term respiratory outcomes of very premature birth (< 32 weeks). Semin. Fetal Neonatal Med. 17, 73–76 (2012).
Jensen, E. A. & Schmidt, B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res. A Clin. Mol. Teratol. 100, 145–157. https://doi.org/10.1002/bdra.23235 (2014).
Stoll, B. J. et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126, 443–456. https://doi.org/10.1542/peds.2009-2959 (2010).
Laughon, M. M. et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am. J. Respir. Crit. Care Med. 183, 1715–1722. https://doi.org/10.1164/rccm.201101-0055OC (2011).
Cassell, G. H. et al. Association of Ureaplasma urealyticum infection of the lower respiratory tract with chronic lung disease and death in very-low-birth-weight infants. Lancet 2, 240–245. https://doi.org/10.1016/s0140-6736(88)92536-6 (1988).
Wang, E. E. et al. Role of Ureaplasma urealyticum and other pathogens in the development of chronic lung disease of prematurity. Pediatr. Infect. Dis. J. 7, 547–551 (1988).
Wang, E. E., Ohlsson, A. & Kellner, J. D. Association of Ureaplasma urealyticum colonization with chronic lung disease of prematurity: Results of a meta analysis. J. Pediatr. 127, 640–644. https://doi.org/10.1016/s0022-3476(95)70130-3 (1995).
Baier, R. J., Loggins, J. & Kruger, T. E. Monocyte chemoattractant protein-1 and interleukin-8 are increased in bronchopulmonary dysplasia: relation to isolation of Ureaplasma urealyticum. J. Investig. Med. 49, 362–369. https://doi.org/10.2310/6650.2001.33902 (2001).
Waites, K. B., Crouse, D. T. & Cassell, G. H. Antibiotic susceptibilities and therapeutic options for Ureaplasma urealyticum infections in neonates. Pediatr. Infect. Dis. J. 11, 23–29. https://doi.org/10.1097/00006454-199201000-00007 (1992).
Patterson, A. M. et al. Ureaplasma urealyticum respiratory tract colonization is associated with an increase in interleukin 1-beta and tumor necrosis factor alpha relative to interleukin 6 in tracheal aspirates of preterm infants. Pediatr. Infect. Dis. J. 17, 321–328. https://doi.org/10.1097/00006454-199804000-00011 (1998).
Eun, H. S. et al. Serological investigation of Ureaplasma urealyticum in Korean preterm infants. Korean J. Pediatr. 56, 477–481. https://doi.org/10.3345/kjp.2013.56.11.477 (2013).
Sethi, S. et al. Isolation pattern and clinical outcome of genital mycoplasma in neonates from a tertiary care neonatal unit. J. Trop. Pediatr. 45, 143–145. https://doi.org/10.1093/tropej/45.3.143 (1999).
Pandey, A. et al. Clinical significance of airways colonization with Ureaplasma urealyticum in premature (< 34 wk) neonates. Indian J. Med. Res. 125, 679–684 (2007).
Paul, V. K. et al. Association of genital mycoplasma colonization with low birth weight. Int. J. Gynaecol. Obstet. 63, 109–114. https://doi.org/10.1016/s0020-7292(98)00135-0 (1998).
Gobec, K. et al. Association between colonization of the respiratory tract with Ureaplasma species and bronchopulmonary dysplasia in newborns with extremely low gestational age: A retrospective study. Croat. Med. J. 64, 75–83. https://doi.org/10.3325/cmj.2023.64.75 (2023).
Katz, B. et al. Characterization of ureaplasmas isolated from preterm infants with and without bronchopulmonary dysplasia. J. Clin. Microbiol. 43, 4852–4854. https://doi.org/10.1128/jcm.43.9.4852-4854.2005 (2005).
Heggie, A. D. et al. Identification and quantification of ureaplasmas colonizing the respiratory tract and assessment of their role in the development of chronic lung disease in preterm infants. Pediatr. Infect. Dis. J. 20, 854–859. https://doi.org/10.1097/00006454200109000-00006 (2001).
Abele-Horn, M. et al. Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetric patients, and gynecological patients with pelvic inflammatory disease. J. Clin. Microbiol. 35, 1199–1202. https://doi.org/10.1128/jcm.35.5.1199-1202.1997 (1997).
Dhawan, B. et al. Evaluation of the diagnostic efficacy of PCR for Ureaplasma urealyticum infection in Indian adults with symptoms of genital discharge. Jpn. J. Infect. Dis. 59, 57–58 (2006).
Stellrecht, K. A. et al. Comparison of multiplex PCR assay with culture for detection of genital mycoplasmas. J. Clin. Microbiol. 42, 1528–1533. https://doi.org/10.1128/jcm.42.4.1528-1533.2004 (2004).
Luki, N. et al. Comparison of polymerase chain reaction assay with culture for detection of genital mycoplasmas in perinatal infections. Eur. J. Clin. Microbiol. Infect. Dis. 17, 255–263. https://doi.org/10.1007/bf01699982 (1998).
Persing, D. H. Polymerase chain reaction: Trenches to benches. J. Clin. Microbiol. 29, 1281–1285. https://doi.org/10.1128/jcm.29.7.1281-1285.1991 (1991).
Blanchard, A. et al. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin. Infect. Dis. 17(Suppl 1), S148–S153. https://doi.org/10.1093/clinids/17.supplement_1.s148 (1993).
Kundsin, R. B. et al. Association of Ureaplasma urealyticum in the placenta with perinatal morbidity and mortality. N. Engl. J. Med. 310, 941–945. https://doi.org/10.1056/nejm198404123101502 (1984).
Eschenbach, D. A. Ureaplasma urealyticum and premature birth. Clin. Infect. Dis. 17(Suppl 1), S100–S106. https://doi.org/10.1093/clinids/17.supplement_1.s100 (1993).
Olomu, I. N. et al. Perinatal correlates of Ureaplasma urealyticum in placenta parenchyma of singleton pregnancies that end before 28 weeks of gestation. Pediatrics 123, 1329–1336. https://doi.org/10.1542/peds.2008-1113 (2009).
Speer, C. P. Chorioamnionitis, postnatal factors and proinflammatory response in the pathogenetic sequence of bronchopulmonary dysplasia. Neonatology 95, 353–361. https://doi.org/10.1159/000209301 (2009).
Gantar, I. Š et al. Prenatal and postnatal risk factors for developing bronchopulmonary dysplasia. Signa Vitae 6, 46–51 (2011).
Lima, M. R. et al. Influence of maternal and neonatal factors on bronchopulmonary dysplasia development. Rev. Assoc. Med. Bras. 57, 391–396. https://doi.org/10.1590/s0104-42302011000400012 (2011).
Hislop, A. A. Bronchopulmonary dysplasia: prenatal and postnatal influences. Pediatr. Pulmonol. 23, 107–109 (2001).
Saxén, H. et al. Chronic lung disease of preterm infants in Finland is not associated with Ureaplasma urealyticum colonization. Acta Paediatr. 82, 198–201. https://doi.org/10.1111/j.1651-2227.1993.tb12638.x (1993).
Ancel, P. Y. et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: Results of the EPIPAGE-2 cohort study. JAMA Pediatr. 169, 230–238. https://doi.org/10.1001/jamapediatrics.2014.3351 (2015).
Isayama, T. et al. Comparison of mortality and morbidity of very low birth weight infants between Canada and Japan. Pediatrics 130, e957–e965. https://doi.org/10.1001/jamapediatrics.2014.3351 (2012).
Jo, H. S. et al. Recent changes in the incidence of bronchopulmonary dysplasia among very-low-birth-weight infants in Korea. J. Korean Med. Sci. 30(Suppl 1), S81–S87. https://doi.org/10.3346/jkms.2015.30.S1.S81 (2015).
de Kleine, M. J. et al. Lower mortality but higher neonatal morbidity over a decade in very preterm infants. Paediatr. Perinat. Epidemiol. 21, 15–25. https://doi.org/10.1111/j.1365-3016.2007.00780.x (2007).
Islam, J. Y. et al. Understanding the short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 192, 134–156. https://doi.org/10.1164/rccm.201412-2142PP (2015).
Brown, E. R., Stark, A. & Sosenko, I. Bronchopulmonary dysplasia: Possible relationship to pulmonary edema. J Pediatr 92, 982–984 (1978).
Zysman-Colman, Z. et al. Bronchopulmonary dysplasia—trends over three decades. Paediatr. Child Health 18, 86–90. https://doi.org/10.1093/pch/18.2.86 (2013).
Funding
This work was supported by Department of Neonatology, The First Affiliated Hospital of Zhengzhou University.
Author information
Authors and Affiliations
Contributions
Study conception and design: H.M.S. Analysis and data interpretation: Q.H., J.Z. and Y.D. Drafting of the manuscript: H.M.S. Substantial revision: H.C., M.A.A., J.C., J.L. Critical revision: X.C. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
This study was approved by the ethical committee of The First Affiliated Hospital of Zhengzhou University with ethical number (2022-KY-0096-002). All methods were performed in accordance with the ethical standards as laid down in the Declaration of Helsinki and its later amendments or comparable ethical standards. All parents were informed about the study objectives and signed the respective consent forms. Informed consent was obtained from their legal guardian(s).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sarwar, H.M.S., Hao, Q., Zhang, J. et al. Clinical significance of Ureaplasma species in bronchopulmonary dysplasia development in preterm infants. Sci Rep 15, 33683 (2025). https://doi.org/10.1038/s41598-025-18692-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18692-6