Abstract
Bioremediation effectiveness of crude oil-polluted soil can be monitored via bacterial enzyme activity. In this study, crude oil-polluted and non-polluted soil samples were used to prepare various treatment media, and petroleum hydrocarbon (PHC) degradation was assessed at different incubation periods and pH levels by measuring catalase activity. The stimulatory effects of kenaf-core combined with 95% rhamnolipid on in vitro PHC degradation were compared against bio-stimulation and bioaugmentation treatments. All treatments showed peak catalase activity on the 90th day of incubation. Among bio-augmented treatments, AZ1T6 (Azikoro-1) exhibited the highest catalase activity (20.19 mL KMnO4 g−1h−1), followed by OL1T6 (Ologbo-1) and OT1T6 (Otukpoti-1) (17.66 mL KMnO4 g−1h−1), with BN3T6 (Benin-3) showing the lowest (17.33 mL KMnO4 g−1h−1). In bio-stimulated treatments, media supplemented with kenaf-core and rhamnolipid (T4) demonstrated the highest catalase activity at day 90. All bio-stimulated treatments had significantly higher catalase activity compared to the negative control (T7) (P < 0.05), though their activity was statistically lower than in bio-augmented treatments (P < 0.05). Optimal degradation occurred at pH values between 6.0 and 8.0. The synergistic combination of kenaf-core and 95% rhamnolipid likely enhanced nutrient availability, stimulating indigenous microorganisms and catalase activity, thereby improving bioremediation efficiency beyond what either treatment achieved alone. The enhanced effectiveness observed when combining kenaf-core and rhamnolipid, relative to their separate use, provides evidence for a novel synergistic interaction.
Similar content being viewed by others
Introduction
The release or accumulation of crude oil and hydrocarbon contaminants in the environment poses a serious threat to ecological systems and human health1. Crude oils and their refined products are primarily composed of hydrocarbons, most of which are biodegradable2. However, more complex hydrocarbon structures degrade more slowly and tend to accumulate partially oxidized intermediary metabolites. The biodegradation of petroleum hydrocarbons by indigenous microorganisms typically occurs slowly due to low microbial population density and activity in contaminated areas3,4. The degradation process can be improved by augmenting with potent microbial strains and stimulating indigenous microbes using mineral nutrients and/or biosurfactants5. Atlas6 underscored bacteria as key agents in bioremediation, since they produce biosurfactants when utilizing both insoluble and soluble substrates7,8. The effectiveness of biodegradation also depends on soil or incubation medium pH; therefore, optimizing pH and addition of nutrients can enhance microbial activity and accelerate degradation.
Nutrient addition, such as kenaf core, is often necessary during bioremediation to facilitate pollutant biodegradation9. Our previous study demonstrated that bio-stimulation with kenaf core enhanced the growth and metabolic activity of indigenous bacteria, thereby improving degradation of crude oil contaminants in polluted soils10. Kenaf (Hibiscus cannabinus L.), an annual fiber crop in the Malvaceae family native to Africa, is utilized in food, non-wood fiber, germination mats, filtration media, animal feed, oil extraction, and paper recycling quality enhancement11,12. The stalks yield two main fibers: the outer “bast” fiber, constituting approximately 40% of biomass, and the inner, whiter “core” fiber, making up about 60%. In addition to kenaf, biosurfactants like rhamnolipids play key roles as bio-stimulants in biotechnology and bioremediation13. These surfactants improve petroleum hydrocarbon biodegradation by increasing substrate availability to microorganisms or facilitating cell-surface interactions that enhance hydrophobic substrate adherence14,15. Notably, bacterial efficacy during bioremediation is often pH-dependent and can be monitored using biomarkers such as catalase activity, which serves as an alternative molecular tool to identify specific bioremediating organisms16.
Our previous studies identified bacterial isolates involved in bioremediation of crude oil-contaminated soils and reported enhanced microbial activities following bio-stimulation with kenaf core10,17. However, there is limited information on the efficacy of kenaf core in bioremediation, particularly its combined effect with rhamnolipid. This study evaluates the effects of bio-stimulation, bio-augmentation, and pH changes on catalase activity of these isolates. Locally produced kenaf core fiber (Ifeken-100) and 95% rhamnolipid were applied as a nutrient bio-stimulant and biosurfactant, respectively. We specifically assessed how enhanced catalase activity influenced the in vitro biodegradation of crude oil in polluted soil samples. The stimulatory effects of kenaf core, rhamnolipid, and their combination on PHC degradation were compared.
This study is novel in directly comparing the effectiveness of bio-stimulation using kenaf core and rhamnolipid with bio-augmentation by specific bacterial consortia under controlled conditions. It also provides empirical data identifying optimal treatment conditions. Importantly, the study demonstrates that combining an abundant agricultural waste (kenaf core) with a potent biosurfactant (rhamnolipid) forms an effective strategy for enhancing bioremediation of crude oil contamination. While many agricultural wastes can provide nutrients for bioremediation, Ifeken-100 kenaf is a uniquely effective bio-stimulant for crude oil contamination due to its specific composition, nutrient-release dynamics, and because it is locally produced and cost-effective. The synergy between kenaf-core and rhamnolipid is a novel finding because it combines a multi-faceted stimulation, and enhanced bioavailability and activity to produce a superior degradation effect not previously documented. Using catalase activity as a biomarker provides a methodological innovation over traditional total petroleum hydrocarbon (TPH) quantification by providing a real-time, functional metric of microbial health and activity, as well as a functional insight as against static measurement. It also serves as early indicator of bioremediation, and a diagnostic tool for soil health.
Materials and methods
Reagents and materials
Di-rhamnolipid (biosurfactant) was purchased from Sigma Aldrich (Germany). Chemical reagents used for preparing the mineral salt medium (MSM), including KH2PO4, Na2HPO4, NaCl, and KCl, as well as nutrient broth, potato dextrose agar (PDA), and nutrient agar (NA), were obtained from SD Fine Chemicals Pvt Limited, SRL Industries Ltd., Merck, and Hi Media Pvt. Ltd. Pure dichloromethane (DCM) for petroleum hydrocarbon extraction was sourced from Merck (India). All other chemicals used were of analytical grade.
Collection and preparation of Kenaf sample
The kenaf crop used (Ifeken-100) is an indigenous variety locally cultivated and processed as described in our previous study10. Taxonomic identification and authentication were performed by Dr. Efosa A. Ogie-Odia, Senior Lecturer, Department of Plant Science and Biotechnology, Ambrose Alli University, Nigeria. No voucher specimen was deposited in a public herbarium. All experimental procedures involving kenaf complied with relevant institutional, national, and international guidelines and legislation.
Soil sample location and collection
Crude oil-polluted and non-polluted soil samples were collected from five locations across three states in southern Nigeria, as previously described10,17. Polluted soils originated from Bayelsa State (Otukpoti and Azikoro; 4.84°N, 6.27°E), Edo State (Ologbo; 6.06°N, 5.66°E), and Benin (6.35°N, 5.63°E). The non-polluted soil was collected from Moor Plantation, Ibadan, Oyo State (7.39°N, 3.5°E).
Screening and isolation of oil-degrading microorganisms from crude-oil contaminated soils
Mineral salt medium (MSM) was prepared following Wu et al.18 by dissolving 0.8 g NaCl, 1.4 g Na2HPO4, 0.27 g KH2PO4, and 0.2 g KCl in one liter of deionized water, then autoclaved at 121 °C for 15 min. Oil-degrading microorganisms were isolated using MSM as described by Churchill et al.19. Briefly, 100 mL of MSM was mixed with 10 g of sieved soil samples and incubated on a rotary shaker at 30 °C and 140 rpm for 48 h. One milliliter of the resulting culture was transferred to 50 mL fresh MSM supplemented with 2% crude oil as the sole carbon and energy source and incubated under the same conditions. Isolation was performed via spread plating; plates were incubated at 30 °C for 72 h. Morphologically distinct colonies were picked and sub-cultured on nutrient agar until pure cultures were obtained, which were preserved on nutrient agar slants for further study. Microbial growth was monitored by measuring optical density at 620 nm using a spectrophotometer20. Strains exhibiting the highest growth density were characterized according to Bergey’s Manual of Systematic Bacteriology21,22.
Authentication of isolated microorganisms
Isolates were authenticated via 16 S rRNA sequencing as we previously reported in17. We utilized the 16 S rRNA gene as a barcode to classify bacteria taxonomically within heterogeneous communities. Segments of this gene were amplified from isolates using PCR, and the products were visualized via gel electrophoresis. Extracted DNA was sequenced and compared to the NCBI database using BLASTn for identification. Phylogenetic analysis involved aligning sequences with close relatives from genetic databases and constructing a phylogenetic tree using MEGA software via the maximum parsimony method. Pure cultures were stored at 4 °C on nutrient agar slants for subsequent experiments.
In-vitro degradation study
In vitro biodegradation of crude oil was conducted as described previously10,17. Briefly, sterilized MSM containing 2% crude oil (0.27 g KH2PO4, 1.4 g Na2HPO4, 0.8 g NaCl, and 0.2 g KCl per liter distilled water) was inoculated with 2 mL of bacterial isolates and supplemented with 3% (w/v) kenaf core and/or rhamnolipid. Incubations were performed at 35 °C on a rotary shaker (Stuart Orbital Model S1500, Japan) at 180 rpm. All treatments were carried out in triplicate according to the experimental design shown in Table 1.
Monitoring of PHC degradation through catalase activity
Catalase activity was measured by titrimetric (volumetric) method as prescribed by Lin et al.24. About 1 mL of each treatment was added to 40 mL of distilled H2O and was shaken for 30 min on a rotary mixer at 30 rpm, followed by addition of 5 mL of 0.3% H2O2. The mixture was allowed to react by shaking for further 10 min at 20 ± 2 °C, after which 5 mL of 3 M H2SO4 was added to stabilize the un-decomposed H2O2. Finally, the mixture was filtered and titrated against 0.02 M KMnO4 after 24 h. Catalase activity was expressed as mL KMnO4g−1 h−1.
The pH of media supplemented with Kenaf core and/or rhamnolipid
The pH of all the treatments was determined at ambient temperature using glass electrode, (Jenway pH meter; Model no: S1500). A mineral salt medium consisting of kenaf core, rhamnolipid, crude oil and various inorganic salts were dissolved in 1000 dm−3 of distilled H2O22. About 100 mL of mineral Salt Medium was dispensed into different round bottom flasks, 3% of kenaf core and/or 3% rhamnolipid were added into the designated flasks for bio-stimulated treatments (Table 2) and the solution was sterilized by autoclaving. About 2 mL of 3 h broth culture (peptone broth) of the selected organism was seeded into the flask designated as bio-augmented treatments as shown in Table 2. The pH of the different treatment media was monitored and recorded at intervals of 15 days.
Statistical analysis
The data obtained were subjected to analysis of variance (ANOVA). Comparison of Mean ± SD was made using a least significant difference at the P < 0.05 probability level by the Statistical Analysis System (SAS) program (SAS Institute, Cary, N.C.).
Results
Catalase activity increased with duration of degradation
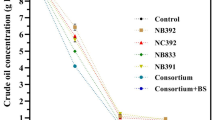
Tables 3, 4, 5 and 6 present catalase activity data for bio-augmented treatments. Catalase activity decreased at day 45 of incubation but progressively increased thereafter with prolonged incubation. Both the inoculated and indigenous bacteria showed reduced growth at day 45. The highest catalase activity was recorded at day 90 for all treatments. Among bio-augmented treatments, AZ1T6 exhibited the highest catalase activity (20.19 mL KMnO4 g−1h−1), followed by OL1T6 and OT1T6 (17.66 mL KMnO4 g−1h−1), while BN3T6 had the lowest activity (17.33 mL KMnO4 g−1h−1) at day 90.
Bio-augmentation enhances catalase activity
Figure 1 illustrates the effect of bio-augmentation on catalase activity during in vitro incubation. All bio-augmented treatments (AZ1T6, OL1T6, OT1T6, BN3T6) exhibited a similar increasing trend in catalase activity over time, reaching the highest levels at day 90. The AZ1T6 treatment consistently yielded the greatest catalase activity among all bio-augmented treatments.
Catalase activity in bio-augmented treatments during in vitro incubation. Bio-augmentation using specific bacterial consortia (AZ1T6, OL1T6, OT1T6, BN3T6) indicate that bio-augmented treatments showed statistically higher catalase activity. It identified the optimal incubation period (90 days) for peak catalase activity across different treatments.
Bio-stimulation enhances catalase activity.
Table 7 presents catalase activity in bio-stimulated treatments. Similar to bio-augmentation results, catalase activity decreased during the first 45 days before steadily increasing with extended incubation. Treatment T4 (supplemented with kenaf core and rhamnolipid) exhibited the highest catalase activity on day 90. The order of catalase activity at day 90 was T4 > T5 > T3 > T7. All bio-stimulated treatments showed significantly higher catalase activity than the negative control (T7) (P < 0.05) but were statistically lower than bio-augmented treatments (P < 0.05).
Alkaline pH favours biodegradation
Table 8 shows pH values during in vitro degradation of crude oil in growth media. A slight pH increase was observed during the first four weeks, followed by a sharp decline at day 45. Results indicate that oil-degrading microbes were most effective within a pH range of 6.0 to 8.0.
Discussion
Catalase role and activity trends
Catalase, a common bacterial enzyme, catalyzes the decomposition of hydrogen peroxide (H2O2) into oxygen and water. Our results demonstrated a significant increase in catalase activity during the first four weeks (day 30) of incubation across all treatments, followed by a sharp decline at day 45 during in vitro degradation. This pattern aligns with findings by Manli et al.18, who reported increased catalase activity in both bio-stimulated and bio-augmented treatments within six weeks, with decline at week seven. Bio-stimulation, involving nutrient or electron donor/acceptor addition, aims to enhance populations or activity of native microorganisms for bioremediation25. Catalase activity mitigates oxidative stress by breaking down hydrogen peroxide, thus protecting living cells during bioremediation.
Catalase activity dynamics and microbial adaptation
A decline in catalase activity was observed at day 45, followed by a progressive increase from day 60 onwards, likely reflecting bacterial adaptation and stabilization within the media. Among bio-stimulated treatments, catalase activity ranked as T7 (negative control) < T3 < T5 < T4 (MSM + kenaf + rhamnolipid + crude oil), with T4 showing the highest and T7 the lowest activity throughout the incubation. Catalase activities in all treatments were significantly higher than the negative control (P < 0.05). Unlike Manli et al.18, who reported higher catalase activity in bio-stimulation than bio-augmentation, we observed that except for T4, bio-stimulated treatments (T3, T5) had lower catalase activities compared to bio-augmented treatments, possibly due to fewer catalase-producing bacteria. The post-six-week increase in catalase activity supports Manli et al.‘s18 observation of steady enzyme activity increase after extended incubation.
Environmental factors influencing catalase activity
Petroleum contamination creates adverse soil conditions such as poor aeration, nutrient immobilization, and lowered pH, which reduce populations of hydrocarbon-degrading microorganisms26,27. Such stress may explain the catalase activity decline observed at day 45. Furthermore, hydrocarbon concentration positively correlates with the abundance of hydrocarbon-utilizing microbes; Achuba and Peretiemo-Clarke26 showed higher counts of culturable degraders facilitate better substrate adaptation. Our findings concur with Adams et al.25, emphasizing organic nutrients as effective stimulants for hydrocarbon biodegradation. The initial catalase activity increase within the first two weeks agrees with Achuba and Okoh’s28 report of elevated catalase activity after 12 days of incubation.
Catalase activity and microbial population growth
The rise in catalase activity after four weeks corresponded to microbial population growth, which enhanced hydrocarbon degradation. Previous studies linked reduced catalase activity to diminished petroleum hydrocarbon biodegradation29. Manli et al.18 reported enzyme activity plateauing during in vitro degradation, while Achuba and Okoh28 attributed fluctuations to metabolic activity changes of specific microorganisms. Our results showed increased catalase activity within 30 days, a decline at day 45, reflecting trends reported for in vitro petroleum hydrocarbon degradation. Findings corroborate Achuba and Okoh’s28 contention that petroleum toxicity alters soil enzymatic activity and biochemistry. Supporting this, Gospodarek et al.30 noted that petroleum derivatives, especially PAHs, negatively impact soil microbial growth and metabolism.
Enzyme Inhibition and microbial community effects
Andreoni et al.31 reported that coating of mineral and organic surfaces on soil particles and microbial cell surfaces can impede enzyme-substrate interactions, negatively affecting enzyme activity. Soil catalase activity reflects the aggregate stable enzyme activities within the soil matrix and the viable microbial population18. Catalase catalyzes hydrogen peroxide hydrolysis releasing oxygen that oxidizes potassium permanganate and thus serves as a proxy for microbial population size and activity. Lin et al.24 noted low catalase activity in soils heavily contaminated with crude oil, which improved markedly following bioremediation. Our findings suggest catalase activity increases as oil concentration declines due to remediation, supporting Lin et al.’s24 recommendation that catalase activity is a sensitive and useful biomarker for monitoring soil bioremediation.
Bioaugmentation and bio-stimulation comparison
Our results showed maximum petroleum hydrocarbon degradation at day 90. Among bio-augmented treatments, AZ1T6 achieved the highest PHC degradation, followed by OT1T6 and OL1T6, with BN3T6 showing the lowest. Bio-augmentation—the addition of potent, specific PHC-degrading microorganisms to augment native populations—is considered essential when indigenous microbes cannot effectively degrade hydrocarbons under contamination stress. Adams et al.25 noted bio-augmentation is critical when indigenous degraders are insufficient or degradation is slow, as seeding reduces lag phase. Effective inoculants must degrade hydrocarbons, maintain genetic stability and viability, survive hostile foreign environments, and compete with native microbes25,32,33. Our findings align with Adetunji et al.34, showing enhanced crude oil degradation via natural biosurfactants (from Pseudomonas aeruginosa) compared to synthetic ones. Contrary to Cai et al.35, we observed significantly superior degradation in bio-augmentation over bio-stimulation, evidencing inoculants’ ability to adapt to environmental changes. Media supplemented with rhamnolipid biosurfactant enhanced degradation throughout the study, corroborating observations by Darvishi et al.36 and Thavasi et al.37., regarding biosurfactants’ efficacy in hydrocarbon biodegradation.
Bio-stimulation performance
Among bio-stimulated treatments, T4 (MSM + kenaf core + rhamnolipid + crude oil) exhibited the highest degradation efficiency, followed by T3 (MSM + kenaf core + crude oil) and T5 (MSM + rhamnolipid + crude oil), which showed comparable results. All bio-stimulated treatments significantly outperformed the negative control (T7). Our findings affirm that petroleum hydrocarbon-degrading microorganisms vary in their hydrocarbon degradation capacities, consistent with Prakash et al.’s38 reports on variable diesel biodegradation among PHC-degrading microbes. The synergistic effect of kenaf core and rhamnolipid nutrients likely stimulated microbial degradation activity, partially agreeing with Manli et al.18, who reported greater total petroleum hydrocarbon degradation by bio-stimulation (60%) than bio-augmentation (34%). In contrast, we recorded higher degradation rates: 82.6% for bio-stimulation and 95.5% for bio-augmentation. This partially contradicts Abdulsalam and Omale’s39 findings, which indicated bio-stimulation (69.2%) was superior to bio-augmentation (65.2%) in hydrocarbon removal.
Bio-stimulation advantages and nutrient synergy
Bio-stimulation alone is widely used in soils where indigenous microorganisms lack access to sufficient nutrients, as nutrient provision stimulates microbial catabolic and anabolic processes necessary for pollutant degradation35. However, given oil toxicity can cause bacterial mortality post-inoculation, our results underscore that inoculation alone does not guarantee microbial survival or activity in the contaminated environment. This aligns with Atlas and Hazen’s40 findings, highlighting nutrient addition as critical for promoting growth and degradative activity of native microbes and suggesting bio-augmentation may be most effective in early pollution stages. Our study also demonstrates that bio-stimulation through organic/inorganic nutrient addition (including oxygen and electron donors/acceptors) effectively enhances bioremediation by increasing microbial populations. Notably, media supplemented with kenaf core and rhamnolipid (T4) yielded significantly higher PHC degradation, likely due to nutrient synergy. Treatments T3 and T5 also showed significantly greater PHC degradation and microbial counts than the negative control, corroborating Lian et al.’s41 report that bio-stimulation increases microbial growth due to the abundance of carbon and energy in PHC pollutants or co-metabolic substrates during incubation.
Supporting studies and metabolic pathways
Previous research demonstrated that cow dung enhanced hydrocarbon degradation by 62.9% in polluted mangrove swamps of the Niger Delta over 70 days42. Our present findings are consistent with Hamzah et al.43, who reported 100% degradation of crude oil within 20 days using sugarcane bagasse and 97% degradation with oil palm empty fruit bunch under similar conditions. The proliferation of hydrocarbon-utilizing bacteria positively correlates with hydrocarbon concentration, facilitating rapid microbial adaptation to contaminated environments44. Petroleum-degrading microorganisms mainly utilize pollutants via two pathways: directly as carbon and energy sources or through co-metabolism with other organic matter. Based on our results, bio-stimulation appears to better support microbial survival than bio-augmentation.
Role of biosurfactants and pH effects
Biosurfactants have been reported to enhance hydrocarbon removal from the environment by increasing bioavailability and thereby facilitating PHC biodegradation45,46. Our data showed bacterial growth in bio-stimulated treatments decreased in the order T4 > T3 > T5 > T7, supporting Banat et al.’s45 findings that combining biosurfactants like rhamnolipid with kenaf core improves accessibility of long-chain hydrocarbons to microbial enzymes. Treatment T4 (MSM + rhamnolipid + kenaf core) also achieved the highest catalase activity and corresponding petroleum hydrocarbon degradation. Panda et al.46 identified the emulsification ability of PHC-degrading bacteria in the presence of surfactants as a key trait enhancing degradation. PHC-degrading microbes differ in their crude oil biodegradation efficiency across pH levels. Our study observed fluctuating pH values between 6.20 and 8.63 during incubation, likely due to acidic metabolite production such as organic acids. Initial pH ranged from 6.20 to 6.53 in the first two weeks, increasing slightly to 6.45–6.80 by day 30, consistent with Rahman et al.’s47 findings that phenanthrene degradation by Alcaligenes faecalis peaks at pH 6.5 and decreases above pH 8.0. We found optimal microbial growth and highest PHC degradation occurred between pH 7.0 and 8.10 at day 90, confirming Vyas and Dave’s and Rahman et al.’s observations that pH 6–8 favors maximum bacterial growth and petroleum hydrocarbon degradation. Vyas and Dave48 also noted lowest microbial growth and degradation at pH 9.0. It is important to recognise that pH influences biodegradation since microbial enzymes function optimally within specific pH and temperature ranges49.
Environmental pH variability and microbial tolerance
In marine environments, pH remains relatively stable between 7.6 and 8.1, thus exerting less influence on biodegradation rates than temperature47. Vyas and Dave48 demonstrated that Marinococcus albus achieves optimal growth and hydrocarbon degradation at 30 °C, with reduced activity at 25 °C and 37 °C. Our results align with previous studies indicating extreme pH values adversely affect microbial hydrocarbon degradation. Most microorganisms prefer pH 6–8 for optimal growth and activity, with deviations retarding microbial proliferation and degradative capabilities50,51. The observed slight pH decrease at day 45 likely reflects acidic metabolite accumulation, potentially impacting microbial survival and enzymatic activity. Overall, PHC degradation increased with incubation time, consistent with findings from Boshui et al.52,53, and our prior reports characterising isolates as hydrocarbonoclastic and effective remediation candidates17.
Conclusion
This study demonstrated that the combination of kenaf core and 95% rhamnolipid synergistically enhanced nutrient availability to indigenous microorganisms, effectively stimulating catalase enzyme production and consequently improving bioremediation efficacy. The combined treatment showed greater hydrocarbon degradation potential than individual bio-stimulation approaches.
Limitations and future directions
A limitation of this study is that the in-vitro experimental design may not accurately reflect real-world conditions like contaminant bioavailability and fluctuating environmental factors. Considering the potential benefit of using kenaf-core, rhamnolipids, and bacterial enzyme activity for bioremediation, potential directions for future research could include scaling up the process and in-situ applications in a controlled lab environment (in-vitro), exploring other agricultural materials, different biosurfactants, and microbes, as well as investigating long-term environmental impacts. Future studies should therefore validate these results under field conditions, incorporate multi-omics approaches to profile microbial communities, and evaluate the cost-effectiveness and sustainability of combined bio-stimulation and bio-augmentation strategies. This will enhance the translational value of laboratory findings for practical application in large-scale remediation of petroleum-contaminated environments.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Erifeta, O. G., Eriyamremu, E. G., Omage, K. & Njoya, K. H. Alterations in the Bio-membrane of libyodrilus Violaceus following exposure to crude oil and its fractions. Int. J. Biochem. Res. Rev. 20, 1–11 (2017).
Prince, R. C. Petroleum and other hydrocarbons biodegradation. In Encyclopedia Environ. Microbiol., 66–74 (2002).
Cerqueira, V. S., Peralb, M. C. R., Camargo, F. A. O. & Bento, F. M. Comparison of the bioremediation strategies for soil impacted with petrochemical oily sludge. Int. Biodeterior. Biodegradation. 95, 338–345 (2014).
Abed, R. M. M., Al-kharusi, S. & Al-Hinai, M. Effects of bio-stimulation, temperature and salinity on the respiration activities and bacteria-community composition in an oil polluted desert soil. Int. Biodeterior. Biodegr. 98, 43–52 (2015).
Jublee, J. & Suparna, M. Evaluation of the bioaugmentation and biostimulation effects on the treatment of refinery oily sludge using 2n full factorial design. Environ. Sci. Processes Impacts. 16, 1889–1896 (2014).
Atlas, R. M. The petroleum biodegradation and oil spill bioremediation. Mar. Poll. Bull. 31, 178–182 (1995).
Fracchia, L., Cavallo, M., Martinotti, M. G. & Banat, I. M. Biosurfactants and bioemulsifiers, biomedical and related applications–Present status, and the future potentials In: Biomedical Science and Engineering Technology, ed. D.N. Ghista (Rijeka: In Tech), ;70–325. (2012).
Mao, X., Jiang, R., Xiao, W. & Yu, J. Uses of the surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 285, 419–435 (2015).
Hesnawi, R. M. & Adbeib, M. M. The effects of the nutrient-source on the Indigenous biodegradation of diesel-fuel contaminated soil. Apcbee Procedia. 5, 557–561 (2013).
Omenna, E. C., Omage, K., Ezaka, E. & Azeke, A. M. Bio-augmentation and bio-stimulation with Kenaf core enhanced bacterial enzyme activities during bio-degradation of petroleum hydrocarbon in polluted soil. Sci. Rep. 14, 8. https://doi.org/10.1038/s41598-023-50882-y (2024).
Han, J. S., Ernest, S., Miyashita, H. & Spielvogel, S. J. Kenaf properties, processing and products. Mississippi State University, Agricultural and Biological Engineering. ;267–283. (1999).
Webber, C. L., Bhardwaj, H. L. & Bledsoe, V. K. Kenaf production: Fibre, Feed, and seed. In Trends in New Crops and New Uses (eds Janick, J. & Whipkey, A.) 327–339 (ASHS, 2002).
Makkar, S. R., Swaranjit, S. C. & Ibrahim, M. B. Advances in utilization of renewable substrates for biosurfactant production. AMB Express. 1 (5), 1–19 (2011).
Donio, M. B. S. et al. BS4, A biosurfactant producing halophilic- bacterium isolated from the solar salt works in India and their biomedical importance 2. In Halomonas Sp 149 (Springer Plus, 2013).
Bai, G. Y., Brusseau, M. L. & Miller, R. M. Biosurfactant enhanced removal of residual hydrocarbon from soil. J. Cont. Hydrol. 25, 157–170 (1997).
Jansson, J. K., Bjorklof, K., Elvang, A. M. & Jurgensen, K. S. Biomarker for monitoring efficacy of bioremediation by microbial inoculants. Environ. Pollution Elsevier. 107, 217–223 (2000).
Omenna, C. E., Omage, K., Ezaka, E. & Azeke, A. M. Tolerance, taxonomic and phylogenetic studies of some bacterial isolates involved in bioremediation of crude oil polluted soil in the Southern region of Nigeria. HELIYON 9, 1–14 (2023).
Wu, M. et al. The bioaugmentation and biostimulation of petroleum-hydrocarbon degradation and the microbial community in petroleum-hydrocarbon contaminated soil. Elsevier Int. Biodeterior. Biodegradation. 107, 158–164 (2016).
Churchill, S. A., Griffin, R. A., Jones, L. P. & Churchill, P. F. Biodegradation rate, and enhancement of hydrocarbons by an oleophilic fertilizer and rhamnolipid biosurfactant. J. Environ. Qual. 24 (1), 19–28 (1995).
Bhoosreddy, W. Manual of Diagnostic Microbiology (Himalaya Publishing House, 1995).
Holt, J. G., Krieg, N. R., Sneath, P. H. A., Stanley, J. T. & William, S. T. The Bergey’s Manual on the Determination of Bacteria. Baltimore MD. USA. ;53–56. (1994).
Churchill, P. F. & Churchill, S. A. Isolation-and characterization of Mycobacterium sp. capable of degrading three- and four-rings aromatic-and aliphatic-hydrocarbons. Appl. Environ. Microbiol. 65, 549–552 (1999).
Marquez-Rocha, P. J., Hernandez-Rodriguez, V. & Lamela, M. T. Biodegradation of diesel oil in soil by a microbial consortium. Water Air Soil. Pollution. 128, 313–320 (2001).
Lin, X. et al. Changes in the microbial-populations and the enzyme activities during the bioremediation of the oil-contaminated soil. Bull. Environ. Contam. Toxicol. 83, 542–547 (2009).
Adams, G. O., Tawari-Fufeyin, P., Okoro, S. E. & Igelenyah, E. Bioremediation: bio-stimulation and bio-augmentation. Int. J. Environ. Bioremediat. Biodegradation. 3 (1), 28–39 (2015).
Achuba, F. I. & Peretiemo-Clarke, B. O. Effects of spent engine oil on the soil catalase and dehydrogenase activities. Int. Agrophys. 22, 1–4 (2008).
Maila, M. P. & Cloete, T. E. The use of biological activity to monitor the removal of fuel contaminants- perspectives to monitoring hydrocarbon contamination. A review. Int. Biodeterior. Biodegradation. 55, 1–8 (2005).
Achuba, F. I. & Okoh, P. N. Effects of the petroleum-products on the soil catalase and dehydrogenase activity. Open. J. Soil. Sci. 4, 399–406 (2014).
Van der-Waarde, J. J., Dijkhuis, E. J., Henssen, M. J. C. & Keuing, S. Enzyme assays as the indicators for bioremediation. In Monitoring and Verification of Bioremediation (eds Hinchee, R. E. et al.) 59–63 (Batelle, 1995).
Gospodarek, J., Milena, R., Barczyk, G. & Nadgórska-Socha, A. The effect of petroleum-derived substances and their bioremediation on soil enzymatic activity and soil invertebrates. Agron. MDPI. 11 (80), 1–20 (2021).
Andreoni, V. et al. Bacterial communities and enzyme activities of the PAHs- polluted soils. Chemosphere 57, 401–412 (2004).
Leahy, J. G. & Colwell, R. R. The microbial degradation of the petroleum- hydrocarbons in the environment. Microbiol. Rev. 54 (3), 305–315 (1990).
Atagana, H. I. Bioremediation of hydrocarbon-contaminated soil using compost-organic manure. Afr. J. Biotechnol. 7 (10), 1516–1525 (2008).
Adetunji, C. O. et al. Characterization and optimization of a rhamnolipid from Pseudomonas aeruginosa with novel biosurfactant activities. Sustainable Chem. Pharm. Elsevier. 6, 26–36 (2017).
Cai, Y. et al. Bioremediation of petroleum hydrocarbons using Acinetobacter sp. SCYY-5 isolated from contaminated oil sludge: Strategy and Effectiveness study. Int. J. Environ. Res. Public Health. 18 (1), 819–825 (2021).
Darvishi, P., Ayatollahi, S., Mowlaa, D. & Niazi, A. Biosurfactant production under the extreme environmental conditions by an efficient microbial consortium, ERCPPI-2. Colloids Surf. B Bio-interfaces. 84 (2), 292–300 (2011).
Thavasi, R., Jayalakshmi, S. & Banat, I. The application of the biosurfactant produced from the peanut-oil-cake by Lactobacillus delbrueckii in the biodegradation of crude-oil. Bioresour Technol. 102 (3), 3366–3372 (2011).
Prakash, A. et al. The biodegradation potential of the petroleum hydrocarbons by bacteria and mixed bacteria consortia from contaminated sites. Turk. J. Eng. Environ. Sci. 38, 41–50 (2014).
Abdulsalam, S. & Omale, A. B. Comparison of bio-stimulation and bioaugmentation techniques for remediation of used motor-oil contaminated soil. Brazilian Archives Biology Technol. 52 (3), 747–754 (2009).
Atlas, R. M. & Hazen, T. C. Crude- oil biodegradation and bioremediation: a Tale of the two worst spills in U.S. History. Environ. Sci. Technol. 45 (16), 6709–6715 (2011).
Lian, J., Wu, J., Ha, Y. & Jia, X. Effect of cotton on several enzymatic activities of the petroleum contaminated soil. Environ. Prot. Eng. 39 (1), 163–182 (2013).
Orji, F. A., Ibiene, A. A. & Dike, E. N. The laboratory scale-bioremediation of petroleum- hydrocarbon polluted-Mangrove swamps in the Niger delta using cow Dung. Malayasian J. Microbiol. 8 (4), 219–288 (2012).
Hamzah, A., Chia-wei, P., Pek-Hoon, Y. & Nurul, H. Oil palm empty-fruit-bunch and sugar cane-bargasse ease bioremediation of the soil artificially-polluted by crude-oil. Soil and sediment contamination. Int. J. 23 (7), 751–762 (2014).
Efsun, D. F. & Olca-Huseyin, S. Variation in the soil enzyme activities in petroleum hydrocarbon contaminated soil. International Bio-deterioration and Biodegradation, 268–275 (2021).
Banat, I. M., Makkar, R. S. & Cameotra, S. S. Potential commercial applications of microbial surfactants. Appl. Environ. Microbiol. 53, 495–508 (2000).
Panda, S. K., Kar, R. N. & Panda, C. R. Isolation and identification of the petroleum hydrocarbon degrading micro-organisms from oil contaminated environment. Int. J. Environ. Sci. 3 (5), 1314–1319 (2013).
Rahman, K. S. M., Rahman, T. J., Lakshmanaperumalsamy, P. & Banat, I. M. Towards efficient crude-oil degradation by mixed bacteria consortia. Bioresour. Technol. 85 (1), 257–261 (2002).
Vyas, T. K. & Dave, B. P. The effect of the crude oil concentration, temperature, and pH on growth and degradation of the crude oil by marine bacteria. Indian J. Mar. Sci. 36 (1), 76–85 (2007).
Chikere, C. B. Culture-Independent analysis of the bacterial community composition during bioremediation of the crude oil polluted soil. Br. Microbiol. Res. J. 2 (3), 187–211 (2012).
Al-Dhabaan, F. A. Morphological, biochemical and molecular identification of petroleum -hydrocarbon-degrading-bacteria isolated from oil polluted soil in Dhabran. Saudi Arabia Saudi J. Biol. Sci. 26, 1247–1252 (2019).
Kour, D. et al. Beneficial microbiomes for bioremediation of diverse contaminated environments for environmental sustainability: present status and future challenges. Environ Sci. Pollut Res. 28, 24917–24939. https://doi.org/10.1007/s11356021-13252-7 (2021).
Boshui, C. B., Nan, Z., Jiang, W., Jiu, W. & Jianhua, F. Biodegradation of the petroleum-hydrocarbons by the Pseudomonas aeruginosa. China Petro Proc. Petrochem. Technol. 14, 66–70 (2012).
Terán-Cuadrado, G., Ortega-Vega, C., Brito, H. A., Quiñones-Murillo, D. & Rojas, M. J. April. Corrigendum to Effectiveness of a bio-catalytic agent used in the bioremediation of crude oil-polluted seawater [Heliyon 7 (4), Article e06926]. Heliyon. 2021;7(8):e07729. doi: 10.1016/j.heliyon.2021.e07729. Erratum for: Heliyon. 2021;7(4):e06926. (2021). https://doi.org/10.1016/j.heliyon.2021.e06926. PMID: 34409189; PMCID: PMC8361057.
Acknowledgements
We acknowledge the Department of Biochemistry, Ambrose Alli University, Ekpoma for providing the necessary laboratory facilities.
Funding
This research was self-funded by the authors.
Author information
Authors and Affiliations
Contributions
ECO, and MAA conceived, designed and performed the experiments; ECO, KO and MAA performed the analysis and interpretation of the data, while KO prepared the draft of the manuscript. All authors have reviewed and approved the final draft of the manuscript. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Consent to participate
Not Applicable.
Ethics approval
The ethical approval for this research was obtained from the Research Ethics Committee of the Department of Biochemistry, Ambrose Alli University, Ekpoma Nigeria.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Omenna, E.C., Omage, K. & Azeke, M.A. Alkaline pH and enhanced catalase activity improve the in vitro bio-remediation of crude-oil polluted soil. Sci Rep 15, 37444 (2025). https://doi.org/10.1038/s41598-025-21353-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-21353-3