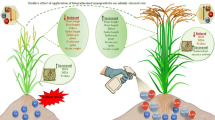
Abstract
Zinc deficiency in rice, a staple food consumed by more than half of the world’s population, poses a major challenge to food safety and nutrition. In this study, the application of biosynthesized zinc oxide nanoparticles (ZnO NPs) was investigated as a sustainable strategy to solve this problem. The laboratory experiment on seed priming was conducted with four concentrations of ZnO nanoparticles (25, 50, 75 and 100 ppm) over three soaking times (12, 18 and 24 h). The priming of 25 ppm ZnO nanoparticles (NP) over a 24-hour period resulted in the highest germination and best growth of the rice seedlings, as evidenced by increased shoot and seedling length and improved overall seedling vigor. The optimized priming treatment (25 ppm ZnO NPs for 24 h), combined with a foliar spray of 100 ppm ZnO NPs applied at the panicle emergence and grain filling stage, was evaluated under field conditions for its effects on plant growth, yield and zinc content in rice. Field trials showed that this integrated approach significantly increased grain yield, straw yield and zinc accumulation in both the grains and straw compared to conventional methods. Biochemical analyzes showed an increase in chlorophyll content, total protein content and antioxidant enzyme activities (SOD, CAT and POX), all of which correlated with improved plant development. Phytohormone profiling showed increased levels of gibberellic acid (GA₃), indoleacetic acid (IAA) and salicylic acid (SA), which contributed to improved vegetative and reproductive performance. In addition, the expression of key zinc transporter genes (OsZIP1.1, OsZIP3, OsNAAT1 and OsYSL14) was significantly modulated in root and flag leaf tissue, indicating improved zinc uptake, translocation and accumulation in the grain. This study highlights the potential of biogenic ZnO NPs as a sustainable and cost-effective strategy for the biofortification of zinc in rice. It offers a novel solution to combat micronutrient deficiencies while improving plant productivity and nutritional quality.
Similar content being viewed by others
Introduction
Malnutrition is a critical problem as the world’s population grows. More than 340 million people suffer from one or more micronutrient deficiencies, including essential nutrients such as vitamin A, iron, iodine and zinc1. Micronutrient deficiency is a serious problem that leads to various health problems. Nutrient deficiency is one of the biggest threats to public health in India. Over 80% of teenagers are affected and 0.5% of deaths in 2016 were due to inadequate nutrition2. Moreover,global climate change manifested through rising temperatures and unpredictable rainfall alongside an increasing global population, is exerting significantpressure on food security and sustainable agricultural systems 83. The ‘Zero Hunger’ target set out in SDG 2 is still a long way off and global progress is insufficient. It is expected that by 2030, more than 37 countries, including India, will not even reach a low level of hunger.
Zinc (Zn), the second most abundant transition metal, is a vital nutrient that is essential for numerous metabolic and cellular reproductive processes3. This essential mineral plays a central role in human health by facilitating enzymatic reactions and strengthening the immune system. Zinc also acts as a powerful antioxidant that protects cells from oxidative damage. Alarmingly, around 20% of the world’s population suffers from zinc deficiency, which has serious health consequences, including stunted growth, impaired wound healing and increased susceptibility to infections4,5,6,7. This problem is particularly pronounced in developing countries, where zinc deficiency is especially prevalent in staple plant foods.
Rice (Oryza sativa L.), a globally cultivated cereal from the Poaceae family, is a basic staple food and an important vehicle for the supply of micronutrients, especially zinc. It contributes significantly to the daily dietary intake of about 70% of the world’s population, especially in developing countries, where rice is the main source of calories and essential minerals8. Rice meets the nutritional needs of three billion people in Asia and contributes to 80% of their calorie intake. Prized for its exceptional nutritional profile, rice consists of 80% carbohydrates, 3% fat, 7–8% protein and 3% fibre and is enriched with important micronutrients such as iron, zinc and B-complex vitamins9. These components are essential for maintaining energy, promoting skin health and strengthening the structural integrity of blood vessels10,11. As living standards rise, consumer demand for high-quality rice fortified with micronutrients important for health is growing11,12. The majority of the population consumes polished rice, a refined variety in which the nutrient-rich layers of the embryo and bran are removed during processing, leaving mainly the starchy endosperm. This can lead to zinc deficiency and malnutrition, especially in regions with high rice consumption.
Rice is particularly susceptible to zinc deficiency due to its shallow root system, which limits its ability to absorb nutrients from deep soil layers. In addition, zinc uptake is reduced under anaerobic conditions due to prolonged flooding. Long-term reliance on rice without adequate zinc intake increases the risk of deficiency, especially among vulnerable populations. Biofortification of rice with zinc through advanced agronomic practices offers a viable solution to this nutritional gap. Zinc is also critical for plant metabolic processes, including hormone biosynthesis and modulation of the stress response, which are important for growth and development13. Consequently, increasing the concentration of zinc in staple foods such as rice is not only a solution to global nutritional problems, but also strengthens plant resilience and agricultural productivity.
Zinc deficiency is a widespread nutritional disorder affecting about 17% of the world’s population, particularly in developing regions where diets are heavily dependent on staple foods with low zinc bioavailability14. Deficiency is a major public health concern as zinc is essential for various physiological functions, including immune response, cognitive development and enzymatic activity15. Chronic zinc deficiency leads to stunted growth, impaired immune function, increased susceptibility to infections and, in severe cases, complications such as delayed wound healing and higher infant mortality. In South Asia, zinc deficiency is particularly alarming due to the high consumption of rice-based foods that do not contain enough zinc16. Over 50% of soils in India, Pakistan, Bangladesh and Nepal are reported to be zinc deficient, resulting in lower zinc intake in staple foods. This has a direct impact on human health. In India alone, over 40% of children under the age of five suffer from stunted growth due to inadequate zinc intake. In addition, zinc deficiency in mothers is a major problem, contributing to complications during pregnancy, low birth weight and higher infant mortality17. Addressing zinc deficiency in South Asia requires sustainable agricultural measures, including biofortification of staple crops such as rice and wheat. To date, zinc fertilizers have traditionally been applied through soil and foliar application, but this is associated with environmental concerns and inefficiencies in nutrient uptake. This study explores the potential of nanotechnology solutions such as ZnO nanoparticles to improve the bioavailability of zinc in rice, providing an innovative and environmentally friendly approach to combat micronutrient malnutrition in the region.
In India, zinc deficiency has become a pervasive problem. About 50% of the cereal growing areas suffer from zinc deficiency. Due to relentless tillage and excessive use of chemical fertilizers, this deficiency is expected to rise to an alarming 63% by the end of 2023. The depletion of zinc status in the soil hinders the uptake capacity of plants and leads to the manifestation of Khaira disease in rice and results in significant yield loss and affects the quality of rice18,19. Zinc fertilizers have long been used as a conventional solution to correct zinc deficiency in crops. However, their overuse increases production costs and often leads to nutrient imbalance in the soil, emphasizing the need for more sustainable crop nutrition strategies. To increase zinc levels in rice, scientists have explored a range of agronomic, biotechnological and conventional breeding approaches. Among these, zinc fertilizer application is still a widely used and relatively cost-effective method to improve zinc content in rice grains20. Nevertheless, the uptake efficiency of conventional zinc fertilizers such as zinc sulfate and zinc nitrate is often limited. These fertilizers are not only ecologically questionable, but can also contribute to long-term soil degradation. Therefore, there is an urgent need for alternative solutions that are cost-effective, environmentally friendly and allow a slow and targeted release of nutrients according to plant needs. In contrast, nano-zinc formulations such as zinc oxide nanoparticles (ZnO NPs) have emerged as an innovative alternative, offering better solubility, controlled nutrient release and improved uptake by plant roots21. Over the past few decades, the utilization of engineered nanomaterials has grown dramatically due to their uniquephysicochemical properties and diverse applications in agriculture and plant science 84. These properties make them a more efficient method of delivering zinc to plants at lower doses. Despite their agronomic benefits, the potential risks of nano-zinc such as accumulation, ecotoxicity and long-term effects on soil health must be carefully considered. Although ZnO NPs show promise for sustainable agriculture, their use must be accompanied by thorough safety assessments to ensure environmental sustainability. In addition, the shallow root system of rice limits the effective uptake and translocation of zinc22. Considering these limitations, the development of innovative and efficient nutrient delivery systems is essential to improve the bioavailability of zinc in rice cultivation.
Biologically synthesized zinc oxide nanoparticles (ZnO NPs) offer a sustainable and economical alternative to bulk zinc fertilizers. ZnO NPs are less affected by soil properties such as texture, colloidal matter and organic content. Their nanoscale size, high specific surface area and improved surface reactivity enable better uptake and utilization by plant roots23. In this study, the application of ZnO nanoparticles via both seed incorporation and foliar spray is investigated as a novel strategy to improve zinc uptake and biofortification of rice. Compared to conventional fertilizers, the ZnO nanoparticles exhibit improved mobility and absorption, resulting in improved plant growth, yield and nutrient quality. By studying their interaction with genes involved in the uptake and transport of zinc, this research also aims to develop an efficient, scalable and environmentally sustainable approach to address zinc deficiency in rice nutrition.
Materials and methods
Synthesis of ZnO nanoparticles
This study focuses on the synthesis of zinc oxide nanoparticles (ZnO NPs) using a biogenic methodology, leveraging the cell lysate of Plant Growth Promoting Rhizobacteria (PGPR) species, Pseudomonas. The bacterial strain was sourced from Navsari Agricultural University, Navsari, India.
Preparation of cell lysate
To collect the cell lysate, a pure culture of Pseudomonas (5 ml) aliquot sourced from the NAUROJI repository at Navsari Agricultural University (NAU), Navsari, Gujarat, was inoculated into 1 L of sterile Nutrient Broth in a 2 L glass conical flask under aseptic conditions. The culture was incubated at 27 °C with constant agitation at 120 rpm using an orbital incubator shaker (Kuhner, Lab Therm LTX) to promote aerobic growth. The cell growth was monitored spectrophotometrically, and cultures were harvested upon reaching the late logarithmic (log) growth phase considered optimal for downstream cell lysis due to peak metabolic activity. The biomass was separated from the culture medium by centrifugation at 5000 rpm for 10 min at 27 °C using a swing-bucket rotor centrifuge (Eppendorf, Model: 5804R). The resulting cell pellet was gently resuspended in Milli-Q water (100 ml per centrifuge bucket) and manually agitated for 10 min to facilitate removal of residual media components. This washing step was repeated thrice, each followed by centrifugation under identical conditions, to ensure the procurement of clean, unadulterated cellular biomass. Subsequently, the purified pellet was resuspended in 100 ml of Milli-Q water in a 500 ml conical flask. Cell disruption was achieved via ultrasonication using a high-powered rod sonicator (Cole Parmer, Model: 04711-75) operated at 500 W. Sonication was performed in three cycles of 10 min each, with 2-minute intervals between cycles to prevent thermal degradation. To enhance lysis efficiency, the sonicated suspension was subjected to mild thermal treatment by heating over a low-flame gas burner for 30 min, after which it was allowed to cool to ambient temperature. The lysate was then filtered through a 0.45 μm membrane using a vacuum filtration unit (Millipore) operating at > 100 psi to remove cell debris and particulate matter. Finally, the filtrate was clarified by a second round of centrifugation at 5000 rpm for 10 min at 27 °C (Eppendorf, Model: 5804R). The resulting supernatant, representing the crude cell-free lysate, was collected and stored under refrigerated conditions for subsequent utilisation in the biosynthesis of zinc oxide nanoparticles (ZnO NPs).
Synthesis of ZnO nanoparticles
The freshly prepared cell lysate was subjected to thermal treatment using a microwave oven (Samsung, ML32 J7055 VB), followed by vigorous agitation on a magnetic stirrer (Tarson, Spinit) for one minute. Zinc sulfate heptahydrate (ZnSO₄·7 H₂O) was employed as the precursor. The compound was incrementally introduced into the preheated cell lysate under sustained stirring until the entirety of 5 g of ZnSO₄·7 H₂O was completely dissolved. The resultant slurry was further heated and stirred until its volume was reduced to 10 mL, yielding a concentrated solution. This concentrated slurry was transferred to a crucible and subjected to calcination in a muffle furnace (Electro-equip, DPC-96) at temperatures ranging between 300 °C and 500 °C for three hours. The final product, characterized as a fluffy white powder, was meticulously ground using a mortar and pestle to achieve a fine particle size, thus producing high-purity ZnO nanoparticles.
Particle size analyzer
The hydrodynamic size, particle size distribution, Polydispersity Index (PdI), aggregation tendencies, and colloidal stability of ZnO nanoparticles synthesized via Pseudomonas spp. were characterized using by Zetasizer ver. 7.04 (Malvern Instrument Ltd, MAL 1098089) at a controlled temperature of 25 °C. The nanoparticle suspension was meticulously diluted with Milli-Q water, centrifuged to eliminate aggregates and extraneous particulates, and subsequently transferred into a pre-cleaned cuvette under controlled conditions to mitigate experimental error. This rigorous preparation ensured the acquisition of precise and reproducible data, offering critical insights into the physicochemical properties and dispersion stability of the ZnO nanoparticles in an aqueous medium.
Characterization of ZnO nanoparticles using SEM
To characterize the synthesized ZnO NPs, a Field Emission Gun-Scanning Electron Microscope (FEG-SEM) (Nova NanoSEM 450, FEI Ltd) was employed, providing detailed insights into the size, shape, and surface morphology of the nanoparticles. The fine ZnO nanoparticle powder was prepared for analysis by blending it with gold particles to enhance conductivity and reduce charging effects during imaging. The sample was then mounted on a conductive stub and sputter-coated with a thin layer of gold, ensuring optimal imaging conditions. Carbon-coated tape was employed for sample preparation, facilitating the adhesion of the powdered nanoparticles to the grid, followed by blotting with absorbent paper to eliminate excess material24. The sample was subsequently dried under a mercury lamp to ensure proper adhesion. The nanoparticles were examined under the FEG-SEM at operating voltages of 1 kV and 1.5 kV, with an accelerating voltage of 200 kV. The combination of low operating voltages and high accelerating voltage was critical for achieving precise imaging without significant sample damage, enabling the detailed examination of nanoscale features.
X-Ray diffraction analysis
To estimate crystalline nature, atoms arrangement, compound formation inside crystal by using XRD instrumentation worked on X-ray diffraction technique(D8 Advance, Bruker)25. The ZnO NPs were prepared by first washing the synthesized sample with ethanol and then rinsing with deionized Milli-Q water to remove any contaminants or debris. Approximately 5 g of the precipitate was collected after synthesis. This sample was then dried in a hot air oven at 65 °C for 2 h to ensure complete moisture removal. Following drying, the sample was crushed using a mortar and pestle to obtain a fine powder. For the analysis, 1 g of the powdered ZnO NPs was placed onto a sample holder and scanned over the 2θ range from 20° to 80°. The scanning was carried out at a step size of 0.02° and a scan rate of 1°/min to achieve a high-resolution diffraction pattern for identifying the crystal structure and phase purity of the ZnO NPs.
Plant materials
The seeds of rice (Oryza sativa L.) variety Jaya were procured from Main Rice Research Station, Navsari Agricultural University, Navsari, Gujarat, India. The seeds of Rice were surface sterilized with 0.01% mercuric chloride (HgCl2) for 1 min followed by rinsing with autoclaved double distilled water (DDW) four times, to remove all traces of HgCl2.
Seed priming of ZnO NPs on rice
The suspension of ZnO NP for different treatments was prepared through mixing the required concentration of nanoparticles treatment as summarized in Table 1 was evaluated in this study. A total of 60 sterilized rice seeds per treatment were soaked in different concentrations of ZnO nanoparticles (25, 50, 75, and 100 ppm) for various durations (12, 18, and 24 h) as outlined in Table 1. After soaking, the seeds were shade-dried and then sown equidistantly on sterilized Petri plates (150 mm × 25 mm) lined with autoclaved germination paper. The experiment was arranged in a Completely Randomized Design (CRD). For each treatment, 20 seeds were placed per Petri plate, and the setup was replicated three times (20 seeds × 3 replicates = 60 seeds per treatment), ensuring consistency with the initial seed count. Seeds were monitored daily until complete germination was observed in the control group. Germinated seeds were counted daily, and evaluations were conducted for germination percentage, speed of germination, and seedling vigour indices based on seedling length and dry weight. Non-primed seeds served as the control.
Field experiment site and design
The best ZnO NPs seed priming treatment evaluated based on physiological observation from laboratory study was further studied under field condition (Table 1). The field experiment was carried out at college farm, N. M. College of Agriculture, Navsari Agricultural University, Navsari during Kharif season (June), 2019 with four different treatments as per Table 2 using Randomized Complete Block Design with five replication and ZnO NPs were applied by foliar spray at panicle emergence and grain filling stage. The rice variety ‘Jaya’ was sown in a nursery at a seed rate of 25 kg per hectare. The 21 days old healthy, uniform seedlings were subsequently transplanted into the main field at a spacing of 20 cm × 15 cm (row × plant) with 1 seedling per hill. Each net plot measured 3 m × 2 m, ensuring an adequate plant population per treatment.
The layout of the field experiment is depicted in the supplementary material. Figure 1 presents the randomised allocation of treatments within the blocks. The green synthesized Zinc oxide nanoparticles (ZnO NPs) were applied by foliar spray at two critical stages viz., panicle emergence and grain filling stage using a hand-held sprayer to ensure uniform coverage. Morphological observations were recorded at 30, 60, and 90 days after transplanting (DAT) from 5 hills as per the sampling technique prescribed by Gomez (1972)26. The standard agronomic practices including fertilization, irrigation, weed control, and pest management were uniformly applied across all treatments to minimize environmental variability.
Morphological analysis
At physiological maturity, five plants were randomly selected from each net plot to evaluate plant growth, grain yield and yield-enhancing traits, including plant height, number of shoots per plant, panicle length, number of panicles per plant, number of filled spikelets per panicle, test weight, grain yield, straw yield and total dry matter production per plant. At the time of harvest, the selected plants were carefully dug up to avoid root loss and thoroughly rinsed with distilled water to remove adhering soil particles. To estimate the total dry mass, the rice plants were harvested from the soil and first dried in the shade for 24 h to reduce surface moisture while maintaining tissue integrity. Immediately after cleansing it properly the plants which were still alive were put in the oven at 105 ℃ temperature for a couple of hours to kill them completely in order to stop any further metabolic activities still going on inside its body which might affect its weight. This was confirmed by two consecutive weighing 24 h apart, which showed no significant difference in mass. The final dry weights were determined using a high-precision analytical balance and expressed per plant (g plant-¹).
Biochemical analysis
Biochemical parameters were determined from flag leaf tissue 24 h after leaf application of ZnO nanoparticles at two crucial growth stages: Panicle emergence and grain filling. Chlorophyll content was quantified by Uv-Vis spectroscopy (Shimadzu 1280, Japan) using 80% acetone (v/v) as extraction solvent. Absorbance was measured at 645 nm and 663 nm using a solvent blank (80% acetone) as a reference27. The true protein content was quantified according to the protocol of Lowry et al. (1951)28, using 0.1 M phosphate as reagent buffer and crystalline bovine serum albumin as reference standard, and the absorbance was accurately measured at 750 nm. The activity of the enzyme superoxide dismutase was determined using the nitroblue tetrazolium (NBT) method29. Catalase activity was analyzed according to the method described by Aebi (1984)30. Peroxidase activity was determined according to the method described by Worthington, Guilbault and Sadar31.
Phytohormone profiling
The phytohormones gibberellic acid (GA3), indoleacetic acid (IAA), jasmonic acid (JA) and salicylic acid were analyzed by ultra-fast liquid chromatography (UFLC; Shimadzu, Japan) according to the method of Li, Wei, Dong, Peng, Liu and Chen32. The leaf tissues (100 mg) were crushed using 0.5 ml of propanol-H2O concentrated HCl (2:1:0.002, v/v/v) and the samples were then shaken at a temperature of 4 °C for 30 min, followed by the addition of 1 ml of dichloromethane and shaking again for another 30 min. The samples were then centrifuged at 5000 rpm for 10 min and the lower organic phase (approximately 1 ml) was transferred to a small beaker, evaporated and dissolved in 1 ml of 80% methanol by vortexing for 5 min. The final samples were filtered through 0.22 μm filters and stored at −20 °C for analysis.
Zn content and uptake in grain and rice straw
The samples were oven-dried at 60 °C for 24 h, powdered by the mechanical grinder and analyzed for zinc composition33. The samples were first digested with HNO3and HClO4, and then, the Zn concentrations of brown rice, straw and hull were determined with an atomic absorption spectrometer.
Zn status of the soil before sowing and after harvest of a crop
To evaluate the effects of the application of ZnO nanoparticles on zinc dynamics in the soil, soil samples were taken from each experimental plot at two points in time: before sowing (baseline) and after harvesting the rice crop. To assess the baseline situation, soil samples were taken at a depth of 30 cm before sowing to determine the initial state of available zinc in the soil. Soil samples were also taken from the same depth after harvest to determine the residual zinc content. All samples were air-dried, sieved and analyzed for available zinc using the DTPA extraction method34.
Statistical analysis
The data obtained by in vitro seed priming experiment was analyzed by a completely randomized design35. The field experiment data was analyzed by randomized block design36.
Molecular analysis
Total RNA was extracted from root and flag leaf tissue 24 h after foliar application of nano-zinc from each treatment using the SpectrumTM plant total RNA kit (Sigma-aldrich, STRN50). For cDNA synthesis, the QuantiTect reverse transcription kit (QIAGEN) with integrated removal of genomic DNA contamination was used according to the manufacturer’s instructions. Expression analysis of Zn transporter genes, i.e. metal phytosiderophore transporter (YSL), metal transporter (IRT) and divalent metal transporter (ZIP) genes, was performed using the QuantiFast™ SYBR® Green PCR kit (QIAGEN) with the ABI 7300 Real-Time Cycler (ABI, USA). Relative quantification of genes was calculated as a fold change in gene expression using elf4A as an endogenous control to normalize gene expression data analyzed using Applied Biosystems 7300 Real-Time PCR System Software SDS v1.4.
Result and discussion
Phytofabrication of zinc oxide nanoparticles (ZnO NPs) as demonstrated by the formation of a white precipitate upon addition of Pseudomonas extract to a zinc sulfate heptahydrate precursor. This microbe-mediated synthesis mimics conventional chemical methods for synthesizing nanoparticles, taking advantage of environmental friendliness and sustainability. Our results highlight the feasibility of this approach for the large-scale production of ZnO NPs and provide an environmentally friendly and efficient alternative for the synthesis of nanoparticles for industrial applications.
Characterization of zinc oxide nanoparticles using nanotrac
In colloidal solution, the particle size distribution of biogenically synthesized nano-zinc ranged from 70.29 to 129.6 nm with a zeta potential of 139.4 mv and a polydispersity index (PDI) of 0.256 (Fig. 1).
Characterization of zinc oxide nanoparticles using SEM
The morphological and topological features of synthesized nano-zinc determined by SEM are shown in Fig. 2. The SEM image result clearly shows that the synthesized nanoparticles have a spherical structure and range from 72 to 150 nm in diameter with an average size of 130 nm ± 5.0 nm. This indicates that the particles have a semi-agglomerated form with several particles being individually crystalline. The result of our data differs from previous findings where ZnO-NP synthesized with Pseudomonas aeruginosa and TEM images showed a smaller size of nano-zinc from 6 to 21 nm with an average of 14.95 ± 3.5 nm37. These drastic size differences in the nanoparticles could be due to the different Pseudomonas species used for nano-zinc synthesis. The particles with a homogeneous distribution can help us to better understand morphological studies and estimate particle sizes.
X-ray diffraction (XRD) analysis
XRD analysis of Pseudomonas-mediated biogenic nano-zinc was used to estimate the lattice parameters, crystallinity and arrangement of atoms. The crystalline nature of the nano-zinc is shown in Fig. 3 and the consistent diffraction signals were assigned to the expressed peaks. The X-ray diffraction (XRD) pattern showed eight prominent diffraction angles corresponding to 2θ values of 31.761°, 34.427°, 36.248°, 47.541°, 56.586°, 62.857°, 67.932°, and 69.060°, confirming the crystalline nature of the synthesized material. A similar result was noted in a previous report indicating that the presence of zinc by Enterobacter cloacae showed XRD peaks with a 2θ value of 68, 56, 36 and 31 positions38. In addition, a small peak due to the presence of metabolites was also identified. According to previous reports, the crystallization of bacterial metabolites such as proteins and organic materials coating the surface of ZnO-NPs could be the cause of the tiny peaks seen at different 2 theta values39.
Seed priming experiment
Seed priming is an effective technology to promote rapid and uniform emergence and achieve high vigor, resulting in better crop establishment and higher yield. Zinc plays a central role in various biochemical processes, such as protein and carbohydrate metabolism, which are crucial during seed germination, including breaking dormancy and increasing germination rates40. Nano-zinc supplementation through seed priming promotes germination metabolism by effectively supplying zinc to the embryo, enabling synchronous and robust establishment41. In addition, zinc supports germination by maintaining cellular structural integrity, which promotes nutrient uptake and accelerates water uptake. Furthermore, zinc helps to increase and immobilize the activity of key enzymes such as protease and amylase, which are responsible for the degradation of proteins and starch into amino acids and simple sugars42,43. These products serve as energy reserves and contribute to the synthesis of new proteins, which are crucial for seed germination and seedling growth.
The results of the present study, shown in Table 3 and Supplementary Fig. 1, clearly indicate that the application of biogenic ZnO nanoparticles (NPs) serves as a strong growth promoter that significantly increases the germination rate and vigor index of rice seedlings. Of the four concentrations of nano-zinc tested, rice seeds treated with a 25-ppm concentration of ZnO nanoparticles for 24 h showed the most significant improvements in both germination and overall seedling development (Figs. 4 and 5). The germination rate peaked under the T9 treatment, while unprimed seeds showed the lowest rate, emphasizing the effectiveness of ZnO priming.
Significantly, the longest root length was observed in seeds treated with 25 ppm ZnO NPs for a 12-h priming duration, while non-primed, non-impregnated seeds exhibited the shortest root length, emphasizing the critical role of nanoparticle intervention. Moreover, seeds primed with 25 ppm ZnO NPs for 24 h attained the greatest shoot length (7.96 cm), seedling length (14.55 cm), seedling vigor index I (1446.89) and seedling vigor index II (2625.78). In stark contrast, unprimed seeds consistently showed the lowest performance in germination, root length, shoot length and overall seedling vigor.
The seed coat of rice is very strong, which delayed seed emergence and seedling growth. The drastically shorter root length in unprimed seeds can be attributed to the lack of pre-soaking or priming treatment, which are essential for accelerating seed hydration and initiating key metabolic processes essential for successful germination. Unprimed seeds remain in a state of dormancy, resulting in delayed radicle and shoot development, inefficient nutrient mobilization and sluggish enzymatic activation. This lack of biochemical stimulation restricts cell division and root tissue elongation, ultimately leading to significantly lower root growth than seeds primed with ZnO nanoparticles. Priming seeds, which leads to more uniform germination, reduces early differences in plant water uptake44.
The seeds can easily take up and utilize the nanoparticles due to their small size and low solubility45. This could be a plausible reason for the increase in SVI in rice treated with nano-zinc46. Overall, priming the seeds with 25 ppm ZnO NP for a period of 24 h proved to be optimal, resulting in maximum percentage of germination, germination speed, shoot length, seedling length, dry weight, seedling growth index I and seedling growth index II (Table 2; Fig. 5). Hence, this treatment was considered for further field evaluation.
Previous studies have shown that nano-zinc has an outstanding effect on the germination and growth of cereals such as wheat, maize and rice47,48,49. ZnO NPs improve seed germination parameters, which is consistent with the results of previous studies that found that ZnO NPs at a dosage of 20 and 40 ppm resulted in earlier emergence and higher germination rate and radicle length in rice seedlings compared to hydroprimed rice seeds50. The effectiveness of NPs to improve seed germination also depends on the plant species. This was confirmed by the results of51 who investigated that the application of ZnO NPs at a dosage of 250 ppm with a soaking period of 72 h stimulated the germination of wheat seedlings by up to 100%, which differs from the results of the present study.
Morphological
To address zinc deficiency in soils, the application of nano-zinc through various supplementation strategies, such as seed incorporation and foliar spray, has been explored to improve plant growth and yield. The comparative effects of different nano-zinc supplementation methods on rice growth performance are shown in Table 4, where significant effects were recorded. The results show that green synthesized nano-zinc, when applied through a combination of seed priming and foliar spray, is most effective in improving plant growth and fruit yield. Plant height and total number of shoots per plant were measured 60 days after transplanting (DAT), 90 days after transplanting and at harvest, and at harvest (Table 4). Since the ZnO NP foliar spray was applied after panicle emergence (i.e. 45 days after harvest), the effects observed at 30 days after harvest were due to ZnO NP seed priming. At this stage, the highest plant height (73.26 cm) and tiller number (6.48) were recorded. However, plant height and tiller count did not vary significantly at 60 or 90 DAT or at harvest.
The combined treatment of seed priming and foliar application resulted in a significant increase in total dry matter production, which correlated with greater panicle length (19 cm), higher number of filled spikelets per panicle (123.60), higher grain yield (6927 kg ha1), higher straw yield (7497 6927 kg ha1) and the highest harvest index (49.63%). The combined treatment is more effective than single applications as it ensures a continuous availability of zinc throughout the growth stages of the plant. The seed priming provides an early nutrient boost and promotes strong root and shoot development, while the foliar spray delivers zinc during the critical reproductive stages. This combination improves nutrient use efficiency, increases stress tolerance and results in better growth and yield compared to either method alone. By targeting key stages of plant development, the dual approach maximizes the uptake and utilization of zinc.
In previous trials, foliar application of nano-zinc was found to improve plant growth, i.e. panicle number, grain filling rate, number of spikelets per spike and grain yield52. In addition, Alsuwayyid et al. (2022) confirmed the positive effect of low concentrations of ZnO NPs on wheat seedlings. The application of nano-zinc to rice increased the production of rice in terms of number of panicles, spikelets per panicle, 1000-grain weight, grain size and dry matter compared to the control23. In addition, the timing and method of application of nanoparticles have a significant impact on plant growth. For example, some reports suggest that foliar application of nano-zinc had a more positive effect on sorghum yield than seed admixture53. However, this differs from our results, possibly because in our study the foliar spray with ZnO NPs was applied later (60 DAT), while the seed primer was applied at an earlier stage.
Biochemical
Zinc is an essential trace element that is involved in various physiological functions in plants, such as chlorophyll synthesis and as an important component of numerous proteins54. In the current study, the influence of nano-zinc oxide (ZnO NPs) on the growth performance and quality of rice was evaluated by analyzing the main biochemical properties. To confirm the role of zinc in the biosynthesis of chlorophyll, protein, plant hormones and the activation of antioxidant enzymes, analyzes were carried out at two important stages of plant development: Panicle emergence and grain filling. The results showed that the chlorophyll and protein content of rice was significantly affected by different treatments under field conditions at both stages (Table 5).
Zinc plays a crucial role in chlorophyll biosynthesis by protecting the sulfhydryl groups in chlorophyll molecules and activating the reaction of PS-II55,56. It also improves the ultrastructure of chloroplasts and the integrity of Rubisco, an important enzyme for photosynthesis, thus ensuring optimal activity. Zinc-based nanoparticles improve photosynthetic efficiency by acting as cofactors for enzymes involved in chlorophyll and carotenoid production and by maintaining chloroplast structure and thylakoid membrane stability, both of which are critical for efficient light absorption and energy conversion. Zinc deficiency in soil hinders chlorophyll synthesis, which negatively affects the nutritional quality of rice56,57.
Compared to single applications, the combined treatment of ZnO NPs for seed priming and foliar application (T4) was most effective in promoting the biosynthesis of chlorophyll a and chlorophyll b during both panicle emergence and grain filling. At the panicle emergence stage, the highest levels of chlorophyll a (0.413 mg/g) and chlorophyll b (0.341 mg/g) were observed in treatment T4, which was significantly higher than the other treatments. During the grain filling stage, T4 continued to show a similar trend, with both chlorophyll a and chlorophyll b content increasing compared to the panicle emergence stage. Chlorophyll a content in T4 was 41.16% higher at the grain filling stage (0.583 mg/g) than at the panicle emergence stage (0.413 mg/g), indicating a notable increase in chlorophyll production as the plant matures. Similarly, chlorophyll b content was 27.86% higher at the grain filling stage (0.436 mg/g) than at the panicle emergence stage (0.341 mg/g). Among the treatments, T4 consistently resulted in the highest chlorophyll levels at both stages, emphasizing its positive influence on chlorophyll accumulation in rice leaves.
In a previous study, nano-zinc supplementation in rice seeds was also found to significantly increase chlorophyll content, which directly correlates with improved photosynthetic performance48. Moreover, under conditions of cadmium toxicity, ZnO NPs increased the synthesis of chlorophyll a, chlorophyll b and total chlorophyll in the shoots of fragrant rice58. Previous studies have also shown that low concentrations of ZnO nanoparticles improve photosynthesis and photosynthetic efficiency, which contributes to improved plant growth, quality and yield, especially in rice20.
Zinc is necessary for the synthesis of RNA polymerase, which plays an important role in the metabolism of plant proteins and blocks the activity of the ribonuclease enzyme, which makes it possible to further stop the process of RNA degradation and increase the synthesis of plant proteins54. This study clarifies the effects of seed priming in combination with a foliar application of nano-zinc on protein synthesis in rice seedlings during the critical stages of panicle emergence and grain filling. The results show that this synergistic treatment significantly increases protein synthesis, reaching peak values of 1.51% at panicle emergence and 1.71% at grain filling, an approximate increase of 43% compared to the control group. The integration of seed priming and foliar application appears to optimize the physiological processes that enhance protein synthesis during these critical growth stages. While the individual applications of seed priming or foliar spraying did not result in statistically significant differences in protein synthesis, both treatments clearly outperformed the control group, highlighting their efficacy.
In addition, zinc, a non-redox transition metal, enhances the activity of enzymes involved in protein synthesis and has a strong affinity for proteins59. Superoxide dismutase (SOD), an enzyme found in mitochondria, chloroplasts and cytoplasm, is a critical chiral center in the antioxidant defense system. It neutralizes ROS by converting superoxide radicals (O2−) into hydrogen peroxide (H2O2) and molecular oxygen (O2). In the current study, the application of nano-zinc in different ways led to an increase in the activity of the SOD enzyme compared to the control. The combined treatment of seed treatment and foliar spray showed a maximum increase in the defense system with a maximum SOD enzyme activity of 29.53 units g-1 protein min-1 at the panicle emergence stage, while a slight decrease in SOD activity (29.13 units g-1 protein min-1) was observed at the grain filling stage. In other ways of applying nano-zinc to rice, such as by seed priming and foliar spray, SOD enzyme activity is lower at both stages, but higher than in the control. At the critical panicle stage, SOD activity is highest, which is due to the role of zinc related to the reproductive development stage and defense against abiotic stress, which promotes metabolic activity and oxidative stress. During the grain filling phase, the oxidative stress of rice plants decreases, resulting in lower SOD activity.
Catalase is an antioxidant enzyme that protects plants under stress conditions by converting H2O2 into oxygen and water60. At the panicle growth stage, the activity of other antioxidant enzymes such as POX (35.55 µM guaiacol min-1 g-1 protein) and CAT (18.88 µM H2O2 min-1 g-1) was highest by the application of biogenically synthesized nano-zinc. The activity of peroxidase (POX) increased significantly in all treatments, with GFS showing slightly higher values than PES. Catalase (CAT) activity also increased slightly, although the differences between treatments were minimal. Both POX and CAT activities showed low variability, indicating consistent responses of the enzymes. The changes in POX activity were more pronounced than the relatively stable CAT activity across the different stages. It is noted that nano-zinc priming altered the activity of antioxidant enzymes in a dose-dependent manner, with SOD, POX and CAT activities increasing by 10%, 38% and 13%, respectively48. Previous reports state that the experimental use of ZnO NPs altered the composition of antioxidant enzymes and the defense system of various plant species such as wheat and maize61. Application of nano-zinc increased the activity of CAT enzymes in leaves and roots of Hordeum vulgare62. Under cold injury condition, the activity of defense-related enzymes such as CAT and SOD was the lowest at 41.6 and 45.8%, respectively. However, the addition of zinc protects the rice plant from low temperatures by significantly increasing the activity of SOD and CAT. However, all three doses of nano-zinc increase the activity of these enzymes depending on the concentration in both the control and stress plants. In contrast to the cold- stressed plants, the highest activity of antioxidant enzymes was found in the control plants63.
Status of zinc in soil before sowing and after harvesting and uptake of Zn in grain and straw at harvest
The zinc content in soil, both before sowing and after harvest, as well as the uptake of zinc in rice grain and straw, was significantly influenced by the application of ZnO nanoparticles (ZnO NPs) (Table 6). The combined treatment (T4: seed priming + foliar spray) recorded the highest pre-sowing soil zinc content (0.80 ppm), followed closely by the foliar spray treatment (T2: 0.77 ppm). After harvest, the zinc concentration in soil increased markedly, with T4 again showing the highest level (2.51 ppm), followed by T2 (2.38 ppm), indicating possible deposition and residual effect of foliar-applied ZnO NPs. Zinc uptake was also enhanced under ZnO NP treatments. In T4, the zinc content in rice grain reached 130.40 g ha⁻¹, while straw showed even higher accumulation at 214.06 g ha⁻¹. This reflects a common trend where zinc preferentially accumulates in vegetative tissues rather than grains. After harvest, the observed increase in soil-available zinc is likely due to foliar-applied nanoparticles being deposited onto soil surfaces, where they become bioavailable through dissolution or redistribution by irrigation and rainfall.
These findings are consistent with previous reports64. observed a similar increase in zinc concentration in rice under a combined nano-priming and foliar spray regime at 50 mg L⁻¹. While their study found a higher contribution from foliar spray alone, our results suggest the synergistic effect of dual application. Furthermore, Mi, Yuan, Wang, Dun, Wang, Yang, Yang, Zhang and Zhang20 demonstrated that although ZnO NP application increases zinc content in rice grains, concentrations in straw and other vegetative tissues remain substantially higher. The soils with DTPA-extractable Zn levels between 0.5 and 1.0 mg kg⁻¹ respond well to zinc fertilization. The pre-sowing levels in our study fall within this responsive range, validating the effectiveness of ZnO NP applications for biofortification.
Efficient methods of increasing the zinc content in grains may boost overall zinc levels. Previous investigation support finding of our study noticed that application of nano zinc at jointing stage enhance the composition of zinc in brown and polished rice52. At basal stage application zinc nanoparticles increase accumulation of zinc with range of 36.48–66.50% in glume and 25.78–48.29% in brown rice23. Raddy, Salimath, Geetha and Shankar65 reported that highest seed and leaf Zn content was found in ZnO nano treatment in seed priming and foliar treatment combinations under the well-watered condition as well as under stress condition in maize. The study by researchers identified that at panicle stage supplementation of zinc nanoparticles in rice enhance translocation rate of zinc metal towards rice grain by improve zinc content in brown rice approximately 13.5–39.4%20.
Collectively, these results underline the potential of ZnO NPs particularly in combined application modes to enhance soil zinc availability, improve plant uptake efficiency, and contribute meaningfully to the mitigation of zinc deficiency in both crops and human nutrition. Future studies should explore seasonal variability, soil-plant Zn fluxes, and long-term environmental impacts to support broader agronomic recommendations.
Phytohormone profiling
Phytohormones are essential bioactive compounds synthesized within plant systems that meticulously regulate various physiological processes, including growth, reproduction, and longevity. Zinc (Zn) plays an indispensable role in several critical metabolic activities, such as the biosynthesis of tryptophan66, a precursor to indole-3-acetic acid (IAA), a prominent auxin that profoundly influences cell elongation, division, and overall plant morphogenesis67. In addition, gibberellins (GAs), another class of crucial phytohormones, govern key developmental events, particularly in the regulation of seed dormancy and germination. Zinc is vital for the functionality of GA 3-oxidase, an enzyme pivotal in converting inactive gibberellin precursors into the bioactive form GA₁, which orchestrates the transition from vegetative to reproductive growth, thereby enhancing flowering and reproductive competency68.
Furthermore, zinc is deeply integrated into the biosynthesis of stress-responsive hormones such as salicylic acid (SA) and jasmonic acid (JA) by activating genes and enzymes central to their respective biosynthetic pathways69. In the jasmonic acid biosynthetic pathway, zinc’s role is critical in the octadecanoid pathway, where it activates lipoxygenase (LOX), an enzyme responsible for catalyzing the oxygenation of linolenic acid into jasmonic acid, a pivotal modulator of plant defense mechanisms and biotic stress responses70. Similarly, zinc facilitates the shikimate pathway, a key metabolic route for the biosynthesis of salicylic acid, by enhancing the activity of enzymes such as isochorismate synthase (ICS), which produces essential precursors for SA biosynthesis, thereby reinforcing the plant’s systemic acquired resistance71.
Recent advancements have underscored the transformative effects of green-synthesized nano zinc oxide (ZnO) nanoparticles on the modulation of key phytohormones, including gibberellic acid (GA₃), indole-3-acetic acid (IAA), jasmonic acid (JA), and salicylic acid (SA) in rice. The combined approach of seed priming and foliar application of ZnO NPs at an optimal concentration of 25 ppm exhibited a significant enhancement in phytohormone biosynthesis, with recorded concentrations of 11.95 µg g⁻¹ for GA₃, 8.03 µg g⁻¹ for IAA, 3.89 µg g⁻¹ for SA, and 5.42 µg g⁻¹ for JA (see Fig. 6). This synergistic application markedly improved hormonal profiles, leading to superior growth performance and developmental outcomes in rice plants. The combined treatment is more effective than individual applications because it ensures continuous zinc availability throughout the plant’s growth stages.
However, the influence of ZnO NPs on the phytohormone levels was notably reduced during the grain-filling stage, likely attributed to the plant’s physiological shift towards maturation and the concomitant increase in ethylene biosynthesis, a hormone intricately involved in the ripening process. Despite this attenuation, the application of nano zinc at 25 ppm was highly effective in promoting the biosynthesis of auxins and gibberellins, leading to significant improvements in flowering and fruit set, as corroborated by morphological assessments. This research provides compelling evidence of the potential of nano zinc in modulating phytohormone production, particularly in cereal crops such as rice. Nevertheless, further investigation is required to fully elucidate the molecular mechanisms by which nano zinc enhances phytohormone biosynthesis and its direct correlation with yield and crop productivity. Notably, findings by Vankova, Landa, Podlipna, Dobrev, Prerostova, Langhansova, Gaudinova, Motkova, Knirsch and Vanek72 revealed that low doses of ZnO NPs stimulated cytokinin (CK) production, while moderate to high concentrations acted as abiotic stressors, inducing upregulation of abscisic acid (ABA) and salicylic acid (SA) levels in Arabidopsis thaliana. This highlights the importance of dose optimization when utilizing nano zinc formulations, as inappropriate concentrations could shift from growth-promotion to stress-induction, underscoring the need for precise calibration to maximize agronomic benefits while mitigating stress responses.
Gene expression
The expression patterns of nine Zn homeostasis related genes (three from the YSL favly of metal-phytosiderophore transporters, two from the NAS family of metal transporters, one from NAAT family and three from the ZIP family of divalent metal transporters were analyzed in flag leaves tissue after 24 h of foliar application of ZnO NP during two reproductive developmental stages: panicle emergence and grain filling relative to eIF4a.
The soil available zinc (Zn2+) is taken up by root membrane transport mechanisms in rice which include phytosiderophores and Zn-regulated transporters and iron (Fe)-regulated transporter-like protein (ZIP) family73,74. In rice plants, several ZIP transporter genes have been reported OsZIP1, OsZIP3 and OsZIP4 were found to be rice Zn transporters induced by Zn deficiency expressed in the vascular bundles in shoots and in the vascular bundles and epidermal cells in roots75,76. In the current investigation, expression pattern of three ZIP family genes viz., OsZIP1, OsZIP3 and OsZIP4 were investigated altered by application of nano zinc oxide in rice plants (see Fig. 7a). The result discovered that OsZIP1.1 expression was influenced by ZnO NP treatment under field condition at panicle emergence stage and grain filling. The T4 recorded higher expression (2.93 Rq) of OsZIP1.1 at panicle emergence stage whereas, this gene was down regulated (0.76 Rq) at grain filling stage. The OsZIP3 expression was higher at panicle emergence stage as compared to grain filling stage. The similar expression of OsZIP3 gene noticed at panicle emergence stage, founded that combined treatment of ZnO NP seed priming and foliar application (T4) recorded higher expression (2.00 Rq). Unfortunately, opposite result observed at grain filling stage, application of nano zinc oxide by seed priming (T3) and combined treatment of seed priming + foliar spray (T4) showed slightly down- regulation of OsZIP3 whereas, foliar application of ZnO NPs up-regulate activity of OsZIP3. The OsZIP4 expression was influenced by ZnO NP treatment under field condition at panicle emergence and grain filling stage. The higher expression (3.63 Rq) was observed in combine treatment of ZnO NP seed priming and foliar application (T4) at panicle emergence stage. At grain filling stage, the treatment T2 reported the highest expression of OsZIP4 gene followed by treatment T4.
The ZIP family genes are induced upon starvation of zinc or iron. At grain filling stage, all ZIP genes except OsZIP4 shows downregulation of rice treated with ZnO NPs combination foliar spray and seed priming due to highest Zn content in T4 treated rice leaves. This finding supported by data of our biochemical analysis of Zn content in different plant part after harvesting. Ramesh et al.. (2003)75 reported novel differential selectivity’s and differential expression of two zinc transporters from rice. The quantitative analysis showed that cells expressing OsZIP1 and OsZIP3 grew at a faster rate than cells expressing OsZIP2 which suggests that gene OsZIP1 and OsZIP3 encoded functional zinc transporters. In study by Deshpande et al., (2018)77 represented that declined the expression of ZIP 1 and ZIP 3 gene day by day after anthesis (DAA) in which highest expression noticed at 7 days after anthesis which continually downregulated by 14 and 21 DAA particularly in wheat. Recent study result opposite to finding of our study shows that zinc fertilizer supplementation in Medicago sativa by foliar spray shows variation in expression of ZIP family genes in which, ZIP3 gene identified downregulation. The downregulation of ZIP3 might be due to plant reduce uptake of zinc in relation with avoid zinc toxicity78.
Across plant cell membranes for the transfer of NA-metal chelates, the YSL family of transporters is a strong contender members of oligopeptide transporters (OPT) family, a poorly understood group of proteins that transports derivatives of amino acids as well as peptides79. In the present study, expression of two YSL genes viz., OsYSL8 and OsYSL14 were analyzed. The OsYSL8 was found to be up-regulated under different treatment at both panicle emergence and grain filling stage (Fig. 7b). At panicle emergence stage, the higher expression (1.91 Rq) of OsYSL8 gene was recorded in combine treatment of ZnO NP seed priming and foliar application (T4). At grain filling stage, the highest expression (4.03 Rq) of OsYSL8 gene was recorded in ZnO NP foliar application treatment (T2). The increased in accumulation of OsYSL14 transcripts was observed for all the treatments at panicle emergence stage whereas it was down-regulated at grain filling stage (Fig. 7b). The higher expression (2.75 Rq) of OsYSL14 gene was recorded in combine treatment of ZnO NP seed priming and foliar application (T4) at panicle emergence stage. At grain filling stage the expression of OsYSL14 gene was down-regulated where lowest expression (0.57 Rq) was observed in combine treatment of ZnO NP seed priming and foliar application (T4). The downregulation of OsYSL14 gene at grain filling stage due to the movement of metals within the plants, further the expression pattern of this gene is restricted to the aerial plant organs. The study correlated with the findings of Sperotto et al.. (2010)80 in which the expression of OsYSL8 was lower in the panicle emergence stage as compared to the grain filling stage.
Nicotinamine (NA) is the major chelator involved in phloem transport of zinc and iron. The nicotianamine synthase (NAS) protein is required for the biosynthesis of NA (Nicotianamine), a non- peptidyl metal chelator that is believed to be a co- substrate of the YSL protein and responsible for translocation of micro-nutrients from plants to grains and enhance nutritional quality81. The OsNAS3.1 gene is expressed in cell involved in long distance transport of Fe (Inoue et al., 2003). The OsNAS3.1 gene was up-regulated under different treatment condition at both panicle emergence and grain filling stage (Fig. 7c). At panicle emergence stage, the higher expression (2.46 Rq) of OsNAS3.1 gene was recorded in ZnONPs seed priming treatment (T2). Opposite to this at grain filling stage, the higher expression (2.06 Rq) of OsNAS3.1 gene was recorded in combine treatment of ZnO NP seed priming and foliar application (T4) indicated more translocation of nano Zinc from leaves to rice grains. The result of this finding consistent with previous finding identified that application of nano zinc at rate of higher than 1 mg zinc per plant enhance expression of NAS1 gene. This is due to under condition of zinc hyper accumulation, zinc redistributed in plant by process of zinc sequestration to detoxify zinc and this process occur in plant vacuoles78.
The OsNAAT1 gene is speculated to participate in biosynthesis of phytosi derohoresrelated to Strategy I of metal uptake in cereal. Variable response in gene expression of OsNAAT was observed among the different ZnO NP treatments at panicle emergence and grain filling stage (Fig. 7d). At panicle emergence stage, the higher expression (1.80 Rq) of OsNAAT gene was recorded in ZnO NP seed priming treatment (T3) whereas at grain filling stage, the higher expression (2.13 Rq) of OsNAAT gene was observed in combined treatment of ZnO NP seed priming and foliar application (T4). Banerjee, Sharma, Verulkar and Chandel82 reported that variation in expression OsNAAT1 gene at maximum tillering stage was found to be correlated to high grain Zn content.
Conclusion
The present study revealed that priming the seed with 25 ppm ZnO NP for a soaking period of 24 h improved the physiological parameters, i.e., percentage germination, seedling length, germination speed, root length, shoot length, seedling vigor index I and seedling vigor index II of rice variety Jaya. The field trial showed that the combination of seed priming with 25 ppm ZnO NP for 24 h soaking and foliar application of 100 ppm ZnO NP at panicle emergence and grain filling stage showed a positive effect on the morphological characteristics of the plant as well as the Zn content in the grain. The observed modulation of genes related to Zn homeostasis, namely OsZIP1.1, OsZIP3, OsZIP4, OsNAAT, OsNAS3.1, OsYSL14 and OsYSL8, supports the transport of Zn reported in the plant. The combined application of a seed treatment of 25 ppm ZnO NP during a 24-hour soaking period and a foliar application of 100 ppm ZnO NP at the time of panicle emergence and grain filling is therefore effective in improving the Zn content in rice grain, husk and straw.
Data availability
The data and materials underlying this research are detailed within the manuscript. For additional inquiries, please contact the corresponding author.
References
Campisi, S. C., Khan, A., Zasowski, C., Bhutta, Z. A. & Malnutrition Textbook of Pediatric Gastroenterology, Hepatology and Nutrition: A Comprehensive Guide to Practice 609–623. (2022).
Addala, V. P., Kurma, V. R., Rao, K., Akasapu, T. M. & Kalyani, M. An observational study on prevalence of micronutrient deficiency in under 5 children in rural areas of Prakasam district. Int. J. Acad. Med. Pharm. 6 (1), 32–37 (2024).
Al-Fartusie, F. S. & Mohssan, S. N. Essential trace elements and their vital roles in human body. Indian J. Adv. Chem. Sci. 5 (3), 127–136 (2017).
Shahzad, Z., Rouached, H. & Rakha, A. Combating mineral malnutrition through iron and zinc biofortification of cereals. Compr. Rev. Food Sci. Food Saf. 13 (3), 329–346 (2014).
Wang, Q., Mei, S., Manivel, P., Ma, H. & Chen, X. Zinc oxide nanoparticles synthesized using coffee leaf extract assisted with ultrasound as nanocarriers for mangiferin. Curr. Res. Food Sci. 5, 868–877. https://doi.org/10.1016/j.crfs.2022.05.002 (2022).
Hotz, C. & Brown, K. H. Assessment of the risk of zinc deficiency in populations and options for its control. (2004).
Salgueiro, M. J. et al. The role of zinc in the growth and development of children. Nutrition 18 (6), 510–519 (2002).
Uddin, M. M., Zakeel, M. C. M., Zavahir, J. S., Marikar, F. M. & Jahan, I. Heavy metal accumulation in rice and aquatic plants used as human food: A general review. Toxics 9 (12), 360 (2021).
Zhao, M., Lin, Y. & Chen, H. Improving nutritional quality of rice for human health. Theor. Appl. Genet. 133, 1397–1413 (2020).
Chaudhari, P. R., Tamrakar, N., Singh, L., Tandon, A. & Sharma, D. Rice nutritional and medicinal properties: A review Article. J. Pharmacognosy Phytochemistry. 7 (2), 150–156 (2018).
Kowsalya, P., Sharanyakanth, P. & Mahendran, R. Traditional rice varieties: A comprehensive review on its nutritional, medicinal, therapeutic and health benefit potential. J. Food Compos. Anal. 114, 104742 (2022).
Wairich, A., Ricachenevsky, F. K. & Lee, S. A Tale of two metals: biofortification of rice grains with iron and zinc. Front. Plant Sci. 13, 944624 (2022).
Hamzah Saleem, M., Usman, K., Rizwan, M., Al Jabri, H. & Alsafran, M. Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Front. Plant Sci. 13, 1033092 (2022).
Lowe, N. M. et al. Preventing and controlling zinc deficiency across the life course: A call to action. Adv. Nutr. 15 (3), 100181 (2024).
Chasapis, C. T., Ntoupa, P. S. A., Spiliopoulou, C. A. & Stefanidou, M. E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 94, 1443–1460 (2020).
Peramaiyan, P. et al. Agronomic biofortification of zinc in rice for diminishing malnutrition in South Asia. Sustainability 14 (13), 7747 (2022).
Wang, H. et al. Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction: a population-based birth cohort study. Sci. Rep. 5 (1), 11262 (2015).
Arunachalam, P., Kannan, P., Prabukumar, G. & Govindaraj, M. Zinc deficiency in Indian soils with special focus to enrich zinc in peanut. Afr. J. Agric. Res. 8 (50), 6681–6688 (2013).
Prasad, R. Zinc biofortification of food grains in relation to food security and alleviation of zinc malnutrition. Current Science. 98(10), 1300–1304 (2010).
Mi, K. et al. Zinc oxide nanoparticles enhanced rice yield, quality, and zinc content of edible grain fraction synergistically. Front. Plant Sci. 14, 1196201 (2023).
Lakhani, K. G., Hamid, R., Motamedi, E. & Marviya, G. A review on plant metabolite-mediated nanoparticle synthesis: sustainable applications in horticultural crops. Front. Nanatechnol. 7, 1545413 (2025).
Patel, P. S., Singh, S. K., Patra, A. & Jatav, S. S. Root dipping, foliar and soil application of zinc increase growth, yields, and grain zinc in rice (Oryza sativa L.) grown in moderate zinc soil of inceptisol order. Commun. Soil Sci. Plant Anal. 53 (15), 1917–1929 (2022).
Zhang, H. et al. The effect of zinc oxide nanoparticles for enhancing rice (Oryza sativa L.) yield and quality. Agriculture 11 (12), 1247 (2021).
Soliman, A. M. et al. Green approach to overcome the resistance pattern of Candida spp. Using biosynthesized silver nanoparticles fabricated by penicillium chrysogenum F9. Biol. Trace Elem. Res. 199, 800–811 (2021).
Ali, I. A. M., Ahmed, A. B. & Al-Ahmed, H. I. Green synthesis and characterization of silver nanoparticles for reducing the damage to sperm parameters in diabetic compared to Metformin. Sci. Rep. 13 (1), 2256 (2023).
Gomez, K. A. Gomez, A. A. Statistical Procedures for Agricultural Research Techniques for field experiments with rice. Int. Rice Res. Inst., John Wiley & Sons, (1984).
Johan, F., Jafri, M., Lim, H. & Maznah, W. W. Laboratory measurement: Chlorophyll-a concentration measurement with acetone method using spectrophotometer. In 2014 IEEE International Conference on Industrial Engineering and Engineering Management, ; IEEE: pp 744–748. (2014).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. biol. Chem. 193 (1), 265–275 (1951).
Zhou, J. Y. & Prognon, P. Raw material enzymatic activity determination: a specific case for validation and comparison of analytical methods—the example of superoxide dismutase (SOD). J. Pharm. Biomed. Anal. 40 (5), 1143–1148 (2006).
Aebi, H. Catalase in vivo. Methods Enzymol. Oxygen Radicals Biol. Syst. 105, 121–126 (1984).
Guilbault, G. G. & Sadar, M. H. Preparation and analytical uses of immobilized enzymes. Acc. Chem. Res. 12 (9), 344–350 (1979).
Li, Y. et al. Simultaneous analysis of multiple endogenous plant hormones in leaf tissue of oilseed rape by solid-phase extraction coupled with high-performance liquid chromatography-electrospray ionisation tandem mass spectrometry. Phytochem. Anal. 22 (5), 442–449 (2011).
Patel, A. C., Patel, K., Dubey, P., Singh, S. & Kaswala, A. Comparative effect of different levels of NADEP manures on nutrients content and quality of different crops grown under certified organic farm. J. Pharmacognosy Phytochemistry. 9 (5), 611–614 (2020).
Lindsay, W. L. & Norvell, W. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 42 (3), 421–428 (1978).
Gomez, K. A. & Gomez, A. A. Statistical Procedures for Agricultural Research (Wiley, 1984).
Panse, V. G. & Sukhatme, P. V. Statistical Methods for Agricultural Workers (Indian Council of Agricultural Research, 1967).
Abdo, A. M. et al. Green synthesis of zinc oxide nanoparticles (ZnO-NPs) by Pseudomonas aeruginosa and their activity against pathogenic microbes and common house mosquito, culex pipiens. Materials 14 (22), 6983 (2021).
Shabani, M. H., Jafari, A., Manteghian, M. & Mousavi, S. M. Green synthesis of zinc oxide nanoparticles using Enterobacter cloacae microorganism and their application in enhanced oil recovery. Sci. Rep. 14 (1), 29409 (2024).
Hassan, S. E. D. et al. Endophytic actinomycetes streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. J. Biol. Inorg. Chem. 24, 377–393 (2019).
Veena, M. & Puthur, J. T. Seed nutripriming with zinc is an Apt tool to alleviate malnutrition. Environ. Geochem. Health. 44 (8), 2355–2373 (2022).
Farooq, M. et al. Morphological, physiological and biochemical aspects of zinc seed priming-induced drought tolerance in Faba bean. Sci. Hort. 281, 109894 (2021).
Bahri, S., Homaei, A. & Mosaddegh, E. Zinc sulfide-chitosan hybrid nanoparticles as a robust surface for immobilization of Sillago Sihama α-amylase. Colloids Surf., B. 218, 112754 (2022).
Gunathunga, C. et al. Germination effects on nutritional quality: A comprehensive review of selected cereal and pulse changes. Journal Food Composition Analysis. 128, 106024. (2024).
Adhikari, B., Dhital, P. R., Ranabhat, S. & Poudel, H. Effect of seed hydro-priming durations on germination and seedling growth of bitter gourd (Momordica charantia). PloS One 16 (8), e0255258. (2021).
Lakhani, K. G., Salimi, M., El Idrissi, A., Hamid, R. & Motamedi, E. Nanocellulose-hydrogel hybrids: A review on synthesis and applications in agriculture, food packaging and water remediation. International J. Biol. Macromolecules. 309, 143081 (2025).
Azim, Z. et al. Potential role of biosynthesized zinc oxide nanoparticles in counteracting lead toxicity in solanum lycopersicum L. Plant. Nano Biology. 2, 100012 (2022).
Itroutwar, P. D. et al. Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L). J. Plant Growth Regul. 39, 717–728 (2020).
Waqas Mazhar, M. et al. Seed nano-priming with zinc oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS One 17 (3), e0264967. (2022).
Adhikary, S. et al. Seed priming with selenium and zinc nanoparticles modifies germination, growth, and yield of direct-seeded rice (Oryza sativa L). Sci. Rep. 12 (1), 7103 (2022).
Sharma, D., Afzal, S. & Singh, N. K. Nanopriming with phytosynthesized zinc oxide nanoparticles for promoting germination and starch metabolism in rice seeds. J. Biotechnol. 336, 64–75 (2021).
Pandya, P., Kumar, S., Patil, G., Mankad, M. & Shah, Z. Impact of ZnO nanopriming on physiological and biochemical traits of wheat (Triticum aestivum L.) seedling. CABI Agric. Bioscience. 5 (1), 27 (2024).
Wang, S. et al. Foliar spraying of ZnO nanoparticles enhanced the yield, quality, and zinc enrichment of rice grains. Foods 12 (19), 3677 (2023).
Poornima, R. & Koti, R. Effect of nano zinc oxide on growth, yield and grain zinc content of sorghum (Sorghum bicolor). J. Pharmacognosy Phytochemistry. 8 (4), 727–731 (2019).
Fan, Y. et al. Zinc affects the physiology and medicinal components of dendrobium nobile Lindl. Plant Physiol. Biochem. 162, 656–666 (2021).
Cakmak, I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant. soil. 302, 1–17 (2008).
Dang, K. et al. Zinc regulation of chlorophyll fluorescence and carbohydrate metabolism in saline-sodic stressed rice seedlings. BMC Plant Biol. 24 (1), 464 (2024).
Ahmed, N. et al. Effect of zinc on chlorophyll contents, gas exchange attributes and zinc concentration in rice. Pak. J. Bot. 54 (1), 17–24 (2022).
Li, Y. et al. ZnO nanoparticle-based seed priming modulates early growth and enhances physio-biochemical and metabolic profiles of fragrant rice against cadmium toxicity. J. Nanobiotechnol. 19, 1–19 (2021).
Maret, W. & Li, Y. Coordination dynamics of zinc in proteins. Chem. Rev. 109 (10), 4682–4707 (2009).
Jomova, K. et al. Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 98 (5), 1323–1367 (2024).
Srivastav, A. et al. Effect of ZnO nanoparticles on growth and biochemical responses of wheat and maize. Plants 10 (12), 2556 (2021).
Azarin, K. et al. Effects of ZnO nanoparticles and its bulk form on growth, antioxidant defense system and expression of oxidative stress related genes in hordeum vulgare L. Chemosphere 287, 132167 (2022).
Song, Y., Jiang, M., Zhang, H. & Li, R. Zinc oxide nanoparticles alleviate chilling stress in rice (Oryza sativa L.) by regulating antioxidative system and chilling response transcription factors. Molecules 26 (8), 2196 (2021).
Ahmad, N. et al. Enhancement of rice zinc content using green synthesized ZnO-NPs by foliar and Nano-Priming applications. Applied Biochem. Biotechnology 197(3), 1906-1922 (2024).
Raddy, R., Salimath, M., Geetha, K. & Shankar, A. ZnO nanoparticle improves maize growth, yield and seed zinc under high soil pH condition. Int. J. Curr. Microbiol. Appl. Sci. 7, 1593–1601 (2018).
Sourati, R. et al. Effects of naphthaleneacetic acid, indole-3-butyric acid and zinc sulfate on the rooting and growth of mulberry cuttings. Int. J. Plant. Biology. 13 (3), 245–256 (2022).
Casanova-Sáez, R., Mateo-Bonmatí, E. & Ljung, K. Auxin metabolism in plants. Cold Spring Harb. Perspect. Biol. 13 (3), a039867 (2021).
Vesty, E. F. Understanding Developmental Processes in early-diverging Plant Model Systems (University of Birmingham, 2017).
Rehman, M. et al. The multifaceted role of jasmonic acid in plant stress mitigation: an overview. Plants 12 (23), 3982 (2023).
Ali, M. et al. Exploring the potential role of hydrogen sulfide and jasmonic acid in plants during heavy metal stress. Nitric Oxide 140-141, 16-29 (2023).
Ahmed, M. et al. The influence of zinc oxide nanoparticles and salt stress on the morphological and some biochemical characteristics of solanum lycopersicum L. Plants. Plants 13 (10), 1418 (2024).
Vankova, R. et al. ZnO nanoparticle effects on hormonal pools in Arabidopsis Thaliana. Sci. Total Environ. 593, 535–542 (2017).
Bashir, K., Ishimaru, Y. & Nishizawa, N. K. Iron uptake and loading into rice grains. Rice 3, 122–130 (2010).
Guerinot, M. L. The ZIP family of metal transporters. Biochim. Et Biophys. Acta (BBA)-Biomembranes. 1465 (1–2), 190–198 (2000).
Ramesh, S. A., Shin, R., Eide, D. J. & Schachtman, D. P. Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiol. 133 (1), 126–134 (2003).
Ishimaru, Y. et al. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J. 45 (3), 335–346 (2006).
Deshpande, P., Dapkekar, A., Oak, M., Paknikar, K. & Rajwade, J. Nanocarrier-mediated foliar zinc fertilization influences expression of metal homeostasis related genes in flag leaves and enhances gluten content in durum wheat. PLoS One 13 (1), e0191035. (2018).
Cardini, A. et al. Transcriptional regulation of genes involved in zinc uptake, sequestration and redistribution following foliar zinc application to medicago sativa. Plants 10 (3), 476 (2021).
Chowdhury, R. et al. Genome-wide Understanding of evolutionary and functional relationships of rice yellow Stripe-Like (YSL) transporter family in comparison with other plant species. Biologia 77, 39–53 (2022).
Sperotto, R. A. et al. Identification of putative target genes to manipulate Fe and Zn concentrations in rice grains. J. Plant Physiol. 167 (17), 1500–1506 (2010).
Aung, M. S. et al. Nicotianamine synthesis by OsNAS3 is important for mitigating iron excess stress in rice. Front. Plant Sci. 10, 451617 (2019).
Banerjee, S., Sharma, D., Verulkar, S. & Chandel, G. Use of in Silico and semiquantitative RT-PCR approaches to develop nutrient rich rice (Oryza sativa L.). (2010).
Ghorbanzadeh Z, Hamid R, Jacob F, Zeinalabedini M,Salekdeh GH, Ghaffari MR. Comparative metabolomics of root-tips reveals distinct metabolic pathways conferring drought tolerance in contrastinggenotypes of rice. BMC Genomics. 152, 24(1), 2023. https://doi.org/10.1186/s12864-023-09246-z
Pu, S., Yan, C., Huang, H.,Liu, S., & Deng, D. Toxicity of nano-CuO particles to maize and microbial community largely depends on its bioavailable fractions. EnvironmentalPollution. 255, 113248 (2019). https://doi.org/10.1016/j.envpol.2019
Acknowledgements
The author, Komal G. Lakhani, gratefully acknowledges the support of the Science and Engineering Research Board (SERB), the Department of Science and Technology (DST), Government of India, and the Confederation of Indian Industry (CII) for the Prime Minister’s Fellowship.
Author information
Authors and Affiliations
Contributions
A.G.: Conducted Wet lab analysis and Statistical Analysis; K.P.S. Contributed to conceptualisation, supervision, Data Validation and resource provision; K.G.L. Conducted Wet lab analysis, and prepared the original draft of the manuscript; R.H. Data Validation, Writing-reviewing and editing. V.B.P., N.K., J.V.P., and N.N.G. provided supervision and software facilities. Authors Aishwarya J. Gamit and Komal G. Lakhani contributed equally as First authors to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gamit, A.J., Lakhani, K.G., Suthar, K.P. et al. Zinc biofortification and yield enhancement in rice with nano- primed seeds and foliar sprays. Sci Rep 15, 36580 (2025). https://doi.org/10.1038/s41598-025-20476-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20476-x