Abstract
Different levels of arterial occlusion pressure (AOP) can influence microcirculatory responses and autonomic nervous system activity, potentially affecting individuals undergoing training methods that incorporate AOP, such as blood flow restriction (BFR) training. Therefore, this study aimed to compare the effects of four distinct AOP levels on microcirculatory responses and autonomic nervous system activity—specifically investigating the relationship between post-occlusive reactive hyperemia (PORH), hemodynamic parameters, and heart rate variability (HRV) metrics. This prospective experimental study involved 30 healthy adults who underwent standardized assessments of heart rate variability (HRV) and post-occlusive reactive hyperemia (PORH) across four arterial occlusion pressure (AOP) levels (40, 80, 100, 130%). Measurements were conducted under controlled environmental conditions and consistent body positioning, enabling an analysis of microcirculatory and autonomic responses under progressive vascular occlusion. The results showed no significant changes in resting flow across AOP levels (p = 0.847), while all other parameters—biological zero, peak hyperemia, time to peak, average NN interval, standard deviation of NN intervals, and heart rate—exhibited significant differences between AOP conditions (all p < 0.001, except low frequency to high frequency ratio p < 0.05). However, for most variables, no significant differences were observed between 100 and 130% AOP (p > 0.999), indicating a possible stabilization of physiological responses at higher occlusion pressures. This study concludes that while increasing arterial occlusion pressure significantly affects multiple physiological parameters, responses tend to stabilize between 100 and 130% AOP, suggesting a threshold beyond which further increases yield minimal additional physiological impact.
Trial registration trial number ISRCTN15418049.
Similar content being viewed by others
Introduction
The investigation into arterial occlusion pressure (AOP) and its effects on both heart rate variability (HRV) and post-occlusion hyperemic responses (PORH) in the microvasculature has seen considerable advancements, especially in elucidating the body’s reactions after blood flow restriction1. For instance, a study aiming to assess HRV indices during high-load and low-load aerobic exercises with and without blood flow restriction (BFR), found that HRV on-kinetics were faster in low-load compared to low-load + BFR and high load, while recovery of HRV indices was delayed in high load compared to both low-load and low-load + BFR2. On the other hand, a study comparing acute autonomic and cardiovascular responses to low and high-load eccentric exercise with and without BFR in 60 men, found no significant differences between groups or interaction effects on cardiovascular variables or autonomic indices; although, an increase in vagal activity was observed during the recovery phase for both low load eccentric + BFR and high load eccentric + BFR, indicating that different loads of eccentric exercise with or without BFR did not cause autonomic or cardiovascular imbalance post-exercise3.
BFR training and ischemic preconditioning (IPC) are widely used in sports4,5. While muscle adaptations in BFR are typically linked to metabolite buildup and a low-oxygen environment6, studies on microcirculatory and sympathetic responses to different body positions7 and AOP are limited8. AOP, often monitored with a Doppler device, typically set between 40 and 80% of individual occlusion pressure values8 with higher AOP used in IPC likely eliciting more pronounced hyperemic responses compared to lower AOP in BFR protocols9. Despite the preliminary studies mentioned above analyzing HRV after AOP in BFR training2,3, research is still lacking on how different AOP levels impact HRV. Previous studies suggested that lower occlusion pressure leads to a more pronounced activation of the sympathetic system, which, in turn, influences HRV10,11. It is therefore expected that particular AOP values can dictate the extent and duration of the hyperemic response, suggesting a relationship between recovery and adaptation processes and the AOP levels employed12.
Post-occlusion hyperemic response (PORH) is a physiological phenomenon characterized by a transient increase in blood flow above baseline levels following the restoration of circulation after an occlusion13. This response is essential for regulating blood flow in tissues based on metabolic demands14, particularly in skeletal muscle during physical activity or flow restriction14,15. The response is governed predominantly by local, endothelium-dependent control within resistance vessels—rather than by direct autonomic commands—with endothelial pathways typically dominating as vessel caliber decreases16. In small arteries and arterioles, endothelium-derived hyperpolarizing factor (EDHF) signaling and activation of Ca2+-activated K+ channels are major mediators of the PORH vasodilation17,18. In human skin specifically, PORH may rely on sensory-nerve–driven axon-reflex contributions alongside endothelial hyperpolarization mechanisms19. Consistent with this, cutaneous PORH shows little or inconsistent involvement of nitric oxide and prostanoids compared with EDHF/sensory mechanisms20,21,22. In skeletal muscle beds, the early hyperemic peak reflects a combination of myogenic relaxation and metabolic vasodilator accumulation (e.g., adenosine), whereas nitric oxide and prostaglandins tend to modulate the later/sustained phase to a lesser degree23,24,25. Additional evidence in humans indicates a role for inwardly rectifying K+ (Kir) channels during reactive hyperemia26. Importantly, while sympathetic/parasympathetic outflow can shape baseline tone, the magnitude and time course of PORH are largely independent of sympathetic control, underscoring its predominantly local origin27,28.
These autonomic responses to occlusion appear to be pressure-dependent10,29,30. Higher AOP levels are associated with reduced HRV, likely due to increased sympathetic nervous system activity under prolonged ischemic conditions10,29,30. In contrast, lower AOP levels may facilitate parasympathetic reactivation, promoting improved HRV and cardiovascular recovery31. Additionally, the PORH response—characterized by a transient surge in blood flow following occlusion release—has been closely linked to the magnitude of AOP, as demonstrated by a study showing that higher occlusion pressures led to more pronounced increases in blood flow during the PORH response, suggesting a dose-dependent relationship32. This hyperemic response reflects the dynamic interplay between sympathetic and parasympathetic regulation, which is crucial for maintaining hemodynamic stability However, while systemic autonomic tone can modulate baseline hemodynamics, PORH itself is elicited predominantly by local endothelial and sensory-nerve mechanisms, with only a limited direct contribution from autonomic outflow to its immediate magnitude and time course17,19.
HRV serves as a valuable non-invasive marker for monitoring these autonomic shifts, helping to elucidate how varying AOP levels modulate cardiovascular control, tissue perfusion, and microcirculatory adaptation in BFR and IPC protocols. For instance, a study33 found that cycles of cuff inflation/deflation altered several spectral HRV indices during stress tests, such as increased very-low-frequency power and changes in detrended fluctuation analysis, indicating modulation of autonomic control linked with occlusion‐related perfusion events. Although established methods exist for assessing microcirculatory function and HRV, the scientific literature still lacks comprehensive comparisons of hyperemic and HRV responses across varying levels of AOP. Analyzing these variables may offer deeper information into the temporal and contextual factors that influence both HRV and post-occlusive hyperemia, particularly during BFR. Therefore, this study aimed to compare the effects of four different AOP levels (40, 80, 100, and 130%) on microcirculatory responses—including resting flow (RF), time to peak (TP), recovery time (TR), biological zero (BZ), and AOP itself—as well as autonomic nervous system activity, assessed via HRV parameters such as the average NN interval (AVNN), standard deviation of NN intervals (SDNN), and the low-frequency to high-frequency ratio (LF/HF).
Material and methods
Study design and setting
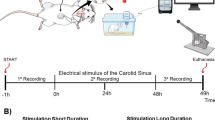
A prospective experimental study took place, with participants visiting Provita Medical Center four times between Monday and Thursday, from 9:00 to 11:00 a.m. Initially, anthropometric measurements were obtained using a Tanita MC-580 M P bioimpedance body composition analyzer (Japan, 2022). Subsequently, participants rested in a seated position for 20 min. On subsequent days, volunteers lay supine and underwent daily assessments of HRV and PORH responses at varying applied AOP levels: 40, 80, 100, and 130%. They remained in these positions for 5 min prior to each assessment. Measurement positions adhered to scientific literature standards to ensure the stability of Laser Doppler Flowmeter (LDF) measurements34,35,36. All assessments were conducted by the same two researchers (a physician and a physiotherapist) in a temperature-controlled room (21.2 ± 0.75 °C; humidity: 54.49 ± 2.5%).
Participants
An a priori power analysis was performed utilizing G*Power software. With a projected effect size of f = 0.50, a significance level set at α = 0.05, and a target statistical power of 0.80, the estimated minimum sample size needed was 28 participants. The study ultimately involved 30 participants, thus providing sufficient statistical power for the analyses conducted.
Thirty healthy volunteers, aged 18 to 30, were recruited via convenience sampling. Based on McKay’s participant classification scheme, the group was level 137. All participants were non-smokers, had an ankle-brachial index (ABI) between 0.9 and 1.2, reported no other health issues, and were not on any medications. They were instructed to refrain from vigorous exercise and alcohol for 24 h before each study session and to avoid ergogenic drinks like coffee and cola for 6 hours. All women were examined during the follicular phase of their menstrual cycle. Exclusion criteria included hypertension (≥ 140/95 mmHg), use of steroids or contraceptives, nicotine dependence, or medications affecting systemic hemodynamics (e.g., β-blockers, calcium antagonists, renin-angiotensin system inhibitors). Participants provided written informed consent after receiving detailed information about the study’s risks and potential benefits and were informed of their right to withdraw at any time. The study received ethical approval from the Polish Society of Physiotherapy (ref. no: 3.03.2024), was registered on 18/04/2024, under clinical trial number ISRCTN15418049, and was conducted in accordance with the principles of the Declaration of Helsinki.
Experiments were conducted with 30 participants (19 men, 11 women). Table 1 summarizes their physical characteristics, presenting the mean values (MEAN) and standard deviations (STD) for age, height, body mass, body mass index (BMI), Ankle-Brachial Index (ABI), and AOP at 100%.
Measurement procedures
-
1.
Following a period of rest in a supine position, thigh circumference was assessed using a centimeter tape, while the depth of the femoral artery in the area covered by the cuff was measured via ultrasound. The circumference of the thigh is a key determinant of the pressure needed to achieve arterial occlusion, particularly in the context of BFR exercise. Several factors, including cuff width and body position, modulate the correlation between thigh circumference and AOP. A clear understanding of these interactions is essential for the precise prescription of BFR, thereby ensuring safety and effectiveness across different exercise and physiotherapy protocols38.
-
2.
HRV data were collected using Polar H10 chest strap sensors, which detect beat-to-beat R-R intervals via ECG-based electrical signal measurement using skin-contact electrodes. The R-R interval data, accurate to within ± 1 ms, were wirelessly transmitted over Bluetooth Low Energy to the HRV Logger software application. This software recorded, timestamped, and stored the data for further HRV analysis, including time-domain, frequency-domain, and non-linear measures. Since HRV is sensitive to posture-related autonomic shifts, the supine position was selected to standardize baseline parasympathetic predominance, which is particularly relevant in the context of BFR research and the study of hyperemic rebound phenomena39.
-
3.
ABI was determined by a skilled physician using a 2D SonoScape P20 B-mode ultrasound scanner (China 20220, linear transducer with a frequency range of 4–18 Hz). To maintain consistency in measurements, the location of the ultrasound probe on the skin was marked following the application of ultrasound gel40.
-
4.
Following skin disinfection of the big toe, the LDF probe was positioned on the plantar skin, aligning its placement with the projection of the nail’s center.
-
5.
The physician monitored the tibial pulse of the dominant leg until complete flow occlusion, placing probes along both arteries at a constant insonation angle of < 60°41. The Doppler window encompassed the entire vessel. Standardizing ankle-brachial index (ABI) measurements is crucial for accurate diagnosis and risk assessment of peripheral arterial disease (PAD) and cardiovascular disease, as well as the safety of BFR33. After 10 min of supine rest, a 13 cm wide pneumatic cuff (Riester ®, Germany) was placed over the dominant thigh’s inguinal region. Cuff pressure was increased from 0 to 100 mmHg, then by 10 mmHg increments until ultrasound no longer detected arterial flow, thus determining individual AOP values. Each pressure level was maintained for 30 s to stabilize blood flow42. Our study employed the standard 5-min occlusion test on the dominant leg43.
-
6.
Post-occlusion responses were evaluated using a PeriFlux System 5000 Laser Doppler Flowmeter (Perimed AB, Järfälla, Sweden), considered a gold-standard device for noninvasive microcirculatory assessment due to its high temporal resolution and reproducibility35. The LDF probe was placed on the plantar skin of the dominant leg, specifically aligned with the center of the nail of the big toe, targeting a skin volume of approximately 1 mm3 at a tissue depth of 2.5 mm.
The PORH protocol involved inducing transient ischemia by inflating a pneumatic cuff (13 cm wide, placed at the inguinal region of the dominant thigh) to various pressure levels expressed as percentages of the participant’s individual AOP, previously determined via Doppler ultrasound44. An automated pneumatic cuff system (Hokanson E20 Rapid Cuff Inflator with AG101 Air Source, Bellevue, WA, USA) was used to apply and precisely maintain the target occlusion pressure. The device automatically inflated the cuff to the desired percentage of AOP (40, 80, 100, or 130%) and released it in a controlled manner to ensure consistent inflation and deflation timing across participants and sessions.
Each occlusion lasted for 5 min, a standardized duration adequate to evoke maximal metabolic and endothelial vasodilatory responses. Baseline resting flow (RF) was measured prior to cuff inflation. Upon rapid cuff deflation, reactive hyperemia ensued, characterized by a transient increase in skin perfusion detected by the LDF at a 32 Hz sampling rate. Parameters extracted from the PORH response included45,46: Rest Flow (RF), The baseline perfusion value prior to occlusion, expressed in perfusion units (PU); Time to Peak (TP): The interval (seconds) from cuff release to the maximum hyperemic perfusion value, reflecting the speed of microvascular reperfusion; Recovery Time (TR): The duration (minutes) required for perfusion to return from peak hyperemia back to baseline resting levels, indicating vascular recovery kinetics; AOP min: The minimum cuff pressure at which resting blood flow begins to decline, marking the threshold for arterial flow restriction; AOP 100%: The cuff pressure corresponding to complete arterial occlusion, confirmed by the absence of detectable arterial flow on Doppler ultrasound; and Biological Zero (BZ): The minimal flow value recorded during full arterial occlusion, representing residual signal or non-flow related noise.
The analysis of HRVconsidered the following measures:
-
AVNN, or Average NN Interval [ms], represents the mean duration in milliseconds between consecutive heartbeats. Longer RR intervals correspond to a lower heart rate, while shorter intervals indicate a higher one. The normal average RR interval (AVNN) typically ranges from 800 to 1000 [ms].
-
SDNN, the Standard Deviation of NN Intervals in milliseconds [ms], is the standard deviation of the RR intervals. For healthy adults, SDNN (Total HRV Variability) norms are: low variability below 20 [ms], medium variability between 20 and 50 [ms], and high variability above 50 [ms]47.
-
LF/HF (Low/High Frequency Ratio) [non-arbitrary unit] represents the ratio of low-frequency (LF) power to high-frequency (HF) power. Normative values for LF/HF (sympatho-parasympathetic balance) are generally: < 1 indicates parasympathetic predominance (relaxation, recovery), 1–3 suggests sympatho-parasympathetic balance, and > 3 indicates sympathetic predominance (stress, activation)48.
Statistical analysis
Statistical analyses were performed based on data distribution. Normality was tested using Shapiro–Wilk; normally distributed data were analyzed with repeated measures ANOVA, while non-normal data used the Friedman test. Post-hoc tests identified specific group differences. Significance was set at α = 0.05 with Bonferroni correction to control Type I error.
Effect sizes were calculated using Hedges’ g, categorized as small (0.0–0.2), medium (0.2–0.8), and large (> 0.8). Positive g values indicate a higher mean in the first group, negative values indicate a lower mean, and effect strength was based on the absolute value. Final conclusions considered both adjusted p-values and effect size magnitudes to assess statistical and practical significance. All statistical analyses were performed using GraphPad Prism software (version 10.1; GraphPad Software, San Diego, CA, USA).
Results
Figure 1 shows the comparison of the distributions of RF, BZ, Rhmax, and TP across different AOP levels (40%, 80%, 100%, and 130%). There were no significant differences in RF values across AOP levels (F(3, 87) = 0.27, p = 0.847; ηp2 = 0.00036). The Friedman test revealed statistically significant differences for BZ (p < 0.001), Rhmax (p < 0.001), and TP (p < 0.001) across AOP levels. Figure 1 presents the pairwise comparisons indicating these significant differences, while Supplementary Material 1 provides detailed p-values and Hedges’ g effect sizes for each comparison.
Specifically, BZ values differed significantly between 40 and 80 (p < 0.001, Hedges’ g = 3.72), 40 and 100 (p < 0.001, g = 9.07), 40 and 130 (p < 0.001, g = 9.10), 80 and 100 (p < 0.001, g = 7.37), and 80 and 130 (p < 0.001, g = 7.42). Similarly, significant differences in Rhmax were observed between 40 and 80 (p < 0.001, Hedges’ g = − 6.06), 40 and 100 (p < 0.001, g = − 8.11), 40 and 130 (p < 0.001, g = − 8.36), 80 and 100 (p < 0.001, g = − 5.44), and 80 and 130 (p < 0.001, g = − 5.60). Significant differences in TP were also found between 40 and 80 (p < 0.001, Hedges’ g = − 2.65), 40 and 100 (p < 0.001, g = − 4.64), 40 and 130 (p < 0.001, g = − 5.44), 80 and 100 (p < 0.001, g = − 3.46), and 80 and 130 (p < 0.001, g = − 4.26).
Figure 2 shows the comparison of the distributions of AVNN, SDNN, LF-HF and HR across different AOP levels (40%, 80%, 100%, and 130%). The Friedman test revealed statistically significant differences across AOP levels for AVNN (p < 0.001), SDNN (p < 0.001), LF/HF ratio (p < 0.005), and HR (p < 0.001). Figure 2 presents the pairwise comparisons indicating these significant differences, while Supplementary Material 1 provides detailed p-values and Hedges’ g effect sizes for each comparison.
Comparison of the distribution of (a) average NN intervals (AVNN) values; (b) Standard Deviation of NN Intervals (SDNN); (c) Low/High Frequency Ratio (LF-HF); and (d) heart rate (HR) across different levels of arterial occlusion pressure (AOP) (40%, 80%, 100%, and 130%). * indicates significant differences in pairwise comparisons.
Significant differences in AVNN were found between 40 and 80 (p < 0.001, Hedges’ g = 3.32), 40 and 100 (p < 0.001, g = 6.01), and 40 and 130 (p < 0.001, g = 6.46). Smaller, yet still significant, differences were also observed between 80 and 100 (p = 0.046, g = 0.60) and between 80 and 130 (p = 0.043, g = 0.65). For SDNN, significant differences occurred between 40 and 80 (p < 0.001, Hedges’ g = − 4.19), 40 and 100 (p < 0.001, g = − 7.88), and 40 and 130 (p < 0.001, g = − 8.46), with smaller but still significant differences found between 80 and 100 (p = 0.046, g = − 1.89) and between 80 and 130 (p = 0.043, g = − 2.00). Significant differences in the LF− HF ratio were found between 40 and 80 (p < 0.001, Hedges’ g = − 5.43), 40 and 100 (p < 0.001, g = − 7.48), and 40 and 130 (p < 0.001, g = − 7.58), as well as between 80 and 100 (p < 0.001, g = − 2.45) and between 80 and 130 (p < 0.001, g = − 2.69). Differences in HR were observed between 40 and 80 (p < 0.001, Hedges’ g = − 0.35), 40 and 100 (p < 0.001, g = − 1.31), 40 and 130 (p < 0.001, g = − 1.47), 80 and 100 (p < 0.001, g = − 0.82), and 80 and 130 (p < 0.001, g = − 0.96), as well as between 100 and 130 (p < 0.010, g = − 0.19).
Discussion
This study shows that varying levels of arterial occlusion pressure (AOP) have distinct effects on both microcirculatory and autonomic responses. While rest flow (RF) remained unchanged across all AOP levels, significant differences were observed in biological zero (BZ), peak hyperemia (Rhmax), time to peak (TP), and heart rate variability (HRV) measures including AVNN, SDNN, LF-HF ratio, and heart rate (HR). The most substantial physiological changes occurred between the lowest pressure (40 mmHg) and higher levels (80–130 mmHg), with large effect sizes across parameters. However, comparisons between 100 and 130% AOP consistently revealed no significant differences, suggesting a plateau or stabilization in microvascular and autonomic responses at higher occlusion pressures.
HRV analysis revealed that increasing AOP significantly affected all examined HRV parameters—AVNN, SDNN, LF-HF ratio, and HR. The most pronounced changes occurred between 40 mmHg and higher AOP levels (80, 100, 130 mmHg), with large effect sizes indicating strong autonomic modulation. However, comparisons between 100 and 130% AOP showed no significant differences across these measures, suggesting a saturation point or plateau in autonomic response at higher occlusion levels. Previous study49 found that greater arterial occlusion pressure in blood flow restriction training increased cardiovascular and metabolic stress, but also mean arterial pressure, indicating high myocardial workload. Moreover, applying higher relative pressures resulted in greatest cardiovascular response in a study comparing different AOP levels50.
The observed saturation point or plateau in autonomic response at higher AOP levels (between 100 and 130% AOP), despite significant changes occurring between 40 mmHg and higher AOP levels (80, 100, 130 mmHg), likely reflects a maximal or near-maximal stimulation of the mechanoreceptors involved in the autonomic response to vascular occlusion51. As AOP increases, baroreceptors and other pressure-sensitive afferent nerve endings within the vasculature are increasingly stimulated4, leading to adjustments in AVNN, SDNN, LF-HF ratio, and HR, indicative of autonomic modulation52. However, these receptors likely have a finite capacity to respond to increasing pressure. Once a sufficiently high pressure was reached (around 100% AOP in this context), further increases in occlusion pressure may not elicit a proportionally greater increase in afferent signaling53. This plateau effect could be due to the receptors reaching their maximal firing rate or the downstream neural pathways becoming saturated in their ability to process and relay the increasing afferent information. Importantly, the pressure at which this saturation occurs is likely more indicative of the diastolic pressure rather than the mean arterial pressure, as diastolic pressure better represents the baseline arterial load sensed by the receptors during the cardiac cycle54.
RF remained unchanged across all AOP levels, indicating that baseline microvascular perfusion is not significantly influenced by moderate variations in external pressure. In contrast, BZ, Rhmax, and TP all showed significant differences between conditions, particularly between lower (40 mmHg) and higher AOP levels. BZ, Rhmax, and TP values plateaued at 100% and 130% AOP, with no significant differences observed between these two levels, suggesting a ceiling effect in vascular occlusion-induced microvascular suppression and subsequent reactive hyperemia. For instance, a previous study found that blood flow response to low-load exercise with and without blood flow restriction is pressure-dependent, with higher pressures reducing resting hyperemia55.
BZ likely reflects tissue compression effects and capillary signal attenuation, which intensify with higher pressures56. The marked changes in Rhmax and TP reflect enhanced PORH, driven by the accumulation of metabolic vasodilators during occlusion and their sudden release upon reperfusion57. The longer TP and higher Rhmax at greater AOP levels suggest a more profound ischemic challenge and stronger rebound vasodilation. However, the absence of further increase beyond 100% AOP implies maximal vasodilatory capacity may have been reached56. These findings align with previous studies indicating that the PORH response is pressure- and duration-dependent55,58 but may saturate once the ischemic stimulus exceeds physiological thresholds.
This study had some limitations that should be acknowledged. First, the sample size and demographic characteristics may limit the generalizability of findings to broader or clinical populations. Additionally, due to recruitment limitations, participant inclusion was unbalanced in favor of men, which should be acknowledged as a study limitation. Second, while multiple AOP levels were tested, the discrete pressure increments (40, 80, 100, 130 mmHg) may not capture subtler thresholds of physiological change or individual variability in pressure tolerance. Third, the design prevents long-term inferences about adaptation to repeated occlusion exposures, such as those encountered BFR training. Additionally, although HRV and microvascular measures were assessed, the study did not include biochemical or neurohormonal markers that could provide deeper insight into the mechanisms underpinning the observed plateau effects at higher AOP levels.
The results of this study suggests that BFR training at pressures between 80 and 100% AOP elicits significant changes in both microcirculatory (BZ, Rhmax, TP) and autonomic (AVNN, SDNN, LF-HF ratio, HR) responses compared to low pressure (40 mmHg), suggesting that moderate to high occlusion pressures are effective in stimulating physiological adaptations relevant to BFR. However, no further significant differences were observed between 100 and 130% AOP across these measures, indicating a plateau in response. This suggests that applying occlusion pressures above 100% AOP may not provide additional benefit in terms of vascular or autonomic stimulation. Additionally, resting flow remained stable across all AOP levels, indicating that baseline perfusion is maintained even at higher pressures.
While this study focuses on BFR training, the findings may also be relevant to clinical settings such as orthopedic surgery, where arterial occlusion cuffs are applied at high pressures for extended periods. Our results suggest that microvascular and autonomic responses reach a plateau around 100% AOP, indicating a limit to physiological adaptation. In surgical cases, patients are often unprepared for such occlusion and reperfusion stresses, which can lead to meaningful hemodynamic changes after cuff release. Understanding the saturation of these responses may help explain some of the cardiovascular impacts observed post-surgery. Additionally, the stable resting flow at moderate pressures suggests that baseline perfusion is maintained during occlusion, but longer or higher pressure applications could exceed this capacity. These findings might inform safer cuff pressure use during surgery and highlight the potential value of preconditioning strategies to improve patient tolerance to vascular occlusion.
Conclusions
The findings show that increasing AOP produces significant changes in Rhmax, TP, BZ, and HRV indices (AVNN, SDNN, LF-HF ratio, and HR)—particularly when comparing low pressure (40 mmHg) to moderate and high pressures (80–100% AOP). However, responses plateaued between 100 and 130% AOP, suggesting a saturation point in both microvascular and autonomic modulation. These results highlight a pressure-dependent relationship up to a physiological ceiling, beyond which further occlusion does not elicit additional response. The results provide new findings into the dose–response characteristics of vascular and autonomic systems under occlusive stress and offers suggestions for optimizing AOP in blood flow restriction applications.
Data availability
All data is available upon request to the corresponding author.
Abbreviations
- AOP:
-
Arterial occlusion pressure
- AVNN:
-
Average of normal-to-normal intervals
- BFR:
-
Blood flow restriction training
- BZ:
-
Biological zero
- HR:
-
Heart rate
- HRV:
-
Heart rate variability
- LF-HF ratio:
-
Low frequency to high frequency ratio
- PORH:
-
Post-occlusive reactive hyperemia
- Rhmax:
-
Peak hyperemia
- SDNN:
-
Standard deviation of normal-to-normal intervals
- TP:
-
Time to peak
References
Cocking, S., Jones, H., Cable, N. T. & Thijssen, D. H. Enhancing sports performance through ischemic preconditioning. Sci. Hormesis Health Longev. https://doi.org/10.1016/B978-0-12-814253-0.00019-X (2019).
Schamne, J. C. et al. Cardiac autonomic responses during and after a single session of aerobic exercise with and without blood flow restriction. Motriz Rev. Educação Física 25, e101936 (2019).
Lemos, L. K. et al. Autonomic and cardiovascular responses on post-eccentric exercise recovery with blood flow restriction at different loads: Randomized controlled trial. Eur. J. Integr. Med. 53, 102148 (2022).
Cristina-Oliveira, M. et al. Clinical safety of blood flow-restricted training?: A comprehensive review of altered muscle metaboreflex in cardiovascular disease during ischemic exercise. Am. J. Physiol. Heart Circ. Physiol. 318, H90–H109 (2020).
Wilk, M. et al. Impact of ischemic intra-conditioning on power output and bar velocity of the upper limbs. Front. Physiol. 12, 626915 (2021).
Lorenz, D. S. et al. Blood flow restriction training. J. Athl. Train. 56, 937–944 (2021).
Hughes, L. et al. Influence and reliability of lower-limb arterial occlusion pressure at different body positions. PeerJ 2018, e4697 (2018).
Crossley, K. W. et al. Effect of cuff pressure on blood flow during blood flow-restricted rest and exercise. Med. Sci. Sports Exerc. 52, 746–753 (2020).
Stanford, D. M., Cupp, B. N., Chatlaong, M. A. & Jessee, M. B. Acute hyperemic response to blood flow restriction and ischemic preconditioning protocols. Med. Sci. Sports Exerc. 55, 48–49 (2023).
Marcinek, A., Katarzynska, J. & Gebicki, J. A new approach to vascular screening: Identification of impaired vascular function using the FMSF technique. Sensors 24, 1721 (2024).
Zhang, Y. Application of blood flow restriction training in lower limb rehabilitation. Theor. Nat. Sci. 50, 24–33 (2024).
Coza, A., Dunn, J. F., Anderson, B. & Nigg, B. M. Effects of compression on muscle tissue oxygenation at the onset of exercise. J. Strength Cond. Res. 26, 1631–1637 (2012).
Morales, F. et al. How to assess post-occlusive reactive hyperaemia by means of laser Doppler perfusion monitoring: Application of a standardised protocol to patients with peripheral arterial obstructive disease. Microvasc. Res. 69, 17–23 (2005).
Rolnick, N. et al. Why blood flow restriction cuff features are an important methodological consideration—A short commentary on “cerebral cortex activation and functional connectivity during low-load resistance training with blood flow restriction: An fNIRS study”. Front. Physiol. 15, 1482816 (2024).
Hughes, L., Paton, B., Rosenblatt, B., Gissane, C. & Patterson, S. D. Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br. J. Sports Med. 51, 1003–1011 (2017).
Ozkor, M. A. & Quyyumi, A. A. Endothelium-derived hyperpolarizing factor and vascular function. Cardiol. Res. Pract. 2011, 1–12 (2011).
Lorenzo, S. & Minson, C. T. Human cutaneous reactive hyperaemia: Role of BK Ca channels and sensory nerves. J. Physiol. 585, 295–303 (2007).
Ozkor, M. A. et al. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation 123, 2244–2253 (2011).
Larkin, S. W. & Williams, T. J. Evidence for sensory nerve involvement in cutaneous reactive hyperemia in humans. Circ. Res. 73, 147–154 (1993).
Zhao, J. L., Pergola, P. E., Roman, L. J. & Kellogg, D. L. Bioactive nitric oxide concentration does not increase during reactive hyperemia in human skin. J. Appl. Physiol. 96, 628–632 (2004).
Hellmann, M., Gaillard-Bigot, F., Roustit, M. & Cracowski, J. Prostanoids are not involved in postocclusive reactive hyperaemia in human skin. Fundam. Clin. Pharmacol. 29, 510–516 (2015).
Dahmus, J. D., Bruning, R. S., Larry Kenney, W. & Alexander, L. M. Oral clopidogrel improves cutaneous microvascular function through EDHF-dependent mechanisms in middle-aged humans. Amer. J. Physiol. Regul. Integr. Compar. Physiol. 305, R452–R458 (2013).
Engelke, K. A., Halliwill, J. R., Proctor, D. N., Dietz, N. M. & Joyner, M. J. Contribution of nitric oxide and prostaglandins to reactive hyperemia in the human forearm. J. Appl. Physiol. 81, 1807–1814 (1996).
Tagawa, T. et al. Role of nitric oxide in reactive hyperemia in human forearm vessels. Circulation 90, 2285–2290 (1994).
Carlsson, I., Sollevi, A. & Wennmalm, A. The role of myogenic relaxation, adenosine and prostaglandins in human forearm reactive hyperaemia. J. Physiol. 389, 147–161 (1987).
Tateno, H. et al. Ca 2+ ionophore A23187 can make mouse spermatozoa capable of fertilizing in vitro without activation of cAMP-dependent phosphorylation pathways. Proc. Natl. Acad. Sci. 110, 18543–18548 (2013).
Cankar, K., Finderle, Ž & Štrucl, M. The effect of α-adrenoceptor agonists and L-NMMA on cutaneous postocclusive reactive hyperemia. Microvasc. Res. 77, 198–203 (2009).
Krishnan, A., Lucassen, E. B., Hogeman, C., Blaha, C. & Leuenberger, U. A. Effects of limb posture on reactive hyperemia. Eur. J. Appl. Physiol. 111, 1415–1420 (2011).
Krajina, I. et al. Two-week low-salt diet improves acetylcholine-induced microvascular dilation in biologically naïve psoriasis patients. Nutrients 17, 693 (2025).
Wang, Y., Wang, Y., Liang, Q. & Wang, Y. Cardiovascular responses and subjective perceive during different positions and resistive exercises in healthy adults. Heart 98, E288–E288 (2012).
Erdmann, S. et al. Indices of heart rate variability as potential early markers of metabolic stress and compromised regulatory capacity in dried-off high-yielding dairy cows. Animal 12, 1451–1461 (2018).
Desanlis, J. et al. Effects of occlusion pressure on hemodynamic responses recorded by near-infrared spectroscopy across two visits. Front. Physiol. 15, 1441239 (2024).
Khaliulin, I. et al. Neuro-autonomic changes induced by remote ischemic preconditioning (RIPC) in healthy young adults: Implications for stress. Neurobiol. Stress 11, 100189 (2019).
Liana, R., Chudański, M. & Katedra, I. P. Standarisation of laser Doppler flowmetry—Own standards. Clin. Diabetol. 10, 58–64 (2009).
Gemae, M. R. et al. Myths and methodologies: Reliability of forearm cutaneous vasodilatation measured using laser-Doppler flowmetry during whole-body passive heating. Exp. Physiol. 106, 634–652 (2021).
Trybulski, R. et al. The effects of combined contrast heat cold pressure therapy on post-exercise muscle recovery in MMA fighters: A randomized controlled trial. J. Hum. Kinet. 94, 127–146 (2024).
McKay, A. K. A. et al. Defining training and performance caliber: A participant classification framework. Int. J. Sports Physiol. Perform. 17, 317–331 (2022).
Wedig, I. et al. Predictors of arterial occlusion pressure in the lower-body across commonly used cuff widths. Physiology https://doi.org/10.1152/physiol.2023.38.S1.573239038 (2023).
Watanabe, N., Reece, J. & Polus, B. I. Effects of body position on autonomic regulation of cardiovascular function in young, healthy adults. Chiropr. Osteopat. 15, 1–8 (2007).
Crawford, F., Welch, K., Andras, A. & Chappell, F. M. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochr. Datab. Syst. Rev. https://doi.org/10.1002/14651858.CD010680.pub2 (2016).
Gifford, J. R. & Richardson, R. S. CORP: Ultrasound assessment of vascular function with the passive leg movement technique. J. Appl. Physiol. 123, 1708–1720 (2017).
Khan, T. H., Farooqui, F. A. & Niazi, K. Critical review of the ankle brachial index. Curr. Cardiol. Rev. 4, 101–106 (2008).
Shirazi, B. R., Valentine, R. J. & Lang, J. A. Reproducibility and normalization of reactive hyperemia using laser speckle contrast imaging. PLoS ONE 16, e0244795 (2021).
Babos, L. Evaluation of microvascular reactivity with laser Doppler flowmetry in chronic kidney disease. World J. Nephrol. 2, 77 (2013).
Ali, S. et al. Standardization and quality control of Doppler and fetal biometric ultrasound measurements in low-income setting. Ultrasound Obstet. Gynecol. 61, 481–487 (2023).
Liana, R., Chudański, M. & Ponikowska, I. Standarisation of laser Doppler flowmetry-own standards. Clin. Diabetol. 10, 58–64 (2009).
García-González, M. A. et al. Normalization of the standard deviation and spectral indices of RR time series for comparison among situations with different mean heart rate. IFMBE Proc. 22, 1357–1361 (2009).
Xu, A. et al. Response of autonomic nervous system to body positions: Fourier and wavelet analysis. Mod. Phys. Lett. B 19, 59–78. https://doi.org/10.1142/S0217984905008025 (2005).
Thomas, H. J., Scott, B. R. & Peiffer, J. J. Acute physiological responses to low-intensity blood flow restriction cycling. J. Sci. Med. Sport 21, 969–974 (2018).
Mattocks, K. T. et al. The effects of upper body exercise across different levels of blood flow restriction on arterial occlusion pressure and perceptual responses. Physiol. Behav. 171, 181–186 (2017).
Spranger, M. D., Krishnan, A. C., Levy, P. D., O’Leary, D. S. & Smith, S. A. Blood flow restriction training and the exercise pressor reflex: A call for concern. Am. J. Physiol. Heart Circ. Physiol. 309, H1440–H1452 (2015).
Mccraty, R. & Shaffer, F. Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob. Adv. Health Med. 4, 46–61 (2015).
Michelini, L. C., O’Leary, D. S., Raven, P. B. & Nóbrega, A. C. L. Neural control of circulation and exercise: A translational approach disclosing interactions between central command, arterial baroreflex, and muscle metaboreflex. Am. J. Physiol. Heart Circ. Physiol. 309, H381–H392 (2015).
Øien, A. H. & Aukland, K. A multinephron model of renal blood flow autoregulation by tubuloglomerular feedback and myogenic response. Acta Physiol. Scand. 143, 71–92 (1991).
Mouser, J. G. et al. Blood flow in humans following low-load exercise with and without blood flow restriction. Appl. Physiol. Nutr. Metab. 42, 1165–1171 (2017).
Hunt, J. E. A., Galea, D., Tufft, G., Bunce, D. & Ferguson, R. A. Time course of regional vascular adaptations to low load resistance training with blood flow restriction. J. Appl. Physiol. 1985(115), 403–411 (2013).
Horn, A. G. et al. Post-occlusive reactive hyperemia and skeletal muscle capillary hemodynamics. Microvasc. Res. 140, 104283 (2022).
Ingram, J. W. et al. The influence of time on determining blood flow restriction pressure. J. Sci. Med. Sport 20, 777–780 (2017).
Author information
Authors and Affiliations
Contributions
RT and MW conceived and designed the analysis, collected the data, performed the analysis, and wrote the paper. JW and GO collected the data and wrote the paper. FK and JM performed data analysis and treatment, and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study received ethical approval from the Polish Society of Physiotherapy (ref. no: 3.03.2024).
Informed consent
All the participantes signed an informed consent form.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Trybulski, R., Krzysztof, F., Muracki, J. et al. A controlled comparative study on the effect of arterial occlusion pressure on immediate sympathetic and hyperemic responses. Sci Rep 15, 41291 (2025). https://doi.org/10.1038/s41598-025-25132-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-25132-y