Abstract
Allergic contact dermatitis (ACD) is a common T cell-mediated hypersensitivity reaction affecting the skin after exposure to an allergen. ACD is managed by antihistamines or steroids and avoiding exposure to allergens. Pentanoate is a short chain fatty acid with a therapeutic potential to modulate lymphocytes to secrete interlukin-10 (IL-10). Although IL-10 producing B cells are known to reduce inflammatory response, their role in ACD remains poorly understood. This study aims to investigate how B cells respond during ACD and how pentanoate influences them. Trinitrochlorobenzen (TNCB) was used to develop an in vivo model of contact hypersensitivity (CHS) in mice. Ear swelling and cellular analysis were used to examine allergic inflammatory reactions. Using flow cytometry, the B and T cell subsets were examined. Data showed that the central memory T cell subset and total CD4 + T cells significantly increased during recurrent CHS responses. Concurrently, there was a significant decrease in regulatory T cell subsets, marginal reduction in regulatory B cells, and naïve T cells during recurrent CHS. In vitro stimulation of B cells with pentanoate lead to their activation to produce IL-10. Adoptive transfer of these pentanoate-stimulated B cells into CHS mouse model diminished inflammatory response significantly. These findings highlight the ability of pentanoate in modulation of CHS responses by enhancing IL-10-production by B cells, indicating its potential therapeutic relevance for allergic contact dermatitis.
Similar content being viewed by others
Introduction
Allergic contact dermatitis (ACD) is an inflammatory skin disease which is antigen-specific, T cell-mediated delayed-type IV hypersensitivity reaction. ACD is specifically associated with CD4⁺ T helper 1 cells and CD8⁺ cytotoxic T cells. Its pathophysiology is two distinct phases: sensitization phase and elicitation phase. In the sensitization phase, allergens penetrate through the stratum corneum and bind to cellular proteins to form a hapten-protein complex which triggers T-cell response. In the skin, dendritic cells (DCs) internalize the allergen and transport the hapten-protein complex to the skin-draining lymph nodes. Allergens are processed by lymph nodes and presented to the naïve CD4 + and CD8 + T cells1,2 leading to their activation, proliferation, and differentiation into effector, helper T (Th) and IFN-γ producing CD8 + T cells. A fraction of these effector T cells differentiates into memory cells, which can proliferate immediately upon re-exposure to the antigen, ensuring a robust immune response3,4,5,6,7,8. In the elicitation phase, upon re-exposure to the allergen the immune response is activated, an effector memory T cells proliferate and migrate from lymph nodes to the site of allergen contact. This migration is mediated by chemokines and cytokines. The release of pro-inflammatory cytokines by these activated T cells like TNF-α and IFN-γ induces apoptosis of keratinocytes, which results in local inflammation and tissue damage9,10,11.
Although ACD is primarily considered a T-cell-mediated condition, B cells, particularly regulatory B cells (Bregs), play an immunosuppressive role in modulation of inflammatory response. They contribute to immune homeostasis and maintain tolerance by decreasing T cell activation and cytokine production12. Bregs secretes IL-10, that reduces the severity of inflammatory diseases13,14,15. Bregs act locally in the skin to suppress CHS responses through the production of IL-10. Their recruitment depends on α4β1-integrin/VCAM-1 interaction, and blocking this axis exacerbates inflammation. B cells can regulate immune responses in different ways. In the skin, B cells exhibit either a pro-inflammatory or anti-inflammatory role, depending on the subset and context. IL-10 + Bregs suppress inflammation in models of psoriasis, ACD, and scleroderma15,16,17,18.
Short-chain fatty acids (SCFAs) are metabolites produced by fermentation of dietary fibers by gut microorganisms19. SCFAs effects immunity, intestinal epithelial barrier and defense functions by regulation of gene expression20. Moreover, SCFAs regulate innate immune cells e.g., macrophages, neutrophils, DCs21 and also dampen pro-inflammatory responses20,22. Studies have shown that SCFAs also play a role in the differentiation of B cells into antibody producing plasma cells and alter the expression of B-cell-related genes (IgGs, IgA, IgJ, Igκ, Igλ, Aicda, Xbp1, Irf4, etc.) by inhibiting histone deacetylases. These genes are involved in the differentiation of B cells and promote transformation of B cells into plasma cells23,24. Several studies have also shown that SCFAs regulate both T and B cells20,22 and particularly Bregs by increasing the production of the anti-inflammatory IL-1024,25,26.
The most commonly studied SCFAs are acetate, propionate and butyrate21. Pentanoate (also known as valerate, C5) is another abundant SCFA with emerging therapeutic potential in immune modulation. While butyrate, has been widely studied, pentanoate remains relatively unexplored in the context of inflammatory diseases. Pentanoate is considered as a potential therapeutic agent for autoimmune and inflammatory diseases because of its ability to induce IL-10 production in lymphocytes and suppress Th17 cells25. However, its specific effects on ACD and B cell regulation are yet to be fully elucidated.
The aim of this study is to investigate the role of B cells in recurrent allergic contact dermatitis and their modulation by SCFA pentanoate. Specifically, we hypothesize that pentanoate enhances B cell mediated IL-10 secretion might reduce inflammatory responses in ACD. Understanding these interactions could provide novel insights into a therapeutic modality for ACD and related hypersensitivity disorders through B cells.
Materials and methods
Ethics and animals
The study protocol was reviewed and approved by the Animal Research Ethics Committee of UAE University, UAE (Approval # ERA-2020-6063 & ERA-2025-6688). All experimental procedures and study protocols were performed in compliance with the care and use of laboratory animals. The study complies with ARRIVE guidelines (https://arriveguidelines.org). 6–8 weeks old C57BL/6J wild type mice were purchased from The Jackson Laboratory and housed in a specific-pathogen-free environment with free access to food and water. Mice were bred and maintained in the Central Animal Facility of UAE University under a 12-h light/dark cycle; Sex- and age-matched mice were used in all experiments.
Mouse model of recurrent contact hypersensitivity (CHS) responses
The allergen 3% 2,4,6-Trinitrochlorobenzene (TNCB) was prepared in acetone. On day 0, for sensitization 3% TNCB was applied to the shaved abdomen of the mice (n ≥ 5). For elicitation, 1% TNCB was applied onto the ear pinna on days 5 for first and on day 30 for second challenge of allergen, respectively (Fig. 1). Ear thickness was measured daily for 3 days after first elicitation (day 5 to day 8) and day 30 to day 33 after second elicitation phase using thickness measuring gauge. For control groups only acetone, the vehicle was applied during the sensitization and recurrent elicitation phases. Mice were humanely euthanized using carbon dioxide at the end of experiments. All experiments were repeated at least three times with five mice per group.
Adoptive transfer of B cells
B cells were isolated from spleens of WT 6–8 weeks old mice using Miltenyi Biotech B cell isolation kit (Bergisch Gladbach, Germany) following the manufacturer’s protocol. isolated B cells were incubated with 2.5 mM CpG adjuvant and 5mM pentanoate in X-Vivo medium and kept in culture for 48 h for Breg expansion. Adoptive transfer of these B cells was performed one day prior to the first elicitation phase (i-e. day 5, Fig. 1), by injecting 3 × 106 pentanoate-stimulated B cells intravenously.
Histology
At the end of CHS experiments (day 33), mice ears were harvested, fixed in 10% formalin and embedded in paraffin for histological analysis. Tissue sections, 3–4 μm thick, were prepared using a rotary microtome and mounted on glass slides. Tissues were stained with hematoxylin and eosin (H&E) using Shandon Instant Hematoxylin Kit (ID:6765015, Thermo ScientificÔ, Waltham, MA, USA) following manufacturer’s protocol.
Flow cytometry analysis
Single-cell suspensions prepared from spleen and ear draining lymph nodes were probed with fluorophore-conjugated antibodies specific for the following proteins: CD45R (B220), CD5, CD1d, CD4, CD25, Foxp3, CD8, CD44, CD62L (all from Invitrogen by Thermo Fisher Scientific, Foster City, CA, USA) following the manufacturer’s protocol. Immunolabeled cell samples were analyzed using BD FACSCanto™ II flow cytometer. Kaluza C V1.1.2 (Beckman Coulter Inc., Kraemer Blvd. Brea, CA, USA) software was used for FACS data analysis.
In vitro cell culture
To prepare single-cell suspension, spleens were harvested, and B cells were isolated by using B cell isolation kit as mentioned before. Cell counting was done by using an TC20 automated cell counter (Bio-Rad Laboratories Inc., CA, USA). B cells were cultured in a 12-well plate and incubated with 2.5mM CpG, with or without the addition of 5mM pentanoate or 5mM pentanoate alone. Cells were harvested after 0, 24, 48 h for real-time PCR.
Real time-PCR analysis
Total RNA was extracted from B cells using the Qiagen RNeasy Mini Kit (ID:74106, Qiagen, Hilden, Germany) by following the manufacturer’s protocol. The quantity and purity of the extracted RNA was measured by NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Then, reverse transcription was done by using iScript cDNA Synthesis Kit (ID:1708891, Bio-Rad, Hercules, CA, USA) following the manufacturer’s protocol. The real-time PCR was performed by using PowerUp™ SYBR Green Master Mix (ID: A25742, Applied Biosystems, Thermo Fisher Scientific, Carlsbad, CA, USA) and all reactions were recorded in QuantStudio™ 5 Real-Time PCR instrument (Applied Biosystems, Thermo Fisher Scientific, Foster City, CA, USA). The following primers were used for Il10 forward, 5′-TGGCCTTGTAGACACCTTGG-3′reverse,5′-AGCTGAAGACCCTCAGGATG-3′; Stat3 forward,5′-GGGCATTTTTATGGCTTTCAAT-3′ and reverse,5′-GTTAACCCAGGCACACAGACTTC-3′; Il4 forward, 5′- GAACTCTAGTGTTCTCATGGAGC-3′ reverse,5′-AGTGATGTGGACTTGGACTCAT-3′; Il13 forward, 5′- CCTGGCTCTTGCTTGCCTT-3′ reverse,5′- GGTCTTGTGTGATGTTGCTCA-3′; Il17a forward, 5′- AAGGCAGCAGCGATCATCC-3′ reverse,5′-GGAACGGTTGAGGTAGTCTGAG − 3′; Ifng forward, 5′- GAGCTCATTGAATGCTTGGC − 3′ reverse,5′- GCGTCATTGAATCACACCTG − 3′. Actb (β-actin), forward,5′-GACGGCCAGGTCATCACTATTG-3′ reverse, 5′-AGGAAGGCTGGAAAAGAGCC-3′27. All results were normalized to the β-actin gene. Samples were tested in duplicates, and the average values were used for quantification by using 2^-ΔΔCt method.
Statistical analysis
GraphPad Prism software version 7.03 (GraphPad Inc., San Diego, CA, USA) was used for the statistical analysis. To calculate statistical significance RT-PCR data were analyzed by using the Student’s t- test (unpaired, two-tailed). Kaluza C V1.1.2 (Beckman Coulter Inc., Kraemer Blvd. Brea, CA, USA) software was used for FACS data analysis. Statistical parameters are reported in each figure legend, where n represents the number of biological replicates. All results are expressed as mean ± SEM, where SEM represents the Standard Error of the mean and NS stands for not significant. Student’s t-tests and Two-way ANOVA were used to evaluate the statistical significance. Values P < 0.05 were considered significant. Statistical significance was indicated in each figure where *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Results
TNCB induced recurrent CHS response
To induce recurrent ACD in mice, the TNCB was applied to the shaved abdomen of mice on day 0 for sensitization followed by elicitation at day 5 and re-elicitation at day 30. For evaluation, ear thickness was measured at multiple time points: before sensitization, as well as before and after both elicitation and re-elicitation. After sensitization, TNCB was applied on ear for elicitation, 24 h later the ear inflammation was observed. Ear thickness peaked at 48 h (***P ≤ 0.001) of post-elicitation and subsequently declined within the next 24 h. In recurrent CHS model, the re-elicitation on day 30 induced a prominent ear thickness as compared to the acute response. In recurrent CHS response a significant increase in ear inflammation was observed, rising from 0.35 ± 0.010 (mean ± SEM) in the acute phase to 0.47 ± 0.013 (***P ≤ 0.001) in recurrent phase (Fig. 2A).
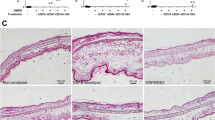
TNCB-induced recurrent CHS response. (A) time-dependent effect of TNCB on ear thickness. (B, C) H&E staining of ear pinna of control and CHS mice, respectively. Note that CHS ear has increased thickness and infiltration with inflammatory cells in the dermis (arrows). The values of ear thickness are expressed as mean ± SEM (n = 12), *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001. Scale bar = 50 μm.
These findings suggest that recurrent CHS phase induces a more severe allergic response as compared to the acute phase consistent with the previous reports showing that reactivation of allergen-specific CD8 + skin-resident T cells can rapidly recruit neutrophils upon challenge28,29,30.
Microscopic analysis of ear tissue samples collected at the end of recurrent CHS responses (at day 33) from control and CHS models revealed some characteristic differences between the two groups (Fig. 2B). Ear biopsy from CHS mice exhibits increased dermal thickness, inflammatory cell infiltration, and structural alterations in response to TNCB exposure as compared to the control. These findings support the inflammatory changes as demonstrated by CHS induced ear thickness (Fig. 2).
Immune cell changes during recurrent CHS response
Following the induction of recurrent CHS responses in mice, the adaptive immune cells were analyzed at day 33 from ear draining lymph nodes using flow cytometry. The results indicate no significant difference in the percentage population of CD45R/B220 + cells (Fig. 3 A) and CD8 + T cells (Fig. 3B). However, a significant increase in CD4 + T cells was observed (P = 0.002) (Fig. 3 C) during CHS responses. These data support the role of CD4 + T cells during recurrent CHS response. The gating strategy for flow cytometry analysis is shown in supplementary Figure S1.
Regulatory B and T cells during recurrent CHS responses
Although we observed an increase in total count of the CD4 + T cells after recurrent CHS responses, the regulatory T cells (Tregs) subset (CD4 + CD25 + Foxp3+) was significantly decreased after recurrent CHS response (*P < 0.05) as shown in Fig. 4A. Tregs are known to play a role in suppression of activity of effector T cell31. Therefore, their reduced numbers may contribute to such inflammatory reactions during recurrent CHS responses. Such apparent reduction in Tregs could also reflect expansion of other CD4⁺ subsets.
Regulatory Immune cell changes during recurrent CHS response. (A) Regulatory T cell subset (CD4 + CD25 + FoxP3 + T cells) was significantly decreased during CHS. (B) Regulatory B cells (B220 + CD5 + CD1d+) showed non-significant marginal decrease during chronic CHS response. *P < 0.05. The values are mean ± SEM(n = 10).
Since the regulatory B220 + CD5+CD1dhigh B cells are reported to produce the cytokine IL-1016, these cells were also analyzed at the end of recurrent CHS responses. Interestingly, Bregs showed a marginal but non-significant decrease in their census (Fig. 4B). These data collectively indicate that regulatory immune cells contribute to development of inflammatory reactions associated with recurrent CHS.
Changes in CD8+ T cell subsets during recurrent CHS response
The subsets of CD8⁺ T cells were analyzed for further investigation at the end of recurrent CHS responses (day 33) in ear draining lymph nodes. Interestingly, CD8⁺ T cell subsets displayed distinct patterns. Naïve T cells (CD8 + CD62L + CD44-) showed a significant decrease in their population (*P < 0.05) (Fig. 5A). An increasing but non-significant trend was observed in effector memory T cells (CD8 + CD62L-CD44+) in CHS mice (Fig. 5B). In contrast, a significant increase in central memory T cells (CD8 + CD62L + CD44+) population was observed (P = 0.009) (Fig. 5C).
CD8 + T cell subsets variations during recurrent CHS responses. (A) CD8 + various subsets, CD8 + CD62L + CD44- (Naïve T cells), (B) CD8 + CD62L-CD44+ (effector memory T cells), C) CD8 + CD62L + CD44+ (central memory T cells). ns = non-significance *P < 0.05, **P ≤ 0.01. The values are mean ± SEM (n = 10).
In vitro B cell treatment with pentanoate modulates IL-10 and STAT3 expression
Previous research has shown specific role of short chain fatty acids pentanoate on B cell differentiation25. Therefore, we performed in vitro B cell differentiation study in the presence of pentanoate and investigated the changes in the expression levels of IL-10 and STAT3 as both are important in elimination of inflammatory response. B cells stimulated with CpG were used as control. IL-10 and STAT3 expressions were observed at 24 h and 48 h post treatment of B cells with pentanoate and CpG (Fig. 6A, B). After 24 h, IL-10 expression was significantly high in pentanoate-treated B cells (P = 0.040), and this elevation in IL-10 level was sustained after 48 h (P = 0.0025) (Fig. 6A). Although in STAT3 expression there was no significant change after 24 h of treatment with pentanoate and CpG, but a trend towards increased expression was observed. After 48 h, the expression of STAT3 was significantly higher in pentanoate-treated B cells (P = 0.007) (Fig. 6B). We show that IL-10 and STAT3 gene expression enhanced transiently when B cells were stimulated with pentanoate alone (Fig. 6C, D), however, due to no further B cell stimulation, their expression decreased. These results collectively indicate that pentanoate induces the expression of IL-10 and STAT3 in B cells. Expression of IL-10 and STAT3 provides information about endogenous mechanisms that limit skin inflammation. Activated STAT3 and increased level of IL-10 are associated with the weak inflammatory response, which highlights their suppressive role in production of pro-inflammatory cytokines32 that can resolve contact hypersensitivity reactions. Therefore, the increase in IL-10 and STAT3 expressions is important in understanding the regulatory mechanism that mitigates CHS symptoms, potentially guiding the development of targeted anti-inflammatory therapies.
Realtime PCR analysis of Il10 and Stat3 gene expression in B cells treated with pentanoate. (A) Isolated B cells were stimulated with CpG or CpG and Pentanoate followed by Il10 gene expression analysis. (B) Gene expression of Stat3 gene. (C and D) Il10 and stat3 genes expression with Pentagnoate alone. Isolated B cells were cultured with Pentanoate alone for a period of 5 min,10 min,15 min. The values are mean ± SEM (n = 8). ***P ≤ 0.001, **P ≤ 0.01,*P < 0.05. ns = non-significance. Unpaired student’s t-test was used for statisitical analysis.
Pentanoate treated B cells attenuated CHS responses
To assess the impact of pentanoate on B cell modulation during recurrent CHS responses, prior to the initial elicitation, pentanoate along with CpG treated B cells were adoptively transferred and ear measurements were taken at three time points (24 h, 48 h, 72 h) following both elicitation events (as shown in experiment scheme in Fig. 7), to evaluate the effect of pentanoate-treated B cells on inflammation. After adoptive transfer of these pentanoate-treated B cells a significant reduction in ear swelling was observed during the acute CHS response at 24 h and 48 h. During recurrent CHS response, again a significant reduction in inflammation was observed at 24 h, it was less pronounced compared to the acute response (Fig. 8). Interestingly, adoptive transfer of unstimulated B cells did not reduce CHS responses (Fig. 8A), indicating that these modulatory effects were specific to the B cells stimulated with CpG and pentanoate.
Adoptive transfer of regulatory B cells, B cells and their effects on ear thickness in recurrent CHS responses. (A) Time-dependent effect of adoptive transfer on ear thickness compared to control CHS. **P ≤ 0.01, ***P ≤ 0.001 by using 2-way ANOVA test. (B) and (C) H&E staining of ear pinna of normal CHS induced mice and adoptive transfer of regulatory B cells in mice at the end of recurrent CHS responses, respectively. Scale bar = 50 μm.
Analysis of ear-draining lymph nodes at day 8 and day 33 following adoptive B cell transfer of B cells revealed a significantly higher frequency of Bregs along with IL-10⁺ Bregs cells and Il10 gene expression in the pentanoate-treated group compared to unstimulated B cell adoptive transfer group and CHS (Supplementary Figure S2 and S3). Gene expression analysis of cytokines (IL-4, IL-13, IL-17 and IFN-γ) in isolated CD4⁺ T cells showed differential expression patterns during the response phase (Supplementary Figure S4).
Discussion
ACD is a common inflammatory T-cell mediated disease and can become distressing. The existing treatment modalities for ACD mainly involve the use of antihistamines or corticosteroids and allergen avoidance. However, a deeper understanding of the disease’s pathophysiology is necessary, particularly regarding the involvement of immune cells (T and B cells) is needed for developing more targeted and effective therapeutic modality with less side effects. This research aims to investigate the pathophysiology and change in population of immune cells during recurrent ACD and modulatory effects of pentanoate on B cells, and its effect during recurrent allergic responses, to explore its potential as a therapeutic strategy to alleviate these reactions. Previous studies have shown that short-chain fatty acids (SCFAs) can modulate immune responses, either by promoting or suppressing the immune response depending on the inflammatory context33. In this study, we demonstrated that SCFA pentanoate mitigate disease severity in an allergen-induced CHS model.
ACD is an inflammatory disease, that is widely studied using contact hypersensitivity mouse model34,35,36. Small chemical haptens like TNCB triggers type IV allergic reactions by modifying proteins to form TNP determinants (trinitrophenyl-modified peptides), these TNP determents activates T cells, known as cytotoxic, IFN-γ producing CD8⁺ TCRαβ⁺ T cells, classically known as Tc1 cells. After skin sensitization, IFN-γ producing T cells mostly resides in lymph nodes proximal to the application site, whereas cytotoxic CD8⁺ T cells may present in all secondary lymphoid organs, including spleen. This indicates systemic priming of cytotoxic T cells instead of localized nature of allergic lesions. After application of TNCB the high frequency of allergen-specific CD8⁺ T cells indicates the rapidity and magnitude of allergic responses11.
In current study, morphometric and microscopic analysis of the ear showed that TNCB successfully induced recurrent CHS responses with a significant increase in ear thickness and infiltration of inflammatory cells. The variations in cellular data during the recurrent CHS response induced by TNCB provides valuable insights into disease progression. A significant increase in total CD4 + T cells population, coupled with decrease in Treg subset (CD4 + CD25 + Foxp3+), indicates that Tregs cells play a negative role in disease progression, while other allergy-specific CD4 + cells were actively involved. Treg cells are important for inducing immunological tolerance and suppression of immune response. The decreased level of Tregs explains the persistent inflammation observed in CHS37. CD4⁺ T cells play dual functions in CHS reactions. Studies reveal that in CHS, CD8⁺ T cells are primary effectors while CD4⁺ T cells play a regulatory role and modulate the intensity and duration of the immune response. CD4⁺ T cells are negative regulators of CHS and suppresses the effector functions of CD8⁺ T cells by producing cytokines like IL-4 and IL-1038. If strong haptens are involved, CD4⁺ T cells primarily act as regulators, and modulate the immune response which results in prevention of excessive inflammation. This regulatory function is mainly because of Tregs (CD4⁺CD25⁺) which suppress the activity of effector T cell by secreting IL-10. Vocanson et al.39 also proposed inhibitory role of CD4⁺ cells in CHS model against CD8 + cells. In this study it was observed that normal mice with CD4 + cells do not develop CHS against weak haptens but CD4⁺ T-cell-deficient mice, develops CHS against weak haptens robustly because of the inflammatory response generated by CD8 + cells39. CD4⁺ T cells suppress the formation of CD8⁺ TRM cells in response to clinically relevant allergens. CD4⁺ T cells like Tregs have potential to limit the development of pathogenic TRM. TRM cells help to recruit and infiltrate neutrophils to the site followed by neutrophil-driven inflammation30,40,41. However, if CD8⁺ T cell population is deficient, CD4⁺ T cells play effector roles, and cause inflammatory response. Therefore, in CHS the role of CD4⁺ T cells is dynamic and context-dependent, which highlights the complexity of immune regulation in allergic contact dermatitis31.
During recurrent CHS responses distinct subsets of CD8 + T cells were observed. A significant reduction in naïve T cells (CD8 + CD62L + CD44-) and increase in central memory T cells (CD8 + CD62L + CD44+), and effector memory T cells (CD8 + CD62L-CD44+) was observed. This shift indicates that more naïve T cells were encountering the allergen, later proliferating, and differentiating into memory T cells and effector memory T cells and establishing a long-lived memory cell population. The CD62L marker distinguishes between central memory (CD62L+) and effector memory (CD62L-) T cells, influencing their homing ability between peripheral lymph nodes and tissues42. Murine Bregs, identified by the B220 + CD5 + CD1d + phenotype, showed a non-significant decreasing trend during recurrent CHS. Bregs are responsible for modulation of immune responses by suppressing the inflammation, inhibiting the CD4 + CD25- effector T cell proliferation, and suppressing Th1 responses. They also secrete IL-10, an anti-inflammatory cytokine that is an anti-inflammatory agent17,43,44. The decreasing trend in Bregs indicates severity of disease and increased inflammation during recurrent CHS responses. Since we observed non-significant differences between the control and recurrent CHS groups, this suggests that Bregs do not proliferate once CHS is established.
The immunomodulatory effects of pentanoate on B cells were examined through the expression of regulatory genes, IL-10 and STAT3. Increased expression of these genes after treatment with pentanoate indicates the role of B cells as an anti-inflammatory agent. Prior studies demonstrated that B cells by overexpression of IL-10 acquire an immunoregulatory phenotype, leading to substantial anti-inflammatory and immunosuppressive effects45,46. STAT3 functions as a critical signal transducer and transcriptional factor that regulates cytokine signaling pathways in lymphocytes and play a central role in B cell-mediated IL-10 production47 enabling B cells to perform anti-inflammatory role. STAT3 inhibition has been shown to reduce IL-10 production by immune cells32,48. STAT3 is not only involved in IL-10 production by Bregs, but also essential for the differentiation and proliferation of both Bregs and Tregs49,50. CpG alone also enhances IL-10 expression through TLR951,52, the combination of CpG with pentanoate enhanced IL-10 gene significantly. These findings suggest that pentanoate exerts anti-inflammatory effects by inducing Bregs mediated expresses of IL-10. The adoptive transfer of pentanoate-stimulated B cells into CHS mouse model prior to the first elicitation results in significant reduction in the inflammatory response during the acute phase. However, mild but still significant effects were observed after 24 h during the recurrent CHS phase whereas unstimulated B cells did not ameliorate CHS responses. Pentanoate enhances IL-10⁺ Breg proliferation by metabolic reprogramming, epigenetic modulation and anti-apoptotic signaling. It activates the AKT/mTOR pathway, and shifts B cell metabolism towards glycolysis, which provides the necessary energy and biosynthetic precursors for proliferation and is essential for IL-10 production. This metabolic rewiring, combined with a partial synergy with the p38 MAPK signaling pathway along with inhibiting histone deacetylase results in IL-10-producing Bregs25,53,54. B cells play a complex regulatory or excitatory role in CHS through distinct subsets and mechanisms55. Upon allergen exposure, peritoneal B-1 cells (antigen-specific B-1 cells) accumulates in lymph nodes and play their role in early inflammation by T cell recruitment and antibodies production (mainly IgM) to elicit CHS responses56,57,58. Interestingly, CD22-deficient (CD22-/-) mice exhibit prolonged CHS responses. While CD22-/- mice have increased numbers of IL-10-producing peritoneal B-1a cells, these cells fail to suppress CHS effectively, due to impaired migration or survival in lymphoid organs. In contrast, adoptive transfer of wild-type peritoneal B-1a cells, but not IL-10-deficient B-1a cells, resolve prolonged CHS in CD22-/- mice, confirming their regulatory function via IL-10 production55.
Nevertheless, our study has some limitations. First, the reduction in Tregs can potentially be a consequence of other CD4⁺ subsets expansion. Therefore, further validation and analysis of these CD4 + T cell subsets is warranted. In addition, small fraction of activated T cells Natural killer cells and plasmacytoid dendritic cells have been shown to express B220 + marker59,60,61. Therefore, other B cell markers like CD19 should also be used in parallel. Furthermore, while our study demonstrates the short-term impact of pentanoate-stimulated B cells on CHS, future studies to evaluate the long-term efficacy of pentanoate-induced Bregs are suggestive.
In conclusion, In ACD, IL-10-producing B cells exhibit anti-inflammatory effects, and pentanoate can effectively modulate B cells into an IL-10-expressing phenotype. These findings highlight the potential of pentanoate as a therapeutic strategy for mitigating the inflammation in ACD. Further studies are needed to evaluate the long-term efficacy of pentanoate-induced Bregs in inflammatory conditions.
Data availability
All data supporting the findings of this study are available within the article and can be obtained from K.M.
References
Gaspari, A. A., Katz, S. I. & Martin, S. F. Contact hypersensitivity. Curr. Protoc. Immunol. 113, 421–427 (2016).
Scheinman, P. L. et al. Contact dermatitis. Nat. Rev. Dis. Primers. 7 (1), 38 (2021).
Sallusto, F. et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401 (6754), 708–712 (1999).
Gaide, O. et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat. Med. 21 (6), 647–653 (2015).
Koh, C. H. et al. CD8 T-cell subsets: heterogeneity, functions, and therapeutic potential. Exp. Mol. Med. 55 (11), 2287–2299 (2023).
Laidlaw, B. J., Craft, J. E. & Kaech, S. M. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat. Rev. Immunol. 16 (2), 102–111 (2016).
Honda, T. et al. Update of immune events in the murine contact hypersensitivity model: toward the Understanding of allergic contact dermatitis. J. Invest. Dermatol. 133 (2), 303–315 (2013).
Azeem, M. et al. Intricate relationship between adaptive and innate immune system in allergic contact dermatitis. Yale J. Biol. Med. 93 (5), 699–709 (2020).
Akiba, H. et al. Skin inflammation during contact hypersensitivity is mediated by early recruitment of CD8 + T cytotoxic 1 cells inducing keratinocyte apoptosis. J. Immunol. 168 (6), 3079–3087 (2002).
Kaplan, D. H., Igyarto, B. Z. & Gaspari, A. A. early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol, 12(2): pp. 114 – 24. (2012).
Martin, S. et al. A high frequency of allergen-specific CD8 + Tc1 cells is associated with the murine immune response to the contact sensitizer Trinitrophenyl. Exp. Dermatol. 12 (1), 78–85 (2003).
Catalan, D. et al. Immunosuppressive mechanisms of regulatory B cells. Front. Immunol. 12, 611795 (2021).
Fillatreau, S. et al. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3 (10), 944–950 (2002).
Katz, S. I., Parker, D. & Turk, J. L. B-cell suppression of delayed hypersensitivity reactions. Nature 251 (5475), 550–551 (1974).
Aira, L. E. & Debes, G. F. Skin-homing regulatory B cells required for suppression of cutaneous inflammation. J. Invest. Dermatol. 141 (8), 1995–2005 (2021). e6.
Yanaba, K. et al. A regulatory B cell subset with a unique CD1dhiCD5 + phenotype controls T cell-dependent inflammatory responses. Immunity 28 (5), 639–650 (2008).
Alrefai, H. et al. NFATc1 supports imiquimod-induced skin inflammation by suppressing IL-10 synthesis in B cells. Nat. Commun. 7, 11724 (2016).
Watanabe, R. et al. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am. J. Pathol. 171 (2), 560–570 (2007).
Wu, W. et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 10 (4), 946–956 (2017).
Yao, Y. et al. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 62 (1), 1–12 (2022).
Correa-Oliveira, R. et al. Regulation of immune cell function by short-chain fatty acids. Clin. Transl Immunol. 5 (4), e73 (2016).
Kespohl, M. et al. The microbial metabolite butyrate induces expression of Th1-associated factors in CD4(+) T cells. Front. Immunol. 8, 1036 (2017).
Kim, M. et al. Gut microbial metabolites fuel host antibody responses. Cell. Host Microbe. 20 (2), 202–214 (2016).
Liu, X. F. et al. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 14, 1186892 (2023).
Luu, M. et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 10 (1), 760 (2019).
Zou, F. et al. Effects of short-chain fatty acids in inhibiting HDAC and activating p38 MAPK are critical for promoting B10 cell generation and function. Cell. Death Dis. 12 (6), 582 (2021).
Sjoblom-Hallen, A. et al. Gene expression profiling identifies STAT3 as a novel pathway for Immunomodulation by cholera toxin adjuvant. Mucosal Immunol. 3 (4), 374–386 (2010).
Funch, A. B. et al. CD8(+) tissue-resident memory T cells recruit neutrophils that are essential for flare-ups in contact dermatitis. Allergy 77 (2), 513–524 (2022).
Schmidt, J. D. et al. Rapid allergen-induced interleukin-17 and interferon-gamma secretion by skin-resident memory CD8(+) T cells. Contact Dermat. 76 (4), 218–227 (2017).
Funch, A. B. et al. Neutrophil infiltration in allergic contact dermatitis to nickel. Br. J. Dermatol. 190 (4), 569–570 (2024).
Saint-Mezard, P. et al. The role of CD4 + and CD8 + T cells in contact hypersensitivity and allergic contact dermatitis. Eur. J. Dermatol. 14 (3), 131–138 (2004).
Williams, L. et al. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J. Immunol. 172 (1), 567–576 (2004).
Trompette, A. et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 15 (5), 908–926 (2022).
Allen, I. C. Contact hypersensitivity models in mice. Methods Mol. Biol. 1032, 139–144 (2013).
Mraz, V., Geisler, C. & Bonefeld, C. M. Dendritic epidermal T cells in allergic contact dermatitis. Front. Immunol. 11, 874 (2020).
Azeem, M. et al. NFATc1 fosters allergic contact dermatitis responses by enhancing the induction of IL-17-producing CD8 cells. J. Invest. Dermatol. 145 (8), 1995–2006 (2025). e5.
Boonpiyathad, T. et al. The role of Treg cell subsets in allergic disease. Asian Pac. J. Allergy Immunol. 38 (3), 139–149 (2020).
Gorbachev, A. V. & Fairchild, R. L. regulatory role of CD4 + T cells during the development of contact hypersensitivity responses. Immunol Res, 24(1): pp. 69–77. (2001).
Vocanson, M. et al. CD8 + T cells are effector cells of contact dermatitis to common skin allergens in mice. J. Invest. Dermatol. 126 (4), 815–820 (2006).
Funch, A. B. et al. CD4(+) T cells inhibit the generation of CD8(+) epidermal-resident memory T cells directed against clinically relevant contact allergens. Contact Dermat. 88 (6), 425–437 (2023).
Funch, A. B. et al. CD8(+) Skin-Resident Memory T Cells Require TCR Signaling for their Persistence in a Mouse Model of Allergic Contact Dermatitis (J Invest Dermatol, 2025).
Yang, S. et al. The shedding of CD62L (L-selectin) regulates the acquisition of lytic activity in human tumor reactive T lymphocytes. PLoS One. 6 (7), e22560 (2011).
Wang, K. et al. TLR4 supports the expansion of FasL(+)CD5(+)CD1d(hi) regulatory B cells, which decreases in contact hypersensitivity. Mol. Immunol. 87, 188–199 (2017).
Franziska, G. et al. NFATc1 abrogation in B cells ameliorates contact hypersensitivity responses. Int. J. Mol. Sci. 26 (17), 8125. https://doi.org/10.3390/ijms26178125 (2025).
Stanic, B. et al. IL-10-overexpressing B cells regulate innate and adaptive immune responses. J. Allergy Clin. Immunol. 135 (3), 771–780 (2015). e8.
Kader, H. A. et al. NFATc1 deficiency in B cells ameliorates atopic dermatitis. Sci. Rep. 15 (1), 25170 (2025).
Deenick, E. K. et al. Signal transducer and activator of transcription 3 control of human T and B cell responses. Front. Immunol. 9, 168 (2018).
Fan, L. et al. Wogonin suppresses IL-10 production in B cells via STAT3 and ERK signaling pathway. J. Immunol. Res. 2020, 3032425 (2020).
Oladipupo, F. O. et al. STAT3 deficiency in B cells exacerbates uveitis by promoting expansion of pathogenic lymphocytes and suppressing regulatory B cells (Bregs) and Tregs. Sci. Rep. 10 (1), 16188 (2020).
Michee-Cospolite, M. et al. Molecular mechanisms driving IL-10- producing B cells functions: STAT3 and c-MAF as underestimated central key regulators? Front. Immunol. 13, 818814 (2022).
Lenert, P. et al. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J. Clin. Immunol. 25 (1), 29–40 (2005).
Sun, C. M. et al. Upon TLR9 signaling, CD5 + B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity 22 (4), 467–477 (2005).
Golpour, F. et al. Short chain fatty acids, a possible treatment option for autoimmune diseases. Biomed. Pharmacother. 163, 114763 (2023).
Kim, C. H. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell. Mol. Immunol. 20 (4), 341–350 (2023).
Nakashima, H. et al. CD22 expression mediates the regulatory functions of peritoneal B-1a cells during the remission phase of contact hypersensitivity reactions. J. Immunol. 184 (9), 4637–4645 (2010).
Szczepanik, M. et al. B-1 B cells mediate required early T cell recruitment to elicit protein-induced delayed-type hypersensitivity. J. Immunol. 171 (11), 6225–6235 (2003).
Itakura, A. et al. An hour after immunization peritoneal B-1 cells are activated to migrate to lymphoid organs where within 1 day they produce IgM antibodies that initiate elicitation of contact sensitivity. J. Immunol. 175 (11), 7170–7178 (2005).
Campos, R. A. et al. Cutaneous immunization rapidly activates liver invariant Valpha14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J. Exp. Med. 198 (12), 1785–1796 (2003).
Marvel, J. & Mayer, A. CD45R gives Immunofluorescence and transduces signals on mouse T cells. Eur. J. Immunol. 18 (5), 825–828 (1988).
Nikolic, T. et al. A subfraction of B220(+) cells in murine bone marrow and spleen does not belong to the B cell lineage but has dendritic cell characteristics. Eur. J. Immunol. 32 (3), 686–692 (2002).
Vosshenrich, C. A. et al. CD11cloB220 + interferon-producing killer dendritic cells are activated natural killer cells. J. Exp. Med. 204 (11), 2569–2578 (2007).
Acknowledgements
This research work is funded by UAE Univeristy grants (UPAR grant G-4960 and UAEU-ZU joint research grant G-5310) to KM. Biorender was used to create Figs. 1 and 7.
Author information
Authors and Affiliations
Contributions
KM: Conceptualization, investigation, Methodology, Software, Funding acquisition, Project administration, Resources, Validation, Writing–review and editing, Writing–original draft. SA, HAK, KA, SS, DS: Data acquisition, formal analysis, Methodology. SS, YW, SK and YAD: Methodology, software, Writing-review and editing. All authors have read and approved the final version of MS to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alhmoudi, S., Kader, H.A., Alia, K. et al. Pentanoate modulates B cells to suppress allergic contact hypersensitivity. Sci Rep 15, 35803 (2025). https://doi.org/10.1038/s41598-025-19886-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19886-8