Abstract
Swarming, or colony reproduction, in honey bees (Apis mellifera) is an indicator of colony-level fitness. The drivers of swarming remain elusive at both the colony and individual bee level. Floral abundance, rapid colony growth, and congestion are colony correlates and partial triggers of swarming but are not singularly causal. The nutritional and physiological state of individual bees within colonies preparing to swarm has been understudied. We hypothesized that vitellogenin (Vg), a phospholipoglycoprotein that influences the honey bee age-based division of labor in individual bees, might also mediate the cascade of physiological and behavioral processes that lead to reproductive swarming. Over two years, we compared vitellogenin(Vg) gene expression levels in age-marked worker bees sampled at various intervals before a swarm (pre-swarming colonies) to samples of same-aged bees collected from non-swarming colonies at the same time intervals. Vg levels were significantly higher in 10- and 14-day old bees from pre-swarming colonies three days prior and within 24 h of swarm issuance. Vg levels normally decrease in 10-14d old bees that are transitioning to the forager behavioral state. We provide a hypothesis for how Vg levels in individual bees might influence the colony-level regulatory processes that lead to swarming. This work may show for the first time, the link between a highly conserved protein associated with individual reproduction across oviparous animal taxa and its function as a mechanism of social reproduction in honeybees colonies.
Similar content being viewed by others
Introduction
Vg, a phospholipoglycoprotein synthesized and stored in the honey bee fat body, is an ancient reproduction-associated protein that provides nutrients to eggs in most oviparous animals. Honey bee queens, who produce hundreds of eggs each day, have high levels of Vg gene expression. Vg levels are also high in the functionally sterile workers, during the period in which they engage in “nursing” behavior. In the social context of a honey bee colony, the Vg produced by workers is still used to provide nutrients to developing young. Instead of provisioning eggs, worker produced Vg is secreted into the hemolymph and transported to the hypopharyngeal glands in the bees’ heads where it is converted into brood food or royal jelly for larval nutrition1.
In eusocial honey bees, Vg has evolved other critical functions as well. Vg is the primary storage protein in honey bees, Vg gene expression levels in individual worker bees are dynamic and age-dependent. Nurse bees, responsible for feeding larvae, typically begin feeding when they are approximately 3 days old and after 12 days move to the outer periphery of the brood nest for food storage tasks, although the exact temporal division of labor can vary depending on colony conditions2. Vg levels are highest in nurse bees and begin to decline significantly as they transition to tasks peripheral to the brood nest3. As workers age further, the levels of juvenile hormone (JH), an endocrine factor, increase concomitantly with a decrease in levels of Vg, which leads bees into the forager behavioral state1. Early life Vg expression is also associated with a forager’s propensity to collect pollen (protein/lipids) or nectar (carbohydrates)4,5. How these associations within individual workers interact with colony-level nutrient stores and needs is still unclear.
Seasonality and environmental conditions, such as pollen availability, also influence Vg dynamics. Its production is highly influenced by the availability of nectar and pollen from flowering landscapes to meet the nutritional needs of colonies6. For instance, in the active spring and summer months, workers from resource-stressed colonies display precocious foraging7. In the late summer and early autumn, the colony produces winter or diutinus bees that accumulate lipid and vitellogenin stores even as field resources decline8. These internal resource stores along with the stored honey, enable the individual workers and colony as a whole to survive the winter months during which no new resources can be collected3,9.
Vg’s known roles in egg/brood provisioning, regulating behavioral maturation and nutrient storage led us to hypothesize that it may be co-opted into the context of swarming, the social reproductive mechanism of a eusocial honey bee colony. Swarming, a seasonal reproductive process, involves about one-half to two-thirds of the colony leaving with the old queen to establish a new nest, leaving a new queen to inherit the parent colony10,11. The bees that leave the colony undergo a short bivouacking stage while scouts locate a new nest site. During this time along with an establishment phase in the new nest, the colony has no access to external nutrient stores11. These conditions could demand a buildup of internal energy stores like that seen in winter bees. Known correlates of swarming include seasonal resource abundance, increased colony size, brood nest congestion, and reduced transmission of queen pheromones12. However, these colony-level factors alone are not sufficient to induce swarming and do not fully explain the physiological and demographic thresholds required for swarming12,13,14,15.
Given Vg’s role in regulating nutritional physiology and worker bee maturation, we hypothesized that this important regulatory protein may be an indicator of the larger processes and signals during swarming preparation. Pre-swarming A. mellifera colonies exhibit delayed increases in JH levels16, suggesting that Vg levels may remain elevated during this period1. We predicted that nurse-age bees (7–14 days old) in pre-swarming colonies would maintain higher Vg levels compared to bees in non-swarming colonies, facilitating a delayed maturation in this age range of bees in colonies preparing to swarm. Our findings draw a new connection between individual age maturation and colony-level reproduction, providing novel insights into the physiological processes underlying swarming.
Materials and methods
Bees
In the summers of 2021 and 2022, Apis mellifera colonies (n = 12 per year) were established at the University of Minnesota from “packages” (10,000 bees with a mated queen in a screened box) purchased from Olivarez Honey Co., California. Each package was hived in a single Langstroth-style deep box containing 10 frames of foundation (no drawn comb) and fed sugar syrup as needed to promote the construction of wax cells and colony growth. Colonies were maintained in the one box to encourage crowding and potential swarming. Colonies were hived on May 7, 2021, and April 30, 2022.
Starting June 1, 2021, and June 2, 2022, a frame of pre-eclosion pupae was removed weekly (2021) or twice weekly (2022) from each colony, caged, and incubated overnight at 33 °C and 50% RH. The next day, newly emerged worker bees (150–200 bees per session) were paint-marked with Testor’s® enamel paint using a unique color for each date and then were returned to their respective colonies. Marked bees were later collected when they were 7 and 14 days old (2021), and 7, 10, and 14 days old (2022), corresponding to known physiological transitions in Vg levels16. Collections were made from June 8 to July 6, 2021, and from June 9 to July 11, 2022, with 20–30 bees collected per age group per colony on each date. Samples were stored at −80 °C.
Colony conditions were monitored weekly to assess indicators of swarming both years, and in 2022, included estimates of colony strength (frames of brood and bees)17. Colony strength was estimated by counting the number of frames completely covered with bees as well as the number of frames with brood (eggs, larvae and/or pupae). Vg expression in bees of known ages was analyzed from pre-swarming and non-swarming colonies each year. We analyzed samples of bees from pre-swarming colonies, collected within 24 h prior to the swarm leaving the colony. Samples of bees collected on the same dates from non-swarming colonies were chosen based on having a similar adult bee population and amount of brood to the pre-swarming colony.
Quantification of vitellogenin mRNA
Samples collected in 2021 and 2022 were analyzed at the USDA-ARS Bee Research Lab in Baton Rouge, LA, using real-time quantitative PCR. RNA was extracted from individual bee abdomens (12 per colony) using the Maxwell RSC 48 SimplyRNA Tissue Kit (Promega). Abdomens were homogenized in 200 µL of SimplyRNA homogenization solution, and debris was removed via centrifugation. RNA extractions were automated using Maxwell RSC 48 cartridges with DNase treatment. The extraction process followed the “Simply RNA Tissue Protocol” and was completed in 52 min.
cDNA synthesis was performed following the protocol described by4. Quantitative real-time PCR was carried out using the Bio-Rad CFX Connect Real-Time System, targeting the vitellogenin (Vg) gene and two reference genes, β-actin and NDUFA8 (see Table 1 for primer details). Each sample was run in triplicate. PCR reactions were performed with a total volume of 10 µL, using SYBR/FAM dye and an annealing temperature of 59 °C. Thermal cycling conditions were as follows: For Vg: 95 °C for 3 min, followed by 40 cycles of 95 °C for 5 s, 57.5 °C for 10 s, and 72 °C for 10 s. For β-actin: 95 °C for 3 min, followed by 40 cycles of 95 °C for 5 s, 52.5 °C for 10 s, and 72 °C for 10 s. For NDUFA8: 95 °C for 3 min, followed by 40 cycles of 95 °C for 5 s, 52.5 °C for 10 s, and 72 °C for 10 s. Relative gene expression was calculated using the ΔΔCt method18.
Statistical analyses
R (Version 2024.09 + 375) was used for comparison of Vg levels in bees from pre-swarming and non-swarming colonies on given dates. Vg expression was calculated with the ΔCT method using the mean CT values of the two references genes for standardization within each sample. Gene expression data were then log 2-transformed to approximate normality. A linear mixed-effects model (LMM) was employed to evaluate the effects of colony type (pre-swarming or non-swarming) and bee age (7, 10, 14 days), and their interaction as fixed effects, on Vg levels. Colony was included as a random effect to account for variability among colonies.
The model was fitted using the lmer() function from the lme4 package. Statistical significance was determined using Type II Wald chi-square tests (Anova() function, car package). Pairwise comparisons and estimated marginal means (EMMs) were computed with the emmeans package, with significance determined at α = 0.05.
To compare the log-transformed Vg levels in bees during the temporal build-up to swarming, non-parametric Jonckheere-Terpstra (JT) tests (R Version 2024.09 + 375) were used to detect monotonic trends across time points (14, 7, 3, and 0 days before swarming). JT tests were performed using the JonckheereTerpstraTest() function from the DescTools package in R. Pairwise comparisons between time points were carried out using Conover’s test (conover.test() function, conover.test package).
Results
Pre-swarm: within 24-hours of swarm
In 2021, six of the 12 colonies swarmed. We analyzed Vg levels in bees from three of the six pre-swarming and three non-swarming colonies. Three swarming colonies were excluded from analysis because they swarmed several days between sampling bouts, or we could not confirm the exact date the swarm issued.
In 2022 four of the 12 colonies swarmed and we compared Vg levels in bees from the four pre-swarming and four non-swarming colonies. We were able to analyze samples from all four because the samples were collected and frozen within 24 h of the known swarm issuance. Estimates of colony strength of the four pairs, taken within one week of the swarm issuance, revealed no significant differences between them: Colonies that swarmed had 7.88 ± 0.65 frames of brood and those that did not swarm had 8.50 ± 0.50 (t(6) = −1.53, p = 0.176); colonies that swarmed had 8.50 ± 0.50 frames of bees vs. 8.12 ± 0.68 in colonies that did not swarm (t(6) = 0.88, p = 0.41).
In 2021, we analyzed 7 and 14 day old stored bees collected within 24 h prior to the swarm and compared them to 7 and 14 day old bees from non-swarming colonies. In 2022, we analyzed 7, 10, and 14 day old stored bees collected within 24 h prior to the swarm. Analysis of year as a factor showed no interaction between year and colony type: swarm vs. no swarm (F(1) = 388, p = 0.203); therefore, data from both years were combined.
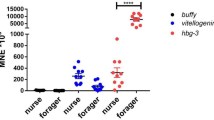
Vg levels were significantly influenced by the interaction between colony type and age group (F = 11.77, df = 2, p = 0.0028). Post-hoc pairwise comparisons showed that Vg levels were not significantly different between 7-day old bees (p = 0.9261) from pre-swarming vs. non-swarming colonies, however the levels were significantly higher in 10- and 14-day-old bees (p = 0.0033 and p = 0.0193 respectively), from pre-swarming colonies vs. non-swarming colonies (Fig. 1).
Among colonies that swarmed, Vg levels did not increase with increasing age of bees; there was no difference among 7-, 10- and 14-day old bees (p = 0.266). However, Vg levels in same age bees from non-swarming colonies significantly decreased in bees older than 7 days (p = 5.58e-08).
Log-transformed relative vitellogenin (Vg) gene expression in honey bee workers from pre-swarming and non-swarming colonies across three age groups: 7, 10, and 14 days from 2021 and 2022 combined. Error bars represent 95% confidence intervals. Different letters indicate significant differences among means (α = 0.05) both among colony types (pre-swarm vs. non-swarm) and across age groups. Significant effects of colony type (F = 4.303, df = 1, p = 0.038) and age group (F = 23.374, df = 2, p < 0.001), as well as a significant interaction between colony type and age group (F = 11.774, df = 2, p = 0.0028) were observed. Vg levels remained the same among bees from pre-swarming colonies at all ages but decreased significantly in 10- and 14-day old bees from non-swarming colonies. A comparison of bees from pre-swarming vs. non-swarming colonies within each age group, showed no significant differences in Vg levels in 7-day old bees (p = 0.9261) but significantly higher levels in both 10-day (p = 0.0033) and 14 day old bees (p = 0.0193), from pre-swarming and non-swarming colonies.
Temporal build-up to swarm
To investigate temporal changes in Vg levels in both pre-swarming and non-swarming colonies, we compared three age groups of worker bees (7-, 10-, and 14-day old) across three time points prior to swarming (14, 7, 3, and 0 days before swarm) from the same colonies. We were not able to analyze all the paired colonies at every time point from pre-swarming and non-swarming colonies. Some of the colonies swarmed early in the experiment and did not have stored, marked bees from 14, 7, or 3 days prior, and in some instances, we did not have enough sampled bees left for a particular age and date. When possible, we analyzed 12 bees from each selected date (Table 2).
Vg gene expression in 7-, 10-, and 14-day-old worker bees from pre-swarming and non-swarming colonies across three time points leading up to swarming. Boxplots show the distribution of log-transformed Vg levels at 7, 3, and 0 days (for 7-day-old bees) or 14, 3, and 0 days (for 10- and 14-day-old bees) prior to swarm issuance. Different letters indicate significant differences (α = 0.05) among days within each colony type; no comparisons were made between pre-swarming and non-swarming colonies. Pre-swarming colonies generally showed increasing Vg levels toward the swarm event, whereas non-swarming colonies exhibited lower and more variable patterns.
7-day-old bees
For pre-swarming colonies the Jonckheere-Terpstra (JT) test revealed the following: Vg expression remained relatively unchanged throughout the experiment (JT = 2902, p = 0.072). For non-swarming colonies, the JT test showed a significant increase in Vg levels (JT = 5064, p < 0.001), with pairwise comparisons revealing significant differences between all time points (p < 0.05) (Fig. 2).
10-day-old bees
In pre-swarming colonies, the JT test showed a significant increase in Vg levels (JT = 3711, p = 3.608e-13). Pairwise comparisons revealed significant differences among all time points (p = 0). In non-swarming colonies, the JT test did not detect a significant difference (JT = 1557, p = 0.8254).
14-day-old bees
For Pre-swarming colonies, the JT test indicated a significant increasing trend in Vg levels over time (JT = 5051, p = 1.449e-08). Pairwise comparisons revealed significant differences among all time points (p = 0). In non-swarming colonies, no significant difference was detected (JT = 2886, p = 0.744 (Fig. 2).
Discussion
Vg is a major driver of individual honey bee longevity and a strong indicator of colony health and nutritional status. High levels of Vg expression in adult bees, coinciding with high pollen intake, are a signal of a healthy physiological state of the colony3,20,21,22,23. Studies have shown that Vg provides resistance to oxidative stress and that the individual life expectancy of honey bees is correlated with levels of Vg24,25. Our findings highlight yet another way Vg influences honey bee individual and colony dynamics, as it supports colony level preparations for reproductive swarming.
We found higher levels of Vg specifically in middle-aged bees (10–14 days old), compared with same age cohort of bees from non-swarming colonies, indicating they are physiologically delayed in maturation, maintaining nurse-like characteristics. The increased levels of Vg in 10-14-day old bees from pre-swarming colonies were observed 3 days prior to swarming. Younger, 7-day old bees had relatively high Vg levels in both pre-swarming and non-swarming colonies, which is typical of younger nurse bees that consume pollen and stimulates their hypopharyngeal glands to secrete brood food for the larvae. The high Vg levels in bees from pre-swarming colonies persisted as bees aged, but dropped in non-swarming colonies, as they do during the normal transition from the nurse to the forager behavioral state. Our results may provide a missing link in the cascade of physiological and environmental factors that lead to reproductive swarming in honey bees.
We hypothesize that Vg may modulate the swarming process as follows. The seasonal reproductive process begins in late spring and early summer, when the rapid increase of floral resource abundance leads to foragers collecting and storing high amounts of pollen, young nurse bees consume the protein and lipid-rich pollen and convert it into Vg which is synthesized and stored in the fat body and secreted into the hemolymph. The Vg is then transported to the hypopharyngeal glands, where it is converted into brood food or royal jelly to feed larvae, supporting rapid population growth. The enhanced food provisioning for the larvae leads to a rapid increase in the number of young workers12,26. As the colony grows, spatial congestion can develop within the hive, leading to a reduction in the effective spread of queen mandibular pheromone (QMP)26. This reduced pheromone distribution is a well-documented factor that stimulates queen rearing, a critical step in the swarming process13. Weeks before a colony swarms, they begin mass queen rearing by creating multiple wax cups for queen larvae on the bottom of combs and provisioning these cups with ample royal jelly for the developing larvae27.
Simultaneously, as the buildup of young, Vg-rich workers occurs, foragers—characteristically low in Vg—begin to curtail their external activities in the period just prior to swarming11. This reduction in foraging activities leads to an excess of foragers within the hive. This seems to confirm the double repressor hypothesis of an external repressor signal coming from foragers1 that was later shown to be ethyl oleate, which delays the transition to foraging behavior via trophalaxis28.The middle aged worker bees in our study had a delayed ontological progression starting three days before swarming, which would coincide with higher numbers of foragers present inside the hive.
At the same time, the buildup of nurse bees consuming high levels of pollen and subsequently maintaining elevated levels of Vg provides an internal repressor that inhibits the age-related transition to foraging. This repressor operates through a negative feedback loop between Vg and JH, where high Vg levels suppress the rise of JH titers. The resulting delay in the transition from nurse to forager ensures that the colony retains a robust population of physiologically younger bees. These patterns align with the delayed endocrine transitions previously reported in honey bees, where a postponed rise in juvenile hormone (JH) titers in older workers was observed16. These bees are essential for feeding the queen and the brood both in the new swarm and within the parent colony. This physiological delay enhances the colony’s survival potential during the critical phases throughout swarming preparation until the successful establishment of the new colony. Colonies that are not preparing to swarm do not exhibit these physiological delays, further supporting the conclusion that Vg dynamics are tightly linked to swarming-specific processes occurring in the days leading up to swarming.
High levels of Vg in workers confer significant advantages to colony reproductive fitness, particularly during the swarming phase29. Beyond its role in provisioning food for the parent colony’s increased brood and queen cells, Vg stored in the fat body of workers contributes to stress resilience and cellular immunity, which are critical for workers transitioning to the new nest site. Additionally, Vg serves a metabolic function, acting as a nutrient reserve that can be broken down to support energy demands during food scarcity or disruption1. During swarming there is a significant food disruption during the bivouac period when bees are scouting for a new nest site and then must build up that nest site and get new foraging stores. These combined functions of Vg highlight its central role in preparing colonies for the challenges of swarming and establishing a new colony.
Our study provides valuable insights into the potential role of Vg as an upstream regulator and physiological marker of the swarming preparation process. We quantified the mRNA expression levels of Vg as a proxy for protein function. Previous research has demonstrated that Vg mRNA levels are often correlated with Vg protein titers1. We recognize that gene expression alone may not fully capture the biochemical pathways involved in swarming preparation. Future studies should measure Vg protein levels directly, juvenile hormone titers, and pollen intake. Additional studies that alter Vg levels or nutrition could further test the causal relationship between Vg expression and swarming behavior. It would also be interesting to study how Vg levels might influence flight endurance during swarming as an energetic reserve.
Finally, it would be valuable to establish a gene expression profile and regulatory network specific to swarming bees. Comparing the swarm-specific profile to known networks and profiles, such as those of foragers versus nurses or winter versus summer bees, could reveal unique regulatory mechanisms underpinning swarming behavior. This approach might also identify key genes and pathways that differentiate swarming bees from their non-swarming counterparts.
Numerous environmental and social cues initiate a cascade of physiological and behavioral changes that prepare the colony for swarming. We found that Vg levels may be a key indicator of these processes as measured by its gene expression in middle aged workers. Our findings highlight the importance of Vg as an indicator of the colony’s physiological state and a modulator of the individual worker state during preparation for swarming. While swarming is ultimately triggered by environmental conditions and colony-level factors, Vg may play an essential role in mediating the internal processes that support colony growth and swarming preparation. The delayed physiological shift in workers ensures the colony retains a robust population of young bees, which are vital for colony fitness during reproductive swarming both for those that swarm and those that are left behind. This study offers a first step in identifying Vg as a molecular signal that modulates the capacity for colony level reproduction. Future studies should focus on the biochemical and biomechanical pathways to further elucidate the novel ways that Vg has been exploited by honey bees.
Data availability
All data supporting the findings of this study have been deposited in Dryad and are available to reviewers via this private link: [https://datadryad.org/share/Ov0hm8hqjSWEgNP8XiHiDT0etH\_RjWaQW3v54SUd0iA](https:/datadryad.org/share/Ov0hm8hqjSWEgNP8XiHiDT0etH_RjWaQW3v54SUd0iA). The dataset DOI is 10.5061/dryad.tb2rbp0dp and will become active upon publication. Please note that the DOI will not resolve to a dataset until publication. To access the data during review, please use the private peer-review link provided above. The point of contact if someone wants to request the data from this study is Katrina Klett, corresponding author.
References
Amdam, G. V., Norberg, K., Hagen, A. & Omholt, S. W. Social exploitation of vitellogenin.. Proc. Nalt. Acad. sci. 100, 1799–1802. https://doi.org/10.1073/pnas.0333979100 (2003).
Seeley, T. D. The Wisdom of the Hive: the Social Physiology of Honey Bee Colonies (Harvard Univ. Press, 2022).
Amdam, G. V. & Omholt, S. W. The regulatory anatomy of honeybee lifespan. J. Theor. Biol. 216, 209–228. https://doi.org/10.1006/jtbi.2002.2545 (2002).
Amdam, G. V., Norberg, K., Fondrk, M. K. & Page, R. E. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees.. Proc. Natl Acad. Sci. 101, 11350–11355. https://doi.org/10.1073/pnas.0403073101 (2004).
Nelson, C. M., Ihle, K. E., Fondrk, M. K., Page, R. E. & Amdam, G. V. The gene vitellogenin has multiple coordinating effects on social organization.. PLoS Biol. 5, e62. https://doi.org/10.1371/journal.pbio.0050062 (2007).
Ricigliano, V. A. et al. Honey bee colony performance and health are enhanced by apiary proximity to U.S. Conservation reserve program (CRP) lands. Sci. Rep. 9, 4894. https://doi.org/10.1038/s41598-019-41281-3 (2019).
Schulz, D. J., Huang, Z. Y. & Robinson, G. E. Effects of colony food shortage on behavioral development in honey bees. Behav. Ecol. Sociobiol. 42, 295–303 (1998).
Fluri, P., Lüscher, M., Wille, H. & Gerig, L. Changes in weight of the pharyngeal gland and haemolymph titres of juvenile hormone, protein and vitellogenin in worker honey bees. J. Insect Physiol. 28, 61–68 (1982).
Mattila, H. R. & Otis, G. W. Dwindling pollen resources trigger the transition to broodless populations of long-lived honeybees each autumn. Ecol. Entomol. 32, 496–505 (2007).
Martin, P. Die steuerung der volksteilung beim Schwärmen der Bienen. Insectes Soc. 10, 13–42 (1963).
Seeley, T. D. Honeybee Democracy (Princeton Univ. Press, 2010).
Grozinger, C. M., Richards, J. & Mattila, H. R. From molecules to societies: mechanisms regulating swarming behavior in honey bees (Apis spp). Apidologie 45, 327–346 (2013).
Winston, M. L., Higo, H. A., Colley, S. & Slessor, K. N. The role of mandibular pheromone and colony congestion in honey bee (Apis mellifera L.) reproductive swarming. J. Insect Behav. 4, 649–660 (1991).
Rittschof, C. C. & Seeley, T. D. The buzz-run: how honeybees signal ‘time to go’. Anim. Behav. 75, 189–197 (2008).
Rangel, J. & Seeley, T. D. The signals initiating the mass exodus of a honeybee swarm. Anim. Behav. 76, 1943–1952 (2008).
Zeng, Z., Huang, Z. H., Qin, Y. & Pang, H. Hemolymph juvenile hormone titers in worker honey bees under normal and preswarming conditions. J. Econ. Entomol. 98, 274–278 (2005).
Delaplane, K. S., van der Steen, J. & Guzman-Novoa, E. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apic. Res. 52, 1. https://doi.org/10.3896/ibra/1.52.1.03 (2013).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108 (2008).
Cameron, R. C., Duncan, E. J. & Dearden, P. K. Stable reference genes for the measurement of transcript abundance during larval caste development in the honeybee. Apidologie 44, 357–366 (2013).
Alaux, C. et al. A ‘landscape physiology’ approach for assessing bee health highlights the benefits of floral landscape enrichment and semi-natural habitats. Sci. Rep. 7, 40568 (2017).
Amdam, G. V., Ihle, K. E. & Page, R. E. Regulation of honeybee worker (Apis mellifera) life histories by vitellogenin. Horm. Brain Behav. 1003–1027 (2009).
Toth, A. L. & Robinson, G. E. Worker nutrition and division of labour in honey bees. Anim. Behav. 69, 427–435 (2005).
Bitondi, M. M. G. & Simões, Z. L. P. The relationship between level of pollen in the diet, vitellogenin, and juvenile hormone titres in Africanized Apis mellifera workers. J. Apic. Res. 35, 27–36 (1996).
Salmela, H. et al. Ancient duplications have led to functional divergence of vitellogenin-like genes potentially involved in inflammation and oxidative stress in honey bees. Genome Biol. Evol. 8, 495–505 (2016).
Seehuus, S. C., Norberg, K., Gimsa, U., Krekling, T. & Amdam, G. V. Reproductive protein protects sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA. 103, 962–967 (2006).
Winston, M. L. & Taylor, O. R. Factors preceding queen swarming in Africanized honey bees (Apis mellifera) in South America. Insectes Soc. 27, 289–304 (1980).
Allen, M. D. The behaviour of honeybees Preparing to swarm. Anim. Behav. 4, 14–22 (1956).
Leoncini, I. et al. Regulation of behavioral maturation by a primer pheromone produced by adult worker honey bees. Proc. Natl. Acad. Sci. USA. 101, 17559–17564. https://doi.org/10.1073/pnas.0407652101 (2004).
Ament, S. A., Wang, Y. & Robinson, G. E. Nutritional regulation of division of labor in honey bees: toward a systems biology perspective. WIREs Syst. Biol. Med. 2, 566–576 (2010).
Author information
Authors and Affiliations
Contributions
K.K. and M.S. conceptualized the study and performed the investigation. All authors contributed to methodology and analysis, and also to manuscript review and editing. K.K. did data curation, prepared figures, and wrote the main manuscript. K.I. and M.S.F provided resources for all lab work. M.S. supervised the project and acquired funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Klett, K., Ihle, K.E., Simone-Finstrom, M. et al. Vitellogenin plays a role in regulating honey bee swarming. Sci Rep 15, 36569 (2025). https://doi.org/10.1038/s41598-025-20547-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20547-z