Abstract
Vitamin A (VA) supplementation has been recommended for pregnant women at-risk of deficiency, and is widely applied to children under 5 in developing countries. We addressed the effect of excessive VA exposure during pregnancy, lactation and first weeks of life on VA, vitamin E and lipid metabolism in rat offspring. Sprague-Dawley female rats were fed either a 2300 IU.kg−1 VA diet (control diet) or a 9858 IU.kg−1 VA diet (vitamin A supplemented diet – VAS diet) and mated with males fed the control diet. Weanlings were fed the same diet as their mothers until they were 8 weeks old. Organs and adipose tissue were collected from half of the offspring after fasting. The other half was force-fed an oil-in-water emulsion containing retinyl palmitate and [1,1,1-13C3]triolein. VAS-fed female and male offspring displayed a significant accumulation of VA in the liver (18 and 32 times more retinyl esters than respective controls, p < 0.0001) and VAS-fed males exhibited higher plasma retinol concentrations (+ 42.8 ± 6.6% compared to control males, p = 0.0011). Fasting plasma triglyceride concentration was higher for both VAS-fed females and males (+ 44.6 ± 6.8%, p = 0.0007 and + 58.8 ± 20.2%, p = 0.0343, compared to their respective controls). Lipid absorption was increased in VAS-fed males (+ 60.9% of [1-13 C]oleate absorption at 1.5 h post-gavage, p < 0.0001). Overall, our data show that VA overexposure disbalances VA status and detrimentally impacts lipid metabolism in offspring.
Similar content being viewed by others
Introduction
VA gathers a family of organic molecules (retinol, retinoic acid and retinal mainly) displaying different biologic activities. VA plays key roles in embryo development, immunity, energy metabolism and vision1. As humans cannot synthetize VA endogenously, it should be provided through the diet. Animal-based foods provide VA as retinyl esters (mainly retinyl palmitate). Plant-based foods provide VA as provitamin A carotenoids (mainly β-carotene, α-carotene and β-cryptoxanthin), which can be metabolized into retinol and derivatives by the human body2.
Between the end of the 20th and the beginning of the 21st century, around 19 million pregnant women and 30% of children aged between 6 and 59 months in Africa and Southeast Asia regions were affected by VA deficiency3,4. This widespread deficiency, primarily caused by malnutrition, leads to a range of serious consequences including night blindness, vision loss and immune system impairments5. The World Health Organization thus recommended a VA supplementation not exceeding 10,000 IU daily or 25,000 IU weekly for pregnant or lactating women in areas where VA deficiency is a severe public health problem6, and up to 200,000 IU every 4–6 months in children7 in regions at risk of deficiency. Recent data confirmed the benefits of VA supplementation, not just in the immediate postpartum period but also when administrated before and/or during pregnancy8,9. However, these guidelines are accompanied by warnings about potential toxicity. Rothman and colleagues reported that 1 out 57 babies had a malformation attributable to a supplementation of more than 10,000 IU of VA per day during pregnancy10. In an observational study, women in the highest quartile of fish oil intake (≥ 11 mL/day), consuming three times the recommended dietary allowance of VA, gave birth to infants with smaller head circumferences and shorter body lengths compared to women with lower intake11. Moreover, excessive maternal VA has been associated with altered offspring metabolism. For instance, a study in Old World monkeys demonstrated that excessive VA increased fetal hepatic retinyl esters storage12. Interestingly, recent findings suggested that both VA and retinoids are transported across the placenta by specific proteins, some of which are also involved in lipoprotein uptake. The expression of these proteins is influenced by the mother’s VA status and dietary intake, creating feedback signals that regulate the uptake of retinoids and potentially the uptake of lipids. This raises the possibility of an interplay between micronutrient and macronutrient metabolism13. Finally, VA has been shown to impair vitamin E intestinal uptake in vitro14and elevated VA intake reduced plasma α-tocopherol levels in rats15,16. Vitamin E plays key roles in the embryo implantation, placental maturation and provides protection against oxidative stress to the fetus and later to the newborn17. Vitamin E is also essential for neonate neurodevelopment18.
Overall, the metabolic consequences of early-life VA overexposure remain insufficiently understood. Therefore, the aim of this study was to determine whether excess VA during pregnancy, lactation and early postnatal development could alter vitamin E status and vitamin A and lipid metabolism in young rats.
Materials and methods
Animal study (flowchart, Fig. 1)
Animals
Animal experiments were conducted in accordance with the European Union Directive 2010/63/EU on the protection of animals used for scientific purposes, in compliance with the ARRIVE guidelines. All experimental procedures were approved by the Ethics Committee of Marseille (France), under agreement number D 12-055-20. Experiments were performed on Sprague-Dawley rats, as this strain has previously been used by our team19,20,21 as well as by other teams22to study VA metabolism. Rats were also preferred to mice because of their larger size, giving us sufficient material for analysis. The methodology was adapted from Antoine et al.19, and the study design is presented in Fig. 1.
16 male Sprague Dawley rats and 24 female Sprague Dawley rats aged 10 weeks (Janvier, Le Genest St Isle, France) were housed under controlled temperature (22–24 °C) and 12-h light/dark cycles (lights on at 8 am), with 3 or 4 animals per litter. Breeding pellets and tap water were provided ad libitum to the animals. One group of 12 females received a control diet (Catalog number #181780-203) containing 2300 IU.kg−1 of VA. The other group of 12 females received VA-supplemented (VAS) diet (Catalog number #1817179-203) containing 9858 IU.kg−1 VA (4-fold excess - still considered a nutritional dose). Both diets were based on the 58M1 - AIN-93M Maintenance Purified Diet developed by Test Diet Limited (London, UK). Diets were isocaloric and contained an equivalent amount of vitamin E (81.6 IU.kg⁻¹). All males were given control diet. After 8 weeks, virgin females were mated (one female with one male). During mating and until the end of lactation period, females were fed either similar control or VAS-diet, both reformulated with macronutrients adapted to gestational requirements (Catalog numbers 1817182-203 and 1817183-203, respectively). During the mating period, males were fed same diet as their female partners. To prevent diet-induced postnatal programming23 litter sizes were adjusted to 10–12 pups on postnatal day 3. Twenty-one days after birth, young rats were weaned. Weanlings were fed same diet as their mothers. At 6 weeks of age, some young rats were randomly selected for this study (control group: n = 14 males and 13 females, and VAS diet-fed group: n = 12 males and 12 females). Other young rats were used in another study20. Selected young rats were weighted, their glycemia was measured and an intraperitoneal glucose tolerance test was performed as described elsewhere19,23,24.
Organ and adipose tissue collection after fasting
At 8 weeks of age, half the young rats (control group: n = 7 males and 6 females, and VAS diet-fed group: n = 6 males and 6 females) were fasted overnight prior to tail-tip blood sampling, which was performed under light isoflurane anesthesia. Subsequently, the animals were euthanized by cervical dislocation under general anesthesia with sevoflurane (8%) before collecting their brain, left kidney, liver, intestine, heart, gastrocnemius muscle, white (i.e., epididymal) adipose tissue (WAT) and brown adipose tissue (BAT). As periovarian adipose tissue was very small in some young females, it was not analyzed further. Brain, kidney, heart and muscle were weighted to evaluate putative developmental issues. Liver and adipose tissue were weighted before further analyses. Intestine was thoroughly rinsed with ice-cold PBS and cut in 3 equal-length segments corresponding to duodenum, jejunum and ileum. All samples were stored in liquid nitrogen until lipid/vitamin analysis.
Postprandial experiments
At 8 weeks of age, the other half of the young rats (control group: n = 7 males and 7 females, and VAS diet-fed group: n = 6 males and 6 females) were fasted overnight and forced-fed 1 mL of an oil-in-water emulsion. This emulsion was made of triolein and an aqueous solution containing 1.4% bovine serum albumin (1:2, v: v), and was supplemented with 3 mg of retinyl palmitate and 2 mg of [1,1,1-13C3]triolein (Sigma Aldrich, Saint-Quentin-Fallavier, France). The mixture was sonicated in a sonication bath (Branson 3510, Branson, Danbury, CO, USA) for 15 min and used for force-feeding within 10 min. Every 90 min for 6 h after force-feeding, blood samples from a tail nick were harvested under light isoflurane anesthesia, poured in tubes containing 50 µL of a 0.109 M sodium citrate solution and immediately centrifuged at 1400 g for 20 min at 4 °C. Plasma was recovered and immediately frozen at − 80 °C.
Six hours after force-feeding, rats were euthanized, and their liver and intestine were promptly harvested and processed as mentioned in the section “Organ and adipose tissue collection after fasting”.
Intestine and plasma lipid content
Intestine segments (i.e., duodenum, jejunum and ileum; ~100 mg) were first homogenized in 800 µL of PBS with two 3 mm-diameter stainless steel balls in 2 ml Eppendorf tubes using a MM301 ball mill (Retsch, Haan, Germany). Lipids were then extracted by the Bligh and Dyer method25and samples were resuspended in isopropanol. Total cholesterol, phospholipid and triglyceride concentrations from plasma and intestine samples were then measured using kits from Biolabo (Maisy, France) according to the manufacturer’s instructions.
VA and E extraction and HPLC analysis
VA (retinol and/or retinyl esters) and vitamin E (α-tocopherol) from plasma (100 µL) and organ samples (~ 20 mg for liver samples and 40 mg for intestine fragments suspended in PBS) were extracted as previously described19using 5-apo-8’-carotenal as an internal standard.
The HPLC system was composed of a LC-20ADSP HPLC Pumps, SIL-20CHT Autosampler and SPD-M20A photodiode array detector (Shimadzu, Champs-sur-Marne, France). Vitamins A, E and 5-apo-8’-carotenal were detected at 325, 290 and 450 nm, respectively. These components were separated using a 250 × 4.6 nm, 5 μm Zorbax Eclipse XDB-C18 column maintained at 35 °C. The mobile phase was made of acetonitrile: dichloromethane: methanol (70:20:10; v: v:v) and the flow rate was 1.8 ml.min−1. Retinol, retinyl palmitate and α-tocopherol were identified by retention time compared with pure (> 95%) standards. Quantification was performed using Chromeleon software (version 6.8) by comparing peak area with standard reference curves. Retinyl esters were identified by their retention times and quantified based on their peak area, adjusted with their molecular extinction coefficients relative to that of retinyl palmitate. Postprandial retinyl ester responses were expressed as area under the curve (AUC) of their plasma concentrations over 6 h. Postprandial retinol responses were calculated by subtracting plasma retinol concentrations after fasting and were expressed as incremental AUC.
[1-13 C]oleate analysis by gas chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS)
Glyceryl triheptadecanoate (C17:0, Sigma Aldrich, Saint-Quentin-Fallavier, France) was used as an internal standard to quantify oleic acid occurring in plasma total lipids (250 ng of C17:0.µL−1) and tissue lipids (7.2 µg.mg−1 of liver and duodenum, 0.5–12 µg.mg−1 of ileum and 5–11 µg.mg−1 of jejunum).
Oleic acid amounts and isotopic enrichments in plasma and tissues were determined as described previously19. Briefly, plasma samples were submitted to direct methylation while for tissue samples (duodenum, jejunum, ileum, liver) a Folch extraction was performed prior to methylation. The total lipid fatty acid methyl esters were then measured using GC-C-IRMS (Isoprime Ltd; Elementar, Lyon, France) with a capillary GC column SP2380 (30 m x 0.25 mm x 0.20 film thickness; Supelco; Sigma-Aldrich; Saint-Quentin-Fallavier, France).
[1–13 C]oleate postprandial response was expressed in concentration (µg/g of tissue and µmol/L of plasma) and as the AUC of [1–13 C]oleate plasma concentrations over 6 h.
Statistical analysis
Results were expressed as mean ± SEM (standard error of the mean). The trapezoidal approximation method was used to calculate all AUC for postprandial concentrations. Differences between control and VAS groups were analyzed by Student’s t test. Beforehand, normality and homoscedasticity were tested by Shapiro-Wilk and F-test respectively. If needed, data were transformed using logarithmic or square root functions and/or Welch’s correction were applied to comply Student’s test conditions. Values of p < 0.05 were considered significant. All statistical analyses were performed using Prism (GraphPad Prism version 8.4.3).
Results
VAS diet modified organ weights and female glucose tolerance
VAS diet didn’t significantly affect total body weight of young rats (Table 1) but it impacted both female and male muscle masses compared to their sex-related control groups as shown in Table 1 (p = 0.0373 and p = 0.0002, for control vs. VAS diets in females and males, respectively). Male left kidney weights were also slightly (−8.3%) but significantly affected (p = 0.0228). Furthermore, VAS diet-fed males presented lighter WAT and heavier BAT than control males (−55.0 ± 6.0%, p = 0.0005 and + 63.5 ± 15.0%, p = 0.0262, respectively).
VAS diet didn’t significantly affect glycemia of young rats (Fig. 2A and D). However, VAS diet-fed females, but not males, displayed a weakened glucose tolerance through time (at 30 min: 170.3 ± 6.4 vs. 147.6 ± 5.3, p = 0.012 and at 120 min: 110.5 ± 3.7 vs. 96.7 ± 2.9 mg.dL−1, p = 0.0075 for VAS and control diet for females respectively; Fig. 2B and E). This response resulted in a higher total glycemia during glucose tolerance test for VAS-fed females (278.7 ± 8.1 vs. 244.6 ± 5.2 mg.h.dL−1 for VAS and control diet respectively; Fig. 2C). No effect on male total glycemia was observed (Fig. 2F).
Young rats glycemia after fasting. Young rats fed either control or VAS diets were tested for their fasting glycemia and glucose tolerance by glucose tolerance test A, D) Fasting glycemia (mg.dL−1); B, C) Glycemia response to glucose tolerance test (mg.dL−1); C, F) Glucose tolerance expressed as the AUC of the glucose tolerance test (mg.h.dL−1), for females and males, respectively. Data are expressed as mean ± SEM for control rats (females n = 13; males n = 14; white symbols) and VAS diet-fed rats (females n = 12; males n = 12; black symbols). Asterisks indicate significant differences between the control and VAS groups: *p < 0.05; **p < 0.01.
VAS diet induced a greater VA status and liver storage
Both female and male young rats from VAS diet-fed groups stored greater amounts of retinol and retinyl esters in their liver (22.0 ± 2.6 vs. 2.4 ± 0.4, p = 0.0006 and 367.9 ± 17.7 vs. 20.4 ± 3.8 nmol.g−1 p < 0.0001, for retinol and retinyl esters in females, respectively and 5.0 ± 0.5 vs. 0.3 ± 0.1, p = 0.0002 and 256.7 ± 17.4 vs. 7.8 ± 2.0 nmol.g−1, p < 0.0001 for retinol and retinyl esters in males, respectively; Fig. 3B-C and G-H). Fasting retinolemia was also significantly superior for male young rats fed VAS-diet compared to males fed control diet (+ 42.8 ± 6.6%, p = 0.0011; Fig. 3F). Same tendency was observed for females, although the difference was not significant (Fig. 3A).
Vitamin status in liver and plasma of fasting young rats. Fasting plasma samples were drawn from the tips of young rat tails. After euthanasia, liver was collected. Retinol, retinyl esters and α-tocopherol were measured by HPLC. A-F) Retinolemia (µmol.L−1); B-G) Liver retinol concentrations (µmol.g−1); C-H) Liver retinyl ester concentrations (µmol.g−1); D-I) Plasma α-tocopherol concentrations (µmol.L−1) E-J) Liver α-tocopherol concentrations (µmol.g−1), for females and males, respectively. Data are expressed as mean ± SEM for control rats (females n = 7; males n = 7; white symbols) and VAS diet-fed rats (females n = 6; males n = 6; black symbols). Asterisks indicate significant differences between the control and VAS groups: *p < 0.05; **p < 0.01; ***p < 0.001; **** p < 0.0001.
The VAS diet affected neither plasma nor liver α-tocopherol concentrations (Fig. 3D-E and I-J).
VAS diet-fed offspring displayed changes in plasma and hepatic lipids
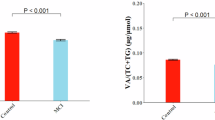
Both female and male young rats from VAS diet-fed groups showed an increase in fasting plasma triglycerides (+ 44.6 ± 6.8%, p = 0.0007 and + 58.8 ± 20.2%, p = 0.0343 for females and males, respectively; Fig. 4A and G). Females also displayed a significant decrease in plasma cholesterol (−21.2 ± 6.5%, p = 0.0337, Fig. 4C and I). No difference was found for phospholipids (Fig. 4E and K).
Lipid status in liver and plasma of fasting young rats. Fasting plasma samples were drawn from the tips of overnight-fasted young rat tails. After euthanasia, liver was collected. Total cholesterol, phospholipid and triglyceride plasma and tissue contents were then measured. A-G) Triglyceride; C-I) Cholesterol; E-K) Phospholipid plasma concentrations (g.L−1) for females and males, respectively. B-H) Triglyceride; D-J) Cholesterol; F-L) Phospholipid hepatic concentrations (mg.g−1); for females and males, respectively. Data are expressed as mean ± SEM for control rats (females n = 7; males n = 7; white symbols) and VAS diet-fed rats (females n = 6; males n = 6; black symbols). Asterisks indicate significant differences between the control and VAS groups: *p < 0.05; **p < 0.01; ***p < 0.001.
In the liver, VAS diet-fed female young rats had a significant reduction of cholesterol and phospholipids concentrations (−21.7 ± 3.0%, p = 0.0246 and − 18.0 ± 3.0%, p = 0.0086 for cholesterol and phospholipids, respectively; Fig. 4D and F), while triglyceride concentrations were not significantly different (Fig. 4B). VAS-fed males showed significantly lower hepatic triglycerides than controls (−81.0 ± 6.0%, p = 0.0103; Fig. 4H). Diet impacted neither hepatic cholesterol nor phospholipids in males (Fig. 4J and L).
VAS diet induced a different response pattern to an oral dose of VA in male young rats
After force-feeding an emulsion containing retinyl palmitate, no significant difference in the postprandial plasma total retinyl ester response, expressed as AUC, was observed for both sexes (Fig. 5B and G). Similarly, the variation in retinolemia during the postprandial period did not differ significantly between the two groups (Fig. 5D and I).
Postprandial plasma responses and final intestinal fragment concentrations of vitamin A in young rats. Young rats were administered an oily emulsion containing retinyl palmitate and [1,1,1-13C3]triolein via force-feeding. Blood samples were collected prior to feeding and every 90 min thereafter from a tail nick. Intestinal fragments were collected after euthanasia. Retinol, retinyl esters were extracted with hexane and quantified by HPLC. A, F) Postprandial plasma retinyl ester concentrations (µmol.L−1); B, G) Postprandial plasma retinyl ester response expressed as area under the curve (AUC) (µmol.h.L−1); C, H) Postprandial plasma retinol responses calculated by subtracting fasting plasma retinol concentrations (Δ retinol) (µmol.L−1); D, I) Δ retinol expressed as area under the curve (AUC) (µmol.h.L−1); E, J) Duodenal, jejunal and ileal vitamin A concentrations (nmol.g−1), in females and males, respectively. Data are expressed as mean ± SEM for control rats (females n = 7; males n = 7; white bars and dashed bars for retinyl esters and retinol respectively) and VAS diet-fed rats (females n = 6; males n = 6; black bars and bold dashed bars for retinyl esters and retinol respectively). Asterisks indicate significant differences in retinol levels between the control and VAS groups: *p < 0.05; **p < 0.01. Hashes indicate significant differences in retinyl ester levels between the control and VAS groups: #p < 0.05; ###p < 0.001.
Females from both VAS- and control-diet groups displayed similar pattern and concentration responses for free and esterified VA forms (Fig. 5A and C). However, a significant difference in the plasma retinyl ester concentrations at 6 h and a tendency toward earlier retinol peak was observed for VAS-fed females (p = 0.1005).
VAS-fed males had a similar retinyl ester response pattern and even though not significant, the curve and therefore the AUC for VAS group tended to be superior (9.1 ± 0.8 vs. 12.3 ± 1.7 µmol.L−1 for control and VAS group, respectively, p = 0.0884, Fig. 5F-G). Postprandial retinol also peaked significantly earlier in VAS-fed males (p = 0.0009; Fig. 5H).
Finally, for both sexes, duodenal retinyl ester concentrations were significantly lower in the control groups than in their VAS-fed counterparts (21.1 ± 2.9 vs. 42.0 ± 3.3 nmol.g−1, p = 0.0005 and 14.0 ± 2.5 vs. 23.8 ± 3.5 nmol.g−1, p = 0.0412, respectively; Fig. 5E and J). Retinol concentration was significantly lower in male ileum of VAS group (−58.1 ± 6.2%, p = 0.0302; Fig. 5J).
VAS diet induced a modification in lipid metabolism in the intestine
After fasting, VAS diet induced globally lower concentrations of lipids in the intestine for both sexes (Table 2). These reductions were significant for both male and female phospholipid concentrations in each intestinal fractions from − 30.0 ± 2.0%, p = 0.006, to −73.2 ± 1.8%, p < 0.0001 for female ileum and male duodenum, respectively. Furthermore, for both sexes, triglyceride concentrations in the duodenum were significantly lower (p = 0.0491 for females and p = 0.0034 for males; Table 2).
At the end of the postprandial period, except in the female ileum, cholesterol concentrations for control groups were lower in the duodenum, jejunum and ileum compared to VAS groups, for males (p < 0.0001 for both duodenum and jejunum and p = 0.0133 for the ileum; Table 2) and females (p = 0.0004 for the duodenum and p = 0.024 for the jejunum; Table 2), respectively. Moreover, triglyceride concentration in the duodenum of VAS-fed young male but not female rats remained lower than in the control groups (p = 0.0305; Table 2). However, it should be noticed that the control group was very heterogenous. Conversely, VAS-fed males had higher levels of ileal triglycerides and phospholipids compared to controls (+ 1386.7 ± 155.4%, p = 0.014 and + 89.5 ± 17.1%, p = 0.0004, respectively).
[1-13 C]oleate postprandial response was partially increased in VAS-fed male offspring
Postprandial [1,1,1-13 C]triolein metabolism was assessed in males only, as a greater impact of VA on plasma and intestinal triglycerides was found in this study, as well as in a previous work19, for this sex. [1-13 C]oleate concentration was significantly higher in the duodenum (+ 137.9 ± 40.1%, p = 0.0081; Fig. 6A) and in the ileum (+ 983.2 ± 349.2%, p = 0.0372) of VAS-fed young males compared to the control males.
Postprandial plasma and tissue [1–13 C]oleate concentrations in male young rats. Young rats were force-fed an oily emulsion containing retinyl palmitate and [1,1,1-13C3]triolein, then euthanized after a 6 h postprandial phase. Blood, duodenal, jejunal and ileal samples, as well as liver samples, were collected. [1-13 C]oleate originating from the administrated labelled triolein was determined by GC-C-IRMS analysis. A) Intestinal concentrations at 6 h (µg.g−1); B) Plasma concentrations during postprandial phase (µmol.L−1); C) Postprandial responses expressed as area under the curve (AUC) (µmol.h.L−1); D) Liver concentrations at 6 h (µg.g−1). Data are expressed as mean ± SEM for control rats (n = 7; white symbols) and VAS diet-fed rats (n = 6; black symbols). Asterisks indicate significant differences between the control and VAS groups: *p < 0.05; **p < 0.01; **** p < 0.000.
Plasma postprandial [1-13 C]oleate concentrations also exceeded those of the control group with the superiority being significant only 1.5 h after force-feeding (2.3 ± 0.4 vs. 3.7 ± 0.3 µmol.L−1, p = 0.0257, for control and VAS-fed male groups, respectively; Fig. 6B-C).
Concomitantly, liver [1–13 C]oleate concentration of VAS male group was higher than that of control group (+ 237.8 ± 30.1 µg.g−1, p < 0.0001; Fig. 6D).
Discussion
VA chronic exposure through maternal supplementation can lead to teratogenic effects in the offspring, when the intake of vitamin A exceeds the critical threshold of 10,000 IU of preformed vitamin A per day10. However, VA excess, even below teratogenic levels, may still cause adverse effects in the offspring11. We thus assessed the effects of chronic high-dose VA supplementation, below teratogenic threshold26, during the perinatal period on VA, vitamin E and lipid metabolism in rat offspring. In this context, mother rats were fed either a VAS or a control diet before and during mating, gestation and lactation phases. Their offspring were then fed the same diets for up to 8 weeks, a critical period for development27 during which VA supplementation is still recommended7.
We first evaluated whether the VAS diet altered VA status in young rats. This status was assessed by two main parameters: the liver VA stores, which account for ~ 80% of total body reserves28, and fasting retinolemia. As expected, hepatic retinyl ester and retinol contents were significantly higher in VAS-fed animals. Despite the tight regulation of retinolemia, plasma retinol levels were elevated for male VAS-fed rats. We hypothesize that females have a greater hepatic VA storage capacity, which would buffer the increase in circulating retinol. Consistently with previous observations in humans and more recently in rats, females tend to exhibit lower retinolemia than males19,20,29. A recent study suggested that hepatic Rbp4 mRNA level could contribute to sex differences in blood retinol concentrations, through a sex hormone-independent mechanism30.
We then evaluated the impact of vitamin A excess on offspring organ weight, lipid distribution and vitamin E status. It was previously shown that VA excess could affect both hepatic and plasma lipid contents31. In particular, it has been suggested that VA excess induced hepatic accumulation of triglycerides, phospholipids and cholesterol through mobilization of fatty acid from adipose tissue31,32. Moreover, is was questioned whether VA excess could lead to hepatic stellate cell lipidosis and liver fibrosis33. Here, although liver weight was not impacted for both sexes, hepatic lipid composition was differently modulated in response to vitamin A excess. VAS-diet induced a significant reduction of hepatic (and plasma) cholesterol as well as hepatic phospholipids in female offsprings. For males, only the triglyceride content was decreased. These observations contrast with a previous study reporting induction of fatty liver by excess vitamin A32.
Adipose tissue is also closely involved in retinoid metabolism, in addition to its role in supporting hepatic retinyl ester storage34. This tissue is a target of retinoid action via Retinoid Acid Receptors (RARs) and Retinoid X receptors (RXRs). In WAT, retinoids can suppress adipogenesis while enhancing thermogenic potential in BAT35. Moreover, BAT has been shown to present high retinoid binding protein mRNA levels34,36. VA excess in rats was reported to induce elevated levels of circulating free fatty acids, due to an impaired utilization of extrahepatic fatty acids, particularly from adipose tissue37. Here, a pronounced lower WAT weight was observed in VAS-fed males compared to control males. A decrease in WAT storage capacity (along with a diminution in hepatic storage capacity) could explain this result, supported by the increase in plasma triglycerides in VAS-fed males, also noticeable for females.
Muscle weight was also lower in VAS-fed males than control males. One study in rats has shown that hypervitaminosis A increased urinary nitrogen excretion and blood urea concentrations, and stimulated hepatic gluconeogenesis due to an elevated circulating amino acids released from muscle proteolysis38. Although we did not directly assess markers of muscle protein turnover, the lower muscle mass in males aligns with these findings. Conversely, muscle mass increased in VAS-fed females, while their glucose tolerance was impaired. We hypothesize that the increased muscle mass may be due to triglyceride accumulation, in line with their higher plasma triglyceride levels. Lipid accumulation could reduce muscle insulin sensitivity, thereby explaining the observed decline in glucose tolerance in females19,32. Further experiments are needed to explore these assumptions, as there is no accumulation of triglycerides in female liver, and to understand the observed sex differences39.
Concerning vitamin E, Blakely and colleagues showed that VA excess reduces plasma α-tocopherol16. This result is consistent with (i) another study reporting increased oxidative stress in offspring, especially in males26 and (ii) an in vitro study showing that vitamin E enhances VA cellular uptake while VA decreases vitamin E uptake14. Given the importance of vitamin E in the development of young individuals, its status was assessed in young rat plasma and liver, to determine whether high-dose VA exposure would have similar effects. Aside from a slight, non-significant decrease in plasma α-tocopherol observed in VAS-fed young rats, no difference was detected in the liver. This may be due to the fact that VA over-supplementation in our study was not as high as that used in the study by Blakely and colleagues16 whose dose was approximately 40 times higher than ours.
The impact of VA deficiency status on the intestinal absorption efficiency of preformed VA and on lipid metabolism were recently assessed19. Insufficient VA levels led to a delayed increase in VA plasma concentrations, suggesting either a delay in intestinal lipid absorption/secretion or a modification in retinyl ester clearance19. In this study, we explored the opposite scenario, in which an excessive VA status is initially present. To investigate this, we force-fed the young rats an oily emulsion containing retinyl palmitate and [1,1,1-13 C]triolein. Postprandial plasma concentration of retinyl esters constitute a marker of intestinal VA absorption19,40. After ingestion as a supplement or as part of a diet, retinyl palmitate undergoes hydrolysis and re-esterification in the digestive tract. It is then transported in the bloodstream, in the form of chylomicron-associated retinyl esters41. No difference in plasma retinyl ester responses was observed in young males and females. Their postprandial patterns were also similar over the 6-hour period following feeding, with a retinyl ester peak at 3 h. In the other hand, in both female and male control groups, the free retinol peak was observed 3-h following force-feeding, whereas for the VAS-fed groups, the peak was earlier in males, with a similar trend among females. This observation aligned with the delayed retinol peak reported in the case of VA deficiency19. This may be attributed to the release of previously absorbed but unsecreted retinol prior to chylomicron assembly with newly formed retinyl esters42. Indeed, it has been stated that lipid-soluble micronutrients can be secreted in the bloodstream over multiple successive postprandial phases43,44, which may have occurred here.
Intestinal mucosa fractions were also collected to compare their VA content at the end of the postprandial phase. Interestingly, in both VAS-fed females and males, retinyl ester contents were higher in the duodenum compared to control groups. In the ileal fraction, retinol levels were lower in the VAS condition than in control condition for both sexes, although this difference was only significant in males. This may reflect enhanced esterification in the proximal region of the intestinal tract to prevent an increase in retinol and retinoic acid, potentially toxic for the organism28.
To explore the impact of VAS-diet on lipid postprandial metabolism, triglyceride, cholesterol and phospholipid intestinal contents were quantified at the end of the postprandial phase. The lipid profile of intestinal fractions differed between the VAS and control groups. The most striking observation was the higher levels of cholesterol found in each intestinal fraction for males, as well as in duodenal and jejunal fractions in females. Moreover, ileal triglyceride content was increased in VAS-fed males. To confirm this observation, we tracked [1-13 C]oleate derived from the [1,1,1-13 C]triolein incorporated into the emulsion. As expected, VAS-fed males displayed a higher ileal [1-13 C]oleate level compared to control males. Surprisingly, the peak plasma concentration of [1-13 C]oleate occurred earlier than that of retinyl esters for the VAS-fed males while this shift was not observed in the control group. [1-13 C]oleate was also found in larger amounts in the liver. These findings confirm modification in postprandial lipid metabolism under VA excess, in line with previous reports31,32,45.
Taken together, our results highlight lipid and VA metabolism alterations in offspring following an excessive exposure to VA. As expected, VAS diet induced exacerbated storage and circulation of VA. At such elevated levels, VA is transported by lipoproteins contributing to its nonspecific delivery and toxic effects associated with hypervitaminosis A45. In particular, male offspring from VAS group displayed abnormal lipid concentrations in serum and tissues, including high triglyceride plasma content, underscoring the detrimental effects of VA excess on lipid metabolism. These changes may be due to epigenetic programming, as fetal and neonatal periods show considerable epigenetic plasticity46. A study revealed that vitamin A supplementation in young rats during the suckling period induced changes in the methylation status of genes involved in adipose tissue differentiation and development47. This may influence offspring both phenotype and susceptibility to pathologies later in life. It is also possible that a VA-related placental dysfunction due to an excessive VA status is linked to some changes observed in our study13. Finally, a recent study highlighted that in mice deficient in intestine-specific homeobox (ISX) transcription factor, mildly increased maternal VA during late gestation and lactation programed energy and lipid metabolism in tissues, which may have implications for human populations where polymorphisms in ISX and ISX target genes involved in VA homeostasis are prevalent48. These findings support the need for cautious monitoring of VA supplementation, as even non-lethal or non-teratogenic excess can lead to adverse metabolic outcomes in offspring. Further research is needed to evaluate whether such outcomes persist later in life.
Data availability
The experimental data that support the findings of this study are available in Recherche Data Gouv with the identifier https://doi.org/10.57745/BHZVXD.
Abbreviations
- AUC:
-
Area Under the Curve
- BAT:
-
brown adipose tissue
- GC-C-IRMS:
-
Gas Chromatography-Combustion-Isotope Ratio Mass Spectrometry
- HPLC:
-
High-Performance Liquid Chromatography
- RBP:
-
Retinol Binding Protein
- VA:
-
Vitamin A
- VAS:
-
Vitamin A Supplemented
- WAT:
-
white adipose tissue
References
Blomhoff, R. Transport and metabolism of vitamin A. Nutr. Rev. 52, 13–23 (1994).
Reboul, E. Absorption of vitamin A and carotenoids by the enterocyte: focus on transport proteins. Nutrients 5, 3563–3581 (2013).
Stevens, G. A. et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Global Health. 3, e528–e536 (2015).
World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Global Database on Vitamin A Deficiency. (2009).
Carazo, A. et al. Vitamin A update: forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients 13, 1703 (2021).
World Health Organization. Recommendations on antenatal care for a positive pregnancy experience. (2023).
World Health Organization. Guideline: vitamin A supplementation in infants and children 6–59 months of age. (2011).
McCauley, M. E., van den Broek, N., Dou, L. & Othman, M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev CD008666 (2015). (2015).
Cruz, S., da Cruz, S. P. & Ramalho, A. Impact of vitamin A supplementation on pregnant women and on women who have just given birth: A systematic review. J. Am. Coll. Nutr. 37, 243–250 (2018).
Rothman, K. J. et al. Teratogenicity of high vitamin A intake. N. Engl. J. Med. 333, 1369–1373 (1995).
Thorsdottir, I., Birgisdottir, B. E., Halldorsdottir, S. & Geirsson, R. T. Association of fish and fish liver oil intake in pregnancy with infant size at birth among women of normal weight before pregnancy in a fishing community. Am. J. Epidemiol. 160, 460–465 (2004).
Mills, J. P., Terasawa, E. & Tanumihardjo, S. A. Ingestion of excessive preformed vitamin A by mothers amplifies storage of retinyl esters in early fetal livers of captive old world monkeys. Comp. Med. 57, 505–511 (2007).
Quadro, L. & Spiegler, E. K. Maternal-Fetal transfer of vitamin A and its impact on mammalian embryonic development. Subcell. Biochem. 95, 27–55 (2020).
Goncalves, A. et al. Fat-soluble vitamin intestinal absorption: absorption sites in the intestine and interactions for absorption. Food Chem. 172, 155–160 (2015).
Blakely, S. R., Grundel, E., Jenkins, M. Y. & Mitchell, G. V. Alterations in β-carotene and vitamin e status in rats fed β-carotene and excess vitamin A. Nutr. Res. 10, 1035–1044 (1990).
Blakely, S. R., Mitchell, G. V., Jenkins, M. Y., Grundel, E. & Whittaker, P. Canthaxanthin and excess vitamin A alter α-Tocopherol, carotenoid and iron status in adult rats. J. Nutr. 121, 1649–1655 (1991).
Debier, C. Vitamin E during Pre- and postnatal periods. in Vitamins & Hormones vol. 76 357–373 (Academic, (2007).
Kolnik, S. & Wood, T. R. Role of vitamin E in neonatal neuroprotection: A comprehensive narrative review. Life 12, 1083 (2022).
Antoine, T. et al. Vitamin A deficiency during the perinatal period and first weeks of life modifies vitamin A and lipid postprandial metabolism in both female and male young rats. Mol. Nutr. Food Res. 65, 2100451 (2021).
Borel, P. et al. β-Carotene bioavailability and conversion efficiency are significantly affected by sex in rats. Mol. Nutr. Food Res. 65, 2100650 (2021).
Borel, P. et al. Vitamin A deficiency during the perinatal period induces changes in vitamin A metabolism in the offspring. The regulation of intestinal vitamin A metabolism via ISX occurs only in male rats severely vitamin A-deficient. Eur. J. Nutr. 62, 633–646 (2023).
Hodges, J. K., Tan, L., Green, M. H. & Ross, A. C. Vitamin A supplementation redirects the flow of retinyl esters from peripheral to central organs of neonatal rats Raised under vitamin A–marginal conditions1. Am. J. Clin. Nutr. 105, 1110–1121 (2017).
Boullu-Ciocca, S., Achard, V., Tassistro, V., Dutour, A. & Grino, M. Postnatal programming of glucocorticoid metabolism in rats modulates high-fat diet-induced regulation of visceral adipose tissue glucocorticoid exposure and sensitivity and adiponectin and Proinflammatory adipokines gene expression in adulthood. Diabetes 57, 669–677 (2008).
Boullu-Ciocca, S. et al. Postnatal Diet-Induced obesity in rats upregulates systemic and adipose tissue glucocorticoid metabolism during development and in adulthood: its relationship with the metabolic syndrome. Diabetes 54, 197–203 (2005).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Schnorr, C. E. et al. The effects of vitamin A supplementation to rats during gestation and lactation upon redox parameters: increased oxidative stress and redox modulation in mothers and their offspring. Food Chem. Toxicol. 49, 2645–2654 (2011).
Black, M. M. & Hurley, K. M. Investment in early childhood development. Lancet 384, 1244–1245 (2014).
Penniston, K. L. & Tanumihardjo, S. A. The acute and chronic toxic effects of vitamin A1234. Am. J. Clin. Nutr. 83, 191–201 (2006).
Olmedilla, B., Granado, F., Blanco, I. & Rojas-Hidalgo, E. Seasonal and sex-related variations in six serum carotenoids, retinol, and α-tocopherol. Am. J. Clin. Nutr. 60, 106–110 (1994).
Moriya, H. et al. Ovariectomy increases Circulating retinol-Binding protein concentrations independently of Sex-Dependent differences in retinol concentrations in rats. J. Nutr. 153, 1019–1028 (2023).
Ahuja, H. C. & Misra, U. K. Hypervitaminosis A and liver phospholipids. Agric. Biol. Chem. 37, 1589–1593 (1973).
Singh, V. N., Singh, M. & Venkitasubramanian, T. A. Early effects of feeding excess vitamin A: mechanism of fatty liver production in rats. J. Lipid Res. 10, 395–401 (1969).
Chen, G., Weiskirchen, S. & Weiskirchen, R. Vitamin A: too good to be bad. Front. Pharmacol. 14, 1186336 (2023).
Tsutsumi, C. et al. Retinoids and retinoid-binding protein expression in rat adipocytes. J. Biol. Chem. 267, 1805–1810 (1992).
Bonet, M. L., Ribot, J., Felipe, F. & Palou, A. Vitamin A and the regulation of fat reserves. CMLS Cell. Mol. Life Sci. 60, 1311–1321 (2003).
Blomhoff, R. Transport and metabolism of vitamin A. Nutr. Rev. 52, S13–S23 (1994).
Ramachandran, C. K., Dileepan, K. N., Singh, V. N. & Venkitasubramanian, T. A. Metabolic Potential of the Adipose Tissue of Rats during Hyper- and Hypovitaminosis A. Proceedings of the Society for Experimental Biology and Medicine 182, 73–78 (1986).
Hillgartner, F. B., Morin, D. & Hansen, R. J. Effect of excessive vitamin A intake on muscle protein turnover in the rat. Biochem. J. 202, 499–508 (1982).
Varlamov, O., Bethea, C. L. & Roberts, C. T. Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. 5, 241 (2015).
Borel, P. et al. Comparison of the postprandial plasma vitamin A response in young and older adults. Journals Gerontology: Ser. A. 53A, B133–B140 (1998).
D’Ambrosio, D. N., Clugston, R. D. & Blaner, W. S. Vitamin A metabolism: an update. Nutrients 3, 63–103 (2011).
Harrison, E. H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta. 1821, 70 (2011).
Reboul, E. Proteins involved in fat-soluble vitamin and carotenoid transport across the intestinal cells: new insights from the past decade. Prog. Lipid Res. 89, 101208 (2023).
Borel, P. et al. Low and high responders to Pharmacological doses of β-carotene: proportion in the population, mechanisms involved and consequences on β-carotene metabolism. J. Lipid Res. 39, 2250–2260 (1998).
Mallia, A. K., Smith, J. E. & Goodman, D. W. Metabolism of retinol-binding protein and vitamin A during hypervitaminosis A in the rat. J. Lipid Res. 16, 180–188 (1975).
de Souza Mesquita, L. M., Mennitti, L. V., de Rosso, V. V. & Pisani, L. P. The role of vitamin A and its pro-vitamin carotenoids in fetal and neonatal programming: gaps in knowledge and metabolic pathways. Nutr. Rev. 79, 76–87 (2021).
Arreguín, A. et al. Dietary vitamin A impacts DNA methylation patterns of adipogenesis-related genes in suckling rats. Arch. Biochem. Biophys. 650, 75–84 (2018).
Srinivasagan, R. et al. Maternal genetics and diet modulate vitamin A homeostasis of the offspring and affect the susceptibility to obesity in adulthood in mice. Am. J. Physiol. Endocrinol. Metab. 327, E258–E270 (2024).
Acknowledgements
The authors thank Corinne Louche-Pelissier (CRNH Rhônes-Alpes) for skillful technical assistance.
Funding
This project received funding from both the AlimH department of INRAE (Paris, France; ANSSD 2016 grant) and from the G.L.N (Groupe Lipides et Nutrition, Paris, France; Research award 2017). Tiffany Antoine received a PhD fellowship from INRAE and CTCPA (Avignon, France). Angélique Berthomé received a PhD fellowship from Région Sud (France) and CTCPA.
Author information
Authors and Affiliations
Contributions
ER, PB and MG designed the study. ER and PB acquired funding. MG and PG conducted the study. ER, CS, MN and TG performed tissue sampling. TA, CS and MN performed vitamin A and lipid analyses. LM and VS performed [1–13 C]oleate analysis. AB, TA and CB did the statistical analyses. ER, AB and TA interpreted the data with PB and RG; ER, AB and TA drafted the manuscript; all authors have read and approved the final manuscript; ER has primary responsibility for the final content of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Berthomé, A., Antoine, T., Meiller, L. et al. Perinatal vitamin A excess impacts vitamin A and lipid metabolism in rat offspring. Sci Rep 15, 34987 (2025). https://doi.org/10.1038/s41598-025-18896-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18896-w