Abstract
Vector-borne diseases (VBDs) account for 17% of the global disease burden, causing approximately 700,000 deaths annually. Among these, dengue is a major public health concern in tropical and subtropical regions, transmitted primarily by Aedes aegypti and Aedes albopictus. This cross-sectional observational study was conducted in Udupi taluk, Karnataka, India, to examine the influence of climatic factors and land use/land cover (LULC) on Aedes distribution during the post-monsoon season. With a 5 km × 5 km grid sampling design, 28 locations (560 households) were surveyed between October and December 2023. Aedes larvae were detected in 71.42% of houses. Of the 425 mosquitoes identified, A. albopictus (57.64%) were more abundant than A. aegypti (23.52%), with the rest belonging to Culex and Armigeres. Climatic factors such as temperature and humidity showed positive correlation with A.albopictus, while A.aegypti showed weak negative correlation. GIS analysis highlighted the Aedes high-density breeding areas, and larval indices maps were developed. Zero-Inflated Negative Binomial (ZINB) regression identified HI as a significant predictor of A. aegypti abundance. LULC mapping indicated Aedes breeding activity concentrated in fragmented urban areas and croplands. The study highlights the importance of integrating climatic and spatial data for targeted vector control.
Similar content being viewed by others
Introduction
According to the World Health Organisation (WHO), vector-borne infections account for 17% of the global disease burden, resulting in 700,000 fatalities annually. Among the Vector-borne diseases (VBDs), mosquito-borne infections like malaria and dengue are estimated to be 219 million and 96 million cases, respectively, across the globe annually1. Mosquitoes that carry infectious agents can transmit the infection for their entire lives under favourable conditions1,2,3. Aedes mosquito species transmit arboviral diseases like dengue, chikungunya, Zika, and yellow fever4. These infections are present in tropics and subtropical regions due to the presence of abundant mosquito vectors, favourable climatic conditions, population movement, and urbanisation1,5,6. Dengue is an important disease with high morbidity and mortality among all the Aedes-borne infections5,6,7,8,9. Due to the absence of a universally effective vaccine and specific antiviral treatments for dengue and chikungunya, disease management remains largely supportive. The limited efficacy and restricted use of existing vaccines further constrain prevention efforts. Therefore, vector control serves as the primary intervention to interrupt Aedes-borne virus transmission and remains central to outbreak prevention strategies6,10,11,12.
Aedes aegypti and Aedes albopictus are the most prevalent species that transmit dengue. Aedes species larval breeding occurs in urban areas in water-holding containers in intra-domestic as well as peridomestic premises of houses, in fridge trays, flower vases, rubber tyres, coconut shells, plastic containers, flowerpots, etc13. The destruction of such breeding habitats would reduce the number of disease cases and transmission rate14. Aedes species are believed to be sensitive to changes in climatic factors, growing populations, and urbanisation15,16. These species are closely associated with humans, making them a significant vector of human pathogens. Since 1950, A. aegypti’s environmental suitability has increased globally by up to 1.5% per decade, and this trend is expected to continue accelerating over the next century17. Between the years 2022–23, India recorded 3,27,449 dengue cases, with 394 deaths. In contrast, chikungunya cases recorded 11,778 cases. Karnataka alone had 11,446 dengue cases in 2023, which included 609 cases from the Udupi district. At the same time, 18 positive chikungunya cases were reported from Udupi during the same period18. These reports pose a public health concern due to their geographical spread and increase in disease burden. Despite these alarming figures, detailed, localised studies have not explored the impact of climatic factors and land use on Aedes mosquito distribution in this region.
Several factors contribute to the expansion of Aedes-borne infections, such as rapid urbanisation, the presence of vectors, population movement, travel and trade, waste management, and climate change, influencing the breeding and proliferation of vectors7,8. Various climatic factors and the changes in vectors, viruses, and hosts are dynamic. Temperature change causes a shift in disease incidence and prevalence, vector biting patterns, transmission frequency, and abundance. Aedes mosquitoes are highly adaptive to temperature variations. An increase in ambient temperature generally accelerates their development rate, shortens the extrinsic incubation period (EIP) of the virus, and can increase biting frequency. However, extreme temperatures may reduce adult mosquito longevity, making temperature both a facilitator and limiter of vector competence and virus transmission. Additionally, a change in humidity is believed to affect the development and lifecycle of Aedes larvae. Humidity is associated with the monsoon season, leading to smaller transmission cycles and rapid spread of infection. Searching and eliminating the containers during the monsoon season is necessary, as they can act as breeding habitats8,19,20.
Numerous research studies have utilised remote sensing (RS) and geographic information systems (GIS) to assess malaria susceptibility, delineate dengue risk zones, and analyse the dynamics of other VBDs across different periods8,21. Public health professionals and environmental scientists increasingly use these technologies to link urban land use with mosquito abundance and malaria prevalence, examining the close association between environmental changes, climate variables, and malaria epidemics. Several studies have shown that spatial and temporal risk factor mapping using surveillance data is crucial for preventing various VBDs like malaria and dengue22. GIS supports overlay and network analysis by documenting spatial parameters, giving epidemiologists access to extensive ecological and climatic data. Visualising epidemiological data in a geographic context and linking it with GIS software aids in prediction and risk profiling8,22. High spatial resolution satellite data offer detailed and real-time digital information as a basis for spatial baseline mapping. These data can be utilised periodically over several years to create precise land-use maps, essential for interpreting low-resolution satellite and socio-economic and geographical data. The choice of a satellite sensor with the appropriate spatial resolution is crucial for specific applications. Recent advancements in accessing remotely sensed vegetation and weather data, coupled with GIS, have opened new avenues for risk assessment, monitoring, and early warning systems8,23,24.
Mapping and predicting early disease outbreak warnings in vector surveillance systems are crucial. The WHO’s global dengue prevention plan emphasises developing predictive models to evaluate dengue outbreak risks. Understanding the spatial distribution of A. aegypti can aid in preventing not only dengue epidemics but also other diseases associated such as chikungunya and Zika outbreaks25. Regression analysis determines the relationship between variables, such as the number of reported cases and temperature. While commonly used in financial contexts, this method is invaluable in predicting factors influencing disease spread based on historical data and research. The primary advantage of regression analysis is its ability to forecast future events based on past trends and patterns26,27,28.
This study discusses the spatial distribution of Aedes mosquitoes in relation to climatic variables and land use/land cover (LULC) patterns in Udupi taluk. It describes the study area and entomological survey design, outlines the data sources and statistical methods used, presents the results of mosquito abundance and statistical modelling, and concludes with implications for targeted vector control using GIS and remote sensing tools.
Methods
Study area
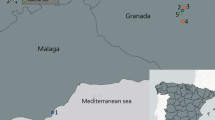
Udupi taluk, located in the southwest region of South India in the Udupi district of Karnataka (Fig. 1a), is characterised by a tropical monsoon climate. It lies between latitudes 13.300°N and 13.550°N and longitudes 74.650°E and 74.850°E, covering an area of 924.13 km². According to the 2011 census, it has a population density of 818 people per km², with 58,086 households29. The region experiences warm temperatures year-round, ranging from 28 °C to 32 °C, and receives an average annual rainfall of approximately 4,119 mm. The high humidity level, averaging 78%, further defines its tropical monsoon climate30. This substantial rainfall and humidity create ideal breeding conditions for mosquito vectors, which pose significant public health challenges. The monsoon season, from June to September, is marked by heavy rainfall, which is crucial for agriculture but also influences the risk of vector-borne diseases due to the proliferation of mosquito breeding sites. In October and November, the post-monsoon season features warmer and damp conditions that provide optimal environments for Aedes mosquito breeding, necessitating heightened vector surveillance and control efforts. From December to February, winter brings cooler and drier conditions that reduce mosquito activity, though residual breeding sites may still pose public health risks. The summer season, from March to May, sees rising temperatures that reduce breeding sites due to evaporation, yet stagnant water from previous monsoons can sustain mosquito populations.
Udupi taluk features a mix of urban and rural landscapes, including residential areas, agricultural fields, forests, and water bodies. These diverse land use patterns influence mosquito distribution and abundance, impacting public health. In urban areas, dense populations and improper waste management can lead to water stagnation, providing breeding grounds for mosquitoes. Agricultural fields, with their irrigation practices, can create standing water that facilitates mosquito breeding.
Historically, Udupi taluk has faced challenges related to vector-borne diseases such as dengue, chikungunya, and malaria31. The high prevalence of Aedes mosquitoes, particularly during and after the monsoon season, underscores the need for targeted public health interventions. Public health initiatives in the region include regular monitoring of mosquito populations and breeding sites to identify and address potential outbreaks, community engagement to educate residents about preventive measures such as eliminating standing water and using mosquito repellents, and environmental management to reduce mosquito habitats.
Udupi taluk serves as a representative model for tropical monsoon regions facing similar public health challenges. The insights gained from this study can inform global strategies for managing Aedes-borne infections in tropical climates. Integrating Geographic Information System (GIS) and remote sensing technologies can be valuable for mapping environmental risk factors, detecting mosquito breeding habitats, and identifying high-risk areas thus enables public health authorities to prioritise resource allocation, such as deploying field teams for source reduction, targeting larvicide application, or planning awareness campaigns, in the most affected or high-risk areas, thereby enhancing the efficiency of vector control programs in regions like Udupi taluk. Given its unique climatic, geographic, and public health characteristics, Udupi taluk is an ideal study area for investigating the impact of climatic factors and LULC on mosquito populations and Aedes-borne disease transmission.
This cross-sectional, observational study examined the relationships between Aedes species abundance, environmental factors, and LULC variables during the post-monsoon period from October 2023 to December 2023. A 5 km X 5 km grid sampling technique was constructed using QGIS software version 3.36.2 to mark the sampling sites systematically. A total of 28 sampling locations were marked at each of the two diagonal corners of the grid cell, ensuring an even distribution across the study area (Fig. 1b).
The grid containing more than 50% of the geographical area belonging to the Udupi taluk was considered for marking the diagonal sampling locations, where each marked location was 2–3 km apart within the administrative boundary, as shown in Fig. 1b. The area belonging to the neighbouring taluk to the same grid was excluded from the study.
(a) Study area – Udupi taluk, (b) 5 × 5 km grid (Map and grid generated using QGIS Version 3.36.2, https://www.qgis.org).
House selection
At each sampling site, 20 houses were chosen for the survey, and a standard survey form was used to record geo-coordinates using the LatLong app, container details, and other specific attributes of mosquito breeding sites.
Data collection
Entomological surveys and larval sampling were carried out between October 2023 and December 2023 to identify the distribution of mosquito fauna in the study region. The observation was made specifically in the sites where stagnant and small collected water originated from storing water for long periods or intermittent rainfall. The marked locations were visited, and the houses were screened for the presence of containers with stored water or discarded containers with stagnant water outside the housing premises. Larvae were collected using pipettes/cups/strainers in plastic containers and transported to the laboratory from all the breeding sites. The procedures and precautions regarding larval collection and transportation were according to the WHO and the National Vector Borne Disease Control Program (NVBDCP) guidelines32. The plastic containers with larvae samples were sealed tightly using mesh cloth and incubated at room temperature (25 °C to 30 °C) so that the larvae could develop into the adult stage. In the mature stage (adult mosquitoes), they were killed using ether. With the help of an aspirator tube, the killed mosquitoes were transferred to the test tubes to carry out morphological identification of species. The mosquito species were confirmed using a stereomicroscope based on the identification keys provided by the WHO33.
Larval indices
Larval indices were estimated as the House Index (HI), Container Index (CI), and Breteau Index (BI)34.
Climatic data
Climate data were collected from meteorological stations for each sampling day at 28 locations. Data included average temperature in degrees Celsius and relative humidity in percentage obtained from the National Aeronautics and Space Administration Prediction of Worldwide Energy Resources (NASA POWER)35. The average rainfall (mm) was also obtained from the Indian Meteorological Department (IMD)36.
Satellite image data
RS and GIS techniques were employed to analyse the influence of LULC on Aedes species distribution. High-resolution multispectral satellite imagery from Sentinel-2 (30 m spatial resolution) was obtained for the study area from Satellite images obtained from Bhuvan (by ISRO37, corresponding to the post-monsoon period (October–December 2023). The images were processed using QGIS version 3.36.2, and a supervised classification method (maximum likelihood algorithm) was applied to derive LULC categories. Training samples were collected through Google satellite reference data, visual interpretation, and ground-truth data from entomological survey sites. Classified land use/cover types included river, lake, forest, grassland, cropland, built-up areas, and barren land. Accuracy assessment was performed using an error matrix and kappa statistics based on randomly selected validation points. The final LULC map was overlaid with mosquito abundance data to identify spatial patterns and breeding hotspots.
Cohen’s Kappa coefficient.
\(\:{\upkappa\:}\:=\frac{{P}_{0}-\:{P}_{e}}{1-{P}_{e}}\) where Po is the observed agreement,
Pe is the expected chance of agreement.
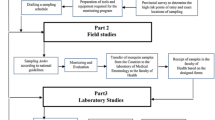
A stepwise methodological framework was followed to conduct the entomological and spatial analysis for Aedes species, as shown in Fig. 2.
Spatial and statistical analysis
Spatial distribution of larval indices was analysed using heatmaps generated in QGIS (v3.36.2) through the Inverse Distance Weighting (IDW) technique, allowing visualisation of mosquito abundance patterns across the study area. In addition, IDW interpolation was also used to produce continuous spatial surfaces of Aedes larval density. IDW is a spatial interpolation technique used in GIS to estimate unknown values at a location based on known values from surrounding locations.
To classify mosquito breeding intensity across the 28 sampled locations, hotspot and coldspot representation was conducted using entomological survey data focused on Aedes breeding sites. Hotspots were defined as locations where Aedes larvae were detected in breeding habitats, indicating active breeding and higher transmission potential. Coldspots are referred to as locations where Aedes larvae were absent, but had conditions such as stagnant water, domestic containers, and the presence of other mosquito species. These hotspot and coldspot zones were overlaid on the LULC map to explore their association with various land use categories.
For statistical analysis, a Zero-Inflated Negative Binomial (ZINB) regression model was applied to investigate the relationship between Aedes mosquito counts and key predictors such as larval indices and environmental variables (e.g., temperature, humidity, rainfall). The model accounted for overdispersion and excess zeros in the count data. Model fit was assessed using the Akaike Information Criterion (AIC). All statistical analyses were performed using R software (version 4.4.3).
Results
Out of 560 houses screened for larvae at 28 locations, 400 houses (71.42%) at 20 locations were found to have Aedes larval presence during the study period. The larval collection was focused on peridomestic habitats only, in close proximity to the households.
Larval indices
The entomological indices recorded in this study, particularly the House Index (HI), Container Index (CI), and Breteau Index (BI), indicate a substantial risk of dengue virus transmission in several locations. According to WHO criteria, HI > 0.05, CI > 0.1, and BI > 20 are considered thresholds for heightened transmission potential. In our study, HI reached 0.6 in some locations, while CI reached 1, far exceeding these benchmarks. Although BI varied, it surpassed 20 in multiple sites. These elevated values, particularly in the post-monsoon period, align with conditions favourable to Aedes mosquito proliferation and point to a need for intensified vector control efforts and community-based source reduction strategies. Figure 3a Larval indices depict the larval indices of our study across 28 locations, showing high values with HI at 0.6% for location 4, CI at 1 for locations 13 and 25, and BI greater than 100 for locations 4 and 18. Figure 3b, c & d for HI, CI and BI illustrate the heatmaps for larval values observed at specific locations.
a. Radar chart showing the larval indices, b. House Index, c. Container Index, d. Breteau Index (Maps generated using QGIS Version 3.36.2, https://www.qgis.org).
Land use/land cover (LULC) classification
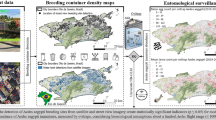
The study area was classified by the supervised classification method and divided into seven classes, i.e., river, pond/lake, forest, grassland, cropland, built-up, and barren land. The error matrix observed an overall accuracy of 98.59%, evaluated based on Cohen’s Kappa coefficient. This Cohen’s kappa value indicates an ‘almost perfect’ agreement between the classified and reference data, suggesting that the classification process was accurate and reliable. Figure 4a and b show LULC maps of 2023 and 2015, respectively. The built-up area was 15.54 sq km (5%) to 29.36 sq km (9.36%) from 2015 to 2023 and also describes the percentage of land use change in each class.
Hotspots and cold spots of aedes breeding within the LULC map
Aedes Larval breeding was detected in 71.43% of the surveyed locations within the study area. When these larval survey locations were overlaid on the LULC maps, it was observed that Aedes larval breeding habitats, i.e. hotspots, were predominantly (42.86%) located in fragmented urban landscapes and agricultural regions. The remaining 28.57% of the locations, which had non-Aedes mosquito breeding activity or absence of Aedes larval breeding, are found in other land cover classes such as forests and grasslands, but these locations had a conducive environment for larval breeding, such as stagnant water in containers, and other domestic mosquito species breeding were observed. Figure 4c hotspots and cold spots for Aedes species suggest favourable conditions for breeding, such as the availability of stagnant water or suitable microhabitats.
a. LULC classification year 2023, b. LULC classification year 2015, c. Hotspots and cold spots of Aedes breeding within the LULC map (LULC Map generated using QGIS Version 3.36.2, https://www.qgis.org).
Aedes species larva abundance
A total of 425 mosquitoes were collected, among which n = 100 (23.52%) were A. aegypti and n = 245 (57.64%) were A. albopictus; the remaining mosquito collection belonged to the genus Culex and Armigeres. Through larval density mapping, a high abundance of A. aegypti was found at locations 17 (n = 17), 18 (n = 18), and 27(n = 29), whereas A. albopictus breeding was observed at locations 1 (n = 22),7 (n = 24),8 (n = 23),17(n = 36) and 28 (n = 19). As shown in Fig. 5 larval density (5a,b) and Aedes abundance (5c) across locations maps. Pie charts represent the relative abundance of A. aegypti and A. albopictus at each site (Fig. 5c). The figure highlights the predominance of A. albopictus across most localities, demonstrating spatial heterogeneity in species composition.
Larval density maps (a) A. aegypti (b) A. albopictus (Maps generated using QGIS Version 3.36.2, https://www.qgis.org).
The survey found significant variability in environmental conditions across different locations, impacting the distribution of Aedes species at a temperature range of 23.4 °C to 26.3 °C, humidity of 70.1% to 88.5%, and intermittent rainfall of 0 to 61 mm. Under these conditions, the data indicated a higher prevalence of A. albopictus than A. aegypti across most surveyed locations (Fig. 5c).
Larval breeding habitats
A wide range of artificial containers contributed to Aedes breeding across surveyed locations, reflecting variability in household water storage practices and waste management.
Figures 6 and 7 illustrates the different types of breeding containers observed during the survey; Location 18 (n = 27) shows a high number of plastic containers, location 4 (n = 21)observed multiple plastic containers, as well as coconut shells, and Location 28 (n = 16), had plastic containers, discarded broken plastic materials and plastic bags with depressions and grooves with stagnant.
Statistical analysis
The impact of environmental factors (temperature, humidity, and rainfall) and larval indices (HI, CI, and BI) on the prevalence of the Aedes mosquito breeding habitats was assessed using Spearman correlation.
The normality of the data was assessed using the Shapiro-Wilk test. As shown in Table 1, temperature and humidity followed a normal distribution (p > 0.05), whereas HI, CI, BI, rainfall, and A. aegypti/A. albopictus counts deviated significantly from normality (p < 0.05). These results, further supported by Q-Q plots (Supplementary file, S1), guided the use of non-parametric methods, including Spearman correlation and Zero-Inflated Negative Binomial (ZINB) regression, for appropriate statistical analysis.
The correlation matrix highlights significant relationships shown in Table 2 between the HI, CI, and BI, which exhibit strong positive correlations. Temperature exhibits a moderate positive correlation with humidity and rainfall among the climatic variables. However, it shows weak or no significant correlations with the breeding indices and Aedes species counts. Meanwhile, humidity strongly correlates with rainfall but shows no significant correlation with Aedes species counts. While A. aegypti shows weak to moderate positive correlations with the breeding indices, it does not significantly correlate with environmental factors such as temperature, rainfall, and humidity. A. albopictus exhibits a strong positive correlation with the HI and medium correlations with the CI and BI, alongside a weak positive correlation with temperature.
ZINB regression model for A. aegypti species
A stepwise regression identified HI and CI as significant predictors in the best-fit model, achieving an AIC of 94.17019. This approach ensures robust modelling, crucial for understanding the factors influencing mosquito populations and disease transmission dynamics. The ZINB regression model was used to analyse the influence of larval indices on the abundance of A. aegypti. The results from the count component of the model (Fig. 8; Table 3) indicate that a one-unit increase in the HI is associated with a statistically significant increase in the log count of A. aegypti by approximately 11.56 (p < 0.05), after adjusting for the CI. This suggests that areas with higher household-level larval positivity are likely to have greater A. aegypti abundance. In contrast, the CI did not show a statistically significant association with A. aegypti abundance in the count model (p > 0.05), indicating that the proportion of infested containers, relative to all inspected water-holding containers, may not directly influence the mosquito abundance.
In the zero-inflation component of the model, HI was associated with an odds ratio of 305.85 for an observation being an excess zero (i.e., no larvae despite favourable conditions). However, this effect was not statistically significant (p > 0.05), implying that HI does not reliably predict the likelihood of zero counts due to structural zeros. Similarly, the effect of CI in the zero-inflation model was also non-significant (p > 0.05). Overall, the model highlights HI as a key predictor of A. aegypti abundance, while the CI appears to have limited explanatory power in both the count and zero-inflation components.
ZINB regression model for A. albopictus species
Stepwise regression identified the HI as the best predictor for A. albopictus breeding, with the selected model yielding the lowest AIC (148.88). However, as shown in Table 4; Fig. 8, the count model revealed that although HI had a positive coefficient (β = 2.33), this effect was not statistically significant (p = 0.109). The wide 95% confidence interval (–0.52 to 5.17) further reflects uncertainty in the estimate.
Similarly, the zero-inflation component of the model indicated that HI did not significantly influence the probability of excess zeros (β = − 261.56, p = 0.365), suggesting that HI alone may not explain the absence of A. albopictus in some areas. This lack of statistical significance may reflect the ecological complexity of A. albopictus, which is known to occupy a wide range of habitats and respond to multiple environmental factors not fully captured in this model.
Discussion
This study aimed to investigate the spatial distribution and ecological determinants of Aedes mosquito breeding in Udupi taluk using entomological surveys, larval indices, and a statistical modelling approach.
Entomological indices and vector risk
The HI, CI, and BI are crucial indicators for assessing the risk of dengue virus transmission. In our study, BI exceeded this threshold in several locations due to the presence of multiple positive containers per household, indicating intense breeding activity38. These elevated indices underscore a heightened risk for arboviral transmission and the urgent need for targeted vector control. Findings of an entomological survey conducted in the state’s capital city corroborate our findings, as they found significant Aedes breeding containers in the peridomestic and intra-domestic habitats, reported a high HI, CI and BI of 0.2, 0.3 and 0.5, respectively, during the pre-monsoon time. Since that study was conducted in collaboration with the state’s health department, it was feasible for them to explore the intra-domestic habitats13. The presence of Aedes larvae in the peridomestic habitats of our study locations indirectly indicates their presence even inside the households. It is well-known that the elimination of breeding sites is the most important step in curbing mosquito-borne diseases. In this context, state-wide entomological surveillance in collaboration with public health authorities could be a timely and effective step, particularly in regions with a high disease burden.
Spatial patterns
IDW interpolation techniques revealed distinct spatial patterns of larval indices and mosquito abundance. The spatial heat maps of larval indices highlight the areas where targeted mosquito control efforts and public health interventions may be needed to reduce the risk of mosquito-borne diseases and protect the community’s health39. Few studies have explored the integration of satellite data for LULC in relation to Aedes proliferation, which can guide towards the preventive, timely measures towards the growing urbanisation and population40,41,42. LULC classification data from 2015 to 2023 revealed a notable increase in built-up areas, which corresponded spatially with identified Aedes hotspot zones, suggesting that rapid urban expansion may be a key driver of enhanced vector breeding potential and dengue transmission risk. Aedes breeding hotspot areas were concentrated in fragmented urban landscapes and croplands. In this study, Aedes breeding hotspots were identified in areas with active larval presence of Aedes species, indicating high transmission potential, while coldspots lacked Aedes larvae but exhibited conducive conditions for future. This highlights the importance of monitoring even the low-risk areas for proactive vector control. A similar study in Maharashtra’s Konkan region linked disease risk zones with rapid urbanisation and land-use changes, aligning with our findings that fragmented and poorly managed landscapes support mosquito proliferation43. This spatial distribution highlights the need for customised intervention strategies based on specific land use patterns.
Climatic influence
The environmental conditions, particularly temperature and humidity, along with prior trends (Table 5), appear to play crucial roles in the distribution of these species44,45. In this study, temperature and humidity appeared to influence Aedes distribution, although not all associations reached statistical significance. According to the WHO, if the annual rainfall exceeds 1500 mm, that region is categorised as a tropical region. As per the previous five-year annual rainfall data (Table 5), the Udupi region receives an average of 4119 mm of rain, which is much higher, and hence, it’s a tropical monsoon zone, an ideal ecological niche for vector breeding46. Several studies have shown that higher rainfall is associated with vector abundance47,48. While this study did not find significant correlations between vector abundance and environmental variables such as rainfall and humidity, these factors may still play an influential role, and this deserves further investigation.
Breeding habitat and anthropogenic activity
A similar study was conducted on 500 household surveys in Udupi taluk from March to August 2012, where the foremost factors for Aedes breeding were coconut shells and uncovered water containers46. This study of the post-monsoon survey revealed a wider diversity of habitats, including plastic buckets, cement tanks, disused tyres, and waste plastic items46. This broader spectrum may reflect environmental and behavioural shifts over the past decade, such as increased urban development, poor waste management, and seasonal differences in water14. These findings highlight the evolving nature of vector breeding ecology in response to changing land use and human practices.
ZINB regression analysis
Traditional count models were insufficient due to excess zero values in the dataset, prompting the use of ZINB regression49. For A. aegypti, HI was a significant positive predictor in the count component, supporting its use as a surveillance tool. However, neither HI nor CI were a significant predictor in the zero-inflation model, suggesting that other unmeasured factors may influence the presence of excess zeros. In contrast, A. albopictus showed no statistically significant associations in the count or zero-inflation components, though HI emerged as the best fit model based on AIC. This may be attributed to the species’ broader ecological tolerance and the influence of unaccounted environmental factors such as vegetation, microclimate, and container types. Similar findings from Florida and Southeast Asia indicate that A. albopictus populations often display unpredictable spatial patterns, highlighting the need for expanded variables such as vegetation cover, shade, socio-economic details and microclimatic conditions in future models28,50,51,52.
Implications for public health and vector control
This study reinforces the critical role of household-level source reduction in Aedes mosquito control. The detection of larvae in peridomestic areas likely reflects breeding activity within intra-domestic spaces, which were not sampled due to access limitations. This underscores the need for comprehensive surveillance encompassing both indoor and outdoor environments. The integration of GIS and RS proved effective for identifying larval hotspots and informing spatially targeted interventions. These tools can enhance the precision of vector control efforts by aligning them with high-risk zones derived from entomological and environmental data. Collaboration with public health authorities is essential, particularly to facilitate access to intra-domestic sites and strengthen entomological surveillance. Expanding the temporal coverage of sampling across multiple seasons and incorporating dengue case data would allow for a more complete understanding of transmission dynamics and seasonality. The observed association between Aedes abundance, climatic variables, and LULC highlights the complex ecological drivers of mosquito proliferation.
The findings provide a basis for evidence-based policymaking aimed at mitigating dengue transmission. Effective strategies may include community engagement for source reduction, enhancing water management practices, and leveraging climate forecasts for proactive vector control. This multi-pronged approach can enhance the effectiveness of dengue control programs and reduce the disease burden in endemic regions like Udupi.
Conclusion
This study highlights the influence of climatic variables and LULC patterns on Aedes mosquito distribution during the post-monsoon period in Udupi taluk. The integration of GIS and RS techniques enabled spatial analysis of larval indices and environmental risk factors, identifying breeding hotspots for targeted vector control. Findings support the use of spatial decision tools in Aedes surveillance programs to enhance vector management strategies.
Limitations
-
The study was limited to a post-monsoon surveillance period (October–December 2023), which may not capture seasonal variations in vector dynamics.
-
Entomological sampling was limited to one Taluk of a district; also, a larger spatial coverage with a larger sample size would increase the statistical reliability of findings. Only climatic and land-use factors were included; other variables such as socio-economic conditions, water management practices, and human movement were not assessed.
-
This study lacks a quantitative measurement of the distance between identified breeding sites and human dwellings. While all breeding containers were found within residential premises, explicitly mapping their spatial proximity could have provided a more nuanced understanding of transmission risk, considering the limited flight range of Aedes mosquitoes.
Future directions
-
Conducting longitudinal studies across multiple seasons to assess temporal variations in Aedes abundance and transmission risk.
-
Integrating socio-economic and behavioural data to improve predictive modelling.
-
Explore the application of machine learning algorithms for real-time outbreak forecasting with the integration of dengue case data.
-
Evaluate intervention outcomes using spatial risk maps to inform adaptive control strategies in high-risk zones.
Data availability
All relevant data are included in the paper.
References
World Health Organisation. Vector-borne diseases. World Health Organisation (2023). https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases. Accessed on 20 Jul 2024
Paul, A. S., Vincent, J. & Saju, C. R. Mohamed Rafi, M. A study on larval indices of Aedes and risk for dengue outbreak in a rural area of Thrissur district. Kerala J. Commun. Dis. 52, 1–6 (2020).
Tripathi, P. & Gupta, R. B. Population dynamics study of mosquitoes in urban and rural areas of Gwalior district, Madhya Pradesh, India. Int. J. Entomol. Res. 2, 103–108 (2017).
Kumar, S. & Agrawal, S. Prevention of vector-borne disease by the identification and risk assessment of mosquito vector habitats using GIS and remote sensing: a case study of Gorakhpur, India. Nanotechnol Environ. Eng. 5, 19 (2020). pp 1–15
Gupta, N., Srivastava, S., Jain, A. & Chaturvedi, U. C. Dengue in India. Indian J. Med. Res. 136, 3 (2012). pp 373–90
Mahmud, M. A. F., Mutalip, M. H., Lodz, N. A., Shahar, H. & Mahmud, C. M. A. F. Study on key Aedes spp breeding containers in dengue outbreak localities in Cheras district, Kuala Lumpur. Int. J. Mosq. Res. 5, 23–30 (2018).
Kumawat, N., Prajapat, R., Meena, P. & Meena, S. Seasonal prevalence of dengue vector mosquito Aedes aegypti Linn in Jaipur City, Rajasthan, India. J. Vector Borne Dis. 60, 421–426 (2023).
Ali, S. A. & Ahmad, A. Spatial susceptibility analysis of vector-borne diseases in KMC using Geospatial technique and MCDM approach. Model. Earth Syst. Environ. 5, 1135–1159 (2019).
Singh, N., Singh, A. K. & Kumar, A. Dengue outbreak update in india: 2022. Indian J. Public. Health. 67, 181–183 (2023).
Shukla, A. et al. Seasonal variations of dengue vector mosquitoes in rural settings of Thiruvarur district in Tamil Nadu, India. J. Vector Borne Dis. 57 (1), [online] (2020). pp. 63–70 http://journals.lww.com/jvbd
Kumawat, R., Singh, K. V., Bansal, S. K. & Singh, H. Use of different coloured ovitraps in the surveillance of Aedes mosquitoes in an arid-urban area of Western Rajasthan, India. J. Vector Borne Dis. 51, 320–326 (2014).
Singh, R. K. et al. Prevalence of Aedes mosquitoes in various localities of Gadchiroli district of Maharashtra state, India. Int. J. Mosq. Res. 2, 38–41 (2015).
Vani, H. C. et al. Unraveling Dengue Dynamics: In-depth Epidemiological and Entomological Analyses in Bengaluru, India. J. Trop. Med. (2024). (2024).
Ferede, G. et al. Distribution and larval breeding habitats of Aedes mosquito species in residential areas of Northwest Ethiopia. Epidemiol. Health. 40, e2018015 (2018).
Martin, J. L. et al. Household and climate factors influence Aedes aegypti presence in the arid City of Huaquillas, Ecuador. PLoS Negl. Trop. Dis. 15 (11), pp. 1–18 (2021).
Varamballi, P. et al. Spatial heterogeneity in the potential distribution of Aedes mosquitoes in India under current and future Climatic scenarios. Acta Trop. 260, 107403 (2024).
Laporta, G. Z. et al. Global distribution of Aedes aegypti and Aedes albopictus in a climate change scenario of regional rivalry. Insects 14(1):49 (2023). pp. 1-18
National Centre for Vector-Borne Disease Control. https://ncvbdc.mohfw.gov.in. (Accessed 25 May 2023).
Impact of climate change on. abundance, distribution, and survival of Aedes species: systematic review. Glob J. Public. Health Med. 1, 579–607 (2022).
Oo, S. S. et al. Seasonal prevalence of Aedes aegypti in semi-urban area of Yangon Region, Myanmar. Adv. Entomol. 8, 107–116 (2020).
Palaniyandi, M. et al. Mapping land use/land cover and malaria mosquitogenic condition, and linking with epidemic transmission in the urban settlements using remote sensing and GIS. J. Entomol. Zool. Stud. 4, 40–47 (2016).
Malone, J. B., Bergquist, R., Martins, M. & Luvall, J. C. Use of Geospatial surveillance and response systems for vector-borne diseases in the elimination phase. Trop. Med. Infect. Dis. 4. https://doi.org/10.3390/tropicalmed4010015 (2019).
Farag, E. A. B. A. et al. Identification and characterisation of mosquitoes from different locations in Qatar in 2017–2019. (2021). Parasite. 2021 28:84. https://doi.org/10.1051/parasite/2021079.
Sithiprasasna, R., Lee, W. J., Ugsang, D. M. & Linthicum, K. J. Identification and characterization of larval and adult anopheline mosquito habitats in the Republic of korea: potential use of remotely sensed data to estimate mosquito distributions. Int. J. Health Geogr. 4 https://doi.org/10.1186/1476-072X-4-17 (2005).
Rahman, M. S. et al. Mapping the Spatial distribution of the dengue vector Aedes aegypti and predicting its abundance in Northeastern Thailand using machine-learning approach. One Health 13: 100358 (2021). pp 1–10. https://doi.org/10.1016/j.onehlt.2021.100358
Parvathy, S., Geetha, P. & Soman, K. P. Novel regression-GIS based approach for the analysis of spread of dengue in Palakkad. Indian J. Sci. Technol. 8, 24 (2015). pp. 1–6. https://doi.org/10.17485/ijst/2015/v8i24/81870,
Dinh, E. T. N. & Novak, R. J. Diversity and abundance of mosquitoes inhabiting waste tires in a subtropical swamp in urban Florida. J. Am. Mosq. Control Assoc. 34, 47–50 (2018).
Yang, B. et al. Modelling distributions of Aedes aegypti and Aedes albopictus using climate, host density and interspecies competition. PLoS Negl. Trop. Dis. 15(3): e0009063, (2021). pp. 1-21
Ministry of Home Affairs, Government of India. Population Udupi. (2022). https://censusindia.gov.in/nada/index.php/catalog/41245
Udupi District Official Website. https://udupi.nic.in (Accessed 25 June 2023).
Chitra, P. & Abhilash, R. Impact of Climate Change on Incidence of Vector-Borne Diseases and Vulnerability Mapping for Karnataka (2015–16) (Environmental Management and Policy Research Institute (EMPRI), Department of Forest, Ecology and Environment, 2016).
Ministry of Health and Family Welfare. Manual on Integrated Vector Management – India. National Centre for Vector Borne Diseases Control (NCVBDC) (2022).
World Health Organisation. Pictorial Identification Key of Important Disease Vectors in the WHO South-East Asia Region (Regional Office for South-East Asia, 2020).
World Health Organisation. Guidelines for Dengue Surveillance and Mosquito Control. Western Pacific Education in Action Series No. 8 (Regional Office for the Western Pacific, 1995).
NASA POWER Project. NASA Prediction of Worldwide Energy Resources. July (2023). https://power.larc.nasa.gov (accessed 1.
India Meteorological Department (IMD. ), Pune. Mar (2023). https://www.imdpune.gov.in (accessed 23.
Indian Space Research Organisation. Bhuvan – Geoportal of ISRO. July (2024). https://bhuvan.nrsc.gov.in/ (accessed 28.
Ngingo, B. L. et al. Aedes aegypti abundance, larval indices and risk for dengue virus transmission in Kinondoni district, Tanzania. Trop. Med. Health 50(1), (2022). pp. 1–8. https://doi.org/10.1186/s41182-021-00395-z
Nagy, A., El-Zeiny, A., Sowilem, M., Atwa, W. & Elshaier, M. Mapping mosquito larval densities and assessing area vulnerable to diseases transmission in nile Valley of Giza. Egypt. Egypt. J. Remote Sens. Space Sci. 25, 63–71 (2022).
Vanwambeke, S. O. et al. Landscape and land cover factors influence the presence of Aedes and Anopheles larvae. J. Med. Entomol. 44, 133–138 (2007).
Lorenz, C. et al. Predicting Aedes aegypti infestation using landscape and thermal features. Sci. Rep. 10, 21688 (2020). pp. 1–11. https://doi.org/10.1038/s41598-020-78755-8
Rwanga, S. S. & Ndambuki, J. M. Accuracy assessment of land use/land cover classification using remote sensing and GIS. Int. J. Geosci. 8, 611–622 (2017).
Fulmali, P. V., Walimbe, A., Mahadev, P. V. & Spread Establishment & prevalence of dengue vector Aedes aegypti (L.) in Konkan region, Maharashtra, India. Indian J. Med. Res. 127, 589–601 (2008).
Cheong, Y. L., Leitão, P. J. & Lakes, T. Assessment of land use factors associated with dengue cases in Malaysia using boosted regression trees. Spat. Spatiotemporal Epidemiol. 10, 75–84 (2014).
Rahman, M. S. et al. Entomological survey for identification of Aedes larval breeding sites and their distribution in Chattogram, Bangladesh. Beni-Suef univ. J. Basic. Appl. Sci. 10, 32 (2021). pp. 1–11. https://doi.org/10.1186/s43088-021-00122-x
Kamath, R., Gupta, R., Chandrasekaran, V. & Pattanshetty, S. Assessment of environmental factors associated with dengue transmission in Udupi taluk. Karnataka J. Sci. Soc. 40, 159 (2013).
Pham, N. T., Nguyen, C. T. & Pineda-Cortel, M. R. B. Time-series modelling of dengue incidence in the Mekong delta region of Viet Nam using remote sensing data. West. Pac. Surveill Response J. 11(1), (2020). pp. 13–21. https://doi.org/10.5365/wpsar.2018.9.2.012
Rozilawati, H. & Adanan Seasonal abundance of Aedes albopictus in selected urban and suburban areas in Penang, Malaysia. Trop. Biomed. 24(1), (2007). pp. 83–94.
Abiodun, G. J. et al. A dynamical and zero-inflated negative binomial regression modelling of malaria incidence in Limpopo province, South Africa. Int. J. Environ. Res. Public. Health 16(11), (2019). pp. 1–19. https://doi.org/10.3390/ijerph16112000
Cavalcante, A. C. P. et al. Spatial modelling of the infestation indices of Aedes aegypti: an innovative strategy for vector control actions in developing countries. Parasit. Vectors 13(1), (2020). pp.1–13. https://doi.org/10.1186/s13071-020-04070-w
Siregar, F. A. & Makmur, T. Survey on Aedes mosquito density and pattern distribution of Aedes aegypti and Aedes albopictus in high and low incidence districts in North Sumatra Province. IOP Conf. Ser. Earth Environ. Sci. 130, (2018). pp. 1–10. https://doi.org/10.1088/1755-1315/130/1/012018
Iqbal, N. T. et al. Identification of co-circulating dengue and South America–origin Zika viruses, Pakistan, 2021–2022. Emerg. Infect. Dis. 31, 1648–1651 (2025).
Acknowledgements
We thank Dr. Asha Kamath, Professor of Applied Statistics and Data Sciences and Ms. Vaishnavi, student of Applied Statistics and Data Sciences, for their invaluable assistance with data analysis. Special thanks to Ms. Raksha Shetty for her indispensable support during the field survey and for facilitating communication with residents in their native language.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal
Author information
Authors and Affiliations
Contributions
PPN carried out the field survey, designed the study, analysed the data, and drafted the manuscript. JPB and SG conceptualized and designed the study, contributed to data analysis, and interpretation, and reviewed the manuscript critically. NBN provided insights about the entomological aspects and design of the study and reviewed the manuscript. PPN, JPB, SG and NBN. approved the manuscript and agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
The Institutional Ethics Committee approved the study (IEC No.: IEC1-123-2023). Informed consent was obtained from the residents of each household before conducting the mosquito breeding site survey.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nayak, P.P., Pai B, J., Govindan, S. et al. Influence of climatic and land use factors on post-monsoon distribution of Aedes mosquito vectors in Udupi taluk. Sci Rep 15, 36649 (2025). https://doi.org/10.1038/s41598-025-20413-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20413-y