Abstract
Psoriasis is a prevalent chronic inflammatory skin disease that significantly reduces patients’ quality of life. Current treatments have limited efficacy and severe side effects, necessitating the development of new drugs. Notopterygii rhizoma et radix (Qiang Huo, QH), a Traditional Chinese Medicine (TCM) herb commonly studied for psoriasis patterns and treatment, requires further clarifications of its pharmacological mechanism. This study first verified the therapeutic effects of QH on Imiquimod (IMQ)-induced psoriasis-like mice and LPS-induced keratinocyte (HaCaT) model. Our study showed QH significantly alleviated skin symptoms, improved pathological changes, inhibited HaCaT proliferation, and reduced inflammation. Network pharmacology was then applied to explore QH’s potential mechanism, revealing its main effects on phosphoinositide-3 kinase/protein kinase-B/mammalian target of rapamycin (PI3K/Akt/mTOR), Erbb, and interleukin-17 (IL-17) signaling pathways. Further experiments using IMQ-induced mice and LPS-induced HaCaT model confirmed QH’s effects on the PI3K/Akt/mTOR pathway. Notably, this is the first study to demonstrate that QH exerts anti-psoriatic effects via modulation of the PI3K/Akt/mTOR pathway, highlighting its multi-compound, multi-target pharmacological nature. QH relieves psoriasis severity in a “multi-compound and multi-target” manner, providing insight into the application of QH in psoriasis treatment. These findings present mechanistic insights into QH’s therapeutic potential and suggest it as a promising multi-target alternative to conventional treatments for psoriasis.
Similar content being viewed by others
Introduction
Psoriasis is a multifaceted, chronic dermatological affliction, distinguished by its episodic nature, inflammatory characteristics, and pervasive systemic impact1. The condition is precipitated by a complex interaction between genetic susceptibilities and environmental factors, including infections, psychological stress, and specific pharmaceuticals2,3. Internationally, the incidence of psoriasis exhibits significant variation, ranging from a low of 0.91% in certain areas To a high of 8.5% in others, underscoring its profound implications for public health4. This disease transcends cutaneous manifestations, impacting the holistic well-being and daily functionality of those affected. It can precipitate a substantial reduction in work productivity due to the physical distress and the time demanded for therapeutic intervention and disease management5. Furthermore, the psychological burden associated with psoriasis is considerable, with patients frequently reporting increased anxiety and depressive symptoms6. The physical symptoms, such as pain, pruritus, and discomfort, can be incapacitating and markedly diminish the quality of life for individuals with the condition7.
Regarding therapeutic interventions, contemporary medical practices provide a spectrum of strategies for managing psoriasis8. Topical therapies, including corticosteroids and vitamin D analogs, typically constitute the initial treatment for mild to moderate cases. In cases of more extensive or severe psoriasis, phototherapy, which entails controlled exposure to ultraviolet radiation, can be effective in mitigating inflammation and retarding the proliferation of skin cells. Systemic immunomodulatory agents9, such as methotrexate and cyclosporine, function by dampening the hyperactive immune response, while biologics target specific immune components implicated in the pathogenesis of the disease10. Despite these therapeutic advancements, several challenges remain7. The side effects of medications can vary from mild to severe, influencing a patient’s adherence to treatment. Over time, some patients may develop resistance to certain medications, requiring a shift in therapeutic strategy. The exorbitant costs of newer biologic therapies can be a barrier for many patients, restricting access to these potentially transformative treatments. Moreover, the variability in individual responses to treatments complicates the identification of the most efficacious treatment protocol for each patient. This variability accentuates the necessity for a tailored therapeutic approach, considering each patient’s distinct genetic profile, environmental influences, and disease severity.
Consequently, it is of paramount importance to conduct an in-depth investigation into the etiology of psoriasis and to identify potent prophylactic and therapeutic interventions. Traditional Chinese Medicine (TCM), embracing a comprehensive approach to medical treatment, holds a significant position in the healthcare systems of Asia and is increasingly recognized in Western nations for its notable healing properties and limited side effects11. In accordance with the foundational tenets of TCM, this system of medicine emerges as a viable alternative for the management and treatment of multifaceted disorders, such as psoriasis12,13. Notopterygii Rhizoma et Radix (QH)14, a TCM herb renowned for its wind-dispelling, dampness-removing, and cold-pain-relieving properties, is extensively utilized in classical formulations for psoriasis treatment, such as the Wind-Expelling and Toxin-Clearing Decoction15. However, the scientific rationale and pharmacological mechanisms underlying QH are not well understood and warrant further exploration.
Broadly speaking, the pharmacological impact of TCM herbs is exerted via a multitude of targets and intracellular signaling cascades, which complicates the identification of the intricate active principles of TCM when relying solely on traditional methodologies16,17,18. Consequently, there is a pressing requirement for the conception and implementation of novel, tailored methodologies. Despite the extensive historical use of QH in TCM formulations for psoriasis, there remains a significant gap in understanding its specific bioactive constituents, molecular targets, and mechanisms of action. This lack of mechanistic clarity hinders its integration into evidence-based therapeutic frameworks. Therefore, the central research question guiding this study is: What are the active compounds and molecular pathways through which QH exerts its therapeutic effects in psoriasis? Given the multicomponent and multitarget nature of TCM, a network pharmacology approach, supplemented by experimental validation, provides a robust and systematic method to decode the complex interactions involved. This integrative strategy is particularly well-suited to bridge traditional empirical knowledge with modern biomedical insights, thereby justifying its application in the current study. This approach has successfully established a solid experimental substrate for the advancement and clinical deployment of QH as a therapeutic agent for psoriasis.
Methods
Preparation and characteristics of QH
Briefly, dried medicinal material of Notopterygii Rhizoma et Radix were ground into powder and extracted by hot water (w: v = 1:20). Heat the mixture To boiling and simmer for 1 h. After the decoction is complete, allow the mixture To cool naturally To room temperature, then filter the decocted mixture using a filter and concentrate the filtrate using a rotary evaporator. Aliquot the concentrate into sterile centrifuge tubes and store as 4℃ in a refrigerator. The preparation method employed here adheres to standard decoction protocols commonly used in TCM studies to reflect clinical usage. Although this extraction method effectively simulates in vivo conditions, further validation of the chemical composition of QH is warranted. In this study, chemical profiling via HPLC or LC-MS was not conducted; however, network pharmacology predictions were based on documented constituents from TCMSP and literature sources. Future work will focus on employing chromatographic techniques to verify the presence and concentration of key bioactive compounds, such as Diosmetin, Phellopterin, and β-sitosterol, to enhance the correlation between extract composition and predicted targets. In addition, to better understand the mechanistic pathways involved, further experiments incorporated PI3K and Akt inhibitors to assess their influence on QH-mediated effects in psoriasis models.
Animals and treatments
Female Balb/c mice, aged 6 to 8 weeks, were accommodated in the vivarium of Nanjing University of Chinese Medicine. Ethical approval for this study was granted by the Institutional Animal Care and Use Committee of Nanjing University of Chinese Medicine, with the application number 202,303–0140, on the 13th of March, 2023. Following a one-week period of environmental acclimation, the mice were randomly assigned to one of six experimental groups, each consisting of eight individuals: (1) a control group; (2) a disease model group; (3) a methotrexate (MTX) group; (4) a low-dose QH treatment group (1.3 g/kg); (5) a medium-dose QH treatment group (2.6 g/kg); (6) a high-dose QH treatment group (5.2 g/kg). Except for the control group, all mice received a daily Topical application of 62.5 mg IMQ on their depilated dorsal skin for a period of seven days. Commencing from the second day post-modeling, the control group mice received Vaseline on their depilated dorsal regions. The QH treatment groups were orally administered QH at low, medium, and high doses, twice daily for seven consecutive days. The oral gavage was conducted using a 0.5% carboxymethyl cellulose (CMC) solution as the vehicle for QH administration, which was prepared fresh daily. The methotrexate group received a methotrexate dosage of 0.98 mg/kg orally, while the blank and model groups were given an equivalent volume of saline solution orally on a daily basis for seven days. After isoflurane anesthesia induction, cardiac puncture was performed To collect blood samples, and the mice were subsequently euthanized via cervical dislocation. Skin tissue samples were collected; some were fixed in a 10% formalin solution for subsequent Hematoxylin-eosin (HE) staining and immunofluorescence assays, while others were placed in pre-chilled phosphate-buffered saline (PBS) for ELISA and quantitative polymerase chain reaction (qPCR) analyses. The oral dose of MTX (0.98 mg/kg/day) was selected based on previously published studies demonstrating its efficacy in short-term murine models of psoriasis, while minimizing systemic Toxicity. Notably, during the 7-day administration period, no signs of gastrointestinal or major organ toxicity were observed in any treatment group, including those receiving MTX or the highest dose of QH (5.2 g/kg/day), as assessed by clinical observations and histological examination of major organs (data not shown). These findings suggest that the selected concentrations of both MTX and QH were well-tolerated over the short-term treatment window. The selection of oral administration for QH was based on its traditional clinical use as a decoction in Chinese medicine, where systemic delivery is standard. This route ensures consistency with pharmacological modeling and reflects clinical translational relevance. In contrast, Vaseline was topically applied in the control group to match the handling and occlusive exposure associated with topical IMQ application, thereby controlling for local skin effects unrelated to the disease model. No Vaseline was applied to QH or MTX groups to avoid potential interference with systemic treatment evaluation. To further elucidate the role of the PI3K/Akt/mTOR pathway in the therapeutic effects of QH, the administration of PI3K and Akt inhibitors (LY294002 and MK-2206, respectively) was incorporated into future experiments. These could help assess if the observed effects would be due to direct modulation of these pathways.
Histological examination
Mice dorsal skin specimens were postfixed in a 4% paraformaldehyde solution, subsequently embedded in paraffin wax, and subjected to hematoxylin-eosin staining. Thereafter, the stained sections were examined microscopically. The assessment included the observation of psoriatic skin pathology, scoring in accordance with the Baker scoring system19, and the quantification of epidermal thickness was performed by measuring the distance from the basal membrane To the stratum corneum using ImageJ software at a magnification of 20x. For each group, five distinct regions of the dorsal skin were measured, and the average thickness was calculated. The Baker scoring system is a semi-quantitative histopathological grading method used to evaluate epidermal alterations, such as hyperkeratosis, parakeratosis, acanthosis, and inflammatory cell infiltration was quantified by counting the number of inflammatory cells within the dermal layers, using a high-power field (40x magnification) for each section. The number of cells was normalized to the area of the tissue section. These methodologies provide a reproducible and objective evaluation of the psoriatic skin pathology.
In vitro cellular cultivation and manipulation
Human keratinocyte HaCaT cells were propagated in Dulbecco’s Modified Eagle Medium, supplemented with 1% antibiotic-antimycotic solution and 10% fetal bovine serum. With the exception of the control group, all experimental groups were exposed to lipopolysaccharide (LPS) at a concentration of 5 µg/ml to replicate the hyperproliferative and inflammatory characteristics of keratinocytes in psoriasis. A comprehensive set of assays was executed, encompassing the assessment of cellular viability using the CCK8 assay, the measurement of inflammatory cytokine expression through qPCR and ELISA, the evaluation of the PI3K/Akt/mTOR signaling cascade via western blot analysis. Diverse concentrations of QH were administered to the cells four hours preceding the addition of LPS. The experimental design also included PI3K and Akt inhibitors (LY294002 and MK-2206) to determine whether inhibition of these pathways alters the cellular response to QH treatment.
Enzyme-linked Immunosorbent Assay (ELISA)
The levels of interleukin-23 (IL-23), IL-17, IL-22, and tumor necrosis factor-α (TNF-α) in both serum and cell supernatant were quantified utilizing commercial ELISA kits from eBioscience, following the manufacturer’s protocol.
Network pharmacology analysis
Data compilation: Comprehensive data on the constituents of QH were sourced from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP)20 and pertinent literature. The aggregated data were then integrated into the ingredient database.
Oral bioavailability (OB) and Drug-Likeness filtering: Oral bioavailability (OB) prescreening refers To the proportion of an orally administered drug dose that achieves entry into systemic circulation, representing a pivotal parameter in the development and clinical utilization of oral pharmaceuticals. The concept of drug-likeness evaluates the structural similarity of compounds To those already recognized for their therapeutic efficacy within the Drugbank database, a crucial assessment conducted during the initial stages of drug discovery. Employing the criteria of an OB value of 30% and a drug-likeness index threshold of 0.18, the selection of prospective compounds was executed, adhering to the meticulous procedures delineated by Wang et al.
Identification of Putative Drug Targets for QH: Potential drug targets for QH were identified using two databases: (1) TCMSP; (2) Swiss Target Prediction21. After eliminating redundant targets, a Total of 386 unique targets were curated.
Compilation of Known Psoriasis-Related Targets: Psoriasis-associated targets were extracted from three databases: (1) GeneCards human gene database22; (2) OMIM database23; (3) DrugBank database24. After redundancy removal, a Total of 2130 targets were selected.
Protein-Protein interaction (PPI) network construction: A network intersection was constructed, encompassing the putative targets of QH and the known psoriasis-related targets, utilizing protein-protein interaction (PPI) data sourced from the String database25. This construction was predicated on a minimum interaction score threshold set at 0.400, specific to Homo sapiens. The resultant PPI network, formatted as TSV, was subsequently imported into Cytoscape for further analysis. Within this platform, the connectivity degree of each node, representing a target, was ascertained employing the NetworkAnalyzer plugin.
Gene ontology (GO)-Biological process (BP) and Kyoto encyclopedia of genes and genomes (KEGG) enrichment analyses: The functional roles of the targets were investigated by cross-referencing them with the Omicshare26 database. A significance threshold was established at a p ≤ 0.05, which was followed by the application of hypergeometric testing to ascertain the enrichment of GO and KEGG pathway terms27,28,29.
Immunofluorescence staining
Immunofluorescence staining was performed following established protocols. Skin tissue sections were fixed, and antibodies targeting p-PI3K, p-Akt, and p-mTOR (Cell Signaling Technology (CST), catalog numbers: p-PI3K #4228, p-Akt #4060, and p-mTOR #5536; all at 1:300 dilution) were applied for staining. Secondary antibodies (goat anti-rabbit IgG H&L, Alexa Fluor® 488, Abcam, ab150077; 1:1000 dilution) specific to the respective antigens were deployed to facilitate the visualization of the aforementioned phosphorylated proteins.
Western blot analysis
Cellular or tissue lysates were generated using a lysis buffer, subsequent To which proteins underwent denaturation and were subjected To electrophoresis on 10% bis-Tris/polyacrylamide gels. The resolved proteins were then transferred onto polyvinylidene fluoride (PVDF) membranes. These membranes were initially blocked to prevent non-specific binding, after which they were incubated with primary antibodies (p-PI3K #4228, p-Akt #4060, p-mTOR #5536, and GAPDH #5174, all from CST, used at 1:1000 dilution) under refrigerated conditions at 4 °C overnight. Subsequently, the membranes were incubated with HRP-conjugated secondary antibodies (goat anti-rabbit IgG-HRP, CST, #7074; 1:10000 dilution) at ambient temperature. The detection of immunoreactive bands was accomplished utilizing an enhanced chemiluminescence detection system (Thermo Fisher Scientific, SuperSignal™ West Pico PLUS, #34577).
Quantitative Real-time Polymerase Chain Reaction (qPCR)
RNA was isolated from cellular samples utilizing the Novogene Total RNA Extraction Kit, which was subsequently reverse transcribed to yield complementary DNA (cDNA). qPCR was performed utilizing the Novogene SYBR qPCR Mix on the Bio-Rad CFX Connect Real-Time PCR Detection System, with data analysis conducted employing the 2−ΔΔCt methodology. The sequences of the primers designed for the amplification of IL-17, TNF-α, IL-22, IL-23, and GAPDH genes are detailed hereinafter:
TCCCACGAAATCCAGGATGC (forward) and GGATGTTCAGGTTGACCATCAC (reverse) for IL-17; CCTCTCTCTAATCAGCCCTCTG (forward) and GAGGACCTGGGAGTAGATGAG (reverse) for TNF-α; GCTTGACAAGTCCAACTTCCA (forward) and GCTCACTCATACTGACTCCGT (reverse) for IL-22; A CTCAGGGACAACAGTCAGTTC (forward) and ACAGGGCTATCAGGGAGCA (reverse) for IL-23; GGAGCGAGATCCCTCCAAAAT (forward) and GGCTGTTGTCATACTTCTCATGG (reverse) for GAPDH.
Psoriasis Area and Severity Index (PASI) scoring system
The severity of psoriasis-like lesions was quantified using the PASI. The PASI score is a commonly used metric to assess the severity of psoriasis based on the extent and intensity of erythema, induration, and desquamation in affected areas of the skin. The body was divided into four regions: head, upper limbs, trunk, and lower limbs. The percentage of affected area (ranging from 0 to 100%) and the severity of erythema, induration, and desquamation (scored from 0 to 4 for each feature) were used to calculate a composite PASI score for each mouse. This PASI score was used to evaluate the degree of lesion severity in the dorsal skin of the mice treated with IMQ, as well as in the QH and methotrexate treatment groups. The PASI score for each animal was recorded daily during the treatment period, and a reduction in PASI score was considered indicative of therapeutic efficacy.
Statistical analysis
The results are depicted as the mean ± standard deviation, and were subjected To statistical analysis using SPSS version 17.0. To determine the presence of statistically significant disparities, an Analysis of Variance (ANOVA) was initially conducted, followed by Dunnett’s post hoc test. A p threshold of less than 0.05 was established to denote statistical significance.
Results
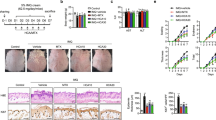
QH ameliorates psoriasis-like lesions and pathological histological changes in IMQ-induced mice
Significantly, when contrasted with the control cohort, the dorsal skin of mice treated with IMQ displayed pronounced psoriasis-like pathologies, including increased epidermal thickness, erythematous changes, and the presence of scales (Fig. 1A). In contrast to the IMQ-only group, the severity of these dermatological lesions was substantially reduced by both QH and MTX interventions (Fig. 1A). The Psoriasis Area and Severity Index (PASI) was utilized to quantify the severity of dorsal lesions, revealing a statistically significant escalation in PASI and aggregate scores post-IMQ treatment (Fig. 1B, p < 0.05). In contrast, sustained QH treatment led to a significant decrease in PASI and aggregate scores (p < 0.05), and it was similarly noted in the MTX cohort (Fig. 1B, p < 0.05).
The ameliorative effects of QH on IMQ-induced psoriasis-like skin lesions in mice. (A) The dorsal skin samples on day 7 post-treatment. (B) The PASI scores for erythema, scaling, thickening, and cumulative assessments. Data are expressed as Mean ± SD for n = 8 mice per group. Statistical significance was determined using ANOVA. Compared to the Model group: *indicates p < 0.05, **indicates p < 0.01, and ***indicates p < 0.001.
Moreover, histopathological analysis disclosed the presence of hyperkeratosis, incomplete keratinization, spinous layer thickening, and inflammatory cell infiltration in the dorsal skin of mice in the IMQ group (Fig. 2A). Compared to the control group, there was a significant increase in epidermal thickness and Baker scores within the IMQ group (Fig. 2B, p < 0.05). Conversely, both MTX and QH treatments significantly ameliorated these histopathological changes, with a marked decrease in epidermal thickness and Baker scores (Fig. 2B, p < 0.05). Notably, the high-dose QH treatment demonstrated the most significant impact on reducing epidermal thickening (Fig. 2B).
QH alleviates histopathological alterations in IMQ-induced psoriatic mice. (A) Representative H&E-stained sections of dorsal skin from each group, highlighting differences in epidermal architecture and inflammatory cell infiltration. Scale bar = 250 μm. (B) Quantitative analysis of epidermal thickness and Baker scores, based on standardized pathological criteria. Data are presented as mean ± SD (n = 8 mice per group). Statistical analysis was performed using one-way ANOVA. Compared to the Control group: ###indicates p < 0.001. Compared to the Model group: *indicates p < 0.05 and ***indicates p < 0.001.
Although our histopathological analysis identified inflammatory cell infiltration in IMQ-induced lesions, no specific immune cell markers (e.g., CD3, CD11c, F4/80) were employed to characterize the infiltrating immune cell subtypes in vivo. Therefore, while QH significantly reduced inflammation and epidermal thickening, the contributions of immune cells to these therapeutic effects could not be conclusively excluded. Further immunophenotyping studies are warranted to delineate whether QH exerts its effects primarily through keratinocyte-intrinsic mechanisms or also via modulation of immune cell activity in the psoriatic microenvironment.
QH ameliorates inflammatory responses in IMQ-provoked psoriasis-Like conditions in mice
Cytokine overexpression is a pivotal element in the etiology of psoriasis30, with the serum concentrations of these inflammatory mediators in mice acting as proxies for disease advancement. As illustrated in Fig. 3, the induction of IMQ treatment led to a marked elevation in the serum concentrations of TNF-α, IL-17, IL-22, and IL-23. Post-intervention with QH, there was a noteworthy decrease in the levels of these pro-inflammatory cytokines (Fig. 3, p < 0.05).
The mitigation of IMQ-induced inflammatory responses in mice by QH. As evidenced by the serum levels of TNF-α, IL-17, IL-22, and IL-23. Data are Mean ± SD for n = 8 mice per group, with ANOVA employed for statistical comparisons. Compared to the Control group: ###indicates p < 0.001. Compared to the Model group: ***indicates p < 0.001.
QH suppresses dysregulated proliferation and inflammatory reactions in LPS-stimulated HaCaT cell lines
In vitro studies conducted To assess the impact of QH on the LPS-mediated aberrant proliferation and inflammatory reactions in Human keratinocytes revealed significant findings. Notably, within the concentration range of 0.5–16 mg/ml, QH did not induce any marked cytotoxicity in the cells, as shown in Fig. 4A. Figure 4B depicts that QH, at varying concentrations, substantially mitigated the LPS-triggered abnormal proliferation of HaCaT cells, with a notably high inhibition efficacy of 40% observed at a concentration of 16 mg/ml (p < 0.05). Consequently, for further experimental investigations, QH concentrations of 4 mg/ml and 8 mg/ml were selected.
The inhibitory effects of QH on the abnormal proliferation and inflammatory reactions in LPS-stimulated HaCaT cell lines. (A) The viability impact of QH on HaCaT cells. (B) The viability effects of QH on LPS-stimulated HaCaT cells. (C-D) The expression (C) and Mrna (D) levels of TNF-α, IL-17, IL-22, and IL-23 in HaCaT cells. Data are Mean ± SD for n = 3 replicates. ANOVA was used to assess differences. Compared to the Control group: ###indicates p < 0.001. Compared to the Model group: *indicates p < 0.05 and ***indicates p < 0.001.
The cytokine profiling analysis in vitro indicated that LPS challenge led to a pronounced increase in both protein and mRNA levels of key inflammatory biomarkers—TNF-α, IL-17, IL-22, and IL-23—in HaCaT cells (Fig. 4C and D). The application of QH, across different concentrations, resulted in a significant suppression of these inflammatory markers (Fig. 4C and D, p < 0.05). These results underscore the potential of QH in significantly alleviating the abnormal proliferative and inflammatory activities in LPS-stimulated HaCaT cells.
Core targets of QH in psoriasis treatment
Significant efforts have been made to elucidate the therapeutic mechanisms of TCM; however, progress at the molecular level has been limited. Given the absence of effective methodologies specifically designed to identify active compounds in medicinal herbs, the integration of oral bioavailability (OB) screening with drug-likeness assessments presents a viable strategy. In this study, a Total of 15 candidate compounds exhibiting favorable values for these parameters were identified from QH (Table S1). Within this suite of compounds, three have been documented for their diverse pharmacological activities and their roles in modulating a spectrum of pathological processes related to dermatologic diseases, such as Diosmetin, Phellopterin, and β-sitosterol31,32,33. Among them, Diosmetin has been reported to exert anti-inflammatory effects by inhibiting IL-6 and TNF-α expression via the NF-κB and MAPK pathways. Phellopterin has demonstrated antioxidant and immunomodulatory activities that may modulate cytokine balance in inflammatory conditions, while β-sitosterol is known for its anti-proliferative properties, potentially reducing keratinocyte hyperplasia through PI3K/Akt pathway modulation. These compounds may act synergistically, and Diosmetin emerges as a particularly influential bioactive due To its known regulatory effects on keratinocyte-mediated inflammation. Further studies concentrating on isolating and testing these individual components in psoriasis models will clarify their precise pharmacodynamic roles. Beyond the identification of the potential bioactive constituents of QH, it is imperative To delineate the therapeutic targets involved. In this study, the putative targets of the candidate compounds were inferred through the amalgamation of chemical, genomic, and pharmacological datasets. A collective Total of 386 putative targets were ascertained for the 15 candidate compounds (Table S2), leveraging information from the TCMSP and the Swiss Target Prediction database.
Psoriasis is recognized as a polygenic predisposition disorder, with gene-environment interactions contributing to its pathogenesis34. In this study, 2130 psoriasis-associated targets were identified from three existing databases. Notably, 128 common putative targets were shared between QH and known psoriasis-related targets, which were identified as candidate targets for QH in the treatment of psoriasis (Fig. 5A and Table S3). Cytoscape was employed to construct the network of active ingredients and candidate targets (Fig. 5B), comprising 144 nodes and 444 edges in total.
The core targets of QH in the treatment of psoriasis. (A) Venn diagram comparing putative QH targets with known psoriasis-related targets. (B) The herb-compounds-targets network. (C) The protein-protein interactions of candidate targets for QH in psoriasis treatment. (D) The Top 20 core targets of QH in psoriasis therapy.
To further identify the core targets of QH in psoriasis treatment, the 128 potential targets were imported into the String database to construct a protein-protein interaction (PPI) network, which included 128 nodes and 1044 edges (Fig. 5C). Using the NetworkAnalyzer tool35 for topological analysis, core targets were selected based on their degree values. To ensure objective and reproducible target prioritization, degree centrality was used as the principal metric during topological analysis in Cytoscape. This parameter reflects the number of direct interactions a node (protein) has in the network, indicating its potential regulatory importance. The Top 20 targets with the highest degree values were selected as core targets for further analysis, under the assumption that they play fundamental roles in the compound-target-disease network. These targets were then subjected to pathway enrichment analysis to identify signaling cascades most significantly associated with QH’s therapeutic effects. Pathways were prioritized based on statistical significance (p < 0.05) and the number of involved core targets, with particular emphasis placed on psoriasis-relevant immune and proliferation-related pathways, such as PI3K/Akt/mTOR. The Top 20 targets ranked by degree were visualized using R 4.2.1 to create a bar chart (Fig. 5D).
Functional annotation of core targets through KEGG and GO pathway enrichment analysis
In the KEGG pathway enrichment analysis conducted on the 20 core targets, a Total of 72 pathways were found to be significantly enriched. The pathways that were most pertinent to psoriasis comprised the PI3K/Akt signaling pathway, ErbB signaling pathway, IL-17 signaling pathway, TNF signaling pathway, and mTOR signaling pathway (Fig. 6A). Within these pathways, the PI3K/Akt signaling pathway was notably prominent, with 10 targets being enriched therein, while the mTOR signaling pathway engaged five targets. The targets predominantly enriched within the PI3K/Akt/mTOR signaling cascade included HSP90AA1, Akt1, JAK2, MTOR, and MAPK1, which justified prioritizing this pathway for further experimental scrutiny.
Functional annotation of core targets through GO and KEGG pathway enrichment analysis. (A) KEGG pathway enrichment analysis on the core targets (see the reference below: Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. and Ishiguro-Watanabe, M.; KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53, D672-D677 (2025)). (B) GO enrichment analysis on the core targets.
In the Gene Ontology (GO) enrichment analysis of the same 20 core targets, the biological processes that were predominantly represented encompassed reproductive processes, immune system processes, responses to stimuli, and metabolic processes. The cellular components were largely confined to protein-containing complexes and cellular anatomical entities, whereas molecular functions were chiefly related to molecular transducer activities and ATP-dependent activities (Fig. 6B).
Nevertheless, while the PI3K/Akt/mTOR signaling pathway was prioritized due to its prominent enrichment and centrality within the network, it is acknowledged that this pathway alone may not fully encapsulate the multifaceted mechanisms underlying QH’s therapeutic effects. Other enriched pathways, such as the IL-17, TNF, and ErbB signaling pathways, may also play significant roles in psoriasis pathophysiology. Further investigations incorporating these pathways, as well as studies exploring downstream cellular responses, such as apoptosis, differentiation, and oxidative stress, are warranted to provide a more comprehensive understanding of QH’s mode of action.
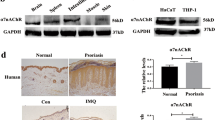
QH modulates the PI3K/Akt/mTOR signaling cascade in skin lesions resembling psoriasis and in LPS-stimulated HaCaT cells
The PI3K/Akt/mTOR signaling cascade, which includes the proteins PI3K, Akt, and mTOR, was recognized as the predominant pathway associated with the action of QH in the realm of psoriasis. Utilizing a triple immunofluorescence staining protocol, specific antibodies were deployed to assess the phosphorylation levels of PI3K, Akt, and mTOR. In comparison to the control cohort, the phosphorylation levels of these proteins were markedly increased in response to IMQ treatment (Fig. 7A). Conversely, QH administration significantly abrogated the phosphorylation of PI3K and Akt in a dose-responsive manner, similar to the effects observed with methotrexate (MTX); nonetheless, MTX showed a less pronounced impact on mTOR phosphorylation compared to QH (Fig. 7A). These observations were further validated through western blot analysis (Fig. 7B).
The modulation of the PI3K/Akt/mTOR signaling pathway by QH in skin lesions resembling psoriasis and in LPS-stimulated HaCaT cells. (A) The expression levels of p-PI3K, p-Akt, and p-mTOR in mouse skin samples via immunofluorescence assay, with intensity analysis shown below. (B) The expression levels of these proteins in mouse skin samples by western-blot assay, with gray-scale analysis on the right. (C) The expression levels of p-PI3K, PI3K, p-Akt, Akt, mTOR, and p-mTOR in LPS-induced HaCaT cells, with gray-scale analysis provided. Data are Mean ± SD for n = 8 in skin tissues or n = 3 in HaCaT cells, with ANOVA used for statistical comparisons. Compared to the Model group: *indicates p < 0.05, **indicates p < 0.01, and ***indicates p < 0.001.
Furthermore, the modulatory impact of QH on the PI3K/Akt/mTOR signaling cascade in LPS-stimulated HaCaT cells was evaluated in vitro. As depicted in Fig. 7A and C, the expression levels of Akt, phosphorylated Akt (p-Akt), PI3K, phosphorylated PI3K (p-PI3K), mTOR, and phosphorylated mTOR (p-mTOR) were scrutinized. Relative to the control group, the overall levels of Akt, PI3K, and mTOR in the LPS group were not significantly altered; however, the phosphorylation ratios of p-Akt/Akt, p-PI3K/PI3K, and p-mTOR/mTOR were notably elevated following LPS stimulation of HaCaT cells (Fig. 7C, p < 0.05). Quantitative analysis indicated that treatment with 4 mg/ml QH led to a significant decrease in the expression levels of p-PI3K, p-Akt, and p-mTOR, with an even more pronounced effect observed at a concentration of 8 mg/ml QH. To further elucidate whether QH directly modulates the PI3K/Akt/mTOR signaling pathways or acts via upstream or parallel pathways, co-treatment assays were conducted using specific inhibitors of PI3K (LY294002) and Akt (MK-2206) in LPS-stimulated HaCaT cells. The results demonstrated that LY294002 and MK-2206 alone significantly decreased the phosphorylation levels of their respective targets. Notably, the combination of QH with either inhibitor did not lead to an additive or synergistic reduction in phosphorylation compared to QH treatment alone, suggesting that QH may exert its effects predominantly through this pathway. These findings indicate that the anti-inflammatory and anti-proliferative actions of QH are, at least in part, mediated by inhibition of the PI3K/Akt/mTOR signaling pathways.
Discussion
In this study, the daily oral administration of QH has been demonstrated to reduce cutaneous manifestations such as erythema, plaque elevation, and scaling, and has shown a remarkable capacity to mitigate pathological changes in psoriatic lesions. Moreover, recent research has shifted the paradigm of psoriasis pathogenesis, highlighting the central role of keratinocytes rather than immune cells alone, thereby unveiling new potential targets for therapeutic intervention36,37,38. Throughout the pathogenesis of psoriasis, there is an escalation in the proliferative capacity of keratinocytes, which results in impaired cellular differentiation, culminating in epidermal hyperplasia and the formation of scales39. Experimental evidence garnered from in vitro assays has demonstrated that QH, administered at two specific concentrations, potently suppresses the LPS-induced aberrant proliferation of HaCaT cells. These results, corroborated by a combination of in vitro and in vivo experimental data, affirm the therapeutic potential of QH in mitigating the dysregulated proliferation of keratinocytes and ameliorating the lesions characteristic of psoriasis.
Keratinocytes play a dual role in the pathogenesis of psoriasis by not only initiating the onset of the disease but also aggravating psoriatic inflammation during its chronic phase40. This is achieved through the maintenance of epidermal hyperplasia via the secretion of diverse cytokines, which amplify local inflammatory responses and sustain the inflammatory cascade. Experimental studies using IMQ in mouse models have demonstrated its ability to induce the production of cytokines such as IL-6, IL-22, and IL-23. Notably, natural compounds found in QH, including Diosmetin, have been shown to effectively reduce the aberrant expression of these inflammatory mediators, thereby mitigating both the onset and progression of psoriasis. Our findings further indicate that QH significantly suppresses the expression of inflammatory cytokines in keratinocytes. Importantly, IL-23 has been implicated in promoting the proliferation and survival of Th17 cells by upregulating IL-6, IL-1β, and TNF-α via a feedback mechanism41. While previous research predominantly emphasized IL-23 derived from immune cells, recent evidence highlights the capacity of keratinocyte-derived IL-23 to activate IL-17-producing immune cells42. IL-17, in turn, acts as a critical mediator linking innate and adaptive immunity, driving keratinocyte secretion of IL-6, IL-22, and TNF-α, inducing epidermal hyperproliferation, enhancing neutrophil maturation and chemotaxis, promoting inflammatory cell aggregation, and establishing a positive feedback loop that perpetuates chronic skin inflammation43.
Network pharmacology, an integrative framework combining systems biology, network analysis, connectivity, redundancy, and pleiotropy, offers a modern approach to drug discovery that accelerates development and improves success rates. This methodology systematically constructs and analyzes the “compound-protein/gene-disease” network. Recently, it has been employed not only for identifying novel therapeutic agents but also for elucidating the mechanisms of traditional prescriptions, repurposing existing drugs, and uncovering potential side effects. In this study, network pharmacology was applied to explore the therapeutic potential and mechanisms of QH in psoriasis treatment. Through database mining, protein-protein interaction (PPI) network construction, and Topological analysis, 20 core targets were identified. KEGG pathway enrichment analysis revealed that these targets were involved in 72 pathways, with the PI3K/Akt/mTOR signaling pathway emerging as the most pivotal. This pathway plays a central role in regulating cytokine-mediated inflammatory responses, particularly in inflammatory skin diseases. In psoriasis, the overactivation of PI3K in serum and lesion tissues leads to Akt activation and subsequent mTOR phosphorylation, driving inflammation, excessive keratinocyte proliferation, and angiogenesis, all of which contribute to disease progression44.
The PI3K/Akt/mTOR signaling axis plays a pivotal role in regulating keratinocyte proliferation, differentiation, and survival, as key cellular processes that are aberrantly activated in psoriatic lesions. Hyperactivation of this pathway in keratinocytes not only fuels epidermal hyperplasia, but also perpetuates inflammation by enhancing the production of pro-inflammatory cytokines. The results of the present study demonstrated that QH significantly attenuated the phosphorylation of PI3K, Akt, and mTOR in both in vivo and in vitro models, particularly in keratinocytes, suggesting that QH could directly disrupt this pathogenic signaling pathway. This modulation may contribute to the suppression of keratinocyte hyperproliferation and cytokine overproduction, positioning QH as a multi-targeted modulator that intervenes at a critical nexus of psoriasis pathophysiology. It is possible that inhibiting PI3K or Akt alone could trigger compensatory feedback loops or activate alternative signaling pathways, which could restore some degree of PI3K/Akt/mTOR signaling, thereby diminishing the expected additive effect of combination treatment. Furthermore, QH could influence upstream or parallel pathways, such as TNF, IL-17, or ErbB signaling, intersecting with the PI3K/Akt/mTOR pathway and could contribute to its therapeutic effects. Consequently, the addition of pathway-specific inhibitors might not further reduce phosphorylation if these other pathways compensate for the inhibition. It is also noteworthy that the inhibitors used in this study (LY294002 for PI3K and MK-2206 for Akt) might not fully inhibit their respective targets under the experimental conditions, and incomplete inhibition could explain the absence of enhanced effects when combined with QH. While the PI3K/Akt/mTOR pathway plays a key role in psoriasis, QH’s mechanism may not be entirely dependent on this pathway. QH might also exert its effects through other signaling networks, such as IL-17 or TNF pathways, which could contribute to the observed therapeutic outcomes independently of PI3K/Akt/mTOR modulation. Therefore, the lack of a synergistic effect between QH and pathway-specific inhibitors does not invalidate the role of PI3K/Akt/mTOR in QH’s action, but rather highlights the complexity of the underlying mechanisms, demonstrating the involvement of multiple, parallel pathways.
While QH demonstrated comparable efficacy to MTX in reducing epidermal thickness, PASI scores, and inflammatory cytokine levels in both in vivo and in vitro models, its mechanistic profile suggests additional advantages. Notably, QH significantly suppressed the phosphorylation of mTOR, an effect that was less evident in the MTX-treated group, indicating a potentially broader modulation of the PI3K/Akt/mTOR axis. Moreover, in contrast to targeted inhibitors, such as rapamycin, which primarily block mTORC1 activity, QH could simultaneously downregulate upstream components, including PI3K and Akt. This multi-target modulation may confirm more comprehensive anti-inflammatory and anti-proliferative effects. Additionally, while biologics targeting IL-17 and IL-23 (e.g., secukinumab and ustekinumab) present a high efficacy, they are costly, administered via injection, and associated with immunosuppressive risks. Hence, QH’s oral bioavailability, safety profile, and low production cost position it as a promising alternative or adjunct, particularly for patients who are refractory to or unable to tolerate conventional systemic or biologic therapies. Further comparative preclinical and clinical studies are warranted to establish the relative therapeutic index and long-term safety of QH vis-à-vis established psoriasis treatments.
Rapamycin, a well-known inhibitor of mechanistic target of rapamycin complex 1 (mTORC1), has demonstrated efficacy in reducing inflammation and suppressing epidermal hyperplasia by targeting mTOR45. Similarly, this study observed that QH significantly reduced the phosphorylation of PI3K, Akt, and mTOR in both IMQ-induced psoriasis-like lesions and LPS-stimulated HaCaT cells. These findings suggest that QH functions as an inhibitory herb of the PI3K/Akt/mTOR pathway, offering a potential therapeutic alternative for psoriasis. Compared to conventional inhibitors, QH may present advantages in terms of cost-effectiveness, safety, and reliability. By targeting the PI3K/Akt/mTOR pathway, QH disrupts the self-perpetuating hyperproliferative inflammatory loops at multiple levels, potentially yielding better therapeutic outcomes for patients who do not respond to standard treatments. Notably, although MTX demonstrated inhibitory effects on PI3K and Akt phosphorylation in both in vivo and in vitro models, its effect on mTOR phosphorylation appeared relatively limited in our triple immunofluorescence staining assays. This observation aligns with previous research, suggesting that MTX primarily exerts its anti-inflammatory action through pathways involving adenosine signaling and suppression of pro-inflammatory cytokines, rather than directly targeting the PI3K/Akt/mTOR axis at all levels46. Moreover, mTOR phosphorylation can be regulated through multiple upstream signals beyond PI3K/Akt, including nutrient status and oxidative stress, which may not be significantly influenced by MTX treatment under our experimental conditions. Therefore, the comparatively weaker suppression of p-mTOR by MTX may reflect its indirect or context-dependent modulation of this pathway, a phenomenon that has also been reported in other inflammatory disease models. In contrast, QH treatment produced a more consistent inhibition across all three nodes (PI3K, Akt, mTOR), indicating that it may exert broader regulatory effects on this signaling cascade.
The pharmacological basis of QH in the treatment of psoriasis is likely attributed to a complex interplay of bioactive compounds identified in the herbal formulation. Diosmetin, Phellopterin, and β-sitosterol have emerged as key candidates for mediating QH’s therapeutic effects in psoriasis. Diosmetin, a flavonoid, has been shown to exert anti-inflammatory effects by inhibiting the expression of IL-6 and TNF-α through the NF-κB and MAPK pathways, which are crucial in psoriasis pathogenesis. Similarly, Phellopterin, a furanocoumarin, has demonstrated antioxidant and immunomodulatory properties, suggesting its potential to modulate cytokine levels and alleviate inflammatory responses in skin lesions. Furthermore, β-sitosterol, a phytosterol, exhibits anti-proliferative effects, likely through the modulation of the PI3K/Akt pathway, which is a critical regulator of keratinocyte proliferation in psoriasis. The therapeutic potential of these compounds has been experimentally validated in several studies. Diosmetin has been shown to inhibit inflammatory cytokine production in animal models and cell lines, supporting its role in QH’s anti-inflammatory effects. Phellopterin has been reported to reduce oxidative stress and cytokine imbalance, which are central to the pathophysiology of psoriasis. β-Sitosterol, in turn, has demonstrated a marked ability To reduce keratinocyte hyperplasia in psoriasis models, aligning with our findings that QH significantly alleviates epidermal thickening in IMQ-induced psoriasis-like lesions. These compounds, acting synergistically within QH, may enhance the therapeutic efficacy of the formulation in managing psoriasis. Further studies isolating and testing individual components of QH will be crucial To clarify their distinct and combined roles in modulating inflammatory pathways. In addition, exploring the pharmacokinetics of these compounds and their tissue distribution following QH administration will provide deeper insights into their bioavailability and therapeutic potential in psoriasis. In support of these findings, network pharmacology predictions, integrating data from the TCMSP and Swiss Target Prediction databases, identified 386 putative targets of QH, with 128 shared targets associated with known psoriasis-related genes. Pathway enrichment analyses revealed that key signaling pathways, including PI3K/Akt, IL-17, and TNF pathways, are significantly involved in QH’s therapeutic effects, providing a mechanistic framework for its action in psoriasis treatment. This supports the hypothesis that QH’s effectiveness in psoriasis may arise from its ability to target multiple interconnected pathways involved in immune response, keratinocyte hyperproliferation, and inflammation. Given the complexity of psoriasis as a polygenic disorder, future experimental validation of these compounds, coupled with detailed studies on immune cell involvement and cytokine profiles, will be necessary to confirm their specific contributions to QH’s clinical efficacy.
It is essential to explore the potential mechanisms by which QH inhibits the PI3K/Akt/mTOR signaling cascade. While our findings clearly demonstrate that QH significantly reduces the phosphorylation of PI3K, Akt, and mTOR in both in vivo and in vitro models, the precise molecular underpinnings remain to be elucidated. One plausible explanation is that specific bioactive constituents of QH, such as Diosmetin or β-sitosterol, may directly bind to upstream regulators or catalytic domains of these kinases, thereby attenuating their activation. Previous in silico docking and pharmacophore studies support this notion, suggesting that flavonoids and phytosterols can occupy ATP-binding pockets or interfere with receptor-mediated activation of PI3K and Akt. Additionally, QH may exert indirect effects by enhancing endogenous negative regulators of the pathway, such as PTEN or AMPK. To confirm these hypotheses, future investigations involving direct binding assays, kinase activity profiling, and co-treatment with selective pathway inhibitors will be critical. Such studies will help determine whether QH functions as a direct modulator of kinase activity or acts through broader upstream signaling mechanisms.
This study highlighted the significant therapeutic potential of QH in ameliorating IMQ-induced psoriasis-like skin conditions. However, several limitations must be acknowledged. Firstly, although the IMQ-induced murine model recapitulates several hallmark features of human psoriasis, including epidermal hyperplasia and inflammatory cytokine upregulation, it does not fully capture the chronic, relapsing nature or the genetic and environmental heterogeneity of the human condition. Consequently, the predictive value of this model for clinical efficacy remains limited. Secondly, the in vitro studies were conducted exclusively in HaCaT cells, which, while useful for assessing keratinocyte-specific responses, lack the dynamic interplay between immune cells and other skin-resident cells that is central to psoriasis pathogenesis. The absence of immune cell–keratinocyte crosstalk in these assays restricts our understanding of how QH may modulate the full spectrum of inflammatory responses in vivo. Thirdly, the multi-component and multi-target nature of TCM formulations, such as QH, poses significant challenges for mechanistic elucidation. While network pharmacology presents a valuable systems-level approach, it cannot fully account for pharmacokinetic factors, synergistic interactions among compounds, or off-target effects that may influence clinical outcomes. Moreover, standardization and quality control of complex herbal preparations remain ongoing challenges for broader clinical adoption. Finally, although we observed significant attenuation of PI3K/Akt/mTOR signaling, it remains unclear whether QH acts primarily via direct inhibition of these kinases, modulation of upstream regulators, or through feedback effects involving cytokine networks and immune modulation. Future studies should integrate direct target validation techniques, such as surface plasmon resonance, isothermal titration calorimetry, or CRISPR/Cas9-mediated gene editing in more complex model systems. To address these translational gaps, future research should employ advanced humanized psoriasis models, such as patient-derived organotypic skin constructs, or integrate T cell–keratinocyte co-culture systems. Additionally, clinical trials assessing pharmacokinetics, safety, and efficacy of QH in diverse patient populations are warranted to substantiate its therapeutic promise and delineate its role alongside existing standard-of-care treatments.
Conclusions
QH demonstrates therapeutic efficacy in managing psoriasis by alleviating the cutaneous manifestations and pathological changes induced by IMQ. This effect is mediated through the suppression of keratinocyte hyperproliferation and inflammatory responses, alongside the regulation of cytokines such as TNF-α, IL-17, and IL-23. The mechanism underlying QH’s action is hypothesized to involve the modulation of the PI3K/Akt/mTOR signaling pathway. These findings provide a foundational basis for integrating TCM with modern approaches to autoimmune disease management, offering new insights into the development of effective therapeutic strategies for psoriasis treatment.
Data availability
The dataset generated and analysed during the current study is available from the corresponding author on reasonable request.
Abbreviations
- TCM:
-
Traditional Chinese Medicine
- HE:
-
Hematoxylin-eosin
- PBS:
-
Phosphate-buffered saline
- qPCR:
-
Quantitative polymerase chain reaction
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
- OB:
-
Oral bioavailability
- PPI:
-
Protein-protein interaction
- GO:
-
Gene Ontology
- BP:
-
Biological Process
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- cDNA:
-
Complementary DNA
References
Wang, A. & Zhang, J. Causal role of immune cells in psoriasis: a Mendelian randomization analysis. Front. Immunol. 15, 1326717. https://doi.org/10.3389/fimmu.2024.1326717 (2024).
To, S. Y. et al. Psoriasis risk with immune checkpoint inhibitors. JAMA Dermatol. https://doi.org/10.1001/jamadermatol.2024.4129 (2024).
Wu, J., Ma, Y., Yang, J. & Tian, Y. Exposure to air pollution, genetic susceptibility, and psoriasis risk in the UK. JAMA Netw. Open. 7, e2421665. https://doi.org/10.1001/jamanetworkopen.2024.21665 (2024).
Mou, Y. et al. Global trends in the incidence of psoriasis from 1990 to 2019. Eur. J. Dermatol. 32, 207–213. https://doi.org/10.1684/ejd.2022.4245 (2022).
Ghezzi, G., Costanzo, A. & Borroni, R. G. Health-Related quality of life in psoriasis: literature review. J. Clin. Med. 13 https://doi.org/10.3390/jcm13164623 (2024).
Kleyn, C. E. et al. Psoriasis and mental health workshop report: exploring the links between psychosocial factors, psoriasis, neuroinflammation and cardiovascular disease risk. Acta Derm Venereol. 100, adv00020. https://doi.org/10.2340/00015555-3375 (2020).
Callea, M. & Pathophysiology Management and quality of life in atopic dermatitis and Psoriasis-A challenge for patients and their families. Child. (Basel). 9. https://doi.org/10.3390/children9050592 (2022).
Narang, T. & Mehta, H. A comorbidity-centered approach to psoriasis management: reflections and future directions. Int. J. Dermatol. https://doi.org/10.1111/ijd.17606 (2024).
James, W. A. et al. Full Guidelines-From the medical board of the National psoriasis foundation: perioperative management of systemic Immunomodulatory agents in patients with psoriasis and psoriatic arthritis. J. Am. Acad. Dermatol. 91, 251e251–251e211. https://doi.org/10.1016/j.jaad.2024.03.008 (2024).
Young, K. Z., Plazyo, O. & Gudjonsson, J. E. Targeting immune cell trafficking and vascular endothelial cells in psoriasis. J. Clin. Invest. 133 https://doi.org/10.1172/JCI169450 (2023).
You, L., Liang, K., An, R. & Wang, X. The path towards FDA approval: A challenging journey for traditional Chinese medicine. Pharmacol. Res. 182, 106314. https://doi.org/10.1016/j.phrs.2022.106314 (2022).
Yang, S., Hu, X. & Wang, Z. Network Pharmacology Analysis of Traditional Chinese Medicine for Treating Psoriasis: Identifying Core Components, Mechanisms, and Dosing Patterns. Altern Ther Health Med PMID: 39038330. (2024).
Xu, J. et al. Effect of fire needle combined with traditional Chinese medicine on psoriasis: A systematic review and meta-analysis. Med. (Baltim). 103, e35832. https://doi.org/10.1097/MD.0000000000035832 (2024).
Hu, J., Na, Y., Xue, J., Gao, S. & Yang, L. A systematic review of the botany, traditional use, phytochemistry, analytical methods, Pharmacological effects and pharmacokinetics of NOTOPTERYGII RHIZOMA ET RADIX. J. Ethnopharmacol. 334, 118589. https://doi.org/10.1016/j.jep.2024.118589 (2024).
Hu, C. C. Exploring the academic thought and medication experience of doctors in the Ming and Qing Dynasties in treating psoriasis based on data mining technology. Beijing University of Chinese Medicine 2021. https://link.cnki.net/doi/10.26973/d.cnki.gbjzu.2021. https://doi.org/10.26973/d.cnki.gbjzu.2021.000655
Zhao, W., Wang, B. & Li, S. Network Pharmacology for traditional Chinese medicine in era of artificial intelligence. Chin. Herb. Med. 16, 558–560. https://doi.org/10.1016/j.chmed.2024.08.004 (2024).
Zhou, Z. et al. Applications of network Pharmacology in traditional Chinese medicine research. Evid. Based Complement. Alternat Med. 2020, 1646905. https://doi.org/10.1155/2020/1646905 (2020).
Wang, S. et al. Network Pharmacological analysis and experimental validation of the mechanisms of action of Si-Ni-San against liver fibrosis. Front. Pharmacol. 12, 656115. https://doi.org/10.3389/fphar.2021.656115 (2021).
Kong, Y. et al. Narciclasine inhibits phospholipase A2 and regulates phospholipid metabolism to ameliorate psoriasis-like dermatitis. Front. Immunol. 13, 1094375. https://doi.org/10.3389/fimmu.2022.1094375 (2022).
Ru, J. et al. TCMSP: a database of systems Pharmacology for drug discovery from herbal medicines. J. Cheminform. 6, 13. https://doi.org/10.1186/1758-2946-6-13 (2014).
Daina, A., Michielin, O. & Zoete, V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 47, W357–W364. https://doi.org/10.1093/nar/gkz382 (2019).
Stelzer, G. et al. The genecards suite: from gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinf. 54 (1 30 31–31), 3033. https://doi.org/10.1002/cpbi.5 (2016).
Amberger, J. S., Bocchini, C. A., Scott, A. F. & Hamosh, A. OMIM.org: leveraging knowledge across phenotype-gene relationships. Nucleic Acids Res. 47, D1038–D1043. https://doi.org/10.1093/nar/gky1151 (2019).
Knox, C. et al. DrugBank 6.0: the drugbank knowledgebase for 2024. Nucleic Acids Res. 52, D1265–D1275. https://doi.org/10.1093/nar/gkad976 (2024).
Szklarczyk, D. et al. The STRING database in 2025: protein networks with directionality of regulation. Nucleic Acids Res. https://doi.org/10.1093/nar/gkae1113 (2024).
Mu, H. et al. OmicShare tools: A zero-code interactive online platform for biological data analysis and visualization. Imeta 3, e228. https://doi.org/10.1002/imt2.228 (2024).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28 (1), 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28 (11), 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51 (D1), D587–D592. https://doi.org/10.1093/nar/gkac963 (2023).
Wang, Q. et al. Cytokine profiles and the relationship of disease severity in patients with psoriasis. Indian J. Dermatol. 67, 204. https://doi.org/10.4103/ijd.ijd_79_22 (2022).
Yang, D., Peng, M., Fu, F., Zhao, W. & Zhang, B. Diosmetin ameliorates psoriasis-associated inflammation and keratinocyte hyperproliferation by modulation of PGC-1alpha / YAP signaling pathway. Int. Immunopharmacol. 134, 112248. https://doi.org/10.1016/j.intimp.2024.112248 (2024).
Zou, J. et al. Phellopterin cream exerts an anti-inflammatory effect that facilitates diabetes-associated cutaneous wound healing via SIRT1. Phytomedicine 107, 154447. https://doi.org/10.1016/j.phymed.2022.154447 (2022).
Prabahar, K., Udhumansha, U., Elsherbiny, N. & Qushawy, M. Microneedle mediated transdermal delivery of beta-sitosterol loaded nanostructured lipid nanoparticles for androgenic alopecia. Drug Deliv. 29, 3022–3034. https://doi.org/10.1080/10717544.2022.2120927 (2022).
Orcales, F. et al. A partitioned polygenic risk score reveals distinct contributions to psoriasis clinical phenotypes across a multi-ethnic cohort. J. Transl Med. 22, 835. https://doi.org/10.1186/s12967-024-05591-z (2024).
Doncheva, N. T., Assenov, Y., Domingues, F. S. & Albrecht, M. Topological analysis and interactive visualization of biological networks and protein structures. Nat. Protoc. 7, 670–685. https://doi.org/10.1038/nprot.2012.004 (2012).
Kshirsagar, S. J. et al. Navigating psoriasis: from immune mechanisms to natural healing approaches. Int. Immunopharmacol. 144, 113626. https://doi.org/10.1016/j.intimp.2024.113626 (2025).
Kamata, M. & Tada, Y. Crosstalk: keratinocytes and immune cells in psoriasis. Front. Immunol. 14, 1286344. https://doi.org/10.3389/fimmu.2023.1286344 (2023).
Lee, Y. G. et al. Natural Product-Derived compounds targeting keratinocytes and molecular pathways in psoriasis therapeutics. Int. J. Mol. Sci. 25. https://doi.org/10.3390/ijms25116068 (2024).
Wang, X. & Lai, Y. Keratinocytes in the pathogenesis, phenotypic switch, and relapse of psoriasis. Eur. J. Immunol. 54, e2250279. https://doi.org/10.1002/eji.202250279 (2024).
Pati, D. et al. Psoriasis pathogenesis: insights from transcriptomics and proteomics studies of keratinocytes. Georgian Med. News 340-341, 205–211. PMID: 37805899 (2023).
Whitley, S. K. et al. Local IL-23 is required for proliferation and retention of skin-resident memory T(H)17 cells. Sci. Immunol. 7, eabq3254. https://doi.org/10.1126/sciimmunol.abq3254 (2022).
Lowes, M. A., Russell, C. B., Martin, D. A., Towne, J. E. & Krueger, J. G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 34, 174–181. https://doi.org/10.1016/j.it.2012.11.005 (2013).
Rioux, G. et al. Development of a 3D psoriatic skin model optimized for infiltration of IL-17A producing T cells: focus on the crosstalk between T cells and psoriatic keratinocytes. Acta Biomater. 136, 210–222. https://doi.org/10.1016/j.actbio.2021.09.018 (2021).
Wang, D. et al. Uncovering the Mechanism of Scopoletin in Ameliorating Psoriasis-Like Skin Symptoms via Inhibition of PI3K/Akt/mTOR Signaling Pathway. Inflammation (2024). https://doi.org/10.1007/s10753-024-02188-y
Buerger, C., Malisiewicz, B., Eiser, A., Hardt, K. & Boehncke, W. H. Mammalian target of Rapamycin and its downstream signalling components are activated in psoriatic skin. Br. J. Dermatol. 169, 156–159. https://doi.org/10.1111/bjd.12271 (2013).
Yan, K. et al. Methotrexate restores the function of peripheral blood regulatory T cells in psoriasis vulgaris via the CD73/AMPK/mTOR pathway. Br. J. Dermatol. 179 (4), 896–905. https://doi.org/10.1111/bjd.16560 (2018).
Acknowledgements
Not applicable.
Funding
This research did not receive any funding.
Author information
Authors and Affiliations
Contributions
CRediT authorship contribution statementYuegang Wei: Conceptualization, Methodology, Resources, Writing – review & editing, Supervision, Funding acquisition.Shun Guo: Conceptualization, Methodology, Resources, Writing – review & editing, Supervision, Funding acquisition. Jia Liu: Validation, Visualization, Writing – original draft.Cheng Tan: Validation, Visualization, Writing – original draft. Jianxin Shi: Formal analysis, Writing – review & editing. Yue Shi: Formal analysis, Writing – review & editing. Qian Zhang: Investigation. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Animal ethics statements
All animal protocols were approved by the Institutional Animal Care and Use Committee of Nanjing University of Chinese Medicine, with the application number 202303–0140. All animal experiments were carried out in accordance with relevant guidelines and regulations. All methods were reported in accordance with ARRIVE guidelines.
Patient consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, J., Tan, C., Shi, J. et al. Exploring the mechanism of Notopterygii rhizoma et radix in the treatment of psoriasis using a network Pharmacology approach and experimental validation. Sci Rep 15, 40422 (2025). https://doi.org/10.1038/s41598-025-20133-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20133-3