Abstract
Basil (Ocimum basilicum L.), a medicinal plant and edible vegetable from the Lamiaceae family, holds significant therapeutic and culinary value. Endophytic bacterial inoculation is an effective strategy for enhancing the essential oil content and metabolic profile of medicinal plants. This study isolated and molecularly identified two endophytic bacteria, and evaluated their effects on the physiological, biochemical, and essential oil traits of basil using a completely randomized design. The bacterial isolates were identified as Microbacterium foliorum Emf1 and Paenibacillus peoriae ER11. Both isolates significantly improved key growth traits, with M. foliorum Emf1 showing the most substantial impact across various indicators. Notable increases compared to the control included root volume (106.79%), root surface area (60.66%), root fresh (71.81%) and dry weight (77.77%), shoot fresh (81.73%) and dry weight (81.48%), leaf fresh (84.54%) and dry weight (83.59%), membrane stability (400.85%), carotenoid content (200.00%), anthocyanin levels (61.90%), essential oil content (101.43%), total phenol (20.46%), and antioxidant activity (56.6%). This research provides novel insights by demonstrating the beneficial effects of these two endophytic bacterial isolates in enhancing basil’s growth performance, biochemical properties and essential oil content.
Similar content being viewed by others
Introduction
Medicinal plants serve as a rich source of antioxidants, containing a wide variety of bioactive phytochemicals such as phenolics, flavonoids, carotenoids, anthocyanins, and vitamins. These secondary metabolites have garnered attention for their potential health benefits, including preventing and treating various diseases1. Among these compounds, phenolics are particularly important due to their antioxidant properties, as they scavenge reactive oxygen species (ROS) and other free radicals2. Plants abundant in phenolic compounds are gaining growing recognition for their applications across various industries, including food, pharmaceuticals, and cosmetics3.
Basil (Ocimum basilicum L.), a perennial medicinal herb from the Lamiaceae family, is widely used both as a culinary and therapeutic plant. The light green, silky leaves contain oil glands that store essential oils, which contribute to the plant’s medicinal properties4. Beyond use as a fresh or dried vegetable, basil is integral to the perfumery, spice, and medical industries5. Phytochemical analysis of the plant has identified numerous important compounds, including eugenol, chavicol, and linalool6, as well as saponins, coumarins, alkaloids, tannins, and anthraquinones7. Essential oils and polyphenolic compounds such as rosmarinic acid, caffeic acid, chicoric acid, and ferulic acid are also key contributors to basil’s health-promoting properties8,9.
The wide range of bioactive compounds in basil supports its broad medicinal profile, which includes anti-cancer, anti-inflammatory, anti-microbial, anti-stress, anti-diabetic, and anti-arthritis effects, along with cardiovascular health benefits10,11. Recent studies have explored the use of endophytic bacteria as biostimulants to enhance the production of bioactive compounds in medicinal plants, promoting eco-friendly agricultural practices12,13.
Endophytic bacteria, capable of colonizing and persisting within plant tissues without causing harm, offer an effective strategy for improving plant growth and enhancing secondary metabolite production14. Exploring novel endophytic isolates and assessing their influence on medicinal plants can boost plant health, improve metabolite synthesis, and reduce reliance on chemical inputs, leading to more sustainable agricultural practices. These bacteria promote plant growth through mechanisms such as nitrogen fixation, siderophore production, and enhanced nutrient uptake, particularly of phosphorus. Additionally, endophytes synthesize plant-specific hormones like auxins and cytokinins, further supporting plant growth15.
Endophytic bacteria can also bolster plant defenses by producing antifungal compounds like antibiotics and hydrogen cyanide, and by reducing pathogen populations through root colonization16,17. Thus, bacterial endophytes are critical in improving plant growth, yield, and the accumulation of valuable phytochemicals18,19,20.
Although basil has been extensively studied for antioxidant properties, the impact of endophytic bacteria on plant growth and bioactive metabolites remains unexplored. This study aims to identify and characterize novel endophytic bacterial isolates, evaluating their effects on basil’s growth, physiology, phenolic and flavonoid content, essential oil content, and antioxidant activity.
Results
Bacterial identification
The two endophytic bacterial isolates, Microbacterium foliorum Emf1 and Paenibacillus peoriae ER11, were conclusively identified by 16 S rRNA gene sequencing and deposited in NCBI GenBank (Emf1: OR342201.1, https://www.ncbi.nlm.nih.gov/nuccore/OR342201.1; ER11: OR342310.1, https://www.ncbi.nlm.nih.gov/nuccore/OR342310.1). Sequence identities to reference strains were 99.40% for Emf1 and 99.84% for ER11, as confirmed using the EzBioCloud 16 S rRNA database. These bacteria were isolated from O. basilicum and P. aucheri plants, both known for their medicinal properties and significance in traditional herbal medicine. The high sequence similarity to known bacterial species suggests that these isolates are likely to possess recognized traits related to plant growth promotion, biocontrol, and stress resistance, typical of their respective genera.
Morphophysiological traits
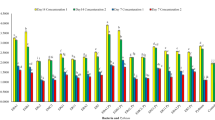
Endophytic bacteria, M. foliorum Emf1 and P. peoriae ER11, significantly enhanced the morphological traits of the plant compared to the control. Emf1 treatment showed the greatest improvements, followed by ER11. Plant height increased by 26.5% in Emf1 and 13.5% in ER11 compared to the control. Shoot fresh weight (FW) and dry weight (DW) were highest in Emf1, with a 75.3% and 81.5% increase, respectively, while ER11 showed a 45.3% increase in FW and 25.3% in DW. Root FW and DW were also significantly improved. Emf1 increased root FW by 71.5% and root DW by 78.4%, while ER11 showed a 39.2% increase in FW and 9.1% in DW. Leaf FW and DW were 84.0% and 85.7% higher in Emf1, and 59.6% and 67.4% higher in ER11, respectively. Additionally, root length, root volume, and surface area increased by 31.7%, 43.6%, and 49.5% in Emf1, respectively, with ER11 showing a 19.0%, 48.8%, and 28.0% improvement compared to the control. Leaf length, width, and surface area also showed significant increases, with Emf1 plants exhibiting 27.5%, 30.4%, and 30.9% higher values, respectively. The control group had the lowest values in all traits (Table 1). Overall, Emf1 treatment led to the most substantial improvements in plant growth and morphology (Fig. 1).
RWC, EL, and MS
Both endophytic bacteria significantly enhanced the examined physiological traits. Emf1 increased RWC by 36.0%, while ER11 led to a 16.1% rise compared to the control (Fig. 2A). Electrolyte leakage decreased by 5.5% with Emf1 and by 0.7% with ER11, indicating enhanced membrane integrity (Fig. 2B). Membrane stability increased most substantially, rising by 400.0% with Emf1 and by 200.0% with ER11 (Fig. 2C). These findings highlight Emf1 as the superior treatment for improving water retention and cellular stability in basil.
The effect of endophytic bacteria (Microbacterium foliorum Emf1 and Paenibacillus peoriae ER11) on the relative water content (A), electrolyte leakage (B) and membrane stability (C) of Ocimum basilicum L. In each panel, means sharing at least one common letter are not significantly different according to Duncan’s multiple range test at p ≤ 0.05. Values represent the mean ± standard error of three replicates.
Chlorophyll, carotenoid, anthocyanin and essential oil content
Inoculation with endophytic bacteria significantly enhanced plant pigment content compared to the control. Emf1 produced the greatest improvements across all measured traits, increasing chlorophyll a by 38.52% and chlorophyll b by 38.27%, while ER11 raised chlorophyll a by 19.25% and chlorophyll b by 14.20%, resulting in total chlorophyll increases of 35.55% and 18.56%, respectively (Fig. 3A-C).
Carotenoid content showed the largest increase, rising by 192.48% in Emf1-treated plants and by 33.08% with ER11 (Fig. 3D). Similarly, anthocyanin content increased by 64.08% with Emf1 and 18.45% with ER11, highlighting the role of endophytic bacteria in promoting pigment biosynthesis (Fig. 3E).
Inoculation with M. foliorum Emf1 increased basil essential oil yield by 101.43%, and P. peoriae ER11 by 48.57%, demonstrating the significant role of these endophytic bacteria in activating plant pathways responsible for secondary metabolite production (Fig. 3F).
The effect of endophytic bacteria (Microbacterium foliorum Emf1 and Paenibacillus peoriae ER11) on the chlorophyll a (A), chlorophyll b (B), total chlorophyll (C), carotenoid (D), anthocyanin (E) and essential oil (F) content of Ocimum basilicum L. In each panel, means sharing at least one common letter are not significantly different according to Duncan’s multiple range test at p ≤ 0.05. Values represent the mean ± standard error of three replicates.
Total phenol and flavonoid content
Inoculation with P. peoriae ER11 significantly increased flavonoid content, whereas no noticeable change in total phenol levels was detected compared with the control. In contrast, M. foliorum Emf1 treatment led to the most substantial enhancements, with total phenol content increasing by 20.46% and flavonoid content rising by 7.692% relative to the control. These findings suggest that M. foliorum Emf1 has a more comprehensive influence on secondary metabolite biosynthesis, potentially contributing to improved plant defense and stress adaptation (Fig. 4A and B).
The ffect of endophytic bacteria (Microbacterium foliorum Emf1 and Paenibacillus peoriae ER11) on the total phenol (A) and flavonoid (B) content of Ocimum basilicum L. In each panel, means sharing at least one common letter are not significantly different according to Duncan’s multiple range test at p ≤ 0.05. Values represent the mean ± standard error of three replicates.
Biochemical traits
CAT, APX and antioxidant activity
The results of this study demonstrate that microbial inoculation can significantly influence antioxidant enzyme activity in plants. Specifically, M. foliorum Emf1 inoculation resulted in a substantial increase in CAT and APX activity, with respective increases of 32.62% and 43.30% compared to the control (Figs. 4B and 5A). In contrast, P. peoriae ER11 significantly enhanced only CAT activity, suggesting that these bacterial isolates have distinct modes of action in modulating plant antioxidant responses.
The results of this study indicate that inoculation with M. foliorum Emf1 significantly enhances antioxidant activity in basil plants, with a recorded increase of 100.7% compared to the control. The highest antioxidant activity (56.6%) occurred in plants inoculated with M. foliorum Emf1, whereas the control showed the lowest activity (28.2%) (Fig. 5C). This substantial enhancement suggests that M. foliorum Emf1 plays a crucial role in modulating the plant’s redox balance and reinforcing its defense mechanisms against oxidative stress.
The effect of endophytic bacteria (Microbacterium foliorum Emf1 and Paenibacillus peoriae ER11) on the catalase (A), ascorbate peroxidase (B) and antioxidant activity (C) of Ocimum basilicum L. In each panel, means sharing at least one common letter are not significantly different according to Duncan’s multiple range test at p ≤ 0.05. Values represent the mean ± standard error of three replicates.
Discussion
M. foliorum has been previously identified for its plant growth-promoting properties, such as phosphate solubilization, auxin production, and stress mitigation, all of which could enhance plant health, particularly under stressful environmental conditions21. In this study, the isolation of M. foliorum Emf1 from basil, a crop valued for its bioactive compounds such as essential oils and phenolics, highlights its potential role in stimulating secondary metabolism pathways, including the production of essential oils and antioxidant compounds. The capacity of M. foliorum to engage in such metabolic pathways suggests that it may offer benefits not only in terms of promoting plant growth but also in enhancing the crop’s phytochemical composition, a valuable trait for medicinal plant production. Similarly, P. peoriae is known for its ability to fix nitrogen, solubilize phosphorus, and suppress pathogens, making it an effective candidate for promoting plant health, particularly in nutrient-poor soils or stressed environments22. The identification of P. peoriae ER11 from Phlomis aucheri further supports its potential role as a biocontrol agent and growth promoter, particularly through its ability to enhance nutrient availability and protect plants from soil-borne pathogens. The significant similarity to known isolates of P. peoriae suggests that this bacterium may have similar biotechnological applications in agriculture, particularly in enhancing crop yields and improving plant resilience under stress conditions. Together, the isolation and identification of these two bacterial isolates contribute valuable insight into the potential role of endophytes in improving plant health, particularly for crops with medicinal or aromatic value. The results suggest that both M. foliorum Emf1 and P. peoriae ER11 are promising candidates for further research into plant growth promotion, biocontrol, and the enhancement of bioactive compounds, which could ultimately contribute to more sustainable agricultural practices and the production of high-quality medicinal plants.
The significant enhancement of basil morphological traits observed in this study after treatment with the endophytic bacteria M. foliorum Emf1 and P. peoriae ER11 highlights the potential of these bacteria to promote plant growth and development. The results suggest that these endophytes may exert their effects through multiple mechanisms, including enhanced nutrient uptake, production of plant growth-promoting substances, and improved stress tolerance. One key mechanism by which these bacteria may enhance plant growth is through the production of phytohormones, such as auxins, cytokinins, and gibberellins, which are known to stimulate root and shoot growth23. The increased root biomass and root length recorded in Emf1-treated plants may be attributed to the production of auxins by these endophytes, which promote root elongation and branching, leading to improved nutrient and water uptake24. This is consistent with previous studies where bacterial endophytes have been shown to enhance root growth and biomass, contributing to overall plant growth25. In addition to hormone production, bacteria can enhance plant growth by improving nutrient availability. P. peoriae ER11 and M. foliorum Emf1 may promote the solubilization of nutrients, such as phosphorus, which is often a limiting factor for plant growth26. Both bacteria may also facilitate nitrogen fixation, particularly in nitrogen-limited soils, leading to an increase in available nitrogen, a key nutrient for plant growth27. The improved root and shoot biomass identified in this study can therefore be attributed, in part, to enhanced nutrient acquisition facilitated by these endophytes.
Another possible mechanism is the enhancement of plant tolerance to abiotic stresses, including salinity, drought, and nutrient deficiencies. Endophytic bacteria have been reported to improve plant stress resilience by producing antioxidant enzymes, which protect plants from oxidative damage28. The increased root and shoot biomass under bacterial treatments could be partially attributed to enhanced oxidative stress tolerance, which allows plants to maintain normal metabolic functions under challenging environmental conditions23. Moreover, bacterial endophytes can produce exopolysaccharides that improve soil structure and water retention, further aiding in stress tolerance29. The recorded increases in leaf area and leaf biomass in Emf1-treated plants suggest that these bacteria may also enhance photosynthetic efficiency, possibly through the regulation of stomatal conductance and increased chlorophyll synthesis30. This is supported by findings that bacterial endophytes can modulate photosynthetic enzyme activity, leading to higher rates of photosynthesis and greater biomass production31. Additionally, the improved leaf morphology may be linked to the ability of M. foliorum Emf1 to produce growth-promoting substances that stimulate cell division and expansion in leaf tissues. In summary, the mechanisms responsible for the enhanced morphological traits in basil following bacterial treatments are likely multifactorial, involving phytohormone production, improved nutrient availability, enhanced stress tolerance, and improved photosynthetic efficiency. These findings are consistent with recent studies highlighting the potential of endophytic bacteria as biotechnological tools for improving plant growth and productivity in both controlled and field conditions32.
The documented improvements in basil’s physiological traits can be attributed to the beneficial roles of M. foliorum Emf1 and P. peoriae ER11 in enhancing plant function and cellular stability. These endophytic bacteria likely contribute to improved water regulation, membrane integrity, and cellular stability through multiple mechanisms, including phytohormone production, enzymatic activity, and metabolic enhancement33. The increase in RWC suggests that both bacterial strains enhance water uptake and retention, with Emf1 showing a stronger effect. This improvement may be linked to bacterial production of indole-3-acetic acid (IAA), which stimulates root growth and enhances water absorption34. Additionally, bacterial interactions may promote the accumulation of osmolytes such as proline and soluble sugars, which contribute to better water retention in plant tissues35. The decrease in EL indicates that both Emf1 and ER11 strengthen cell membrane integrity, possibly through enhanced production of antioxidant enzymes such as superoxide dismutase (SOD), CAT, and peroxidase (POD)36. These enzymes help maintain membrane stability by reducing oxidative damage and lipid peroxidation, ultimately leading to lower ion leakage37. The significant improvement in membrane stability (MS), especially the 400.0% increase with Emf1, suggests that this strain plays a key role in reinforcing cellular structures. This could be attributed to its ability to enhance secondary metabolite production, such as phenolic compounds and flavonoids, which contribute to membrane stabilization38. Additionally, Emf1 may influence lipid composition and protein expression in the membrane, further enhancing its structural integrity39. Overall, Emf1 demonstrated superior effectiveness compared to ER11, likely due to its stronger influence on water retention, membrane protection, and metabolic regulation. These findings highlight the potential application of Emf1 as a plant-growth-promoting bacterium for improving basil’s physiological performance.
Bacterial inoculation led to a notable rise in chlorophyll content, which demonstrated the positive influence of endophytic bacteria on photosynthetic efficiency and pigment biosynthesis. This improvement can be attributed to several interconnected physiological and biochemical mechanisms. One primary factor is the ability of plant growth-promoting bacteria (PGPB) to enhance nitrogen assimilation and iron uptake, both of which are critical for chlorophyll biosynthesis40. M. foliorum Emf1 exhibited the greatest impact on chlorophyll accumulation, likely due to its ability to increase the bioavailability of essential nutrients such as nitrogen (N), magnesium (Mg), and iron (Fe), which are key components of the chlorophyll molecule. Additionally, these bacteria may induce the upregulation of genes involved in the biosynthetic pathway of chlorophyll, including glutamyl-tRNA reductase and protochlorophyllide oxidoreductase, leading to increased pigment production41.
Carotenoids, which serve as accessory pigments in photosynthesis and play a critical role in photoprotection, exhibited a remarkable increase in bacterial-treated plants, particularly in the Emf1 treatment. Carotenoids function as antioxidants by quenching singlet oxygen and dissipating excess energy through the xanthophyll cycle, preventing photooxidative stress42. The substantial increase in carotenoid content suggests that endophytic bacteria contributed to the upregulation of carotenogenesis-related genes, such as phytoene synthase (PSY) and ζ-carotene desaturase (ZDS), leading to higher carotenoid accumulation43. Moreover, these bacteria are known to produce phytohormones such as indole-3-acetic acid (IAA), which can enhance chloroplast development and promote the synthesis of carotenoids by modulating the expression of light-responsive genes44. The significantly higher carotenoid levels in Emf1-treated plants suggest that this strain had a stronger impact on stimulating the antioxidant system, thereby improving the plant’s ability to cope with oxidative stress.
Anthocyanin accumulation was also significantly enhanced by bacterial treatments, with the highest increase observed in Emf1-treated plants. Anthocyanins, flavonoid compounds responsible for red, blue, and purple pigmentation, are crucial for protecting plants against oxidative stress, UV radiation, and pathogen attacks45. The recorded enhancement of anthocyanin production in this study suggests that endophytic bacteria stimulated the phenylpropanoid pathway, which regulates flavonoid biosynthesis. This could be due to bacterial-induced upregulation of key enzymes such as phenylalanine ammonia-lyase (PAL) and chalcone synthase (CHS), which are essential for anthocyanin biosynthesis46. Additionally, the increase in anthocyanin content may be linked to the bacteria’s ability to trigger ROS signaling, leading to the activation of transcription factors such as MYB and bHLH, which regulate anthocyanin biosynthetic genes47. Given that Emf1 had a stronger impact than ER11 in increasing anthocyanin content, it is possible that this strain more effectively triggered these regulatory pathways, enhancing stress tolerance mechanisms in the plant.
Overall, the documented enhancements in chlorophyll, carotenoid, and anthocyanin content highlight the multifaceted role of endophytic bacteria in regulating plant physiological traits. These bacteria likely acted through a combination of nutrient mobilization, phytohormone production, and activation of stress-responsive pathways, ultimately leading to enhanced pigment biosynthesis and improved plant resilience. The differential response detected between ER11 and Emf1 treatments suggests that specific bacterial strains have varying capacities to influence plant metabolism, with Emf1 demonstrating superior effectiveness in promoting pigment accumulation.
These results are consistent with previous studies that highlight the ability of PGPB to enhance essential oil production by improving nutrient uptake, inducing plant defense mechanisms, and modulating phytohormonal balance27,48.
One of the key mechanisms by which these endophytic bacteria enhance essential oil biosynthesis is their role in improving nutrient availability. It has been reported that Paenibacillus and Microbacterium species contribute to phosphorus solubilization and nitrogen fixation, both of which are crucial for plant metabolism and the synthesis of secondary metabolites21,22. Additionally, these bacteria may produce signaling molecules such as volatile organic compounds (VOCs) that trigger the production of essential oils in medicinal plants49.
Furthermore, the differential impact of M. foliorum Emf1 and P. peoriae ER11 on essential oil content suggests that their mode of action may vary. The more pronounced effect observed in M. foliorum Emf1-treated plants could be attributed to its superior ability to enhance plant systemic resistance, increase antioxidant enzyme activity, or upregulate genes involved in the biosynthesis of terpenoids, which are key components of basil essential oil50,51. Previous research has shown that bacterial endophytes can upregulate terpene synthase genes, leading to higher accumulation of bioactive compounds in essential oils14.
Additionally, the significant increase in essential oil content aligns with the broader role of endophytic bacteria in mitigating environmental stresses. Stress conditions such as drought and salinity often reduce essential oil production; however, PGPB can help plants cope with these stresses by modulating osmolyte accumulation, reducing oxidative stress, and maintaining cellular homeostasis48,52. The ability of M. foliorum Emf1 and P. peoriae ER11 to enhance essential oil biosynthesis under controlled conditions suggests their potential for improving basil productivity in suboptimal environments.
Inoculation with M. foliorum Emf1 led to increased phenolic and flavonoid contents, indicating that this isolate may trigger crucial metabolic pathways associated with plant defense and stress response. Several studies have reported that plant growth-promoting rhizobacteria (PGPR) can induce systemic resistance and stimulate secondary metabolite biosynthesis by modulating phytohormone levels, increasing nutrient availability, or triggering oxidative stress responses27,48. Phenolic compounds, particularly flavonoids, are critical secondary metabolites that contribute to plant tolerance against environmental stressors and pathogen attacks by acting as antioxidants and signaling molecules53,54. The notable increase in total phenols and flavonoids in response to M. foliorum Emf1 treatment suggests that this isolate may act as an elicitor, enhancing the plant’s biochemical defense mechanisms.
On the other hand, P. peoriae ER11 selectively enhanced flavonoid accumulation without affecting total phenol content. This suggests that P. peoriae ER11 may be influencing specific branches of the phenylpropanoid pathway rather than inducing a broad-spectrum response. Flavonoids play essential roles in plant defense, including antimicrobial activity, UV protection, and scavenging of ROS55. The selective increase in flavonoids suggests that P. peoriae ER11 might modulate key regulatory enzymes such as chalcone synthase (CHS) or flavone synthase (FNS), which govern flavonoid biosynthesis56.
The differential effects of the two bacterial isolates may be attributed to variations in their modes of action, including their ability to produce phytohormones, release VOCs, or induce specific signaling pathways within the host plant57. PGPRs have been shown to upregulate defense-related genes and activate plant signaling cascades such as salicylic acid (SA)- and jasmonic acid (JA)-mediated pathways, which influence phenolic metabolism49. Future research should explore the molecular mechanisms underlying these responses, particularly focusing on the expression of key genes in the phenylpropanoid pathway and the role of microbial metabolites in triggering these metabolic shifts.
The increased activity of CAT and APX in response to M. foliorum Emf1 inoculation suggests that this isolate plays a critical role in enhancing the plant’s oxidative stress defense mechanisms. CAT and APX are key components of the enzymatic antioxidant system, responsible for scavenging ROS and maintaining redox homeostasis52,58. The significant increase in both enzyme activities implies that M. foliorum Emf1 enhances the plant’s ability to detoxify hydrogen peroxide (H₂O₂), thereby improving stress resilience. The ability of PGPR to induce antioxidant enzyme activity has been well-documented, with studies showing that bacterial inoculation can mitigate oxidative damage caused by environmental stressors such as salinity, drought, and heavy metals59,60.
On the other hand, P. peoriae ER11 selectively enhanced CAT activity without significantly affecting APX levels. This suggests that P. peoriae ER11 may primarily regulate H₂O₂ detoxification through the CAT pathway, whereas M. foliorum Emf1 may employ a broader range of antioxidant responses. The specificity of these responses may be linked to differences in bacterial signaling molecules, such as VOCs and phytohormones, which differentially regulate the expression of stress-responsive genes57,61.
The differential activation of antioxidant enzymes by these bacterial isolates highlights their potential application as bioinoculants for improving plant stress tolerance. The ability of M. foliorum Emf1 to enhance both CAT and APX activity suggests a strong protective effect against oxidative stress, which could be beneficial in improving plant resilience under adverse environmental conditions. Meanwhile, the selective enhancement of CAT by P. peoriae ER11 indicates that its role may be more specific, potentially complementing other stress adaptation mechanisms.
Endophytic bacteria, particularly PGPB, have been widely reported to improve plant antioxidant capacity through multiple mechanisms, including the upregulation of antioxidant enzyme activity, production of secondary metabolites, and modulation of phytohormonal pathways48,60. The significant increase in antioxidant activity following M. foliorum Emf1 inoculation suggests that this bacterium may stimulate the biosynthesis of antioxidant compounds, including phenolics, flavonoids, and other secondary metabolites involved in oxidative stress mitigation52. This aligns with previous studies demonstrating that PGPRs can trigger the accumulation of antioxidants by activating plant defense-related genes and enhancing enzymatic and non-enzymatic antioxidant pathways27.
The substantial improvement in antioxidant activity could be attributed to M. foliorum Emf1’s ability to induce the production of ROS-scavenging enzymes, such as CAT and APX, as observed in this study. Enhanced antioxidant activity helps plants mitigate oxidative damage caused by environmental stresses, including salinity, drought, and pathogen attacks57,58. Moreover, endophytic bacteria are known to influence the synthesis of phenylpropanoid-derived antioxidants, which contribute to plant resilience and improved secondary metabolite profiles53,54.
Conclusion
This study highlighted the pivotal role of M. foliorum Emf1 and P. peoriae ER11 in enhancing plant growth, physiological performance, and biochemical responses in basil plants. Among the two, M. foliorum Emf1 emerged as the most effective, markedly improving root development, biomass accumulation, essential oil content, and key physiological parameters. Notably, this bacterial isolate also induced significant increases in antioxidant enzyme activities, total phenolic and flavonoid contents, and overall antioxidant capacity, indicating its potential to bolster plant resilience against oxidative damage. Although P. peoriae ER11 also demonstrated growth-promoting effects, particularly in root architecture and flavonoid synthesis, its influence on antioxidant defense mechanisms was comparatively more targeted. The demonstrated ability of both endophytic bacterial isolates to modulate critical physiological and biochemical pathways underscores their promise as effective bioinoculants for sustainable agriculture. These findings offer valuable insights into the complex interactions between endophytes and host plants and highlight the potential of microbial applications in enhancing the yield and phytochemical quality of medicinal and aromatic species. Furthermore, the results suggest that Emf1 and ER11 could be developed into bioformulations for field applications, providing a practical approach to improve crop productivity and stress resilience. Future research should aim to elucidate the molecular and metabolic frameworks governing these beneficial associations and validate their agronomic potential under diverse environmental conditions.
Materials and methods
Isolation of endophytic bacteria
Two plant species, Ocimum basilicum and Phlomis aucheri, were collected from a basil cultivation field adjacent to Ilam Airport, Ilam, Iran (33.5853° N, 46.405° E; 1342 m a.s.l.), and from the natural habitat of P. aucheri in Derak Mountain, west of Shiraz, Fars, Iran (29.6342° N, 52.3940° E; 2278 m a.s.l.) for the isolation of endophytic bacteria. The plant material of P. aucheri was identified by Dr. Mohammad Reza Parishani Foroushani, a plant taxonomist from the Department of Biology, Faculty of Science, Shahid Chamran University of Ahvaz, Iran, and deposited in the herbarium of the same department under the voucher number HSCU1100.
Healthy root samples were collected from each plant species, thoroughly cleaned, dried, and cut into 2–3 cm segments. To ensure effective decontamination, the samples underwent a series of disinfection treatments using 70% ethyl alcohol for 1 min, followed by a 5% sodium hypochlorite solution for 5 min. After each treatment, the samples were rinsed five times with dH2O to remove any residual disinfectants. Subsequently, the samples were immersed in a 1% mercury chloride solution for 2 min and then rinsed again. The final step involved plating the processed root samples on nutrient agar medium. Following a 14-day incubation period, a single dominant colony from each plant sample was isolated for further analysis62.
For bacterial cultivation, both nutrient agar and nutrient broth media were used. The bacterial isolates were stored at −70 °C in a 25% glycerol solution for long-term preservation. To identify the isolated bacteria, polymerase chain reaction (PCR) targeting the 16 S rRNA gene region was performed. The primers 27 F and 1492R were used for amplification63. The PCR products were visualized using agarose gel electrophoresis, and samples exhibiting distinct bands were selected for sequencing.
Disinfection of seeds, germination test, and experimental design
The cultured bacterium was initially transferred to nutrient broth and incubated with shaking for 24 h at 250 rpm. The bacterial cells were harvested by centrifugation (10,000 × g for 5 min at 4 °C), followed by washing with doubled distilled water (ddH2O). The cell concentration was adjusted to 5 × 10^7 CFU/ml.
The seeds were disinfected by soaking in 2.5% sodium hypochlorite (NaOCl) for 5 min, followed by treatment with 70% ethanol for 1 min. The seeds were then washed five times with ddH2O, dried, and soaked in sterile ddH2O for the control treatment or in the bacterial suspension for the inoculation treatments for 1 h. The treated seeds were placed on sterile Petri dishes containing two pieces of wet filter paper and monitored daily for germination and moisture content over 14 days, with three replicates. Germination was considered successful if the root length exceeded 2 mm. The experiment was performed in triplicate, with 20 seeds per replication. Counting continued until no further germination was observed over three consecutive counts. Data were analyzed using SPSS version 9.1, and Duncan’s test was applied to compare the means at a 5% significance level.
A greenhouse experiment was conducted to evaluate the impact of bacterial inoculation on the morphophysiological and biochemical properties, as well as the essential oil content, of basil. The study followed a completely randomized design with three replications. The sterilized seeds were soaked for 1 h in bacterial suspension (inoculation treatments) or sterile ddH2O (control). The seeds were then planted in plastic pots filled with a 1:1 mixture of farm soil and sand (Table 2). The plants were treated twice with 30 ml of bacterial suspension at the aforementioned concentration when they were 14 and 30 days old. Growth was regularly monitored, and the plants were harvested at 60 days for subsequent analysis.
Morphophysiological traits
Five pots from each treatment were randomly selected to measure the specified traits. Plant height and root length were measured with a ruler, accurate to 1 mm. Root volume was determined by immersing the roots in dH2O in a 1-liter graduated cylinder. Root surface area was calculated using the method described by Atkinson (1980)64. Fresh weights of the shoot, root, and leaf were recorded using a precision digital scale (0.001 g). After drying in the shade for 7 days, their dry weights were measured too. Roots were thoroughly washed with dH2O and weighed after excess moisture had been removed using paper towels. During the flowering stage, three leaves were collected from each plant at a height of 8–12 cm above the crown. Leaf length and width were measured, and leaf surface area was determined using graph paper.
RWC, EL, and MS
In the morning, three basil plants were selected from each experimental treatment. One of the youngest fully developed leaves was randomly chosen from each plant and immediately placed in an ice bath. The fresh weight of the leaf was measured, and after 24 h of immersion in a Falcon tube containing 10 ml of dH2O at 25 ± 2 °C, the turgor weight of the leaf was determined. Dry weight was measured following the method described by Galmes et al. (2007)65. The relative water content (RWC) was calculated using the formula:
For electrolyte leakage (EL) measurements, 5 fresh leaf discs (0.5 cm² each) were washed three times for 2–3 min with dH2O and then floated in a Falcon tube containing 10 ml of dH2O. After vortexing for 30 s, the initial electrical conductivity (EC0) of each sample was recorded. EL was measured in the solution after 24 h of floating at 25 ± 2 °C. The samples were then subjected to 90 °C in a Bain-Marie for 2 h, and the final electrical conductivity (EC2) was recorded66. EL was calculated using the formula:
Membrane stability (MS), which is inversely related to ion leakage, was calculated using the formula described by Anjum et al. (2003).
Chlorophyll, carotenoid, anthocyanin and essential oil content
Chlorophyll a, b, and total chlorophyll content were measured using the method described by Porra (2002)67 while anthocyanin content was quantified following the procedure outlined by Sims and Gammon (2002)68. Carotenoid content was assessed according to the method of Lichtenthaler and Welburn (1983)69. A 500 mg leaf sample was homogenized with 5 ml of 80% acetone and centrifuged at 13,000 × g for 15 min (Prismr, Labnet, USA). The supernatant was then adjusted to a final volume of 10 ml with 80% acetone. Absorbance was measured at 663 nm, 470 nm, 646.6 nm, 663.6 nm, 647 nm, and 537 nm using a spectrophotometer (Specord 50, Analytic Jena AG, Germany). The absorbance values were used to calculate the content of chlorophylls, anthocyanins, and carotenoids in milligrams per gram of fresh weight using the appropriate equations.
In the above relations, A is the absorption wavelength of the spectrophotometer.
The plants were carefully dried in a controlled environment with appropriate temperature and ventilation. After removing any contaminants, yellow leaves, and stems, the plant Material was ground to enhance the quality of the essential oil. A 50-gram sample was then prepared for essential oil extraction, which was carried out via a three-hour distillation process using a Clevenger apparatus in a 1-liter flask filled with water. The essential oil percentage (in microliters per gram of dry weight) was calculated using a graduated Clevenger tube, according to the Equation70:
Total phenol and flavonoids content
The total phenol content was determined using the Folin-Ciocalteu method with slight modifications. To prepare the sample, 200 mg of ground fresh leaves was mixed with 10 ml of 95% ethanol and stored in the dark at 25 ± 2 °C for 24 h. Afterward, 1 ml of the liquid above the sediment was diluted with 1 ml of methanol in a separate microtube. From this solution, 200 µl was added to 1000 µl of 50% Folin reagent and incubated in the dark for 5 min. The mixture was then combined with 800 µl of 7.5% NaHCO3 and incubated for 2 h. The absorbance of each sample was measured at 765 nm. A standard curve was constructed using different concentrations (12.5 to 800 µg/ml) of gallic acid. Total phenol content was calculated in mg/g fresh weight (FW) using the gallic acid curve71.
To measure flavonoid content, 0.05 g of fresh leaf tissue was powdered using liquid nitrogen and homogenized in 3 ml of 80% methanol. The mixture was heated in a water bath at 70 °C for 3 h. After centrifugation, the supernatant was adjusted to 3 ml using 80% methanol. From this solution, 1 ml was transferred to a 5 ml microtube, followed by the addition of 250 µl of 1 M CH3CO2K and 250 µl of 10% AlCl3 solution. Absorbance was measured at 415 nm, and flavonoid content was calculated in mg/g FW using the quercetin standard curve72.
Biochemical traits
Preparation of enzyme extract
To create a uniform mixture, 1 gram of frozen leaf sample was ground with 100 mM KH2PO4 buffer (pH 7.4) in a 10:1 extraction ratio (1 g of leaf with 10 ml of buffer) using a Chinese mortar. The resulting mixture was transferred to 15 ml falcon tubes and centrifuged at 10,000 × g for 20 min at 4 °C. The clear supernatant was collected, transferred into marked tubes, and stored at −80 °C.
CAT, APX and antioxidant activity
Catalase (CAT) activity was measured following the method outlined by Aebi (1984)73 with minor modifications. A 100 µl enzyme extract was mixed with 400 µl of 50 mM Na3PO4 buffer (pH 7.0) and 300 µl of 20 mM H2O2. The decrease in absorbance at 240 nm was recorded for 2 min. The spectrophotometer was zeroed with 3 ml of dH2O. Enzyme activity was calculated in µmol of H2O2 per minute per gram of fresh weight (FW) using an extinction coefficient of 39.4 mM−1cm−1.
To prepare the reaction complex (2 ml), 0.5 ml of 100 mM sodium phosphate buffer (pH 7.0), 0.5 ml of 1 mM ascorbate, 0.5 ml of 0.1 mM EDTA, 0.02 ml of 10 mM H2O2, 0.38 ml of ddH2O, and 0.1 ml of enzyme extract were combined. The absorbance was measured at 290 nm before and 1 min after the reaction commenced. The ascorbate peroxidase (APX) enzyme’s inactivation coefficient was determined to be 2.8 mM−1cm−1. Finally, one unit of activity was defined as the amount of enzyme, that oxidizes 1 µmol ascorbate per min in 1 g of fresh weight74.
To measure antioxidant activity, 150 µL of the sample extract was mixed with 2 mL of a 0.02 g DPPH solution. The absorbance of the mixture was immediately recorded at 517 nm (At0). The samples were then incubated for 30 min at room temperature in the dark, after which the absorbance was measured again at 517 nm (At30). The antioxidant activity was calculated using the following formula75:
At0: absorption of solution at zero time and At30: absorption of solution at 30 min.
Data availability
The 16 S rRNA gene sequences of *Microbacterium foliorum* Emf1 and *Paenibacillus peoriae* ER11 are available in NCBI GenBank under accession numbers OR342201.1 ([https://www.ncbi.nlm.nih.gov/nuccore/OR342201.1](https:/www.ncbi.nlm.nih.gov/nuccore/OR342201.1)) and OR342310.1 ([https://www.ncbi.nlm.nih.gov/nuccore/OR342310.1] (https:/www.ncbi.nlm.nih.gov/nuccore/OR342310.1)). Other data from this study are available upon reasonable request.
References
Xu, D. P. et al. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int. J. Mol. Sci. 18, 96. https://doi.org/10.3390/ijms18010096 (2017).
Haseloff, R. F., Blasig, I. E., Meffert, H. & Ebert, B. Hydroxyl radical scavenging and antipsoriatic activity of benzoic acid derivatives. Free Rad Biol. Med. 9, 111–115. https://doi.org/10.1016/0891-5849(90)90113-W (1990).
Plaskova, A. & Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 10, 1118761. https://doi.org/10.3389/fnut.2023.1118761 (2023).
Sestili, P. et al. The potential effects of Ocimum Basilicum on health: a review of Pharmacological and toxicological studies. Expert Opin. Drug Metab. Toxicol. 14, 679–692. https://doi.org/10.1080/17425255.2018.1484450 (2018).
Abou El-Soud, N. H., Deabes, M., El-Kassem, A., Khalil, M. & L. & Chemical composition and antifungal activity of Ocimum Basilicum L. essential oil. J. Med. Sci. 3, 374. https://doi.org/10.3889/oamjms.2015.082 (2015).
Lal, R. Breeding for new chemotypes with stable high essential oil yield in Ocimum. Ind. Crops Prod. 59, 41–49. https://doi.org/10.1016/j.indcrop.2014.04.047 (2014).
Rubab, S. et al. Phytochemical and antimicrobial investigation of methanolic extract/fraction of Ocimum Basilicum L. Biocatal. Agric. Biotechnol. 31, 101894. https://doi.org/10.1016/j.bcab.2020.101894 (2021).
Lee, J. & Scagel, C. F. Chicoric acid found in Basil (Ocimum Basilicum L.) leaves. Food Chem. 115, 650–656. https://doi.org/10.1016/j.foodchem.2008.12.075 (2009).
Tarchoune, I. et al. Changes in the antioxidative systems of Ocimum Basilicum L.(cv. Fine) under different sodium salts. Acta Physiol. Plant. 34, 1873–1881. https://doi.org/10.1007/s11738-012-0985-z (2012).
Verma, R. S., Padalia, R. C., Chauhan, A. & Thul, S. T. Exploring compositional diversity in the essential oils of 34 ocimum taxa from Indian flora. Ind. Crop Prod. 45, 7–19. https://doi.org/10.1016/j.indcrop.2012.12.005 (2013).
Shahrajabian, M. H., Sun, W. & Cheng, Q. Chemical components and Pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 23, 1961–1970. https://doi.org/10.1080/10942912.2020.1828456 (2020).
Ryan, R. P., Germaine, K., Franks, A., Ryan, D. J. & Dowling, D. N. Bacterial endophytes: recent developments and applications. FEMS Microbiol. Lett. 278, 1–9. https://doi.org/10.1111/j.1574-6968.2007.00918.x (2008).
Singh, N. A. & Jain, R. Diversity and bioactive potential of endophytic bacteria from High-Value medicinal plants. In Bacterial Endophytes for Sustainable Agriculture and Environmental Management (eds Singh, A. K. et al.) 45–69 (Springer, 2022). https://doi.org/10.1007/978-981-16-4497-9_3.
Hernández-Pacheco, C. E., del Carmen Orozco-Mosqueda, M., Flores, A., Valencia-Cantero, E. & Santoyo, G. Tissue-specific diversity of bacterial endophytes in Mexican husk tomato plants (Physalis Ixocarpa brot. Ex Horm.), and screening for their multiple plant growth-promoting activities. Curr. Res. Microb. Sci. 2, 100028 (2021).
Chaudhary, P., Agri, U., Chaudhary, A., Kumar, A. & Kumar, G. Endophytes and their potential in biotic stress management and crop production. Front. Microbiol. 13, 933017. https://doi.org/10.1016/j.crmicr.2021.100028 (2022).
Eid, A. M., Salim, S. S., Hassan, S. E. D., Ismail, M. A. & Fouda, A. Role of endophytes in plant health and abiotic stress management. In Microbiome in Plant Health and Disease: Challenges and Opportunities (eds Kumar, V. et al.) 119–144 (Springer, 2019). https://doi.org/10.1007/978-981-13-8495-0_6.
Purwanti, E., Waskito, H. & Sa’diyyah, I. The effect of organic fertilizer types and endophyte bacteria application on the productivity of hot pepper (Capsicum ascalonicum). IOP Conf. Ser. : Earth Environ. Sci. 653, 012063. https://doi.org/10.1088/1755-1315/653/1/012063 (2021).
Zhou, J. Y. et al. Endophytic Pseudomonas induces metabolic flux changes that enhance medicinal sesquiterpenoid accumulation in Atractylodes lancea. Plant. Physiol. Biochem. 130, 473–481. https://doi.org/10.1016/j.plaphy.2018.07.016 (2018).
Aeron, A., Maheshwari, D. K. & Meena, V. S. Endophytic bacteria promote growth of the medicinal legume Clitoria Ternatea L. by chemotactic activity. Arch. Microbiol. 202, 1049–1058. https://doi.org/10.1007/s00203-020-01815-0 (2020).
Almuhayawi, M. S. et al. S. Bacterial endophytes as a promising approach to enhance the growth and accumulation of bioactive metabolites of three species of Chenopodium Sprouts. Plants. 10, 2745. (2021). https://doi.org/10.3390/plants10122745
Corretto, E. et al. Comparative genomics of Microbacterium species to reveal diversity, potential for secondary metabolites and heavy metal resistance. Front. Microbiol. 11, 1869. https://doi.org/10.3389/fmicb.2020.01869 (2020).
Goswami, D., Dhandhukia, P. & Thakker, J. N. Expanding the horizons for the use of Paenibacillus species as PGPR for sustainable agriculture. In Bacilli and Agrobiotechnology (eds Islam, M. T. et al.) 281–307 (Springer, 2016). https://doi.org/10.1007/978-3-319-44409-3_12.
Timofeeva, A. M., Galyamova, M. R. & Sedykh, S. E. How do plant growth-promoting bacteria use plant hormones to regulate stress reactions? Plants. 13, 2371. (2024). https://doi.org/10.3390/plants13172371
Martínez-Arias, C. et al. Stem endophytes increase root development, photosynthesis, and survival of elm plantlets (Ulmus minor Mill). J. Plant. Physiol. 261, 153420. https://doi.org/10.1016/j.jplph.2021.153420 (2021).
Adeleke, B. S., Babalola, O. O. & Glick, B. R. Plant growth-promoting root-colonizing bacterial endophytes. Rhizosphere 20, 100433. https://doi.org/10.1016/j.rhisph.2021.100433 (2021).
Chen, Q., Song, Y., An, Y., Lu, Y. & Zhong, G. Soil microorganisms: their role in enhancing crop nutrition and health. Diversity 16, 734. https://doi.org/10.3390/d16120734 (2024).
Khoso, M. A. et al. Impact of plant growth-promoting rhizobacteria (PGPR) on plant nutrition and root characteristics: current perspective. Plant. Stress. 11, 100341. https://doi.org/10.1016/j.stress.2023.100341 (2024).
Siddique, S., Naveed, M., Yaseen, M. & Shahbaz, M. Exploring potential of seed endophytic bacteria for enhancing drought stress resilience in maize (Zea Mays L). Sustainability 14, 673. https://doi.org/10.3390/su14020673 (2022).
Ameen, M., Mahmood, A., Sahkoor, A., Zia, M. A. & Ullah, M. S. The role of endophytes to combat abiotic stress in plants. Plant. Stress. 100435. https://doi.org/10.1016/j.stress.2024.100435 (2024).
Habermann, E. et al. Inoculation with plant growth-promoting bacteria mitigates the negative impacts of 2° C warming on the photosynthesis, growth, and nutritional value of a tropical C4 grassland under field conditions. Sci. Total Environ. 967, 178769. https://doi.org/10.1016/j.scitotenv.2025.178769 (2025).
Das, D. et al. Harnessing endophytes: innovative strategies for sustainable agricultural practices. Discov Bact. 2, 1. https://doi.org/10.1007/s44351-025-00011-z (2025).
Eid, A. M. et al. Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: an overview. Plants 10, 935. https://doi.org/10.3390/plants10050935 (2021).
Santoyo, G., Moreno-Hagelsieb, G., del Carmen Orozco-Mosqueda, M. & Glick, B. R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 183, 92–99. https://doi.org/10.1016/j.micres.2015.11.008 (2016).
Spaepen, S., Vanderleyden, J. & Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 31, 425–448. https://doi.org/10.1111/j.1574-6976.2007.00072.x (2007).
Etesami, H. & Beattie, G. A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 9, 148. https://doi.org/10.3389/fmicb.2018.00148 (2018).
Bashan, Y. & de-Bashan, L. E. How the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. Adv. Agron. 108, 77–136. https://doi.org/10.1016/S0065-2113(10)08002-8 (2010).
Glick, B. R. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 963401. (2012). https://doi.org/10.6064/2012/963401 (2012).
Hashem, A., Tabassum, B. & Abd_Allah, E. F. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 26, 1291–1297. https://doi.org/10.1016/j.sjbs.2019.05.004 (2019).
Grover, M. et al. PGPR mediated alterations in root traits: way toward sustainable crop production. Front. Sustain. Food Syst. 4, 618230. https://doi.org/10.3389/fsufs.2020.618230 (2021).
Mishra, A. & Tailor, S. Plant-microbe interactions in photosynthesis, nutrient acquisition, and plant growth. In: Plant-Microbe Interaction-Recent Advances in Molecular and Biochemical Approaches. Academic Press. pp. 421–434. (2023). https://doi.org/10.1016/B978-0-323-91875-6.00019-0
Ermis, H., Guven-Gulhan, U., Cakir, T. & Altinbas, M. Effect of iron and magnesium addition on population dynamics and high value product of microalgae grown in anaerobic liquid digestate. Sci. Rep. 10, 3510. https://doi.org/10.1038/s41598-020-60622-1 (2020).
Demmig-Adams, B. & Adams, W. W. III Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New. Phytol. 172, 11–21. https://doi.org/10.1111/j.1469-8137.2006.01835.x (2006).
Wang, P., Ji, S. & Grimm, B. Post-translational regulation of metabolic checkpoints in plant tetrapyrrole biosynthesis. J. Exp. Bot. 73, 4624–4636. https://doi.org/10.1093/jxb/erac203 (2022).
Glick, B. R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 169, 30–39. https://doi.org/10.1016/j.micres.2013.09.009 (2014).
Steyn, W., Wand, S., Holcroft, D. & Jacobs, G. Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New. Phytol. 155, 349–361. https://doi.org/10.1046/j.1469-8137.2002.00482.x (2002).
Zhang, Y., Butelli, E. & Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant. Biol. 19, 81–90. https://doi.org/10.1016/j.pbi.2014.05.011 (2014).
Nakabayashi, R. et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant. J. 77, 367–379. https://doi.org/10.1111/tpj.12388 (2014).
Morales-Cedeño, L. R. et al. Plant growth-promoting bacterial endophytes as biocontrol agents of pre-and post-harvest diseases: fundamentals, methods of application and future perspectives. Microbiol. Res. 242, 126612. https://doi.org/10.1016/j.micres.2020.126612 (2021).
Santoro, M. V., Zygadlo, J., Giordano, W. & Banchio, E. Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita). Plant. Physiol. Biochem. 49, 1177–1182. https://doi.org/10.1016/j.plaphy.2011.07.016 (2011).
Iijima, Y. et al. The biochemical and molecular basis for the divergent patterns in the biosynthesis of terpenes and phenylpropenes in the peltate glands of three cultivars of Basil. Plant. Physiol. 136, 3724–3736. https://doi.org/10.1104/pp.104.051318 (2004).
Ayaz, M. et al. Exploring plant growth promoting traits and biocontrol potential of new isolated Bacillus subtilis BS-2301 strain in suppressing Sclerotinia sclerotiorum through various mechanisms. Front. Plant. Sci. 15, 1444328. https://doi.org/10.3389/fpls.2024.1444328 (2024).
Hasanuzzaman, M. et al. Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9, 681. https://doi.org/10.3390/antiox9080681 (2020).
Verma, N. & Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants. 2, 105–113. https://doi.org/10.1016/j.jarmap.2015.09.002 (2015).
Dehghanian, Z. et al. Reinforcing the bulwark: unravelling the efficient applications of plant phenolics and tannins against environmental stresses. Heliyon 8 https://doi.org/10.1016/j.heliyon.2022.e09094 (2022).
Falcone Ferreyra, M. L., Rius, S. P. & Casati, P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front. Plant. Sci. 3, 222. https://doi.org/10.3389/fpls.2012.00222 (2012).
Hernández, I., Alegre, L., Van Breusegem, F. & Munné-Bosch, S. How relevant are flavonoids as antioxidants in plants? Trends Plant. Sci. 14, 125–132. https://doi.org/10.1016/j.tplants.2008.12.003 (2009).
Vacheron, J. et al. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant. Sci. 4, 356. https://doi.org/10.3389/fpls.2013.00356 (2013).
Gill, S. S. & Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant. Physiol. Biochem. 48, 909–930. https://doi.org/10.1016/j.plaphy.2010.08.016 (2010).
Ma, Y., Dias, M. C. & Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant. Sci. 11, 591911. https://doi.org/10.3389/fpls.2020.591911 (2020).
Neshat, M. et al. Plant growth promoting bacteria (PGPR) induce antioxidant tolerance against salinity stress through biochemical and physiological mechanisms. Physiol. Mol. Biol. Plants. 28, 347–361. https://doi.org/10.1007/s12298-022-01128-0 (2022).
Dutta, P. et al. Molecular interaction between plants and trichoderma species against soil-borne plant pathogens. Front. Plant. Sci. 14, 1145715. https://doi.org/10.3389/fpls.2023.1145715 (2023).
Aravind, R., Antony, D., Eapen, S. J., Kumar, A. & Ramana, K. Isolation and evaluation of endophytic bacteria against plant parasitic nematodes infesting black pepper (Piper nigrum L). Indian J. Nematol. 39, 211–217 (2009).
Frank, J. A. et al. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 74, 2461–2470. https://doi.org/10.1128/AEM.02272-07 (2008).
Atkinson, D. The distribution and effectiveness of the roots of tree crops. Hortic. Rev. (1981).
Galmés, J., Flexas, J., Savé, R. & Medrano, H. Water relations and stomatal characteristics of mediterranean plants with different growth forms and leaf habits: responses to water stress and recovery. Plant. Soil. 290, 139–155. https://doi.org/10.1007/s11104-006-9148-6 (2007).
Zhao, Y., Aspinall, D. & Paleg, L. Protection of membrane integrity in medicago sativa L. by Glycinebetaine against the effects of freezing. J. Plant. Physiol. 140, 541–543. https://doi.org/10.1016/S0176-1617(11)80785-6 (1992).
Porra, R. J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res. 73, 149–156. https://doi.org/10.1023/A:1020470224740 (2002).
Sims, D. A. & Gamon, J. A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 81, 337–354. https://doi.org/10.1016/S0034-4257(02)00010-X (2002).
Lichtenthaler, H. K. & Wellburn, A. R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc Trans. 11, 591–592. https://doi.org/10.1042/bst0110591 (1983).
Kapoor, R., Giri, B. & Mukerji, K. G. Improved growth and essential oil yield and quality in foeniculum vulgare mill on mycorrhizal inoculation supplemented with P-fertilizer. Bioresour Technol. 93, 307–311. https://doi.org/10.1016/j.biortech.2003.10.028 (2004).
Rahim, N. A., Roslan, M. N. F., Muhamad, M. & Seeni, A. Antioxidant activity, total phenolic and flavonoid content and LC–MS profiling of leaves extracts of Alstonia angustiloba. Separations 9, 234. https://doi.org/10.3390/separations9090234 (2022).
Akkol, E. K., Göger, F., Koşar, M. & Başer, K. H. C. Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem. 108, 942–949. https://doi.org/10.1016/j.foodchem.2007.11.071 (2008).
Aebi, H. Catalase in vitro. Meth Enzymol. 105, 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3 (1984).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell. Physiol. 22, 867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232 (1981).
Bondet, V., Brand-Williams, W. & Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH. Free radical method. LWT - Food Sci. Technol. 30, 609–615. https://doi.org/10.1006/fstl.1997.0240 (1997).
Acknowledgements
This research was partially supported by the Deputy of Research and Technology at Ilam University, Ilam, Iran.
Author information
Authors and Affiliations
Contributions
M. Bagnazari, M.R. Alymanesh, and A. Azizi designed and experimented. F. Ghanbari collected and analyzed the data. M. Bagnazari and M.R. Alymanesh wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Data analysis
Data were analyzed using SPSS (version 26), with Duncan’s test (P ≤ 0.05) applied for mean comparison. Graphs were generated using Microsoft Excel.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bagnazari, M., Alymanesh, M.R., Ghanbari, F. et al. Isolated endophytic bacteria promoted growth, essential oil content, and antioxidant activity in Basil (Ocimum basilicum L.). Sci Rep 15, 36205 (2025). https://doi.org/10.1038/s41598-025-20218-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20218-z
Keywords
This article is cited by
-
Endophytic Micrococcus sp. TCR-5 ameliorates drought stress in rice plants and improves soil enzymatic activity
Annals of Microbiology (2025)