Abstract
Biomaterials are engineered to interact with biological systems for treating, enhancing, or replacing organs, tissues, or functions. Bioactive glasses show strong potential in bone regeneration, drug delivery, soft tissue repair, and wound healing. So, numerous attempts have been made to fabricate transparent glasses within the glassy composition (45-x)SrO − 45SiO2 − 10K2O − xAl2O3 (0 ≤ x ≤ 6 mol%) for biomedical applications. X-ray Diffractometer was performed and confirmed the amorphous behaviour of the sintered glasses, indicating a short-range order structure. Further, the density (ρ) of the glasses was measured based on the Archimedes principle, and an increment was obtained from 3.0945 to 3.3465 g/cm3 with increasing Al2O3 content. Additionally, numerous properties, like molar volume, oxygen packing density, and field strength, were calculated and found to be improved, including structural stability. Moreover, to study structural and functional groups within the glassy matrix, FTIR spectroscopy was performed. Furthermore, to study the optical behaviour of fabricated glasses, UV-visible spectroscopy was executed. Using Tauc’s plots, the energy band gap was determined and found to be decreased from 4.94 to 4.79 eV with increasing Al2O3 content. The MTT assay revealed dose-dependent cytotoxicity against cancer cells, with IC50 values decreasing from ~ 121 µg/ml to ~ 99 µg/ml, respectively. Among all samples, 39SrO-45SiO2-10K2O-6Al2O3 (SrKS6A) demonstrated the highest biocompatibility and anticancer efficacy, turning it into a contender for biomedical applications. The incorporation of Al2O3 improved the structural, optical, and biocompatibility characteristics of the glasses, positioning SrKS6A as the most promising composition for advanced photonic and biomedical uses.
Similar content being viewed by others
Introduction
Biomaterials are designed to interact with biological systems and are utilized for the treatment, enhancement, or replacement organs, tissues, or bodily functions1. Bioactive glasses (BGs) offer considerable potential in bone regeneration, drug delivery systems, soft tissue repair, and wound healing2. Biomaterials have evolved from simply interacting with the body to actively influencing biological processes, with the goal of promoting tissue regeneration1. Moreover, BGs possess antibacterial capabilities and can promote an anti-inflammatory response2,3,4. They are also frequently integrated into multifunctional composites in the field of biomaterial engineering5. Larry Hench discovered a bioactive glass system composed of 46.1SiO2-24.4CaO-26.9Na2O-2.6P2O5 (in mol%), which was named 45S5 and known as Bio-glass. This discovery led to the development of new areas such as bioactive glass, glass ceramics, hydroxyapatite (HAp), and calcium phosphates6. There are some techniques for the synthesis of bioactive glass, but the commonly used ones are the melt-quench technique and sol-gel method7,8. The melt-quench technique is generally better for high-volume, transparent, mechanically strong materials and also cost-effective9. Bioactive glasses are widely used in dental and orthopedic treatments because of their biocompatibility, non-toxic and non-inflammatory characteristics, and their ability to support angiogenesis, osteoconductive and osteo-inductive properties10,11. In addition, bioactive glasses trigger a unique biological response, forming a hydroxycarbonate apatite (HCA) layer more quickly than other bio-ceramics, which allows for faster bonding with living tissue12,13,14. Alumina (Al2O3) is considered an intermediate in binary oxide glasses because it exhibits dual behavior: it can function as both a glass former and a glass modifier, it enhances the hardness and toughness of the glass, and it makes the material durable against corrosion. However, Al2O3 solely cannot form glass on its own15. Advancements in the composition of 45S5 glass, particularly through the addition of new ions and modifications to the SiO2 glass former, have played a vital role in enhancing the functionality and applications of BGs16,17. Silicon (Si) plays a crucial role in boosting bone mineral density and improving bone strength18. SrO acts as a network modifier in glass materials, improving their bioactive properties and influencing key processes like cell proliferation, differentiation, and mineralization. This enhances the material’s effectiveness in tissue engineering applications. Additionally, SrO has been shown to promote osteogenic activity, which is essential for bone healing19,20. It contributes to improving bone microarchitecture and increasing bone mineral density21. However, K2O is a flux, a temperature-reducing agent, it enhances the glass resistance, refractive index, and transparency, resulting in brilliant clarity22. Yeliz et al.23 revealed the role of Al2O3 doping on the antibacterial features of 45S5 bioactive glass and they reported that alumina acts as a stabilizer within the glassy structures by eliminating non-bridging oxygens (NBOs), which reduces glass dissolution and thereby enhances its antibacterial properties. Melchers et al.24 explored the impact of aluminum ion incorporation on the bioactive composition of mesoporous bioactive glass (MBG). They reported that doping MBG with small amounts of Al2O3 (0.5 and 1 mol%) did not significantly impact its bioactivity and might even result in a slight enhancement. Furthermore, they concluded that a small quantity of Al2O3 could be used to enhance the mechanical properties of bioactive glasses without restricting their capability for tissue engineering applications. Mohini et al.25 synthesized B2O3-SiO2-P2O5-Na2O-CaO bio-glass by incorporating varying concentrations of Al2O3 through a melt quenching method and reported that the bioactivity of the glass samples enhanced with the raised concentrations of Al2O3. Further, El-Kheshen et al.26 fabricated a glass composition (65-x)P2O5.20CaO.15Na2O.xAl2O3 which showed the addition of Al2O3 boosted the bioactivity of glass and improved the long-term stability of the implant required for bone defect repair. This improvement is attributed to the increased strength of the glass. Furthermore, Tripathi et al.27 fabricated numerous glass compositions 42SiO2-34CaO-6P2O5-(18-x)SrO-xAl2O3 (x = 0, 0.5, 1.0, 1.5, and 2.5) with an increase in alumina content and showed an enhancement in density, elastic modulus, and compressive strength. The ability of osteosarcoma cells to grow on the surface of these bioactive glasses confirmed their biocompatibility, enhancing the suitability of these materials for clinical applications such as bone implants and healing. Several studies have investigated the effect of Al₂O₃ incorporation on different bioactive glass systems. In the system 45Na₂O–xAl₂O₃–(55 − x)P₂O₅ glasses, density increased with Al₂O₃ content and compressive strength while maintaining non-toxic behavior. Then samples remained biocompatible. A contrasting trend was observed in the 44.5P₂O₅–44.5CaO–(11–x)Na₂O–xAl₂O₃ system, where density decreased despite an increase in strength, indicating structural rearrangements. In the 45SiO₂–24.5CaO–(24.5 − x)Na₂O–6P₂O₅–xAl₂O₃ glass system, Al₂O₃ addition improved mechanical strength, though density and biological outcomes were not fully reported. Overall, Al₂O₃ incorporation generally enhances mechanical performance and maintains non-toxicity, though its effect on density may vary depending on the glass matrix28,29,30. Although bioactive glasses like 45S5 have demonstrated excellent bioactivity, their mechanical strength and long-term stability remain limited for broader clinical applications. While prior studies have explored Al₂O₃ doping to enhance mechanical and biological properties, few have focused on developing highly transparent, dense, and durable SrO-based glasses with systematic Al₂O₃ variation. Thus, there is a need to synthesize and comprehensively characterize new bio-glasses to optimize both structural integrity and biological performance.
The prime objective of this research is to synthesize crystal-clear, high-strength, and durable bioactive glasses and to investigate their biological activities. To achieve this, Al2O3 doped transparent glasses in a glass system (45-x)SrO.45SiO2.10K2O.xAl2O3 (x = 0, 2, 4, and 6) were produced via a melt-quench technique. Various characterizations, including density, XRD, FTIR, ultraviolet-visible spectroscopy, SEM, EDS, XPS, TEM and MTT assay, were conducted to analyze the properties of the developed glasses.
Experimental procedures
Fabrication of glass
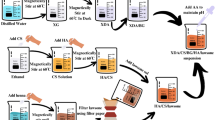
Numerous glasses within the glass composition (45-x)SrO-45SiO2-10K2O-xAl2O3 (x = 0, 2, 4, and 6 mol%) were synthesized using the melt-quenching technique with varying concentrations of Al2O3. To prepare the glass compositions, highly pure analytical reagent (AR) grade oxides were used as raw materials, including SrCO3 (99%), SiO2 (99%), K2CO3 (99%), and Al2O3 (99%). For each composition, a batch weighing 20 g was measured by a digital balance with an accuracy of 0.0001 g. Further, material was mixed with the help of a mortar and pestle using acetone as a liquid medium for 4 h and then dried out in open air. The mixture was kept in a highly pure alumina crucible and subjected to a programmable melting furnace at 1450 °C for 30 min. After melting, the molten material was rapidly quenched in a brass mold and shifted to a muffle furnace at 475 °C for 3 h to eliminate the residual stress. The resulting glass samples were obtained at room temperature and named SrKS0A, SrKS2A, SrKS4A, and SrKS6A respectively, details are enlisted in Table 1. The entire synthesis process of glass using the melt quench method is shown in Fig. 1.
XRD measurements of the glasses
For XRD measurements, the prepared glasses were converted into fine powder through an agate mortar and pestle. XRD patterns were recorded on the powdered samples with a “Rigaku Ultima X-ray diffractometer” employing Cu-Kα1 radiation having a \(\:\lambda\:\) of the order of 1.5406 Å to verify the amorphous nature. The scans were executed over a 2θ range of 200 to 800, with the X-ray tube executed at voltage of 40 kV and a current of 40 mA.
Physical properties of the fabricated glasses
The densities of the developed bulk glasses were precisely measured using Archimedes’ principle, and distilled water has been used as an immersion liquid based on the following method31. To ensure accuracy, density measurements for all the fabricated glasses were conducted three times, and the results were averaged.
The density (ρ) of each developed bulk glass sample, measured in g/cm3, is determined based on the following parameters: W1, W2, W3, and W4 are the weights (g) of the unfilled relative density (RD) bottle, weights (g) of the RD bottle with the glass specimen, weights (g) of the RD bottle filled with distilled water and the glass sample, and weights (g) of the RD bottle filled with the distilled water, respectively. The density of the immersion liquid (ρw) is 1 g/cm3, as it is distilled water. Herein, average molecular weight (AMW), molar volume (Vm), oxygen packing density (OPD), oxygen molar volume (Omv) and ionic concentration (Ic) were calculated for all the synthesized glasses in their different units like g/mol, cm3/mol, g-atom/L, cm3/mol, and ions/cm,3 using the following empirical formulas, respectively31,32,33.
Here, Xi represents the composition of the glass in terms of mole percent, Wi denotes the molecular weight of the ith oxide in g/mole, n refers to the total amount of oxygen atoms in the composition, and (NA) is Avogadro’s number, valued at 6.02214076 × 1023.
Moreover, the inter-ionic separation (ri), and the polaron radius (rp) would be estimated using the succeeding empirical formulas31,33,34,35,36.
In the end, field-strength (Fs) of the glasses will be determined through an empirical relation37.
Here, Z represents the charge of dopant ion (Al3+).
Fourier transform infrared (FTIR) spectroscopy
The FTIR spectra of the fabricated glasses SrKS0A, SrKS2A, SrKS4A, and SrKS6A were noted at room temperature, covering a wavenumber range of 400–3500 cm− 1 using a JASCO FT/IR-5300 spectrometer. The glass samples were crushed into fine powder and mixed with KBr powder in a precise proportion of 1:100. The resulting mixtures were then pressed using a hydraulic press at approximately 7 tons to form clear, homogenous discs. Further, these transparent discs were utilized to record FTIR spectra of all the samples.
UV-visible spectroscopic measurements
The glass absorption edge provides information on its band structure, optical bandgap, and transitions. UV-visible spectra were obtained using a Thermo Scientific Evolution 201 spectrophotometer (240–1100 nm, 0.2 nm resolution). About 1 mg of crushed material was dispersed in 1 ml of DMSO for sample preparation. Spectra were averaged from three measurements and plotted. The optical bandgap (\(\:{E}_{g}\)) was calculated from Davis-Mott/Tauc plots, linking photon energy (hν) to the absorption coefficient (α)31,34.
Here, p represents the transition type: p = 2 for indirect and p = 1/2 for direct transitions39,40. hν is the photon energy, represents the glass energy bandgap, B is a material constant, and the absorption coefficient is \(\:\alpha\:=\left(\frac{4\pi\:k}{\lambda\:}\right)\).
Optical properties
Molar refractivity (Rm) of the developed glasses is primarily determined by (Vm) and energy bandgap (\(\:{E}_{g}^{ind}\)), as expressed through the next empirical formula.
Next, the molar polarizability (αm) of the glass material is directly related to Rm and can be determined using the succeeding Eq.
Here, NA denotes Avogadro’s number (6.0221 × 1023).
The metallization value (Mc), optical basicity (∧), reflection loss (RL), optical electronegativity (χ), and electron polarization (αe) were determined using the corresponding equations33.
Here, \(\:{E}_{g}^{ind}\) represent the indirect energy band gaps.
Refractive index (η) is a key optical parameter that describes the refractive behavior of a transparent material in relation to a vacuum. The refractive index of all the fabricated glass samples was theoretically determined using an empirical formula that depends on the optical bandgap35,37,38.
Furthermore, the theoretical optical dielectric constant (ε) of all the synthesized glasses can be projected using the mentioned non-linear relation37.
Scanning electron microscopy (SEM) and Energy-dispersive spectroscopy (EDS)
The surface morphology of the synthesized SrKSA glass samples was investigated using a field emission scanning electron microscope (FESEM; JSM-7601, JEOL, Tokyo, Japan). High-resolution micrographs were captured at a magnification of ×15,000 from carefully polished specimens. Prior to imaging, small sections of each sample were affixed to copper (Cu) stubs using conductive carbon adhesive. To prevent surface charging under the electron beam, the specimens were coated with a thin platinum (Pt) layer using a sputter coater (JEOL Auto Fine Coater, EC-32010CC, Tokyo, Japan). Furthermore, energy-dispersive X-ray spectroscopy (EDS) was conducted to perform both qualitative and quantitative analyses of the elemental composition. Spectral data were acquired by scanning the entire surface of each sample, and the results were interpreted to assess the distribution of constituent elements.
Transmission electron microscopy (TEM) measurements
TEM analysis was carried out to thoroughly investigate the microstructural or non-crystalline behavior of the SrKS6A synthesized glass sample. The examination was performed using a JEOL 2100 F microscope operating at an acceleration voltage of 200 kV. The analysis included TEM imaging to observe detailed structural features.
X-ray photoelectron spectroscopy (XPS) measurements
XPS is a vital technique used to analyse the chemical composition and electronic states of material surfaces. In this study, a K-alpha XPS instrument from Thermo-Fisher Scientific was employed to examine the surfaces of both the undoped SrKS0A glass and the SrKS6A glass sample doped with the highest concentration of 6 mol% of Al2O3. For the measurements, pellets were prepared from powdered glass, each being 10 mm in diameter and 1 mm thick.
10 cell culture and cell viability assessment using the MTT assay
The cytotoxicity of SrKS0A, SrKS2A, SrKS4A, and SrKS6A samples was assessed on the cervical cancer cell line HeLa using dimethyl thiazolyl tetrazolium bromide (MTT) assay. The HeLa cell line and the intermediates necessary for the cultivation of cells were acquired from NCCS, Pune, and Sigma Aldrich. The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 1% antibiotic-antimycotic solution and 10% fetal bovine serum (FBS). The cells were initially seeded in 25cm3 culture flasks at a density of 0.7 × 106, thereafter incubated overnight in a carbon dioxide (CO2) incubator at 37 °C with 5% of CO2. After the HeLa cells achieved 80–90% confluency, medium was discarded, and the cells were subjected to trypsinization for subsequent seeding in 96-well plates at a density of 1 × 10441. Following a 24-hour period, the cells were exposed to different concentrations of the test samples (0, 20, 40, 60, 80, 100, 120, and 140 µg/ml) in triplicate and then incubated for 24 h in a CO2 incubator. Each well was administered with 10 µl of 5 mg/mL MTT dissolved in phosphate buffered saline (PBS) (Himedia, USA). Following a 2-hour incubation period, the supernatant from each well was discarded. Subsequently, 100 µl of DMSO was added to each well to dissolve the formazan crystals and was gently agitated42. The proportion of cellular toxicity was determined in relation to the control (0 µg). Trivedi et al.43 reported that the optical density (OD) of the resultant-colored solution was assessed using a multiwell plate reader at a designated wavelength of 540 nm (BioTek, USA). The OD values of the treated cells were examined in relation with the values of the control group (with no treatment), and analysis was performed using GraphPad Prism v.644. The cell proliferation part was calculated using the formula:
The aforementioned formula facilitates the assessment of the relative quantity of viable cells in the treated samples in relation to the control, hence elucidating the impact of the treatments on cell proliferation.
Dual-fluorescence viability study
The cells (1 × 105) were seeded in 12-well plates and incubated overnight. Following that, cells were exposed to a 100 µg/ml concentration of test samples, SrKS0A, SrKS2A, SrKS4A and SrKS6A for 24 h. After removing media from the treated cells, they were washed with 1X PBS, and then 300 µl (100 µM) of Acridine Orange (AO) and Propidium Iodide (PI) solution (1:1) was added and incubated for 15 min at room temperature. The cells were rinsed twice with 1X PBS after the dye was removed and examined at 200X magnification using a Nikon Eclipse Ti2 fluorescent microscope. ImageJ (Fiji) software was used to analyze the pictures41.
Results and discussions
XRD investigation
The recorded XRD outlines of the samples within compositions (45-x)SrO-45SiO2-10K2O-xAl2O3 (x = 0, 2, 4, and 6) with varying doping concentrations of Al2O3, i.e., SrKS0A, SrKS2A, SrKS4A, and SrKS6A, are depicted in Fig. 2. It clearly indicates that the material is amorphous, displaying a disordered atomic structure without any distinct or sharp peaks. The observed broad peak suggests the absence of long-range order, confirming the successful fabrication of bulk non-crystalline (amorphous) glasses.
Physical parameters analysis
Several parameters (ρ, AMW, Vm, OPD, Omv, N, ri, rp, and Fs) for SrKS0A, SrKS2A, SrKS4A, and SrKS6A were calculated using Eqs. (1–9) and listed in Table 2. Density can be defined as a basic parameter that measures the amount of mass in a given substance volume, which is influenced by atomic mass, atomic packing arrangement, and coordination number45. Figure 3(a) demonstrates the variation in ρ and Vm as the Al2O3 concentration (mole%) increases. Density increases as the Al2O3 content increases and is calculated to be from 3.0945 to 3.3465 g/cm3. This may be due to the fact that Al3+ ions could have occupied interstitial sites within the glass network27. Additionally, density and molar volume are reciprocal to each other. The molar volume of the glasses was decreased from 28.2712 to 26.1126 cm3/mol with the augmentation of Al2O3 content. The reduction in Vm is attributed to an increase in NBO count that binds the excited electrons46. The OPD values increased, as shown in Fig. 3(b), from 51.2887 to 60.1241 g-atom/l as the Al2O3 content increased in the glassy system, with rising density demonstrating a direct correlation between them47. Furthermore, the Omv values decrease from 19.4974 to 16.6322 cm3/mol48. Besides this, Ic and ri were evaluated and plotted in Fig. 3(c) with respect to Al2O3. The Ic values seem to be increased with the addition of dopant as 1.3455 × 1023, 2.8943 × 1023, and 4.6313 × 1023 ions/cm3 for SrKS2A, SrKS4A, and SrKS6A glasses, respectively. The ri values revealed a decrement trend with measurements of 1.9514, 1.5117, and 1.2925 Å. This reduction may be due to the increasing values of N, as ri is inversely proportional to the cube root of N. An analogous pattern was seen for the values of rp as shown in Fig. 3(d), with 7.8630, 6.0913, and 5.2078 Å values for SrKS2A, SrKS4A, and SrKS6A, respectively47,49. But the Fs values rise with increased doping concentration of Al2O3 from 1.6713, 2.6950, and 3.6870 Å−2 for SrKS2A, SrKS4A, and SrKS6A glasses, respectively. The reduction in rp is accompanied by a corresponding increase in Fs, as field strength is inversely proportional to the square of the polaron radius37,50.
a Density, and Molar volume, b Oxygen packing density and Oxygen molar volume, c Ion concentrations and Inter-nuclear distance, and d Polaron radius and Field strength with respect to concentrations of Al2O3 for all the synthesized glasses SrKS0A, SrKS2A, SrKS4A, and SrKS6A in the SrKSA glassy system respectively.
FTIR spectra analysis
Figure 4 illustrates the FTIR spectra of the bioactive glass samples SrKS0A, SrKS2A, SrKS4A, and SrKS6A, with corresponding peak assignments detailed in Table 3, covering the wavenumber range of 400–2000 cm− 1. Several transmission peaks observed between 650 and 900 cm− 1 are attributed to Si-O, O-Si-O, Si-O-Si bending, and Si-O symmetric stretching vibrations46,47. A band around 580 cm− 1, whose intensity decreases with increasing Al2O3 content, is also evident, alongside another peak at 780 cm− 1. A prominent peak at 1261 cm− 1, associated with O-C-O bending vibrations, disappears upon the incorporation of Al2O348. Similarly, a broad band at 1250 cm− 1, initially wider in the absence of Al2O3, becomes narrower with its addition. Broad absorption bands at 1473, 1483, 1487, and 1495 cm− 1 are linked to carbonate (CO32−) groups, likely due to the presence of K2CO3 and SrCO3 in the composition49. Minor shoulders around 1641 and 1645 cm− 1 correspond to C = C stretching vibrations50. A band at 1600 cm− 1, along with others around 2400 and 2900 cm− 1, also shows a decrease in intensity with increasing Al2O3 content. Characteristic peaks in the 2300–2500 cm− 1 range are attributed to O-H stretching and bending vibrations, indicating the presence of residual water molecules6,28. Additionally, broad bands between 2800 and 3000 cm− 1 are assigned to O-H vibrations, hydrogen bonding, and surface-adsorbed water, resulting from the hygroscopic nature of K2CO36. While the addition of Al2O3 lead to significant changes in the FTIR spectra, it acts as an intermediate (modifier and network former) oxide within the glass network.
UV -visible and optical properties studies
UV-visible spectroscopy was employed to study the absorption characteristics of the fabricated samples (SrKS0A, SrKS2A, SrKS4A, and SrKS6A). The UV-visible absorption spectra exhibited a bathochromic shift (red shift), indicative of changes in the electronic structure with varying Al2O3 content (Fig. 5a). Tauc’s plots (Fig. 5b) using Eq. (10) were utilized to determine the nature of the indirect inter-band transitions, revealing valuable insights into the optical properties of the materials. Figure 5(c) illustrates a decreasing trend in band gap values, which declined from 4.94 eV to 4.79 eV with increasing Al2O3 concentration, suggesting the incorporation of Al2O3 influences the glass network’s structural and electronic properties. Additionally, key optical parameters derived from UV-visible data, including absorption edge and energy band gap, were analyzed and tabulated in Table 4. This systematic variation in the band gap with Al2O3 doping highlights the tunable nature of the fabricated glasses, which is critical for tailoring materials for specific optical and electronic applications.
Various parameters (Rm, αm, RL, χ, αe, Λ, Mc, η, and ε) for all the synthesized glasses SrKS0A, SrKS2A, SrKS4A, and SrKS6A in the SrKSA glassy system with different concentrations of Al2O3 (mol%), respectively. The parameters were calculated from pre-derived \(\:{(E}_{g}^{ind}\)) values obtained from Tauc’s plots, from equations (11 to 19). Figure 6(a) represents the plot among Rm and the rising content of Al2O3 (mol%). The values reveal that with increasing Al2O3, the Rm values decreased like 14.22, 14.12, 13.66, and 13.33 cm3/mol, respectively. The decrement in Rm is ascribed to its direct dependence on Vm, which also decreases with the increasing content of Al2O3. The value of \(\:{\alpha\:}_{m}\) indicates the electron count detected under an applied electric field and is directly influenced by \(\:{R}_{m}\), exhibiting a similar trend. As shown in Fig. 6(a), \(\:{\alpha\:}_{m}\) it decreased from 5.64 to 5.29 Å3 as the concentration of Al2O3 increased. Additionally, based on calculated values of \(\:{R}_{L}\) and \(\:\chi\:\) (Table 4), a graph in Fig. 6(b) is generated to analyze their behaviors as the concentration of Al2O3 increases. Al2O3 has a refractive index of about 1.76, which is higher than that of typical glass materials like silica, whose refractive index is around 1.45. As the proportion of Al2O3 increases, the refractive index (η) of the material also increases, leading to the observed incremental increase in reflection loss (RL) values such as 0.503, 0.505, 0.0507, and 0.51151. The power of an atom to attract electrons toward itself within a molecule is designated as electronegativity. Linus Pauling, who developed the electronegativity scale, suggested that in a compound or alloy formed by atoms with different electronegativity values, the atom with greater electronegativity attracts the shared pair electron more effectively49,56. The χ values were observed in a decreasing trend: 1.33, 1.32, 1.31, and 1.29, respectively, and the decreasing trend may indicate a weakening of an atom’s ability to attract electrons in a chemical bond, weakening the nucleus control over electrons, which makes properties like polarizability and optical basicity more pronounced. The increase in αe (2.3–2.34) and \(\:{\Lambda\:}\) (1.036–1.056) shown in Table 4 is logically linked to the decrease in χ due to the reduced nuclear attraction at lower electronegativity, facilitating greater electron cloud distortion and electron-donating ability. The negative gradient observed in the linear dependence reflects this inverse relationship displayed in Fig. 6(c). The Mc values were used to determine the non-metallic/metallic characteristics of materials, as depicted in Fig. 6(d) with Al2O3 content. The metallization Mc values might decrease from 0.497 to 0.489 with the higher concentration of Al2O3, signaling a transition toward non-metallic behavior due to its insulating nature56,57. The calculated η values, including SrKS0A, SrKS2A, SrKS4A, and SrKS6A, are listed in Table 4. Furthermore, Fig. 5(c) presents a graph showing η as a function of Al2O3 concentration (in mole%). The η values increase from 2.009 to 2.032 on increasing the doping weightage of Al2O3. The increase in η values is likely due to a combination of higher material density, increased polarizability, and structural changes in the glass that enhance its interaction with light, resulting in a higher refractive index. Additionally, the refractive index increases significantly due to alteration in the stoichiometry of the batches and the strain (internal) produced within the material49,56,57,58,59. The optical dielectric constant values, \(\:\epsilon\:\) (Table 4), were determined using Eq. (19) and are shown in Fig. 6(d). The increase in \(\:\epsilon\:\) values from 4.036 to 4.13 with an increase in Al2O3 concentration is mainly due to enhanced polarizability, stronger network formation, and the non-linear relationship with the refractive index. These factors collectively lead to a greater ability of the material to polarize in response to an electric field, resulting in a higher optical dielectric constant49,56,57.
Variations of a Molar refractivity and Molar polarizability, b Reflection loss and Electronegativity, c Electron polarizability and Optical basicity, and d Metallization value and TPA with respect to increased concentrations of Al2O3 (mol%) for all the synthesized glasses SrKS0A, SrKS2A, SrKS4A, and SrKS6A in the SrKSA glassy system respectively.
SEM and EDS analysis
The FESEM test was successfully completed to assess the surface morphology of all the fabricated glasses in the SrKSA glassy system doped with Al2O3. Figure 7 (a-d) shows the SEM micrographs at a fixed magnification of 15,000 for SrKS0A, SrKS2A, SrKS4A, and SrKS6A glass samples, respectively. The microstructural analysis revealed the amorphous nature of the prepared glasses because there is a complete absence of nucleation and growth, which means that no grain and grain boundaries (crystallization) were found within the SEM images of the glass samples37. Furthermore, these results are well consistent with the results of XRD analysis which showed the short-range ordered structure (Fig. 2) and again confirmed the amorphous behavior of the all-prepared glass samples.
The EDS analysis was conducted to evaluate the elemental composition of the synthesized glasses in the fabricated glassy system. Figure 8 shows the EDS spectra for each sample, while Fig. 7 (SEM images) was used to collect average elemental data, with the corresponding weight% and atomic% values listed in Table 5. The EDS spectra confirmed the presence of all expected elements based on the respective glass formulations. For the undoped SrKS0A glass, the major elements identified were strontium (Sr = 46.82%), oxygen (O = 33.31%), potassium (K = 11.74%), silicon (Si = 2.68%), and carbon (C = 5.45%). As the Al2O3 content increased in the doped samples (SrKS2A, SrKS4A, and SrKS6A), the aluminum (Al) content increased correspondingly, while the Sr content decreased. Due to the close proximity of the kinetic energies of Sr (1.81 keV) and Si (1.74 keV), overlapping peaks were observed in the glass samples. However, a clear shoulder growth in Al peaks supported the trend. These findings are in good agreement with the intended glass compositions.
TEM analysis
The microstructure of 6 mol% Al2O3-doped glass (SrKS6A) was examined by TEM at various magnifications, as shown in Figs. 9 (a-c). Figure 9 (a) displays a low-magnification TEM image clearly revealing glassy regions comprising the embedded nano-crystallites. The morphology indicates a heterogeneous distribution of irregularly shaped particles with amorphous behavior. The variation in contrast confirms the coexistence of an amorphous glassy matrix with crystalline domains. Some faceted crystalline grains, measuring approximately 60–200 nm, are visible, suggesting partial crystallization as a result of Al2O3 incorporation into the glassy network. Figure 9 (b, c) highlights the boundary regions between the amorphous and crystalline phases at higher magnification. Blurred areas correspond to the disordered amorphous matrix, while sharp, well-defined regions indicate nanocrystalline inclusions. This observation supports that Al2O3 promotes localized nucleation and growth of crystallites within the glassy host. TEM analysis thus demonstrates that the incorporation of Al2O3 into the glass system facilitates partial crystallization, resulting in the formation of nano-crystallites embedded within the large amorphous matrix.
XPS analysis
XPS analysis was conducted to investigate the electronic states and elemental composition of an undoped glass sample (SrKS0A) and a glass sample doped with the highest concentration of 6 mol% Al2O3 (SrKS6A). The survey spectrum (Fig. 10a) of SrKS0A sample confirms the presence of Sr, K, Si, O, and C in the glass. The high-resolution X-ray photoelectron spectroscopy (HRXPS) Sr 3d spectrum (Fig. 10b) clearly shows the characteristic 3d5/2 and 3d3/2 states60. Similarly, the K 2p spectrum (Fig. 10c) displays a well-defined spin–orbit doublet, with K 2p3/2 at ~ 292–293 eV and K 2p1/2 at ~ 295–296 eV. Deconvolution reveals contributions from surface K2CO3/KOH species, formed through interactions between potassium oxides and atmospheric CO₂/moisture. This confirms that potassium exists both as a network modifier within the glass and as surface carbonate/hydroxide species, a common feature in alkali-containing glasses61,62. The Si 2p spectrum (Fig. 10d) exhibits three distinct peaks: elemental Si (~ 101 eV), SiO₂ (~ 102 eV), and silicon suboxides (Si⁺, Si²⁺, Si³⁺) between 102 and 104 eV respectively. These peaks reflect various oxidation states of silicon, with binding energy increasing in higher oxidation states, thereby distinguishing pure silicon, fully oxidized SiO2, and partially oxidized forms63,64. The C 1s spectrum (Fig. 10e) originates mainly from surface adventitious carbon, showing a dominant C–C/C–H peak near 284.6 eV and minor signals from C–O and C = O groups due to surface contamination63,65,66. The O 1s spectrum (Fig. 10f) contains two main peaks: one near 531 eV associated with lattice oxygen in mixed oxides (SiO2, Al2O3) and another around 532 eV corresponding to surface hydroxyl groups (− OH)63,67,68.
Further, the XPS survey spectrum of the SrKS6A glass (Fig. 11a) confirms the presence of Sr, Si, O, K, Al, and C, validating the successful incorporation of all intended components into the glass network. The absence of unexpected peaks reflects the high purity of the sample. Similar to SrKS0A glass sample, the HRXPS spectra of SrKS6A glass were further deconvoluted to identify the chemical states of individual elements. The HRXPS spectra confirm the expected chemical states of the elements: the Sr 3d (Fig. 11b) shows distinct 3d5/2 and 3d3/2 peaks60, while the K 2p (Fig. 11c) doublet indicates K⁺ as both a network modifier and surface K₂CO₃/KOH species61,62. The Si 2p spectrum (Fig. 11d) reveals elemental Si, SiO₂, and suboxides, reflecting multiple oxidation states63,64. The C 1s peak (Fig. 11e) mainly arises from adventitious carbon with minor oxidized species63,65,66. The O 1s spectrum (Fig. 11f) differentiates lattice oxygen in oxides (~ 531 eV) from surface hydroxyl groups (~ 532 eV)63,67,68. The Al 2p spectrum (Fig. 11g) displays a peak at ~ 74 to 75 eV, confirming Al³⁺ in tetrahedral (AlO4) coordination, which contributes to charge balance and cross-linking within the glass network69. Overall, HRXPS deconvolution verifies the expected oxidation states of the constituent elements. Si and Al act as network formers, while Sr²⁺ and K⁺ serve as modifiers generating non-bridging oxygens (NBOs). The distribution of O 1s states further highlights the coexistence of bridging and non-bridging oxygen species, underscoring their role in shaping the local bonding environment of the fabricated glasses.
Cell viability
In the MTT assay, formazan crystals are a purple-hued product resulting from the reduction of the water-soluble tetrazolium salt MTT. The counts of formazan crystals generated are related to the live cell number, which was found to be decreased with the increasing concentrations of composites SrKS0A, SrKS2A, SrKS4A, and SrKS6A. The biocompatibility of SrKS0A, SrKS2A, SrKS4A, and SrKS6A was evaluated using the MTT assay for a range of concentrations from lowest to highest. The data highlights were distinctly illustrated in Figs. 12 and 13 (A-E). Consequently, these data unequivocally indicated that, in comparison to control cells, the cancer cells viability diminishes significantly with increasing doses of all the composites. Half-maximal inhibitory concentration (IC50) measures the potency of various compounds in pharmacological research. Hence, the IC50 for SrKS0A, SrKS2A, SrKS4A, and SrKS6A was calculated as ~ 121 µg/ml, ~ 113 µg/ml, ~ 109 µg/ml, and ~ 99 µg/ml respectively, when treated on HeLa cancer cells. Consequently, all composites exhibit favorable outcomes in the biocompatibility assessment; nevertheless, SrKS6A is the more efficacious material, as it achieved a 50% inhibitory rate of cancer cells at around IC50 of ~ 99 µg/ml, which is comparatively lower than other composites. These results indicate that, as the proportion of Al2O3 increases, the inhibitory concentration for cervical cancer cells decreases, leading to 6 mol% of Al2O3 (SrKS6A) being more biocompatible than the other Al2O3 proportions (0, 2, and 4 mol%).
Bar graph showing the cell viability (%) of cervical cancer HeLa cells treated with different concentrations (20–140 µg/ml) of SrKS0A, SrKS2A, SrKS4A, and SrKS6A. Values are expressed as a percentage, where 100% represents control. The results are shown as mean ± SD (Statistical significance, *p < 0.05, **p < 0.01, ***p < 0.001).
AO/PI dual-fluorescence viability study
AO/PI fluorescence microscopy double labeling revealed that live cells with intact membranes exhibited green fluorescence, whereas compromised and dead cells exhibited yellow-orange and red fluorescence, respectively. Three randomly selected microscopic fields were used to count the number of viable and membrane-compromised/dead cells in each sample. The results were expressed as the ratio of alive to dead cells. In comparison to the control (untreated) group, this study showed that all bio-glasses (SrKS0A, SrKS2A, SrKS4A, and SrKS6A) had more dead cells (Fig. 14). Notably, the SrKS6A sample induced cell death in highest percentage of HeLa cells, indicating its better cytotoxicity and potent anti-cancer activity.
The fluorescent images illustrate a Effect of SrKS0A, SrKS2A, SrKS4A, and SrKS6A on HeLa cells at 100 µg/ml of concentration, as demonstrated by Acridine Orange and Propidium Iodide double staining. Dead and viable (living) cells are indicated by red and green fluorescence, respectively. b A graph displaying the proportion of live and dead cells after treatment. Statistical significance: **p < 0.01, ***p < 0.001; data are shown as mean ± SEM.
Conclusions
Various transparent glasses have been fabricated within the composition (45 − x)SrO − 45SiO2 − 10K2O − xAl2O3 (x = 0, 2, 4, and 6 mol%) successfully. XRD patterns confirmed the amorphous behaviour of the synthesized glasses, indicating a disordered atomic structure without any long-range order. The density (ρ) increased from 3.0945 to 3.3465 g/cm³ with increasing Al2O3 content, likely owing to the incorporation of Al3+ ions into the glassy network. Molar volume (Vm) decreased with Al2O3 addition due to more NBOs, while oxygen packing density (OPD) increased, indicating denser glass matrices. Ion concentration (Ic) increased, with internuclear distance (ri) and polaron radius (rp) decreasing, enhancing atomic packing and field strength (Fs), thus improving structural stability. The FTIR spectra confirmed the presence of functional groups and vibrations associated with Si − O, O − Si − O, and carbonate groups. The optical bandgap (Eg) values decreased from 4.94 to 4.79 eV with increasing Al2O3 content, showing a bathochromic shift. Optical properties like molar refraction (Rm) and electronic polarizability (αm) decreased, while refractive index (η) and optical dielectric constant (ε) increased, indicating structural densification and enhanced polarizability. Metallization criterion (Mc) values decreased, confirming a transition toward non-metallic behaviour with higher Al2O3 content. MTT assay results demonstrated dose-dependent cytotoxicity against cancer cells, with the IC50 values decreasing from ~ 121 µg/ml for SrKS0A to ~ 99 µg/ml for SrKS6A. The IC50 value is the half maximal inhibitory concentration of alumina glasses against the cervical cancer cell line. The lower the IC50 value, the higher the inhibitory potential of the samples. AO/PI dual staining of HeLa cells demonstrated that SrKS6A exhibited maximum percentage dead cells compared to all other samples, indicating its better efficacy. This suggests that reinforcing the glasses with alumina decreases the cell viability of cancer cells. Therefore, SrKS6A exhibited the most effective biocompatibility and anticancer potential among the samples, making it a suitable candidate for biomedical applications. Increasing Al2O3 content enhanced the structural, optical, and biocompatibility properties of the fabricated glasses, with SrKS6A emerging as the most suitable composition for advanced applications in photonics and biomedicals. The enhancement in density, oxygen packing density, and field strength, along with the decrease in molar volume and optical bandgap, reflects a denser, more tightly packed glass network with improved structural stability and controlled optical properties. These structural and optical modifications contribute to increased biocompatibility and stronger anticancer activity, as evidenced by the lower IC50 values observed with higher Al₂O₃ content.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
O’brien, F. J. Biomaterials & scaffolds for tissue engineering. Mater. Today. 14 (3), 88–95. https://doi.org/10.1016/S1369-7021(11)70058-X (2011).
Saha, S. et al. Prospects of antibacterial bioactive glass nanofibers for wound healing: an in vitro study. Int. J. Appl. Glass Sci. 11 (2), 320–328. https://doi.org/10.1111/ijag.15029 (2020).
Zheng, Z. K. et al. Synthesis of copper-containing bioactive glass nanoparticles using a modified Stöber method for biomedical applications. Colloids Surf. B. 150, 159–167. https://doi.org/10.1016/j.colsurfb.2016.11.016 (2017).
Pantulap, U., Arango-Ospina, M. & Boccaccini, A. R. Bioactive glasses incorporating less-common ions to improve biological and physical properties. J. Mater. Sci. Mater. Med. 33, 1–41. https://doi.org/10.1007/s10856-021-06626-3 (2022).
Gerhardt, L. C. & Boccaccini, A. R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 3 (7), 3867–3910. https://doi.org/10.3390/ma3073867 (2010).
Shweta, S. K. et al. Fabrication of bioactive transparent glass ceramics 55SiO2–25Na2O-(15-x)CaO–5P2O5-xZrO2 (0 ≤ x ≤ 6): physical, structural and in vitro cell viability insights for biomedical applications. Ceram. Int. 50 (9), 14550–14570. https://doi.org/10.1016/j.ceramint.2024.01.368 (2024).
Moghanian, A., Zohourfazeli, M. & Tajer, M. H. M. The effect of zirconium content on in vitro bioactivity, biological behavior and antibacterial activity of sol-gel derived 58S bioactive glass. J. Non-Cryst Solids. 546, 120262. https://doi.org/10.1016/j.jnoncrysol.2020.120262 (2020).
Duminis, T. Evidence for new fluoride coordination in SiO2-Al2O3-P2O5-CaO/SrO-CaF2 compositions used in ion-leachable ionomer-type cements: A 19F MAS-NMR investigation. Mater. Lett. 359, 135919. https://doi.org/10.1016/j.matlet.2024.135919 (2024).
Guo, R., Xu, K., Zhao, Z., Niu, C. & Li, H. Effect of the ratio of R=[Na2O]/[B2O3] on the structure, ag species, and antibacterial activity of ag-doped borosilicate glass. J. Non-Cryst Solids. 588, 121610. https://doi.org/10.1016/j.jnoncrysol.2022.121610 (2022).
Huang, Y., Wu, C., Zhang, X., Chang, J. & Dai, K. Regulation of immune response by bioactive ions released from silicate bioceramics for bone regeneration. Acta Biomater. 66, 81–92. https://doi.org/10.1016/j.actbio.2017.08.044 (2018).
Zhai, W. et al. Silicate bioceramics induce angiogenesis during bone regeneration. Acta Biomater. 8 (1), 341–349. https://doi.org/10.1016/j.actbio.2011.09.008 (2012).
Hench, L. L., Splinter, R. J., Allen, W. C. & Greenlee, T. K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 5 (6), 117–141. https://doi.org/10.1002/jbm.820050611 (1971).
Bellucci, D., Anesi, A., Salvatori, R., Chiarini, L. & Cannillo, V. A comparative in vivo evaluation of bioactive glasses and bioactive glass-based composites for bone tissue repair. Mater. Sci. Eng. C. 79, 286–295. https://doi.org/10.1016/j.msec.2017.05.062 (2017).
Naruphontjirakul, P., Li, M. & Boccaccini, A. R. Strontium and zinc Co-Doped mesoporous bioactive glass nanoparticles for potential use in bone tissue engineering applications. Nanomaterials 14 (7), 575. https://doi.org/10.3390/nano14070575 (2024).
Hashimoto, H. et al. Structure of alumina glass. Sci. Rep. 12 (1), 516. https://doi.org/10.1038/s41598-021-04455-6 (2022).
Wen, C. et al. Effects of B2O3 on the structural evolution and biological behavior of Borate bioactive glasses by sol-gel and melting methods. Ceram. Int. 50 (22), 47864–47875. https://doi.org/10.1016/j.ceramint.2024.09.131 (2024).
Logrado, M., Youngman, R. E., Aitken, B. G. & Eckert, H. Network modifier effects in multiple network former glasses: NMR results on the system 70SiO2–7.5P2O5–(22.5–x)Al2O3–xNa2O (0 ≤ x ≤ 17.5). J. Phys. Chem. C. 127 (34), 17269–17284. https://doi.org/10.1021/acs.jpcc.3c04478 (2023).
Dong, M. et al. Biological silicon stimulates collagen type 1 and osteocalcin synthesis in human osteoblast-like cells through the BMP-2/Smad/RUNX2 signaling pathway. Biol. Trace Elem. Res. 173, 306–315 (2016).
Özarslan, A. C. & Yücel, S. Comprehensive assessment of SrO and CuO co-incorporated 50S6P amorphous silicate bioactive glasses in vitro: revealing bioactivity properties of bone graft biomaterial for bone tissue engineering applications. Ceram. Int. 49 (9), 13940–13952. https://doi.org/10.1016/j.ceramint.2022.12.276 (2023).
Li, Y. et al. 45S5 bioglass works synergistically with SiRNA to downregulate the expression of matrix metalloproteinase-9 in diabetic wounds. Acta Biomater. 145, 372–389. https://doi.org/10.1016/j.actbio.2022.04.010 (2022).
Gavinho, S. R. et al. Influence of the addition of zinc, strontium, or magnesium oxides to the bioglass 45S5 network on electrical behavior. Materials 17 (2), 499. https://doi.org/10.3390/ma17020499 (2024).
Fernandes, H. R. et al. The role of K2O on sintering and crystallization of glass powder compacts in the Li2O–K2O–Al2O3–SiO2 system. J. Eur. Ceram. Soc. 32 (10), 2283–2292. https://doi.org/10.1016/j.jeurceramsoc.2012.02.003 (2012).
Elalmış, Y. Effect of Al2O3 doping on antibacterial activity of 45S5 bioactive glass. J. Turk. Chem. Soc. Sect. A: Chem. 8 (2), 419–428. https://doi.org/10.18596/jotcsa.835912 (2021).
Melchers, S., Uesbeck, T., Winter, O., Eckert, H. & Eder, D. Effect of aluminum ion incorporation on the bioactivity and structure in mesoporous bioactive glasses. Chem. Mater. 28 (10), 3254–3264. https://doi.org/10.1021/acs.chemmater.5b04117 (2016).
Mohini, G. J., Krishnamacharyulu, N., Baskaran, G. S., Rao, P. V. & Veeraiah, N. Studies on influence of aluminium ions on the bioactivity of B2O3–SiO2–P2O5–Na2O–CaO glass system by means of spectroscopic studies. Appl. Surf. Sci. 287, 46–53. https://doi.org/10.1016/j.apsusc.2013.09.055 (2013).
El-Kheshen, A. A., Khaliafa, F. A., Saad, E. A. & Elwan, R. L. Effect of Al2O3 addition on bioactivity, thermal and mechanical properties of some bioactive glasses. Ceram. Int. 34 (7), 1667–1673. https://doi.org/10.1016/j.ceramint.2007.05.016 (2008).
Tripathi, H., Rath, C., Kumar, A. S., Manna, P. P. & Singh, S. P. Structural, physico-mechanical and in-vitro bioactivity studies on SiO2–CaO–P2O5–SrO–Al2O3 bioactive glasses. Mater. Sci. Eng. C. 94 (1), 279–290. https://doi.org/10.1016/j.msec.2018.09.041 (2019).
Jeznach, O. et al. New calcium‐free Na2O–Al2O3–P2O5 bioactive glasses with potential applications in bone tissue engineering. J. Am. Ceram. Soc. 101 (2), 602–611 (2018).
Manupriya, Thind, K. S., Singh, K., Sharma, G. & Rajendran, V. Influence of addition of Al2O3 on physical, structural, acoustical and in-vitro bioactive properties of phosphate glasses. Phys. Status Solidi (a). 206 (7), 1447–1455 (2009).
Mohamad, H., Yan, P., Ibrahim, N. F. & Noor, S. N. F. M. December. Effects of alumina (Al2O3) addition on mechanical property of fabricated melt-derived bioactive glass. In AIP Conference Proceedings (Vol. 1791, No. 1). (AIP Publishing, 2016).
Shweta, C. et al. Influence of carbon nanotubes reinforcement on the structural feature and bioactivity of SiO2–Al2O3–MgO–K2CO3–CaO–MgF2 bioglass. Appl. Phys. A. 127 (7), 545. https://doi.org/10.1007/s00339-021-04708-1 (2021).
Fredholm, Y. C., Karpukhina, N., Law, R. V. & Hill, R. G. Strontium containing bioactive glasses: glass structure and physical properties. J. Non-Cryst Solids. 356, 2546–2551. https://doi.org/10.1016/j.jnoncrysol.2010.06.078 (2010).
Madheshiya, A. et al. Synthesis, physical, optical and structural properties of SrTiO3 borosilicate glasses with addition of CrO3. Bull. Mater. Sci. 46 (1), 34. https://doi.org/10.1007/s12034-022-02871-6 (2023).
Kumari, S. et al. Structural, mechanical and biological properties of PMMA-ZrO2 nanocomposites for denture applications. Mater. Chem. Phys. 295, 127089. https://doi.org/10.1016/j.matchemphys.2022.127089 (2023).
Abdel Wahab, E. A. & Shaaban, K. H. Structural and optical features of aluminum lead Borate glass doped with Fe2O3. Appl. Phys. A. 127 (12), 956. https://doi.org/10.1007/s00339-021-05062-y (2021).
Salinigopal, M. S., Gopakumar, N., Anjana, P. S. & SureshKumar, B. Structural, optical and dielectric properties of aluminoborosilicate glasses. J. Electron. Mater. 49, 695–704. https://doi.org/10.1007/s11664-019-07674-w (2020).
Mishra, R. K. et al. Doping impacts of La2O3 on physical, structural, optical and radiation shielding properties of (30-x)BaCO3–30TiO2–40SiO2-xLa2O3 (0 ≤ x ≤ 6) glasses for optoelectronic applications. Phys. Scr. 98 (10), 105918. https://doi.org/10.1088/1402-4896/acf539 (2023).
Mukherjee, S., Rananaware, P., Brahmkhatri, V. & Mishra, M. Polyvinylpyrrolidone-curcumin nanoconjugate as a biocompatible, non-toxic material for biological applications. J. Clust Sci. 34, 395–414. https://doi.org/10.1007/s10876-022-02230-9 (2023).
Dimitriadis, K., Tulyaganov, D. U., Gioti, C., Karakassides, M. A. & Agathopoulos, S. Effect of Al2O3 on microstructure, thermal, and physicomechanical properties, and biomineralization of Na2O/K2O-CaO-MgO-SiO2-P2O5-CaF2 glasses for dental applications. J. Mater. Eng. Perform. 32 (17), 7895–7904 (2023).
Abubakar, G. et al. Enhanced broadband-visible photoluminescence properties of gold nanoparticle-dispersed bismuth borosilicate glasses. J. Mater. Sci. : Mater. Electron. 35 (20), 1423 (2024).
Parveen, S. et al. Enhanced therapeutic efficacy of Piperlongumine for cancer treatment using nano-liposomes mediated delivery. Int. J. Pharm. 643, 123212. https://doi.org/10.1016/j.ijpharm.2023.123212 (2023).
Sachan, A. et al. Synergistic effects of Na2O on structural, physical, optical, and bioactive properties of SrO-SiO2-ZrO2 glasses for biomedical applications. Biomed. Phys. Eng. Express. 11, 015051. https://doi.org/10.1088/2057-1976/ada15e (2025).
Trivedi, P. et al. Anti-Cervical cancer potential of (E)-3-(Substitutedbenzylidene)-1, 8-naphthyridine derivatives: A combined molecular modelling and Pharmacokinetic study. Russ J. Gen. Chem. 95, 135–1377. https://doi.org/10.1134/S1070363225600523 (2025).
Kumar, S., Parveen, S., Swaroop, S. & Banerjee, M. TNF-α and MMPs mediated mucus hypersecretion induced by cigarette smoke: an in vitro study. Toxicol. Vitro. 92, 105654. https://doi.org/10.1016/j.tiv.2023.105654 (2023).
Shweta, S. K. et al. Structural, morphological and mechanical insights from La2O3 doped machinable silicate glass ceramics for biomedical applications. Ceram. Int. 49 (6), 8801–8819. https://doi.org/10.1016/j.ceramint.2022.11.031 (2023).
Rosmawati, S., Sidek, H. A. A., Zainal, A. T., Mohd, H. & Zobir Effect of zinc on the physical properties of tellurite glass. J. Appl. Sci. 8 (10), 1956–1961 (2008).
El-Rehim, A. F. A., Zahran, H. Y., Yahia, I. S., Ali, A. M. & Shaaban, K. S. Physical, radiation shielding and crystallization properties of Na2O-Bi2O3-MoO3-B2O3-SiO2-Fe2O3 glasses. Silicon 14, 405–418. https://doi.org/10.1007/s12633-020-00827-1 (2022).
Shweta, P. et al. Fabrication, structural, and physical properties of alumina doped calcium silicate glasses for carbon dioxide gas sensing applications. J. Non-Cryst Solids. 583, 121475. https://doi.org/10.1016/j.jnoncrysol.2022.121475 (2022).
Geidam, I. G. et al. Optical characterization and Polaron radius of Bi2O3 doped silica borotellurite glasses. J. Lumin. 246, 118868. https://doi.org/10.1016/j.jlumin.2022.118868 (2022).
Calahoo, C. & Wondraczek, L. Ionic glasses: structure, properties and classification. J. Non-Cryst Solids. 8, 100054. https://doi.org/10.1016/j.nocx.2020.100054 (2020).
Shweta, C. R. et al. Synthesis, physical and mechanical properties of lead strontium titanate glass ceramics. Phys. B Condens. Matter. 615, 413069. https://doi.org/10.1016/j.physb.2021.413069 (2021).
Hongxu, C., Azis, R. A. S., Zaid, M. H. M., Matori, K. A. & Ismail, I. Influence of sintering temperature on structure, physical, and optical properties of wollastonite based glass-ceramic derived from waste eggshells and waste soda-lime-silica glasses. Sci. Sinter. 56, 89–103. https://doi.org/10.2298/SOS230701041H (2024).
Shido, T. & Iwasawa, Y. Regulation of reaction intermediate by reactant in the water-gas shift reaction on CeO2, in relation to reactant-promoted mechanism. J. Catal. 136 (2), 493–503. https://doi.org/10.1016/0021-9517(92)90079-W (1992).
Linker, R., Shmulevich, I., Kenny, A. & Shaviv, A. Soil identification and chemometrics for direct determination of nitrate in soils using FTIR-ATR mid-infrared spectroscopy. Chemosphere 61 (5), 652–658. https://doi.org/10.1016/j.chemosphere.2005.03.034 (2005).
Mehta, N., Gaëtan, J., Giura, P., Azaïs, T. & Benzerara, K. Detection of biogenic amorphous calcium carbonate (ACC) formed by bacteria using FTIR spectroscopy. Spectrochim Acta Mol. Biomol. Spectrosc. 278, 121262. https://doi.org/10.1016/j.saa.2022.121262 (2022).
Zhukovsky, M. et al. Dielectric, structural, optical and radiation shielding properties of newly synthesized CaO–SiO2–Na2O–Al2O3 glasses: experimental and theoretical investigations on impact of tungsten (III) oxide. Appl. Phys. A. 128 (3), 205. https://doi.org/10.1007/s00339-022-05328-z (2022).
Bhemarajam, J., Syam Prasad, P., Mohan Babu, M., Özcan, M. & Prasad, M. Investigations on structural and optical properties of various modifier oxides (MO = ZnO, cdo, bao, and PbO) containing bismuth Borate lithium glasses. J. Compos. Sci. 5 (12), 308. https://doi.org/10.3390/jcs5120308 (2021).
Mishra, R. K., Avinashi, S. K., Shweta, S., Kumari, C. R. & Gautam Synergistic effect of Fe2O3 doping on physical, structural, optical, and radiation shielding characteristics of the glasses in a system (30-x)BaO–30TiO2–40SiO2–xFe2O3 (0 ≤ x ≤ 6) for optoelectronic applications. J. Inorg. Organomet. Polym. 34, 1379–1402. https://doi.org/10.1007/s10904-023-02897-1 (2024).
Mishra, R. K., Singh, R., Avinashi, S. K. & Gautam, C. R. Fabrication of ZrO2 doped (30-x)BaO-30TiO2-40SiO2-xZrO2 (0 ≤ x ≤ 6) glasses: enhanced physical, optical and radiation shielding characteristics for optoelectronics applications. J. Inorg. Organomet. Polym. https://doi.org/10.1007/s10904-024-03304-z (2024).
Mhareb, M. H. A. et al. XPS analysis, structural, physical and radiation shielding performance of innovative lead-free boro-tellurite glasses: A comprehensive experimental study. J. Science: Adv. Mater. Devices. 10 (2), 100879 (2025).
Liu, H., Liu, S., Kang, J., Yue, Y. & Jiang, X. Effectively reduce the softening temperature and enhance the wettability of Na2O-CaO-P2O5 glasses for the sealing of aluminum alloys via a modulation of K2O/Na2O ratio. J. Alloys Compd. 1014, 178817 (2025).
Sawyer, R., Nesbitt, H. W. & Secco, R. A. High resolution X-ray photoelectron spectroscopy (XPS) study of K2O–SiO2 glasses: evidence for three types of O and at least two types of Si. J. Non-cryst. Solids. 358 (2), 290–302 (2012).
Mishra, R. K. et al. Unveiling of physical, structural, morphological, and electrical properties of Fe2O3 doped (30-x)BaO•30TiO2•40SiO2•x[Fe2O3], (0 ≤ x ≤ 6) glass-ceramics potential for energy storage devices. Ceram Int (2025).
Nesbitt, H. W. et al. Yan. Bridging, non-bridging and free (O2–) oxygen in Na2O-SiO2 glasses: an X-ray photoelectron spectroscopic (XPS) and nuclear magnetic resonance (NMR) study. J. Non-Cryst Solids. 357 (1), 170–180 (2011).
Wei, J., Liu, B., Zhang, X. & Song, C. One-pot synthesis of N, S co-doped photoluminescent carbon quantum Dots for Hg2+ ion detection. New. Carbon Mater. 33 (4), 333–340 (2018).
Alghamdi, Y. G., Krishnakumar, B., Malik, M. A. & Alhayyani, S. Design and Preparation of biomass-derived activated carbon loaded TiO2 photocatalyst for photocatalytic degradation of reactive red 120 and Ofloxacin. Polymers 14 (5), 880 (2022).
Banerjee, J., Bojan, V., Pantano, C. G. & Kim, S. H. Effect of heat treatment on the surface chemical structure of glass: oxygen speciation from in situ XPS analysis. J. Am. Ceram. Soc. 101 (2), 644–656 (2018).
Mishra, R. K., Avinashi, S. K., Shweta, S., Kumari, C. R. & Gautam Synergistic effect of Fe2O3 doping on physical, structural, optical, and radiation shielding characteristics of the glasses in a system (30-x)BaO–30TiO2–40SiO2–xFe2O3 (0 ≤ x ≤ 6) for optoelectronic applications. J. Inorg. Organomet. Polym Mater. 34 (3), 1379–1402 (2024).
Djebaili, K., Mekhalif, Z., Boumaza, A. & Djelloul, A. X. P. S. XPS, FTIR, EDX, and XRD analysis of Al2O3 scales grown on PM2000 alloy. J. Spectrosc. 2015 (1), 868109 (2015).
Acknowledgements
Authors acknowledged the central facility of powder X-Ray Diffraction at Department of Physics and Cell Culture Facility in the Molecular and Human Genetics Lab (MHG), Department of Zoology, under the Centre of Excellence scheme by the Government of Uttar Pradesh University of Lucknow, Lucknow. DBT-BUILDER research grant to MHG lab in Department of Zoology is also duly acknowledged.
Funding
This proposed work was financially supported by HRDG-CSIR (Pusa, New Delhi) for Senior Research Fellowship under NET-JRF scheme vide file no. 09/0107(12949)/2021-EMR-I. PS and SM acknowledge the research fellowships from UGC (File No. 211610006888 and CSIR (File No. 09/0107(13395)/2022-EMR-I)), New Delhi respectively.
Author information
Authors and Affiliations
Contributions
Akash Sachan and Rajat Kumar Mishra: Conceptualization, Methodology, Data curation, Formal analysis, Writing-Original Draft, Funding acquisition. Sarvesh Kumar Avinashi: Investigation, Formal analysis. Shweta, Rakhi, Priya Sharad and Shireen Masood: Data curation, Formal analysis, Writing-Original Draft. Alok Kumar Rai and Bhoomika Yadav: Data curation, Formal analysis, Writing-Original Draft. Monisha Banerjee and Chandkiram Gautam: Conceptualization, Methodology, Supervision, Writing-Reviewing and Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sachan, A., Mishra, R.K., Shweta et al. Synthesis of Al2O3-Reinforced SrO–SiO2–K2O glasses with enhanced optical, and biological properties for biomedical applications. Sci Rep 15, 36607 (2025). https://doi.org/10.1038/s41598-025-20343-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20343-9