Abstract
Paeoniflorin (PF), one of the active components of Paeoniae radix, has antitumor activity in different cancers, including glioblastoma (GBM), which is mediated by its effect on autophagy. However, its underlying mechanism remains unclear. CCK-8, colony formation, scratch, and Transwell assays were performed to determine the effects of PF on GBM cell viability, proliferation, migration, and invasion. PI/RNase and Annexin V-FITC were used to detect cell cycle progression and apoptosis. Protein levels were detected by western blotting. The generation of autophagy flow in GBM cells was observed by transmission electron microscopy and LC3B-GFP plasmid transfection. PF induced autophagy and apoptosis in a dose-dependent manner in two human GBM cell lines (U87 and U118), inhibited cell proliferation, migration, and invasion, and caused cell cycle arrest. Further investigation of the mechanism underlying PF-mediated autophagy and inhibition of GBM cell growth showed that PF upregulated the autophagy-related proteins LC3B and P62 and downregulated P-AKT and P-mTOR, which may be involved in the regulation of autophagy. Treatment with an activator of AKT restored the expression of these proteins. The results indicate that PF induces autophagy and apoptosis through the AKT/mTOR pathway, suggesting its potential as a novel treatment for GBM.
Similar content being viewed by others
Introduction
Glioblastoma (GBM), the most common malignant primary brain tumor, is highly invasive and associated with a poor prognosis. Despite the availability of several treatments including surgery, radiotherapy, chemotherapy, and electric field therapy, the 5-year overall survival rate of grade IV glioma patients is 6.8% 1,2,3. Malignant progression and recurrence are the main determinants of the poor prognosis of GBM patients. Therefore, new and effective glioma treatment strategies are urgently needed.
Paeoniflorin (PF), a component of Paeoniaceae family plants such as Paeonia lactiflora, has been used medicinally in China for more than 1500 years4,5. PF has multiple functions, such as neuroprotection6, anti-allergy activity, repair injury7, and antitumor effects. Studies show that PF has antitumor effects in glioma8,9,10,11, although the underlying anti-glioma effect of PF remains unclear.
Autophagy is a hot research topic in the field of biomedicine. The autophagy-lysosome pathway is the main process mediating the clearance of denatured organelles and soluble macromolecules in the cytoplasm12. The autophagy-lysosome pathway transports damaged13, denatured, or unnecessary proteins and organelles to the lysosome for digestion and degradation by a series of enzymes, thus facilitating the cell’s own metabolism and energy renewal14. Autophagy dysfunction can lead to major diseases such as cancer, diabetes, neurodegenerative diseases, and immune disorders. Autophagy is thus a vital process to ensure cell homeostasis and survival. Microtubule-associated protein light chain 3 (LC3-II) binds to the intracellular autophagy vacuolar membrane and regulates its formation. LC3-II is a known marker of the autophagic vacuole membrane15. In addition, p62 plays an important role in selective autophagy16,17. Therefore, the expression of LC3 and P62 can reflect the autophagy level to a certain extent.
We hypothesized that PF may regulate the growth of GBM cells by inducing autophagy. To prove this hypothesis, we investigated the effects of PF on the proliferation, apoptosis, migration, and invasion of GBM cells, as well as its effect on autophagy. The results confirmed that PF induced GBM cell autophagy and suggested that this effect was mediated by the AKT/mTOR pathway. The present results indicate that PF may be an effective agent for GBM therapy, providing a potentially novel strategy for the treatment of GBM.
Materials and methods
Reagents and cell culture
All experiments were performed in accordance with protocols approved by the Institutional Ethics Committee of Nanchang University and the Military General Hospital of Beijing PLA. PF was obtained from Sigma-Aldrich (St. Louis, MO, USA), catalog number P0038, with a purity of ≥ 98% as confirmed by HPLC. Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). LC3-GFP plasmids were purchased from Addgene. AKT, P-AKT, mTOR, P-mTOR, MMP2, MMP9, Bax, Bcl-2, cleaved caspase-3, cyclin B1, CDK1, P62, LC3B, and β-actin antibodies were purchased from Proteintech (Chicago, IL, USA), and the AKT activator (SC79) was obtained from Beyotime.
The U87 and U118 cell lines were acquired from the Chinese Academy of Medical Sciences (Beijing, China) and cultured in high-glucose DMEM (supplemented with 10% FBS and 1% penicillin-streptomycin).
Cell viability assay
Cells were treated with different concentrations of PF for 24, 48, and 72 h, followed by 10 µL CCK8 and incubation at 37 °C for 1 h. Cells were analyzed on a microplate reader.
Colony formation assay
Approximately 2,00 cells (U87 and U118) were seeded in a 6 cm culture dish and treated with PF at 10, 20, and 40 µM for 15 days. The colonies were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet, and counted.
Wound healing assay
A wound-healing assay was performed to assess GBM cell migration in response to PF. Approximately 5 × 105 cells were seeded in a 6 cm culture dish. When cells reached 80–90% confluence, the bottom of the culture dish was scored with the tip of a sterile pipette. Then, the bottom of the culture dish was rinsed with PBS to remove the separated cells, and the culture medium was replaced with medium containing different concentrations of PF. Image J software was used for image processing.
Transwell chamber invasion assay
To evaluate cell invasion ability, 5 × 104 cells were seeded into the upper wells of Matrigel-coated Transwell plates (Corning, USA) in 200 µL DMEM supplemented with 1% FBS. The lower chamber was filled with 600 µL DMEM containing 20% FBS and the indicated concentrations of PF. After 24 h, the cells invading through the membranes were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet, and photographed under a microscope. Cells were counted in three randomly selected regions.
Cell cycle analysis
U87 and U118 cells were incubated overnight in 6-well dishes (1–1.5 × 105 /well) and treated with different concentrations of PF. The cells were collected and fixed in 70% ethanol for 24 h. Cells were resuspended in PBS and incubated with propidium iodide (PI)/RNAase staining buffer (BD, USA). Finally, the results were detected using the Accuri C6 flow cytometer (BD, USA).
Cell apoptosis analysis
Cells were cultured in 6-well plates and treated with 10 or 20 µM PF for 24 h, followed by 500 µL of binding buffer, 5 µL of PI, and 5 µL of FITC-conjugated anti-Annexin V antibody. Apoptosis was analyzed using the Accuri C6 flow cytometer (BD, USA) within 1 h.
Western blotting
Cells were lysed with RIPA lysis buffer (CWBIO, China), and 30 µg of protein were separated by 10% SDS-polyacrylamide gel electrophoresis, followed by transfer to PVDF membranes. The membranes were blocked with 5% BSA for 1 h, incubated with specific primary antibodies overnight at 4 °C, washed, and incubated with secondary antibodies. Protein expression was measured using an ECL kit (Pierce, Rockford, IL, USA).
Transmission electron microscopy
U87 and U118 cells were seeded in a 10 cm culture dish (5 × 105 cells/dish) and allowed to adhere overnight. Cells were then treated with PF at the indicated concentrations for 24 h. The culture solution was then discarded and cultures were washed 1–2 times with PBS at room temperature. A special fixative for electron microscopy was added and cells were fixed at 4 °C for 30 min. The cells were gently scraped out with a cell scraper and transferred to a centrifuge tube. The cells were fixed at 4 °C for 2 h. After centrifugation, they were washed three times with buffer solution (10 min each time) and stored at 4 °C. The samples were dehydrated and embedded with acetone and epoxy resin. Then, ultrathin sections were prepared and observed using a transmission electron microscope (Jeol Jem-1010 electron microscope, Japan).
GFP-LC3 transfection
U87 and U118 cells transfected with LC3-GFP plasmids were treated with PF and the LC3B-GFP dots were visualized. The dots were considered to be autophagic.
Statistical analysis
GraphPad Prism 9.0 was used for data analysis. The results are presented as the mean values ± standard deviation (SD). Differences between two groups were assessed using the Student’s t test, and differences between three or more groups were assessed by ANOVA. P < 0.05 was considered statistically significant.
Results
PF suppresses GBM cell proliferation
The effect of PF on GBM cell growth was investigated using the CCK-8 assay. As shown in Fig. 1a and b, PF inhibited U87 and U118 cell growth in a dose- and time-dependent manner. The half maximal inhibitory concentration of PF for U87 and U118 cells was approximately 20 µM in 24 h. The anchorage independent growth of GBM cells was examined using colony formation assays. The results showed that the colony formation ability was significantly lower in PF-treated GBM cells (U87 and U118) than in the control group (Fig. 1c, d). These data confirmed that PF suppressed the growth of GBM cells.
PF inhibits GBM cell migration and invasion
The effect of PF on the motility of U87 and U118 GBM cells was examined using wound healing and Transwell assays to assess migration and invasion abilities. Treatment of cells with 10, 20, and 40 µM PF inhibited cell migration into the wound (Fig. 2a–d). The number of U87 and U118 cells infiltrating the membrane (Fig. 2B) was lower in the PF-treated group than in the control group (Fig. 2e, f). PF treatment downregulated the expression of MMP2 and MMP9, confirming that PF inhibited the migration and invasion of glioma cells (Fig. 2g).
PF modulates GBM cell apoptosis and inhibits cell cycle progression
The effect of PF on cell apoptosis was evaluated using the PI-FITC-Annexin assay, which showed that PF treatment increased the rate of apoptosis (Fig. 3a, b). PI staining and flow cytometry to assess cell cycle progression showed that PF induced glioma cell arrest in G2/M phase (Fig. 3c, d). Cyclin-dependent kinases (CDKs) control the cell cycle in eukaryotes18, and Cyclin B1 is highly expressed in the G2/M boundary19. To confirm the effect of PF on cell cycle arrest and apoptosis, the expression of cyclin B1, CDK1, Bax, Bcl-2, and cleaved caspase-3 was detected by western blotting. PF downregulated CDK1, cyclin B1, and Bcl-2 and upregulated cleaved caspase-3 and Bax in a dose-dependent manner (Fig. 3e). Taken together, these results indicate that PF induced cell cycle G2/M arrest and apoptosis.
PF enhances autophagic activity in GBM cells
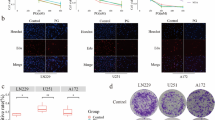
Transmission electron microscopy (TEM) detected a greater number of autophagosomes in the PF treatment group than in the control group (Fig. 4a, b). A stable cell line expressing LC3B-GFP was established to evaluate the effect of PF on autophagy, and the number of autophagosomes in glioma cells treated with PF was detected by immunofluorescence. The number of GFP-LC3 spots and autophagosomes in GBM cells increased significantly after PF treatment (Fig. 4c, d). Western blot analysis of the expression of the autophagy-related proteins LC3 and P62 showed that PF upregulated the expression of LC3-II and downregulated the expression of P62 (Fig. 4e, f). These results confirmed the role of PF in inducing autophagy of GBM cells.
PF regulates GBM cells through the AKT/mTOR pathway
The AKT/mTOR pathway plays an important role in autophagy regulation. Studies show that PF modulates autophagy via the AKT/mTOR pathway in breast cancer and prostate cancer20,21. We explored the mechanisms underlying PF-induced autophagy by analyzing the activity of the AKT/mTOR pathway to determine its possible involvement in the effect of PF in GBM cells. The results showed that PF decreased the levels of phosphorylated Akt and mTOR (Fig. 5a, b). These results suggest that PF suppressed the growth of GBM cells by inducing autophagy via the AKT/mTOR pathway.
SC79 rescues PF-Induced autophagy
To further study the effect of PF on regulating the AKT/mTOR signaling pathway, GBM cells were treated with the AKT activator SC79, and the protein expression and phosphorylation of AKT and mTOR, as well as the expression of the autophagic markers P62 and LC3B-II were detected. The results showed that SC79 activated AKT/mTOR signaling and reversed the regulatory effect of PF on autophagy of GBM cells (Fig. 6a–d). We observed a significant increase in the number of LC3B points in the PF + SC79 group compared with the PF group (Fig. 7a, b). Taken together, these results support that PF inhibits GBM cell proliferation and growth via the AKT/mTOR pathway.
Discussion
Chinese herbal medicine has become an alternative resource for the discovery of new antitumor drugs. Screening new effective drugs for the treatment of glioma with Chinese herbal medicine has become a research hotspot. PF, a monoterpene glucoside, extracted from the root of Paeonia lactiflora Pall, has various biological activities such as neuroprotection, repair injury, and anti-inflammatory, immunomodulatory, and anticancer effects. GBM refers to tumors derived from glial cells of the brain and is the most common primary intracranial malignant tumor. Currently, the clinical treatment of glioma includes maximum safe tumor resection and postoperative radiotherapy combined with chemotherapy and electric field therapy. The prognosis of glioma patients remains poor despite the use of individualized combined therapy. Therefore, new strategies for the treatment of GBM are urgently needed. In the present study, we demonstrated that PF exerts anti-glioma effects by regulating autophagy through the modulation of the AKT/mTOR pathway.
Cell proliferation is the most important feature in higher organisms, and the infinite proliferation potential of tumor cells is the hallmark of malignancy. PF suppresses the proliferation of prolactinoma cells22 and inhibits breast cancer cell proliferation by regulating Notch-1 signaling23. In this study, PF inhibited the growth of GBM cells in a dose-dependent manner.
Increased invasion and migration abilities, which are characteristics of malignant tumors, play a key role in the development of glioma. Therefore, inhibiting the invasion and migration of tumor cells is an effective treatment. PF inhibits the migration and invasion of liver cancer cells24. Consistent with previous reports, we showed that PF inhibits glioma cell migration and invasion.
The cell cycle is a basic process of cell life. Apoptosis is an autonomous extinction process regulated strictly by cell signals. The regulation of the cell cycle and apoptosis plays a vital role in maintaining homeostasis in the body. Therefore, blocking tumor cell cycle progression and inducing tumor cell apoptosis are the objectives of tumor therapy. PF induces apoptosis and cycle arrest in B-cell acute lymphoblastic leukemia cells and osteosarcoma cells25,26. In this study, PF caused cell cycle arrest in G2/M phase by downregulating cyclin B1 and CDK1.
Autophagy refers to the process by which damaged, denatured, or senescent macromolecules are degraded by lysosomes in response to external stimuli as well as the self-digestion of organelles. Normally, autophagy is considered as a self-protection mechanism of cells, which plays an important role in the regulation of cell survival and cell death. However, excessive upregulation of autophagy-related genes can also cause cell death. PF activates the autophagy-lysosome pathway and promotes α-synuclein degradation in the treatment of Parkinson’s disease27. PF regulates bone formation by regulating the AKT-mTOR autophagy pathway28. In this study, PF upregulated the expression of LC3B and downregulated P62, suggesting that PF can regulate autophagy of glioma cells. However, the underlying mechanism remains unclear.
The AKT/ mTOR signaling pathway is involved in the process of autophagy29,30,31 and regulates tumor development32. Li et al. reported that PF regulates the AKT/mTOR pathway33. In this study, PF inhibited phosphorylated AKT and mTOR in glioma cells. Treatment with SC79 (an AKT activator) reversed the effect of PF on the AKT/mTOR pathway and LC3B. These findings suggest that the mechanism by which PF inhibits GBM cell proliferation involves the AKT/mTOR pathway and the modulation of autophagy. PF can both enhance and inhibit autophagy. PF protects against intestinal ischemia/reperfusion by decreasing apoptosis and promoting autophagy34. However, Carvedilol inhibits autophagy and promotes apoptosis in hepatic stellate cells35. These studies indicate that both autophagy and apoptosis are involved in the anti-glioma mechanism of PF.
This study represents an extension of our previous work on Arctigenin, further exploring natural compounds that target the AKT/mTOR pathway and induce autophagy in glioblastoma. While our findings with Paeoniflorin (PF) provide additional mechanistic insights and broaden the preclinical evidence base, we acknowledge that the core signaling axis investigated—AKT/mTOR—is similar to our earlier study. This overlap may limit the perceived novelty of the present work.
Although both Paeoniflorin (PF) and Arctigenin have been shown to inhibit glioblastoma proliferation via suppression of the AKT/mTOR signaling pathway and induction of autophagy, several distinctions underscore the novelty and additional value of the present study. First, PF exerts broader biological effects, as evidenced by its ability to modulate apoptosis- and cell cycle-related proteins, including Bcl-2, Bax, cleaved caspase-3, cyclin B1, and CDK1. These markers were not evaluated in our previous study on Arctigenin, and their inclusion here provides a more comprehensive understanding of PF’s anti-glioblastoma mechanisms. Second, we employed more rigorous approaches to validate autophagy in this study, including LC3B-GFP plasmid transfection and transmission electron microscopy (TEM), offering ultrastructural and dynamic evidence of autophagosome formation. These advanced methods go beyond the techniques used in our prior work. Third, PF differs from Arctigenin in its pharmacological profile: PF is a principal bioactive compound in Paeonia lactiflora, with well-established clinical usage, a favorable safety profile, and documented blood–brain barrier permeability. These properties suggest stronger translational potential for PF in glioblastoma therapy. Collectively, these distinctions indicate that PF not only shares a similar signaling axis with Arctigenin but also offers new mechanistic insights and preclinical evidence, thereby enriching the therapeutic landscape of glioblastoma treatment.
PF is well tolerated in vivo11,36. Crossing the blood-brain barrier is a prerequisite for the treatment of GBM. PF can penetrate the blood-brain barrier to reach the hippocampus and maintain a certain concentration in rats37. One limitation of this study is that the effect of PF was not verified using animal experiments. This will be addressed in future studies. In conclusion, we showed that PF regulates autophagy at least partly by regulating the AKT/mTOR pathway. However, the data do not exclude the possibility that other molecules or pathways are involved in controlling the autophagy of GBM cells in response to PF stimulation.
PF suppresses GBM cell proliferation. (a, b) A CCK-8 assay was used to detect the viability of U87 and U118 cells at different time points and in the presence of different doses of PF. (c, d) U87 and U118 cells were cultured for 15 days after treatment with different doses of PF and then stained with 0.1% crystal violet. Colonies with > 50 cells were counted. ∗P < 0.05 vs. the control group. Data are presented as the mean ± SD. (n = 3)
PF suppresses GBM cell migration and invasion. (a–f) Wound-healing and Transwell assays were performed to detect U87 and U118 cell migration and invasion after treatment with different doses of PF for 24 h. (Bar, 100 μm) ∗P < 0.05 vs. the control. Data are presented as the mean ± SD. All experiments were repeated three times. (g) MMP2/9 protein expression was evaluated by western blotting in cells treated with different concentrations of PF for 24 h. Data are presented as the mean ± SD. ∗P < 0.05 vs. the control group. (n = 3)
PF regulates GBM cell apoptosis and cell cycle progression. (a, b) Effect of PF on GBM cell apoptosis and quantitative analysis. (c, d) Effect of PF on cell cycle progression. The percentage of cells in each cell cycle phase is shown. (e) Effect of PF on Bcl-2, Bax, cleaved caspase-3, cyclin B1 and CDK1. Data are presented as the mean ± SD of three independent experiments. (n = 3)
PF enhances autophagic activity in GBM cells. (A) Representative transmission electron microscopy images illustrating autophagosomes (red arrows) and (B) semi-quantitative analysis of autophagosomes in each group (scale bar = 100 nm; magnification, ×50000 and ×70,000) (n = 5). (C) U87 and U118 cells were transfected with LC3B-GFP plasmids and the LC3B-GFP puncta were observed under a fluorescence microscope. (Bar, 100 μm) (D) Number of LC3B-GFP puncta per cell. (E–F) U87 and U118 cells were treated with different concentrations of PF, and whole cell lysates were subjected to LC3B-II and P62 immunoblotting. β-Actin was used as the control. Data are presented as the mean ± SD. ∗P < 0.05 vs. the control group. (n = 3)
PF regulates the AKT/mTOR pathway. (a) U87 and U118 cells were treated with different concentrations of PF for 24 h, and the protein expression levels of mTOR, AKT, phosphorylated mTOR, and phosphorylated AKT were analyzed; β-actin was used as the control. (b) ImageJ software was used for statistical analysis of the western blotting results. Data are presented as the mean ± SD. ∗P < 0.05 vs. the control group; #P < 0.05, PF + SC79 group vs. the PF group. (n = 3)
The AKT agonist SC79 reversed the regulatory effect of PF on AKT/mTOR pathway and autophagy of GBM cells. GBM cells were pretreated with the AKT agonist SC79 to analyze the regulation of the AKT/mTOR pathway and autophagy by PF. (A) Western blot analysis of the AKT/mTOR pathway. B. Quantitative analysis was conducted to analyze the results depicted in (B). (C) Western blot analysis of autophagy markers. (D) Quantitative analysis was conducted to analyze the results depicted in (C). Data are presented as the mean ± SD. ∗P < 0.05 vs. the control group; #P < 0.05, PF + SC79 group vs. the PF group. (n = 3)
The AKT agonist SC79 reverses the PF-induced activation of autophagy. (A) U87 and U118 glioma cells were transfected with LC3B-GFP plasmids, and LC3B puncta formation was assessed by fluorescence microscopy. Representative images of LC3B-GFP puncta in each treatment group are shown. (B) Quantification of LC3B puncta per cell from (A). Data are presented as the mean ± SD. (Bar, 100 μm).∗P < 0:05 vs. the control group;#P < 0.05, PF + SC79 group vs. the PF group. (n = 3)
Data availability
The datasets used and analyzed during the current study are available from the corresponding author, Ruen Liu, upon reasonable request. Please contact Ruen Liu at liuruen@pku.edu.cn for data access.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Mishchenko, T. A. et al. Far-Red fluorescent murine glioma model for accurate assessment of brain tumor progression. Cancers (Basel). 14. https://doi.org/10.3390/cancers14153822 (2022).
Liu, D. et al. Nanotechnology Meets glioblastoma multiforme: emerging therapeutic strategies. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. e1838 https://doi.org/10.1002/wnan.1838 (2022).
Wang, X. Z. et al. The multifaceted mechanisms of Paeoniflorin in the treatment of tumors: State-of-the-Art. Biomed. Pharmacother. 149, 112800. https://doi.org/10.1016/j.biopha.2022.112800 (2022).
Han, X. et al. Paeoniflorin ameliorates airway inflammation and immune response in ovalbumin induced asthmatic mice: from oxidative stress to autophagy. Phytomedicine 96, 153835. https://doi.org/10.1016/j.phymed.2021.153835 (2022).
Wang, L. et al. The neuroprotective effects of Paeoniflorin against MPP(+)-induced damage to dopaminergic neurons via the Akt/Nrf2/GPX4 pathway. J. Chem. Neuroanat. 122, 102103. https://doi.org/10.1016/j.jchemneu.2022.102103 (2022).
Chen, Y. F., Wu, K. J. & Wood, W. G. Paeonia lactiflora extract attenuating cerebral ischemia and arterial intimal hyperplasia is mediated by Paeoniflorin via modulation of VSMC migration and Ras/MEK/ERK signaling pathway. Evid. Based Complement. Alternat Med. 2013 (482428). https://doi.org/10.1155/2013/482428 (2013).
Ouyang, J. et al. Paeoniflorin exerts antitumor effects by inactivating S phase kinase-associated protein 2 in glioma cells. Oncol. Rep. 39, 1052–1062. https://doi.org/10.3892/or.2017.6175 (2018).
Yu, G. et al. Paeoniflorin Inhibits Hepatocyte Growth Factor- (HGF-) Induced Migration and Invasion and Actin Rearrangement via Suppression of c-Met-Mediated RhoA/ROCK Signaling in Glioblastoma. Biomed Res Int 9053295, (2019). https://doi.org/10.1155/2019/9053295 (2019).
Nie, X. H. et al. Paeoniflorin inhibits human glioma cells via STAT3 degradation by the ubiquitin-proteasome pathway. Drug Des. Devel Ther. 9, 5611–5622. https://doi.org/10.2147/dddt.S93912 (2015).
Gao, Z. W. et al. Paeoniflorin elicits the anti-proliferative effects on glioma cell via targeting translocator protein 18 KDa. J. Pharmacol. Sci. 145, 115–121. https://doi.org/10.1016/j.jphs.2020.10.004 (2021).
Zhang, J. & Ney, P. A. Autophagy-dependent and -independent mechanisms of mitochondrial clearance during reticulocyte maturation. Autophagy 5, 1064–1065. https://doi.org/10.4161/auto.5.7.9749 (2009).
Al-Bari, M. A. A. & Xu, P. Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann. N Y Acad. Sci. 1467, 3–20. https://doi.org/10.1111/nyas.14305 (2020).
Parzych, K. R. & Klionsky, D. J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 20, 460–473. https://doi.org/10.1089/ars.2013.5371 (2014).
Kaushik, S., Massey, A. C., Mizushima, N. & Cuervo, A. M. Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol. Biol. Cell. 19, 2179–2192. https://doi.org/10.1091/mbc.e07-11-1155 (2008).
Komatsu, M. et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131, 1149–1163. https://doi.org/10.1016/j.cell.2007.10.035 (2007).
Lee, Y. & Weihl, C. C. Regulation of SQSTM1/p62 via UBA domain ubiquitination and its role in disease. Autophagy 13, 1615–1616. https://doi.org/10.1080/15548627.2017.1339845 (2017).
Gorman, L. M. et al. Phylogenetic analysis of cell-cycle regulatory proteins within the symbiodiniaceae. Sci. Rep. 10, 20473. https://doi.org/10.1038/s41598-020-76621-1 (2020).
Pillon, A., Dare-Shih, J., Fong, J., Fidalgo da Silva, E. & Porter, L. A. Dissecting the roles of the tuberin protein in the subcellular localization of the G2/M Cyclin, Cyclin B1. PLoS One. 17, e0272741. https://doi.org/10.1371/journal.pone.0272741 (2022).
Yang, H. et al. Histocompatibility minor 13 (HM13), targeted by miR-760, exerts oncogenic role in breast cancer by suppressing autophagy and activating PI3K-AKT-mTOR pathway. Cell. Death Dis. 13, 728. https://doi.org/10.1038/s41419-022-05154-4 (2022).
Ma, X., Ren, H., Zhang, Y., Wang, B. & Ma, H. LncRNA RHPN1-AS1 Inhibition induces autophagy and apoptosis in prostate cancer cells via the miR-7-5p/EGFR/PI3K/AKT/mTOR signaling pathway. Environ. Toxicol. https://doi.org/10.1002/tox.23656 (2022).
Wei, Y., Zhou, X., Ren, L., Wang, C. & Li, Y. The prolactin-release inhibitor Paeoniflorin suppresses proliferation and induces apoptosis in prolactinoma cells via the mitochondria-dependent pathway. J. Cell. Biochem. 119, 5704–5714. https://doi.org/10.1002/jcb.26752 (2018).
Zhang, J. et al. Paeoniflorin influences breast cancer cell proliferation and invasion via Inhibition of the Notch–1 signaling pathway. Mol. Med. Rep. 17, 1321–1325. https://doi.org/10.3892/mmr.2017.8002 (2018).
Liu, H., Zang, L., Zhao, J., Wang, Z. & Li, L. Paeoniflorin inhibits cell viability and invasion of liver cancer cells via Inhibition of Skp2. Oncol. Lett. 19, 3165–3172. https://doi.org/10.3892/ol.2020.11424 (2020).
Jin, L. B. et al. Paeoniflorin induces G2/M cell cycle arrest and caspase-dependent apoptosis through the upregulation of Bcl-2 X-associated protein and downregulation of B-cell lymphoma 2 in human osteosarcoma cells. Mol. Med. Rep. 17, 5095–5101. https://doi.org/10.3892/mmr.2018.8464 (2018).
Qin, X. et al. Paeoniflorin induces apoptosis and cycle arrest in B-cell acute lymphoblastic leukemia cells by inhibiting SENP1/c-Myc signaling pathway]. Zhongguo Zhong Yao Za Zhi. 47, 3312–3319. https://doi.org/10.19540/j.cnki.cjcmm.20220309.401 (2022).
Sun, X. et al. ASICs mediate the modulatory effect by Paeoniflorin on α-synuclein autophagic degradation. Brain Res. 1396, 77–87. https://doi.org/10.1016/j.brainres.2011.04.011 (2011).
Yang, L. et al. Paeoniflorin attenuates Dexamethasone-Induced apoptosis of osteoblast cells and promotes bone formation via regulating AKT/mTOR/Autophagy signaling pathway. Evid. Based Complement. Alternat Med. 2021 (6623464). https://doi.org/10.1155/2021/6623464 (2021).
Xu, M. et al. Mycoplasma Bovis inhibits autophagy in bovine mammary epithelial cells via a PTEN/PI3K-Akt-mTOR-dependent pathway. Front. Microbiol. 13, 935547. https://doi.org/10.3389/fmicb.2022.935547 (2022).
He, Y. et al. Effects of 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione on autophagy and the PI3K/AKT/mTOR signaling pathway in human cholangiocarcinoma QBC939 cells. J. Gastrointest. Oncol. 13, 1423–1432. https://doi.org/10.21037/jgo-22-298 (2022).
Jalouli, M. et al. Allethrin promotes apoptosis and autophagy associated with the oxidative Stress-Related PI3K/AKT/mTOR signaling pathway in developing rat ovaries. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms23126397 (2022).
Sousa, D., Pereira, S. S. & Pignatelli, D. Modulation of autophagy in adrenal tumors. Front. Endocrinol. (Lausanne). 13, 937367. https://doi.org/10.3389/fendo.2022.937367 (2022).
Li, P. P. et al. BAFF/BAFF-R involved in antibodies production of rats with collagen-induced arthritis via PI3K-Akt-mTOR signaling and the regulation of Paeoniflorin. J. Ethnopharmacol. 141, 290–300. https://doi.org/10.1016/j.jep.2012.02.034 (2012).
Wen, J. et al. Paeoniflorin protects against intestinal ischemia/reperfusion by activating LKB1/AMPK and promoting autophagy. Pharmacol. Res. 146, 104308. https://doi.org/10.1016/j.phrs.2019.104308 (2019).
Meng, D. et al. Carvedilol attenuates liver fibrosis by suppressing autophagy and promoting apoptosis in hepatic stellate cells. Biomed. Pharmacother. 108, 1617–1627. https://doi.org/10.1016/j.biopha.2018.10.005 (2018).
Wang, Z. et al. Paeoniflorin inhibits migration and invasion of human glioblastoma cells via suppression transforming growth factor β-Induced Epithelial-Mesenchymal transition. Neurochem Res. 43, 760–774. https://doi.org/10.1007/s11064-018-2478-y (2018).
He, X. et al. Determination of Paeoniflorin in rat hippocampus by high-performance liquid chromatography after intravenous administration of paeoniae radix extract. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 802, 277–281. https://doi.org/10.1016/j.jchromb.2003.11.040 (2004).
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (Grant No. 82074174).
Author information
Authors and Affiliations
Contributions
Haima Li: Study design, Experiment Execution, data analysis, Writing the initial draft of the article.Jia Ouyang: Study design , Statistical analysis and revise the manuscript.Yan Zhang: Statistical analysis, data analysis, supervised data, revise the manuscript. Zihao Zhang: Statistical analysis, data analysis, supervised data, revise the manuscript.Mingyang Sun: Statistical analysis, supervised data, revise the manuscript.Ruen Liu: Study design, revise the manuscript, Provide funding.Chao Qian: Study design, revise the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, H., Ouyang, J., Zhang, Y. et al. Paeoniflorin inhibits glioblastoma proliferation and promotes autophagy through the AKT/mTOR pathway. Sci Rep 15, 37897 (2025). https://doi.org/10.1038/s41598-025-21772-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-21772-2