Abstract
To explore the effectiveness and safety of surgical treatment for patients with Posner-Schlossman syndrome (PSS) accompanied by optic nerve Changes. A retrospective analysis was conducted on 15 cases (15 eyes) of glaucoma secondary to PSS who underwent trabeculectomy from January 2018 to March 2023 at Chongqing Aier Eye Hospital. Preoperative and postoperative conditions, as well as intraocular pressure (IOP) during postoperative recurrences, were compared. 15 eyes underwent trabeculectomy, with diffuse and mildly elevated filtering blebs postoperatively, without Choroidal detachment or macular edema. The preoperative IOP was 30.91 ± 11.75 mmHg (mean ± SD), and patients used 2.60 ± 0.91 types of antiglaucoma medication. At 12 months postsurgery, the mean IOP was 12.09 ± 2.33 mmHg, and no antiglaucoma medications were used thereafter (both P < 0.05). Nine eyes experienced recurrence of PSS, with IOP during recurrences being lower than preoperative levels. Except for one eye with an IOP of 24 mmHg, the other eight eyes had IOPs not exceeding 21 mmHg. Trabeculectomy is a safe and effective treatment for patients with PSS during episodes with optic nerve damage, and it can reduce the peak IOP during recurrences, preventing further optic nerve damage from high IOP.
Similar content being viewed by others
Introduction
Posner-Schlossman syndrome, also known as glaucomatous Cyclitic crisis, was first reported by Posner and Schlossman in 1948. It predominantly affects middle-aged individuals, presenting with increased IOP (mostly in one eye), keratic precipitates on the posterior cornea, open angles, and generally unaffected or mildly weakened vision1,2,3. It was previously considered a self-limiting disease with mild symptoms and signs that did not require surgical treatment. However, it has been increasingly reported that some patients experience recurrent episodes or complications such as open-angle glaucoma, which can lead to vision loss, visual field loss, and enlarged cup-to-disc ratios as signs of optic nerve damage4. There is controversy regarding trabeculectomy in treating patients with PSS. The opposing view holds that performing surgery during the acute phase can exacerbate postoperative scarring, leading to surgical failure. The supporting view argues that the new filtration pathway helps drain inflammatory cells from the anterior chamber, thereby alleviating the condition. Currently, there are few reports on the surgical treatment of PSS both domestically and internationally3. Fifteen patients with glaucoma secondary to PSS from January 2018 to September 2023 at Chongqing Aier Eye Hospital were enrolled, and trabeculectomy was conducted to assess the safety and effectiveness of surgical treatment in patients with optic nerve damage during episodes.
Materials and methods
This was an open-label clinical study conducted in accordance with the guidelines of the World Medical Association of Helsinki. Informed consent was obtained from all subjects and this study was approved by the Institutional Review Board of Chongqing Aier Eye Hospital [IRB-AF(SOP-016)−04−05.1].
Patients
The PSS diagnostic criteria were (1) unilateral and recurrent attack, (2) transient episodes of IOP elevation with blurred vision, (3) mild anterior chamber inflammation and/or hoar and white suet-shaped keratic precipitates (KPs), and (4) open anterior chamber angle without iris synechia. The inclusion criteria of the study were adult patients diagnosed with PSS, and fulfillment the following conditions5:
-
1.
PSS Recurrent attacks: ≥ 2 documented episodes.
-
2.
Clear optic-nerve damage defined as EITHER.
-
•
an inter-eye cup-to-disc ratio (C/D) difference ≥ 0.2, OR.
-
•
a C/D ratio ≥ 0.6 in the affected eye, combined with reproducible visual-field loss.
-
3.
In eyes not meeting the above cup-to-disc criteria, trabeculectomy is indicated if, during a PSS flare, IOP remains > 21 mmHg despite the use of ≥ 3 topical IOP-lowering agents and reproducible visual-field loss is present.
Patients were enrolled only if they fulfilled Criterion 1 together with either Criterion 2 or Criterion 3.
Patients who had previously undergone any intraocular surgery or completed < 12 months of follow-up were excluded from the analysis.
Preoperatively, ganciclovir ophthalmic gel was instilled into the affected eye four times daily up to the day before surgery, prednisolone acetate eye drops were administered four times daily until one day prior to surgery, and topical intraocular-pressure-lowering medications were used as required.
Surgical method
Fifteen eyes of 15 patients with PSS were recruited and underwent ophthalmic examinations, including slit-lamp examination, gonioscopy, corneal endothelial microscope, fundus photography, IOP, Visual acuity, Visual-field examination and retinal nerve fiber layer (RNFL) assessment. All patients underwent trabeculectomy performed by an experienced surgeon (Wang Wei) under subconjunctival anesthesia. The surgeries were performed using a Limbus-based Conjunctival flap. After dissecting a 4× 3 mm scleral flap, intraoperative MMC was applied for 3 min using a surgical sponge soaked in MMC solution (0.2 mg/ml), followed by thorough rinsing with a balanced salt solution. A block of tissue located anterior to the scleral spur (1.0 × 3.0 mm) was removed, followed by a peripheral iridectomy. The scleral flap and Conjunctival incision were sutured sequentially using 10− 0 nylon. At the end of surgery, tobramycin-dexamethasone ointment and ophthalmic gel of atropine were applied to the operated eye. Postoperatively, ganciclovir ophthalmic gel was instilled into the operated eye four times daily for 4 weeks, and prednisolone acetate eye drops were administered four times daily, tapering by one drop per week over 4 weeks.
Follow-up and assessment of surgical outcomes
IOP was recorded during the attack before administering medications; after administering medications (preoperative); at 1 d, 1 month, 6 months, and 12 months postoperatively; and at postoperative PSS recurrence. Simultaneous visual acuity and use of IOP-lowering medications were also assessed. Changes in the corneal endothelial cell density of the affected eye and the other eye were measured, and intraoperative and postoperative complications were monitored.
The primary outcome was success, which was defined as IOP ≤ 18 mmHg, ≥ 6 mmHg, and ≥ 20% decrease in IOP from the preoperative level, with no secondary procedures at 12 months. Complete success (CS) was defined as success without any glaucoma medications, and qualified success (QS) was achieved using glaucoma medications6. Adverse events included but were not limited to VA loss ≥ 10 letters, numerical hypotony, hypotony + VA loss ≥ 10 letters at the same visit, anterior chamber bleeding, hypotony maculopathy, choroidal detachment, bleb leakage, endophthalmitis, and secondary glaucoma procedures.
Statistical analysis
Statistical analyses were performed using SPSS version 23 (IBM Corp., Armonk, NY, USA). Quantitative data are expressed as the mean ± standard deviation (x ± s). Paired t-tests were used to compare preoperative and postoperative data with normal distribution, and paired Wilcoxon tests were used to compare data without normal distribution. Statistical significance was set at P < 0.05.
Results
Fifteen patients (15 eyes) with recurrent PSS underwent trabeculectomies. The shortest duration before surgery was 6 months, the longest was 300 months, and the average duration was 71 ± 77.19 months. One patient had a cup-to-disc ratio (vertical diameter) of 0.3, and the Cup-to-disc ratios of the remaining 14 patients ranged from 0.6 to 1.0. With medications, the average IOP was 30.91 ± 11.75 mmHg preoperatively. The naked visual acuity ranged from hand motion to 0.25, and the best-corrected visual acuity ranged from hand motion to 1.0. Four eyes before surgery had an IOP less than 21 mmHg after treatment with medications, however, they had significant signs of optic nerve damage (cup-to-disc ratio ≥ 0.7, MD <−12 dB, average GCC thickness = 58.5 ± 12.12 μm), and 11 eyes had preoperative IOP > 21 mmHg after receiving medications. The patients’ baseline characteristics before surgery are detailed in Table 1.
Comparison of preoperative and postoperative IOP
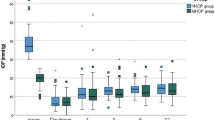
The preoperative IOP was 46.40 ± 10.64 mmHg, which decreased to 12.31 ± 2.63 mmHg at 6 months and to 12.09 ± 2.33 mmHg at 12 months postoperatively (both P < 0.05). Complete success was defined as IOP ranging from 6 to 18 mmHg without medication. The changes in IOP at each time point are summarized in Fig. 1; Table 2. The patients exhibited a 60.9% reduction in IOP, 100% decrease in IOP-lowering medication use, and 100% Complete success rate at 12 months postoperatively.
Adverse events
Intraoperative anterior chamber bleeding occurred in three eyes with small amounts (1–2 mm), which were absorbed over time within one month postoperatively. All filtering blebs were well-formed, diffuse, and mildly elevated, with no leakage, choroidal detachment, macular edema, or visual-acuity loss of ≥ 10 letters.
Comparison of IOP and the use of IOP-lowering medications during PSS recurrence before and after surgery
PSS recurred in nine eyes within 12 months postoperatively, with one eye having a peak IOP of 24 mmHg and the other eight eyes with IOP less than 21 mmHg (7–16 mmHg) during recurrence. The 9 patients with recurrence received an average of 2.56 ± 0.68 types of IOP-lowering medications during preoperative episodes. IOP was significantly lower in postoperative recurrences than in preoperative episodes (12.67 ± 4.97 mmHg vs. 44 ± 9.24 mmHg) (Fig. 2). Accordingly, no IOP-lowering medications were used during postoperative recurrence.
Comparison of corneal endothelial cell density
Preoperatively, the average corneal endothelial cell density (ECC) in the affected eye was 2425.93 ± 467.58 (cells/mm2) and 2850.53 ± 239.47 (cells/mm2) in the fellow eye (Fig. 3). The corneal endothelial cell density in the affected eye was significantly lower than that in the fellow eye.
Discussion
Initially, PSS was believed that PSS is a self-limiting disease with a good prognosis that does not significantly affect visual function. However, an increasing number of studies have shown that patients with recurrent PSS develop glaucomatous optic neuropathy. Owing to the mild nature of its symptoms, it is often overlooked until glaucomatous optic nerve damage occurs. Previous studies have reported that approximately 26.4–67% of patients with PSS develop secondary glaucoma due to prolonged recurrent episodes4,5,7,8 and that patients with optic nerve and visual field damage require long-term use of several IOP-lowering medications to Control IOP. For some patients with PSS and a significant increase in IOP during episodes and a high IOP during remission, it is important to determine whether they have combined primary open-angle glaucoma. Studies have reported that the incidence of PSS combined with primary open-angle glaucoma is 3.17%9, and among the 15 patients undergoing surgery in this study, 3 had Combined bilateral primary open-angle glaucoma. Three patients with bilateral primary open-angle glaucoma had been on chronic monotherapy with a single prostaglandin analogue instilled in both eyes to control IOP within 21mmHg. In all three, the fellow eye showed a cup-to-disc ratio ≥ 0.6 and localized retinal nerve fiber layer thinning. Two of these fellow eyes exhibited paracentral scotomata, whereas the remaining eye displayed a nasal-step visual-field defect. In the remission of these patients, the IOP was high in both eyes, requiring IOP-lowering medications. When the affected eye experienced a PSS attack, its IOP was significantly higher than that of the other eyes. The following signs were present in the affected eye: greyish-white creamy keratic precipitates, ciliary body swelling, and damage to the visual field and optic nerve.
The etiology of PSS remains unknown. Studies have suggested that herpes virus infection, allele heterogeneity, changes in cytokine levels in the aqueous humor, and vascular endothelial dysfunction can lead to PSS. Recurrent inflammation and high IOP damage the trabecular meshwork, which accelerates the apoptosis of ganglion cells and leads to glaucomatous damage3,10,11,12,13,14,15.The cohort we collected, however, comprised individuals with recurrent PSS who had become “seasoned patients” and extremely anxious. During an acute episode of PSS, anti-inflammatory drugs, antiviral medications, and ocular hypotensive agents are routinely used. Inflammation and high intraocular pressure decrease corneal endothelial cell density. Our study showed that corneal endothelial cell density in PSS-affected eyes was significantly lower than that in fellow eyes, which is consistent with previous reports5,16,17. Some studies have shown that patients with PSS who are positive for aqueous humor cytomegalovirus have a higher risk of endothelial cell loss and glaucoma filtration surgery18. Among the 15 patients in this study, four underwent aqueous humor testing, and one was positive for cytomegalovirus. Cytomegalovirus has an affinity for the trabecular meshwork and corneal endothelial cells, leading to increased resistance in the aqueous humor outflow pathway and a marked decrease in the number of corneal endothelial cells19, which can exacerbate the condition of patients with PSS. Some patients reduce the use of IOP-lowering medications after adding antiviral medications during episodes18. In this study, patients still had a high IOP after receiving preoperative medications. Uncontrolled IOP with medication is a clear indication for surgical intervention in patients with PSS. During episodes of PSS, intraocular inflammatory cytokines are upregulated, and the risk of filtering bleb fibrosis increases, which is not conducive to controlling IOP with filtration surgery. However, in this study, we found that the patients did not have obvious scarring of the filtering bleb within one year after surgery; thus, no antiproliferative drugs were injected postoperatively. This may be because trabeculectomy promotes the outflow of inflammatory mediators, thereby downregulating the intraocular inflammatory reactions. There were no intraoperative or postoperative complications such as choroidal hemorrhage or shallow anterior chamber. Nine eyes recurred, but the IOP during recurrences was significantly lower than that during the preoperative episodes. Therefore, we believe that performing trabeculectomy for IOP reduction during episodes of PSS is safe and effective when IOP control is poor, and can effectively decrease the peak IOP during postoperative recurrences, thereby preventing further optic nerve damage due to high IOP. Typically, patients with PSS experience a cross-phenomenon of IOP during and between episodes. That is, the IOP of the affected eye significantly increases and becomes higher than that of the contralateral eye during the acute attack phase. In contrast, IOP of the affected eye was lower than that of the contralateral eye during the intermission phase. Due to trabecular meshwork damage, patients with multiple recurrences suffer from abnormal IOP regulation during intermission periods, leading to a lack of IOP cross-phenomenon and optic nerve damage similar to POAG20. This point was not verified in our experiment and guides us to further explore t
Data availability
Data are available upon request. Data were acquired from the corresponding author upon request.
References
Posner, A. & Schlossman, A. Syndrome of glaucomato-cyclitic crises. Am. J. Ophthalmol. 31(6), 735 (1948).
Posner, A. & Schlossman, A. Syndrome of unilateral recurrent attacks of glaucoma with cyclitic symptoms. Arch. Ophthal. 39(4), 517–535 (1948).
Roly, M. & Kumar, P. Posner-Schlossman syndrome. Surv. Ophthalmol. 62 (3), 277–285 (2016).
Jap, A., Meenakshi Sivakumar, M. & Chee, S-P. Is Posner Schlossman Syndrome Benign?? Ophthalmol. ;108(5):913–918. (2001).
Jin-Ho, K., Ji-Young, L. & Jin, A. C. Long-term prognosis for glaucoma in patients with Posner-Schlossman syndrome. Graefes Arch. Clin. Exp. Ophthalmol. 259 (12), 3757–3767 (2021).
Shaarawy, T. Guidelines on Design and Reporting of Glaucoma Surgical Trials. World Glaucoma Association (2009).
Kass, M. A., Becker, B. & Kolker, A. E. Glaucomatocyclitic crisis and primary open-angle glaucoma. Am. J. Ophthalmol. 75 (4), 668–673 (1973).
Raitta, C. & Vannas, V. Glaucomatocyclitic crisis. Arch. Ophthalmol. 95(4), 608–612 (1977).
Zheng, Z. H., Chuan, W. B. & Xiong, Z. Unilatteral primaly open angle glaucoma and bilatteral glaucomatocyclitic crisis. Chin. J. Misdiagnosis. 1, 334–336 (2001).
Chee, S-P. & Jap, A. Presumed Fuchs heterochromic iridocyclitis and Posner-Schlossman syndrome: comparison of cytomegalovirus-positive and negative eyes. Am. J. Ophthalmol. 146 (6), 883–9e1 (2008).
Hedayatfar, A. & Chee, S-P. Posner-Schlossman syndrome associated with cytomegalovirus infection: a case series from a non-endemic area. Int. Ophthalmol. 34 (5), 1123–1129 (2014).
Li, J. et al. Aqueous cytokine changes associated with Posner-Schlossman syndrome with and without human cytomegalovirus. PLoS One. 7 (9), e44453 (2012).
Jun, Z. et al. Human leukocyte antigens-B and -C loci associated with Posner-Schlossman syndrome in a Southern Chinese population. PLoS One. 10 (7), e0132179 (2015).
Saori, O., Toshihiro, I., Keiichiro, I., Eri, T. & Hidenobu, T. Factors influencing aqueous Proinflammatory cytokines and growth factors in uveitic glaucoma. PLoS One. 11 (1), e0147080 (2016).
Su-Chin, S. et al. Peripheral vascular endothelial dysfunction in glaucomatocyclitic crisis: a preliminary study. Invest. Ophthalmol. Vis. Sci. 51 (1), 272–276 (2009).
Nozomi, I. et al. Involvement of autotaxin in the pathophysiology of elevated intraocular pressure in Posner-Schlossman syndrome. Sci. Rep. 10 (1), 6265 (2020).
Nozomi, I., Megumi, H., Toshikatsu, K. & Makoto, A. Effects of ROCK inhibitors on apoptosis of corneal endothelial cells in CMV-positive Posner-Schlossman syndrome patients. Invest. Ophthalmol. Vis. Sci. 61 (10), 5 (2020).
Chien-Chia, S. et al. Clinical outcomes in cytomegalovirus-positive Posner-Schlossman syndrome patients treated with topical ganciclovir therapy. Am. J. Ophthalmol. 158 (5), 1024–31e2 (2014).
Kazuichi, M. et al. Characteristics of cases needing advanced treatment for intractable Posner-Schlossman syndrome. BMC Ophthalmol. 17 (1), 45 (2017).
Guo, H. & Zhou, H. The characteristic of intraocular pressure dynamic change in patients with glaucomatocyclitic crisis. Int. Ophthalmol. 39 (8), 1819–1825 (2018).
Acknowledgements
The authors express their gratitude to EditSprings (https://www.editsprings.cn) for providing expert linguistic services.
Funding
This research was conducted independently without external funding support.
Author information
Authors and Affiliations
Contributions
XY was responsible for the overall content. YW and YM collected data. WW and YW performed surgeries. XY and YW contributed to the data quality control. XY, YW, and YQ contributed to study design and quality control. YW, YM, and BH analyzed the data. XY and YW drafted the manuscript. YQ revised the manuscript accordingly.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All studies were reviewed and approved by the Medical Ethics Review Committee of the Chongqing Aier Eye Hospital, Chongqing, China (approval no. 202502020).
Provenance and peer review
Not commissioned; externally peer reviewed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, Y., Wang, W., Ma, Y. et al. One-year outcomes of trabeculectomy with mitomycin C in secondary glaucoma due to posner-schlossman syndrome. Sci Rep 15, 35725 (2025). https://doi.org/10.1038/s41598-025-19643-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19643-x