Abstract
This study compared liposomal bupivacaine (LB) and conventional bupivacaine hydrochloride (BH) for postoperative pain management among 50 female patients undergoing uniportal thoracoscopic lung surgery. The patients were randomized to LB (n = 25) or BH (n = 25) groups based on the intercostal nerve blocks they received post-surgery. Outcomes included intraoperative vital signs, pain scores (static/dynamic visual analog scale [VAS]), and adverse reactions (nausea, vomiting, and dizziness) assessed at 6, 24, 48, and 72 h. Intraoperative vital signs (heart rate [HR], blood pressure, oxygen saturation, and bispectral index) and anesthetic/vasoactive drug administration exhibited no significant differences between groups. Postoperatively, the LB group demonstrated superior short-term analgesia, with significantly lower static and dynamic VAS scores at 6 and 24 h in the LB group compared to the BH group [3(3,4) versus 4(4,4); 4(4,4) versus 5(4,6)] (p < 0.05). Between 48 and 72 h, pain scores equalized between groups. Additionally, LB reduced nausea incidence after 24 h (p < 0.05), with no severe adverse reactions reported in either group. Trend analysis revealed peak pain and adverse reactions at 24 h, which subsequently declined in both groups. Although both anesthetics exhibited similar safety profiles, LB offered enhanced early pain relief and fewer nausea-related adverse effects. These findings suggest that LB improves short-term postoperative pain management without compromising safety. Further studies should explore its cost-effectiveness and long-term outcomes.
Similar content being viewed by others
Introduction
Thoracoscopic surgery has recently gained widespread adoption in the treatment of lung diseases, especially for early-stage non-small cell lung cancer (NSCLC), because of its minimally invasive nature and favorable postoperative recovery outcomes1. Uniportal thoracoscopic surgery is associated with reduced intraoperative trauma, shorter operation, and hospital stay, and decreased postoperative complications compared to conventional open surgery. However, postoperative pain management remains a considerable challenge that affects patient recovery2. Postoperative pain substantially affects recovery rates and the patient’s quality of life, especially after thoracoscopic surgery, where severe chest wall pain is commonly observed3. A previous study demonstrated that approximately 80% of patients experience moderate to severe pain within the first 6 h after surgery4. Inadequate management of postoperative pain can compromise patient comfort and result in severe complications, including atelectasis, hypoxemia, and pulmonary infections5. Consequently, developing effective pain management strategies is essential for ensuring optimal postoperative recovery.
Bupivacaine, a commonly utilized local anesthetic, is essential in postoperative pain management across several surgical procedures6. Bupivacaine effectively impedes nerve impulse conduction by inhibiting sodium channels in peripheral nerves, thereby providing local anesthesia7. Although effective, the duration of action of conventional bupivacaine hydrochloride is limited, frequently necessitating supplementary analgesia postoperatively to ensure adequate pain management8. Furthermore, conventional bupivacaine has been associated with adverse effects in some patients, including nausea, vomiting, and hypotension, highlighting the need to address issues related to analgesia duration and drug safety9. Liposomal bupivacaine, an innovative long-acting local anesthetic, has recently garnered research interest for postoperative pain management. Unlike conventional bupivacaine, liposomal bupivacaine employs liposomal technology to encapsulate the drug within lipid vesicles, enabling its gradual release over an extended period10. A previous study demonstrated that liposomal bupivacaine offers superior postoperative analgesia for various surgical procedures. It is characterized by extended pain relief, reduced postoperative opioid consumption, and fewer adverse effects11. These attributes render it an ideal option for long-term postoperative pain management.
Postoperative pain management in female patients has distinct challenges12. Previous studies have demonstrated that women typically experience more intense postoperative pain compared to men due to a combination of physiological, psychological, and social factors13,14. Physiologically, hormonal fluctuations, including changes in estrogen and progesterone levels, can affect pain tolerance in women. For instance, pain perception and responsiveness in women vary significantly during the menstrual cycle15. Furthermore, women exhibit different neural mechanisms in pain transmission compared to men, leading to increased susceptibility to chronic and persistent postoperative pain16. Women are psychologically more susceptible to experiencing anxiety and depression postoperatively, which can exacerbate pain perception17. Additionally, social and cultural norms influence these disparities. Societal norms may lead to women underreporting pain or prematurely discontinuing pain management, leading to inadequate pain control18.
This highlights the need for safer and more effective postoperative analgesic treatment specifically tailored for female patients. Liposomal bupivacaine, with its longer analgesia and fewer postoperative adverse effects, may solve these challenges. However, despite its potential benefits, the efficacy of liposomal bupivacaine in female patients, especially for thoracoscopic surgery, remains insufficiently investigated. Accordingly, this study aimed to compare the analgesic efficacy and safety of liposomal bupivacaine and conventional bupivacaine hydrochloride in female patients undergoing uniportal thoracoscopic lung surgery to provide new insights for enhancing postoperative pain management in this population.
Methods
Study participants
This study included 50 female patients who underwent uniportal thoracoscopic lung surgery at the First Affiliated Hospital of Guangxi Medical University between January 2024 and August 2024. The inclusion criteria were as follows: (1) Female patients aged between 18 and 65 years; (2) female patients who met the clinical indications for thoracoscopic lung surgery and were willing to undergo the procedure; (3) female patients eligible for general anesthesia; (4) female patients who voluntarily agreed to participate in the study and signed informed consent. The exclusion criteria were as follows: (1) Female patients with severe cardiovascular, hepatic, or renal dysfunction or other severe systemic diseases; (2) female patients allergic to bupivacaine or other local anesthetics; (3) female patients with a history of thoracoscopic or other related thoracic surgeries; (4) female patients with a history of severe postoperative or anesthesia-related complications; (5) female patients with a history of chronic neuropathic pain; (6) patients who were pregnant, breastfeeding or menstruating at the time of the surgery. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (No. 2023-S572-01), and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki (2013).
Study design
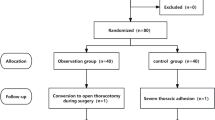
This study employed a random number table to allocate patients randomly to two groups: the liposomal bupivacaine group (LB group, n = 25) and the conventional bupivacaine hydrochloride group (BH group, n = 25). Preoperatively (day 0), the patients were screened based on the inclusion and exclusion criteria, and corresponding numbers were assigned based on the random number table. On the surgery day, an assistant who was unaware of the study protocols drew the designated number from an envelope and prepared the anesthetic agents according to the assignment. An independent nerve blocker who was not involved in the anesthesia procedure and postoperative assessment was informed of the group allocation. The nerve blocker prepared the drugs required for the study based on the randomization results and independently performed the nerve block after the administration of anesthesia. All patients, surgeons, anesthesiologists, and follow-up staff were blinded to group allocation, and statistical analysis was conducted by a researcher who was unaware of the groupings.
Anesthesia management and monitoring
All patients were directed to fast from food for 8 h and from fluids for 4 h before surgery. Upon entering the operating room, a peripheral intravenous line was established, and Ringer’s solution was infused. The patient’s position was adjusted, and anesthesia monitoring commenced. The monitoring parameters included non-invasive blood pressure, electrocardiogram, pulse oxygen saturation (SpO2), bispectral index (BIS), respiratory rate, and end-tidal carbon dioxide (PETCO2). All patients received oxygen using a nasal cannula with end-tidal CO2 monitoring (100% O2, 3 L/min). After preparing all anesthesia equipment, anesthetic drugs, and emergency medications, anesthesia induction commenced. The appropriate dose of liposomal bupivacaine or conventional bupivacaine hydrochloride was calculated based on the patient’s weight and clinical condition. Immediately post-surgery, intercostal nerve blocks were performed, targeting the third to eighth intercostal spaces on the operated side (comprising six injection sites). All surgeries were performed by a team of experienced surgeons to minimize technical discrepancies. Patients in the LB group received local injections of liposomal bupivacaine (20 mL: 266 mg) under thoracoscopic guidance. We injected 266 mg of bupivacaine liposomes into the thoracic cavity according to the established protocol. The solution was injected in equal volume into the third to eighth intercostal spaces. The bupivacaine hydrochloride group received conventional bupivacaine hydrochloride (5 mL: 37.5 mg) injections. Each injection was administered in a dose of 2 mL, with regular aspiration to verify blood return, hence minimizing the risk of inadvertent intravascular injection. The anesthesia protocol comprised propofol 0.4 mg/kg, sufentanil 0.4–0.6 µg/kg, and rocuronium 0.6 mg/kg for induction, and maintenance with propofol 3–5 mg/kg/h, remifentanil 0.1–0.2 µg/kg/min, and rocuronium 5–10 µg/kg/min. The dosages of propofol and remifentanil were adjusted during the procedure based on blood pressure, HR, and BIS, and intermittent intravenous sufentanil was administered as needed to maintain stable vital signs and BIS values between 40 and 60. Postoperative analgesia was administered by intravenous pentazocine 30 mg and patient-controlled analgesia (PCA) comprising sufentanil 50 µg, pentazocine 60 mg, ondansetron 16 mg, and 0.9% saline to a volume of 300 mL, with a background rate of 2 mL/h and a patient-controlled bolus dose of 6 mL/h, with a lockout interval of 15 min. Adverse reactions during anesthesia, including respiratory depression, bradycardia, hypertension, hypotension, allergic reactions, and severe pleural reactions, were recorded and treated promptly as per protocol.
Data collection
Collected intraoperative data included vital signs, the amount of anesthetic drugs used, and the volume of vasoactive medications administered. Postoperative data included VAS and NRS pain scores at rest and during movement at 6, 24, 48, and 72 h after surgery, and the occurrence of adverse reactions (nausea, vomiting, urinary retention, orthostatic dizziness, non-orthostatic dizziness, and local anesthetic allergy) at 6, 24, 48, and 72 h after surgery.The original data of this study can be found in Table S1.
Statistical analysis
The sample size was calculated based on the primary outcome: the 24-h VAS pain score. According to previous research, the VAS scores for 24-h pain were 4.55 ± 0.52 and 5.36 ± 1.03, respectively. Assuming a type I error rate (α) of 0.05, a type II error rate (β) of 0.2, and accounting for a 10% loss to follow-up, a minimum of 18 participants per group was required to achieve sufficient statistical power.
The statistical package for the social sciences software (version 27.0) was utilized for data analysis. Normality tests were performed for all measurement data, with normally distributed data presented as mean ± standard deviation (SD). Inter-group comparisons were conducted using independent sample t-tests, and within-group comparisons were performed using repeated measures analysis of variance (ANOVA). For non-normally distributed data, results were presented as median (P25, P75), and inter-group comparisons were conducted using the Mann–Whitney U test. Repeated measures ANOVA was utilized for continuous data following a normal distribution. Categorical data were expressed as frequency and percentage (%), and comparisons between groups were performed using the chi-square test. A p < 0.05 was considered statistically significant.
Results
Intraoperative vital signs monitoring
The vital signs (invasive blood pressure [IBP], HR, peripheral oxygen saturation [SpO2], and BIS) of patients in both groups were monitored before injection of liposomal bupivacaine or bupivacaine hydrochloride, 30 min post-injection, and at the end of the surgery. The comparison revealed no statistically significant variations in vital signs between the two groups before and after nerve block (all p > 0.05). Additionally, no significant differences were observed in the consumption of sufentanil, remifentanil, and the administration of vasoactive drugs during surgery between the two groups (all p > 0.05). Table 1 presents the results.
Postoperative pain control and complications comparison
The static VAS scores at 6 and 24 h postoperatively were significantly lower in the LB group compared to the BH group (p < 0.05). At 48 and 72 h postoperatively, no significant difference was observed in static VAS scores between the two groups. For dynamic VAS and NRS scores at 6, 24, and 48 h postoperatively, the LB group exhibited significantly lower scores than the BH group (p < 0.05). However, no significant differences were observed in the static VAS, dynamic VAS, and NRS scores at 72 h postoperatively (p > 0.05). These results are presented in Tables 2, 3 and 4.
When comparing postoperative adverse reactions between LB and BH groups, the number of patients experiencing nausea at 24 h postoperatively was significantly lower in the LB group than in the BH group (p < 0.05). No cases of allergic reactions to local anesthetics were reported in either group. Table 5 presents these findings.
In both groups, static VAS, dynamic VAS, and NRS scores exhibited an upward trend within the first 24 h postoperatively, peaking at 24 h, followed by a downward trend thereafter. The highest number of postoperative adverse reactions in both groups was observed within the first 24 h, with a marked reduction in adverse reactions after 24 h.
Trends in postoperative pain control and complications between LB and BH groups
Herein, significant differences were observed between the LB and BH groups based on postoperative pain control and complications. Static VAS scores were higher at 6 h post-surgery and gradually decreased over time. The LB group consistently exhibited lower scores than the BH group, with a significant difference observed at 24 h post-surgery (Fig. 1a). Regarding dynamic VAS scores, the LB group exhibited lower scores at 6 h post-surgery, and the scores improved over time. However, the BH group’s scores peaked at 24 h post-surgery, followed by a decrease. At 48 and 72 h, the LB group exhibited significantly lower scores during deep breathing, cough, and ambulation than the BH group (Figs. 1c–e), indicating better control of movement-related pain in the LB group. NRS scores decreased at 6, 24, 48, and 72 h, with a peak at 24 h post-surgery. Afterward, the scores declined more significantly, with the LB group maintaining significantly lower NRS scores at all time points than the BH group (Fig. 1b). PCA drug residuals gradually decreased over time, and the LB group exhibited a significantly faster decline in drug residuals than the BH group. At 72 h post-surgery, the LB group exhibited significantly lower drug residuals than the BH group (Fig. 1f), indicating reduced analgesic drug requirements in the LB group. Nausea reached its peak at 24 h post-surgery and gradually decreased. The LB group exhibited significantly lower nausea scores compared to the BH group (Fig. 1g), indicating that the LB group was more effective in reducing nausea.
Comparison of postoperative pain control and complications between LB and BH groups. The trends in static VAS scores (a), NRS scores (b), dynamic VAS scores for deep breathing (c), coughing (d), and ambulation (e), PCA drug residuals (f), and nausea (g) are indicated at postoperative 6, 24, 48, and 72 h. Statistical significance is indicated by *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Discussion
Recent studies on postoperative pain management after thoracic surgery have predominantly focused on the implementation of multimodal analgesia and personalized management strategies19,20,21. Peripheral nerve blocks, a form of regional analgesia, have demonstrated considerable benefit in alleviating postoperative pain, diminishing opioid administration, and reducing the risk of complications22. Effective postoperative pain management improves patient compliance with postoperative functional exercises and reduces the incidence of complications, including pulmonary infections, atelectasis, and deep vein thrombosis, thereby expediting recovery23. Furthermore, it reduces the incidence of chronic pain, mitigates the risk of drug dependence and related adverse effects (including nausea, constipation, and respiratory depression) associated with opioid use, and improves patient satisfaction during hospitalization24.
This study demonstrated that in female patients undergoing single-port thoracoscopic lung surgery, the administration of liposomal bupivacaine correlates with reduced pain scores within 48 h postoperatively, compared to the use of conventional bupivacaine hydrochloride for intercostal nerve blocks. This indicates that liposomal bupivacaine may provide enhanced analgesic effects during the early postoperative period. The mechanisms underlying the analgesic effects of liposomal bupivacaine and bupivacaine hydrochloride at various postoperative time intervals require additional investigation. The microencapsulation technology of liposomal bupivacaine facilitates the sustained distribution of continuous, dynamic analgesic coverage, maintaining effective local anesthetic concentrations in the neuro-rich thoracic region. Compared to conventional bupivacaine hydrochloride, it possessed an enhanced capacity to deliver pain relief within 24 h of administration. The pharmacokinetics of liposomal bupivacaine exhibit a biphasic pattern, with an initial peak occurring within 1 h of administration, followed by a second peak approximately 12–36 h later25. Meanwhile, compared with conventional bupivacaine hydrochloride, liposomal bupivacaine demonstrated a reduced peak plasma concentration, attributable to its sustained-release characteristics. Bupivacaine hydrochloride exhibited a higher peak plasma concentration and an increased propensity to induce early central nervous system toxicity symptoms. Subsequently, drug concentrations begin to diminish. This phenomenon may serve as the underlying mechanism responsible for its postoperative analgesic efficacy and safety. A review by Dinges HC et al.10, comprising 23 randomized controlled trials, revealed a significant decrease in average pain scores at 24 h postoperatively in the liposomal bupivacaine cohort. Furthermore, Corsini EM et al.26 conducted a long-term follow-up study on patients with early-stage non-small cell lung cancer, demonstrating that liposomal bupivacaine effectively reduces the incidence of postoperative complications, including pneumonia and acute respiratory distress syndrome. Additionally, a retrospective study by Wayne B. Bauerl et al.27 demonstrated that the Enhanced Recovery After Surgery protocol, combined with liposomal bupivacaine nerve blocks, significantly reduces opioid consumption. Liposomal bupivacaine offers enhanced analgesic efficacy in pain-sensitive female patients compared to conventional local anesthetics and significantly reduces the incidence of postoperative complications and opioid usage. The incidence of adverse reactions was markedly lower in the LB group than in the BH group, indicating a superior safety profile for liposomal bupivacaine.
The U.S. Food and Drug Administration approved the LB injection suspension, known as EXPAREL, in 2011. Research indicates that it exhibits limited diffusion and minimal absorption into the lymphatic system and systemic circulation after local injection, thereby mitigating adverse reactions associated with elevated plasma concentrations28. Alan David Kaye et al.29 reported that EXPAREL offers prolonged analgesic effects, significantly enhancing postoperative pain management. Various international guidelines recommend the integration of liposomal bupivacaine into multimodal analgesia protocols to enhance patient recovery30. This study demonstrated that the utilization of liposomal bupivacaine for intercostal nerve blocks can more effectively alleviate early postoperative pain in female patients compared to conventional local anesthetics. The analgesic effect of bupivacaine liposomes in the early postoperative period is crucial for managing chronic postoperative pain. Severe acute postoperative pain is a well-established risk factor for chronic postsurgical pain (CPSP)31, as it can induce neuroplastic changes, including central sensitization—a process whereby repeated nociceptive input enhances pain perception and results in persistent hypersensitivity post-injury recovery32. By attenuating this acute pain, LB may disrupt the process of central sensitization, thereby reducing the risk of CPSP. This hypothesis will be validated in our future research. LB is associated with a lower incidence of nausea reaction, indicating that its administration to female patients may be associated with a lower degree of risk. The findings advocate for the incorporation of liposomal bupivacaine into personalized treatment plans to enhance recovery in female patients undergoing thoracic surgery, thereby offering new insights into postoperative pain management.
This study has some limitations: (1) the relatively small sample size limited the generalizability of the results, necessitating larger, multicenter clinical trials to enhance the reliability and applicability of the findings. (2) The limited follow-up period hindered a comprehensive comparison of pain score trends between LB and BH groups. Consequently, there is an absence of reliable evidence regarding the long-term analgesic efficacy of liposomal bupivacaine following surgical interventions. (3) Individual patient differences, including psychological status, pain tolerance, and previous medication history, which could influence analgesic efficacy, were not considered. (4) Different resection methods of single-port thoracoscopic partial lung resection may exert different degrees of effect on postoperative pain, complicating the comparison of primary outcomes. These factors might have confounded the results; therefore, future research should implement more rigorous controls for these potential confounding variables.
Conclusion
This study demonstrated that LB offers superior early postoperative analgesic effects compared to conventional BH for female patients undergoing thoracoscopic lung surgery. LB is associated with a lower incidence of postoperative nausea. This evidence enhances postoperative pain management strategies for female patients.
Data availability
Data is available from the corresponding author on reasonable request.
References
Pan, J. M., Watkins, A. A., Stock, C. T., Moffatt-Bruce, S. D. & Servais, E. L. The surgical renaissance: Advancements in Video-Assisted thoracoscopic surgery and Robotic-Assisted thoracic surgery and their impact on patient outcomes. Cancers (Basel). 16 (17), 3086 (2024).
Feray, S. et al. PROSPECT guidelines for video-assisted thoracoscopic surgery: A systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia 77 (3), 311–325 (2022).
Grosen, K., Laue Petersen, G., Pfeiffer-Jensen, M., Hoejsgaard, A. & Pilegaard, H. K. Persistent post-surgical pain following anterior thoracotomy for lung cancer: A cross-sectional study of prevalence, characteristics and interference with functioning. Eur. J. Cardiothorac. Surg. 43 (1), 95–103 (2013).
Gjeilo, K. H., Oksholm, T., Follestad, T., Wahba, A. & Rustøen, T. Trajectories of pain in patients undergoing lung cancer surgery: A longitudinal prospective study. J. Pain Symptom Manage. 59 (4), 818–828e1 (2020).
Soto, R. G. & Fu, E. S. Acute pain management for patients undergoing thoracotomy. Ann. Thorac. Surg. 75 (4), 1349–1357 (2003).
Piacherski, V. & Muzyka, L. Comparison of the efficacy of 0.5% isobaric bupivacaine, 0.5% levobupivacaine, and 0.5% hyperbaric bupivacaine for spinal anesthesia in lower limb surgeries. Sci. Rep. 13 (1), 2736 (2023).
Scholz, A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br. J. Anaesth. 89 (1), 52–61 (2002).
Deng, W. et al. Synthesis of nanocapsules blended polymeric hydrogel loaded with bupivacaine drug delivery system for local anesthetics and pain management. Drug Deliv. 29 (1), 399–412 (2022).
Peng, F. et al. Interaction between ropivacaine and a Self-Assembling peptide: A nanoformulation for Long-Acting analgesia. Int. J. Nanomed. 17, 3371–3384 (2022).
Dinges, H. C. et al. The analgesic efficacy of liposomal bupivacaine compared with bupivacaine hydrochloride for the prevention of postoperative pain: A systematic review and meta-analysis with trial sequential analysis. Reg. Anesth. Pain Med. 46 (6), 490–498 (2021).
Hamilton, T. W. et al. Efficacy of liposomal bupivacaine and bupivacaine hydrochloride vs bupivacaine hydrochloride alone as a periarticular anesthetic for patients undergoing knee replacement: A randomized clinical trial. JAMA Surg. 157 (6), 481–489 (2022).
Shamim Seth, U. et al. Postoperative analgesia in modified radical mastectomy patients after instillation of bupivacaine through surgical drains. Cureus 14 (4), e24125 (2022).
Tolver, M. A., Strandfelt, P., Rosenberg, J. & Bisgaard, T. Female gender is a risk factor for pain, discomfort, and fatigue after laparoscopic groin hernia repair. Hernia 17 (3), 321–327 (2013).
Sodhi, N., Qilleri, A., Aprigliano, C. & Danoff, J. R. One size does not fit all: Women experience more pain than men after total knee arthroplasty. J. Arthroplasty. 40 (4), 880–886 (2025).
Kvalem, I. L., Dahr Nygaard, I. M., Træen, B., Ivanova, A. & Dahlgren, C. L. Menstrual attitudes in adult women: A cross-sectional study on the association with menstruation factors, contraceptive use, genital self-image, and sexual openness. Womens Health (Lond). 20, 17455057241249553 (2024).
Eyring, J. B., Crandall, A. & Magnusson, B. M. A modified menstrual attitudes scale: heteronormative attitudes, Sexism, and attitudes toward menstruation in male and female adults. Arch. Sex. Behav. 52 (4), 1535–1547 (2023).
Miller, M. M. et al. Differential effect of patient weight on pain-Related judgements about male and female chronic low back pain patients. J. Pain. 19 (1), 57–66 (2018).
McCammon, E. et al. Exploring young women’s menstruation-related challenges in Uttar Pradesh, India, using the socio-ecological framework. Sex. Reprod. Health Matters. 28 (1), 1749342 (2020).
Manworren, R. C. Multimodal pain management and the future of a personalized medicine approach to pain. AORN J. 101(3): (2015). 307–318.
Nassif, G. J. & Miller, T. E. Evolving the management of acute perioperative pain towards opioid free protocols: A narrative review. Curr. Med. Res. Opin. 35 (12), 2129–2136 (2019).
Makkad, B. et al. Practice advisory for postoperative pain management of thoracic surgical patients: A report from the society of cardiovascular anesthesiologists. J. Cardiothorac. Vasc Anesth. 39 (5), 1306–1324 (2025).
Guerra-Londono, C. E. et al. Assessment of intercostal nerve block analgesia for thoracic surgery: A systematic review and Meta-analysis. JAMA Netw. Open. 4 (11), e2133394 (2021).
Montgomery, R. & McNamara, S. A. Multimodal pain management for enhanced recovery: Reinforcing the shift from traditional pathways through Nurse-Led interventions. AORN J. 104 (6S), S9–S16 (2016).
Sertcakacilar, G. et al. Regional anesthesia for thoracic surgery: A narrative review of indications and clinical considerations. J. Thorac. Dis. 14 (12), 5012–5028 (2022).
Hu, D., Onel, E., Singla, N., Kramer, W. G. & Hadzic, A. Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin. Drug Investig. 33 (2), 109–115 (2013).
Corsini, E. M. et al. Liposomal bupivacaine intercostal block is important for reduction of pulmonary complications. Ann. Thorac. Surg. 112 (2), 423–429 (2021).
Bauerle, W. B. et al. Impact of liposomal bupivacaine on enhanced recovery after surgery protocol for lung resection. Ann. Thorac. Surg. 119 (1), 219–226 (2025).
Knight, R. et al. Study of Peri-Articular anaesthetic for replacement of the knee (SPAARK): Study protocol for a patient-blinded, randomised controlled superiority trial of liposomal bupivacaine. Trials 20 (1), 732 (2019).
Kaye, A. D. et al. Exparel for postoperative pain management: A comprehensive review. Curr. Pain Headache Rep. 24 (11), 73 (2020).
Joshi, G. P., Hawkins, R. J., Frankle, M. A. & Abrams, J. S. Best practices for periarticular infiltration with liposomal bupivacaine for the management of pain after shoulder surgery: Consensus recommendation. J. Surg. Orthop. Adv. 25 (4), 204–208 (2016).
Chapman, C. R. & Vierck, C. J. The transition of acute postoperative pain to chronic pain: An integrative overview of research on mechanisms. J. Pain. 18 (4), 359e1–359e38 (2017).
Nijs, J. et al. Central sensitisation in chronic pain conditions: Latest discoveries and their potential for precision medicine. Lancet Rheumatol. 3 (5), e383–e392 (2021).
Acknowledgements
The authors cordially thank the participants.
Funding
This study was supported by the Beijing Hongyi Medical Development Foundation (HY20220037-A-44), Guangxi Key Research and Development Program (GuiKe-AB25069017), the Joint Project on Regional High-Incidence Diseases Research of Guangxi Natural Science Foundation (2024GXNSFAA010101, 2024GXNSFBA010032), the Youth Science Foundation of Guangxi Medical University (GXMUYSF202420), Guangxi Medical and Health Appropriate Technology Development and Promotion Application Project (S2021098), Guangxi medical and health appropriate technology development and popularization application project (S2022070), the National Key Clinical Specialty Construction Project, Guangxi Medical And Health Key Discipline Construction Project and Guangxi Key Clinical Specialty Construction Project.
Author information
Authors and Affiliations
Contributions
Dongsheng Lu, Zehao Huang and Huajian Peng, Jianji Guo wrote the main manuscript text, Zhanyu Xu, Nuo Yang prepared figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University [NO.2023-S572-01].
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lu, D., Huang, Z., Peng, H. et al. Clinical outcomes of liposomal and conventional bupivacaine in postoperative pain management in female patients following thoracoscopic lung surgery. Sci Rep 15, 40601 (2025). https://doi.org/10.1038/s41598-025-24214-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-24214-1