Abstract
Coleoid cephalopods have the most elaborate camouflage system in the animal kingdom. This enables them to hide from or deceive both predators and prey. Most studies have focused on benthic species of octopus and cuttlefish, while studies on squid focused mainly on the chromatophore system for communication. Camouflage adaptations to the substrate while moving has been recently described in the semi-pelagic oval squid (Sepioteuthis lessoniana). Our current study focuses on the same squid’s complex camouflage to substrate in a stationary, motionless position. We observed disruptive, uniform, and mottled chromatic body patterns, and we identified a threshold of contrast between dark and light chromatic components that simplifies the identification of disruptive chromatic body pattern. We found that arm postural components are related to the squid position in the environment, either sitting directly on the substrate or hovering just few centimeters above the substrate. Several of these context-dependent body patterns have not yet been observed in S. lessoniana species complex or other loliginid squids. The remarkable ability of this squid to display camouflage elements similar to those of benthic octopus and cuttlefish species might have convergently evolved in relation to their native coastal habitat.
Similar content being viewed by others
Introduction
Camouflage is a common term describing a capacity to avoid detection by visual seekers1. It is an important defense tactic for terrestrial and aquatic animals2,3,4,5. Coleoid cephalopods, such as octopuses, squid, and cuttlefish, express rapid dynamic camouflage by altering coloration, texture, posture, and movement in response to visual stimulus in their surroundings6,7,8,9. Currently, cephalopod camouflage studies have focused mainly on benthic species of cuttlefish and octopus. Cuttlefish such as Sepia officinalis and Sepia pharaonis exhibit background matching, disruptive patterning, masquerading, countershading, and mimicry7,8,10,11,12,13,14. Background matching in octopuses has been observed in situ15 or in the field16. Textural camouflage achieved by octopuses and cuttlefish is often via papillae, which squid lack17.
Cephalopod body patterns are modulated by neurally controlled chromatophores, iridophores, and leucophores distributed throughout their bodies9,18. Unlike pigment elements found in other color-changing animals, cephalopod chromatophores are muscular pigment sacs innervated directly by motor neurons9,19,20,21,22,23. Iridophores, also neurally controlled, refract light, allowing the animal to create spots of variable color24,25. Leucophores are flattened, elongated cells containing clear, colorless granules that reflect and scatter ambient light24,26,27,28. Although squids generally have less leucophores compared with octopus and cuttlefish, certain regions of the squid’s body have higher densities of leucophores, which may be covered or exposed by the chromatophores. Cephalopods combine the chromatic expression of these chromatophores, iridophores, and leucophores to yield a wide repertoire of body patterns that match visually diverse environments such as coral reefs9,24,26.
Cephalopods can alter their body shape, distorting the outline of their body and enhancing camouflage. Types of camouflage behavior that have been frequently observed in cephalopods include 1) "camouflage in motion," where the animal camouflages while moving (e.g.29,30,31), and 2) "situational motionless camouflage," where the animal actively selects a site and camouflages at that site without moving from it (e.g.16). Motionless camouflage in cuttlefish and octopuses has frequently been described, but squid camouflage has rarely been studied20,31. Because the habitats of many species of squid are pelagic, direct observation in nature and laboratory observation remain challenging, and studies of camouflage behavior in squid species are limited. Mesopelagic deep-sea squid, Onychoteuthis banksii, was reported to control pigmentation and transparency based on ambient light conditions to yield optimal countershading32. Staudinger et al.33 discovered that motionless situational camouflage to the substrate is exclusively associated with a disruptive pattern in the longfin squid, Doryteuthis pealeii. A semi-pelagic species, the Caribbean reef squid, Sepioteuthis sepioidea is considered having the greatest repertoire of disruptive components for a teuthoid17. It has been reported to exhibit background matching by mottled pattern, countershading and translucency; disruptive coloration against soft coral; masquerading to soft coral; and potential mimicry to striped parrotfish17. It is a sister species to oval squids34.
Oval squids, Sepioteuthis lessoniana Férussac in Lesson, 1830, are widely distributed in temperate and tropical waters ranging from northern Japan to Australia and from Hawaii to the Mediterranean35,36,37,38. They form a species complex of at least three distinct cryptic species39,40 that have not yet been formally defined and classified. In this study, we have focused on S. lessoniana sp.2, also called "Shiro-ika" or "white-squid", which has the widest geographical and ecological distribution of the three currently recognized cryptic species of oval squid41. We use the name white-squid in this publication to distinguish it clearly from other members of the S. lessoniana species complex that are behaviorally different42. White-squid’s habitat ranges from shallow water in reefs at a depth of 1 m or less to depths of 100 m along the coastal environment, with substrate including seagrass beds, coral reefs, and sandy bottom38.
In such highly biodiverse zones, predator–prey interactions are frequent. As a defense mechanism against predation, white-squid is known to form schools with over 200 individuals43. Schooling behavior is observed in natural habitats and artificial conditions44. Although there is no comprehensive ethogram of white-squid, previous studies45 suggest that they possess a repertoire of body patterns comparable in breadth to phylogenetically related species such as Sepioteuthis australis with 48 body pattern components associated with reproductive behavior46, and S. sepioidea with 57 described body pattern components47,48,49,50,51.
Although behavior in related species has been reported, studies of behavior within the S. lessoniana species complex remain scarce. Furthermore, it is often very difficult or even impossible to determine, which member of the species complex has been actually studied, because the authors are rarely reporting that. Previous studies have primarily focused on schooling, mating, lateralization, and hunting43,45,52,53,54,55,56,57.
Squids are an important source of prey for many marine predators, including fish, mammals, and sea birds58,59,60. They demonstrate anti-predatory strategies, including jetting away, inking, and deimatic display using body patterns and postures, following standard and customary heuristic classifications of cephalopod behavior19,61,62,63,64,65. It has been reported that even pelagic squids such as Illex sp.66, Doryteuthis pealeii33, and Todarodes pacificus67 rest on a substrate in the wild. Such behavior suggests that squid camouflage to substrate behavior merits further investigation. Unlike the pelagic and deep-sea species with little to no substrate in their natural environments, the visual complexity and diversity of white-squid’s habitat offer a wide variety of opportunities to hide from their predators. In the field, camouflage to substrate behavior has been observed anecdotally in the related squid, S. sepioidea17 and the white-squid’s ability to camouflage to substrate has been reported by Nakajima et al.31.
In this study, we assume that the correlation of the white-squid body pattern with the substrate is produced by camouflage behavior. We describe behavioral components related to the camouflage to substrate of the white-squid observed in captive environment and characterize the squid’s ability to camouflage to the surrounding environment. Unlike many other squids, white-squid can be bred in captivity over multiple generations68,69,70 and thus represents an attractive model species for studying the distinctive biology of loliginid squids.
Methods
Animal maintenance
Experiments were conducted in accordance with the 2006 guidelines for Proper Conduct of Animal Experiments of the Science Council of Japan and were approved by the Committee for Care and Use of Animals at the Okinawa Institute of Science and Technology, an AAALAC-accredited facility, under protocol no. 2016-137. In addition, this study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). All methods were carried out in accordance with the guidelines and regulations for this study, and no violations of ethical conduct were reported. We used the second and third-generation laboratory-bred juvenile/adult white-squid in years 2018 and 2019.
Egg casings of white-squid were sampled in August 2017 from an intertidal seagrass bed in Tancha village on the west coast of Okinawa Island, Ryukyu Archipelago, Japan. The eggs were transported to the Okinawa Institute of Science and Technology Graduate University Marine Science Station (MSS) near the beach. A flow-through system was used for squid keeping over multiple generations. Indoor tanks were lit with natural light from windows and fluorescent lights programmed to turn on at 9:00 and off at 18:00, regardless of natural light conditions, and outdoor tanks were illuminated by natural light. All tanks were fed by ocean water pumped through a plumbing system with a sand filter. The tank water reflected the seawater conditions of the surrounding shallow sea that are recorded by the Japan Meteorological Agency (https://www.jma.go.jp/). Temperature, pH, salinity, ammonia, nitrite, and nitrate levels in the tanks were constantly monitored. On some occasions during the Summer, chillers were used to clip temperature at 30 °C during the heatwaves without typhoons.
White-squid were fed four times a day, with food type according to their growth: i) from hatching up to 60 days: live brackish mysid Neomysis japonica; ii) from 3-day-old to approximately 100-day-old: frozen larval Japanese anchovy Engraulis japonicus and ghost shrimp Palaemonetes paludosus; and ii) squid older than 30 days: frozen subadult and adult silver-stripe round herring Spratelloides gracilis and occasionally live tiger prawn Marsupenaeus japonicus. Additionally, squids were fed with mysid, larval anchovy, or ghost shrimp, appropriate to their size. Live ghost shrimp and tiger prawns were used to enhance the animals’ feeding and hunting motivation, to provide extra flexibility and enrichment for the subjects, and to diversify their diet. Perished subjects, waste, and food remains were removed from the tank after every feeding session and upon discovery.
Data acquisition
The recordings of camouflaging to substrate in multiple tanks were conducted on two successive generations of captive-bred subjects between December 2018 and November 2019. In response to disturbances around the tank, the squid was observed moving down the water column and hovering a few centimeters above the substrate (we defined this as Hovering) or sitting directly on the substrate (we defined this as Sitting; Figs. 1, 2).
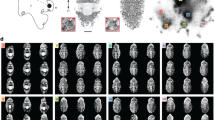
Examples of white-squid Sitting behavior. (A) Two animals with fully extended fins and curled arms overlap in an open space. The overlapping effect creates complexity by merging chromatic components. (B) Three animals with body patterns ranging from dark uniform to disruptive are stacked next to an algae-covered rock, giving the impression of collective camouflage and/or masquerade with the rock. (C) An animal uses live rocks as protective shelter, showcasing a dark mottled body pattern that blends with its surroundings. (D) An animal rests at the bottom of the tank with its fins fully extended and transparent, overlaying chromatic components on the fins over the substrate, resulting in a gradual transition from the substrate to a non-transparent chromatic expression of the Disruptive pattern (see Supplementary Video 1). (E) Two animals position themselves between a rock and an array of wooden branches, revealing a light-mottled body pattern. (F) Two animals are stacked atop one another with uniformly dark body patterns, creating a visual fusion that obscures contour detection. (G) An example of masquerade, where an animal displaying a dark mottled body pattern has positioned itself next to an algae-covered rock, with Raised arms (see sideview in the Supplementary Video 2, around the time 2:20–3:20).
To observe the subject’s behavior during feeding and tank cleaning, we used GoPro HERO6, Olympus TG -4, Olympus TG-5 (Olympus Corporation, Japan), and Sony Alpha 9 (Sony Corporation, Japan). Here, we focus on observing camouflage when the subjects are in a static position and displaying an acute body pattern lasting more than 5 s. Photographs and video recordings without motionless camouflage were excluded from further analysis. Then, we excluded duplicates and extracted records of a record containing a motionless camouflage to substrate behavior. We did not track individuals, but following our keeping protocols, we know that over 50 adult and semi-adult squid individuals were subjects in these observations. In total, 66 high-resolution photographs and 42 HD videos of squid motionless camouflage to substrate behavior resulted in a sample size of N = 216 events examined in this study.
We organized recorded motionless camouflage to substrate behaviors of white-squid (Table 1) based on existing body pattern descriptions of cephalopods, which have been categorized by visual observation into components such as chromatic, postural, locomotor, and body pattern display10,51,71. We recorded where the individual subject was located relative to other subjects, e.g., alone; with another individual nearby; touching another individual; piling on top of one other; in an open space; adjacent to an object in the tank; or masquerading to an object in the tank.
Data analysis
Staudinger et al. 33 report that motionless situational camouflage to the substrate is exclusively associated with disruptive body patterns in the longfin squid Doryteuthis pealeii. We noticed that while camouflaging to the substrate, white-squid displays a variety of body patterns, not solely disruptive (Figs. 1, 2 and 3). To examine the relationship of locomotor components to Disruptive body pattern in white-squid, we split our squid photographs into two groups, Disruptive and non-disruptive body patterns (Fig. 4). We then compared the association of the Disruptive body pattern with Sitting and Hovering (Supplementary Files 1, 2).
Differentiation between Disruptive body pattern and Mottled body pattern. Related squid, S. sepioidea, have been observed displaying only a disruptive body pattern when resting on the substrate 17. However, the recorded images reveal a more complex chromatic expression of the white-squid, ranging from Disruptive to Mottled and Uniform. To describe the differences between Disruptive and Mottled patterns, we created a template for a fully disruptive body pattern that includes two transverse mantle bars, anterior and posterior mantle bars, and a head bar. When these components divide the body into ten areas with high contrast and complete coverage across the entire width of the animal, we categorized it as Disruptive based on visual inspection. The degree of area coverage within the Disruptive body pattern delineates three levels of mottled patterns. The light-mottled body pattern exhibits little to no dark chromatic components on the fins, with small darker blotches visible on the mantle. The medium-mottled body pattern displays more blotches on the fins, with dark chromatophores expanding in the disruptive zone. The dark mottled body pattern appears overall dark, reducing the contrast of transverse bars. (A) Sampled original images. (B) Dark chromatic components extracted from the original images. (C) Assessment of the area coverage by the dark chromatic components within the disruptive zone.
Disruptive pattern analysis from dark chromatic components area coverage area. The diagram illustrates the distribution of dark chromatic component area coverage while the animal exhibits a disruptive body pattern (A). Colors in the central image (B) correspond with merged Fig. 3C. Areas a, b, and c appear highly informative and are utilized for the pattern’s quantitative analysis (C).
The Red–Green–Blue (RGB: 0–255) values were imported into R console Version 4.4.1 (R Core Team, 2024). The data was further organized based on random variable individuals (selected high-quality images of whole squids, n = 75) and dependent variable zones (zone a-c; Fig. 4). The Brightness of each data point is calculated (Formula 1) based on greyscale brightness conversion72. The ratio of RGB color components (R%, G%, or B%) was obtained by getting the percentage of each primary color (R, G, or B) in the total RGB sum.
Distributions of dependent variables (R, G, B, Brightness, R%, G%, and B%) were validated using the Shapiro–Wilk test (Table 2) and visualized in Ternary plot (Fig. 5). To examine the Disruptive pattern (light–dark alternating coloration among the zones) the RGB and Brightness values were tested using nested repeated non-parametric test (Friedman test; Table 2), with Nemenyi pairwise post-hoc test for each value category (Table 3).
To observe the color threshold values of Disruptive (Positive) and non-disruptive (Negative) pattern, the Receiver Operating Characteristic (ROC) was utilized (pROC package:73). The statistical test hoped to detect the threshold values (Brightness) separating the Positive and Negative behaviors, by validating it through the Area Under the Curve (AUC, probability of correct prediction), Sensitivity (True Positive Rate), Specificity (True Negative Rate) and Youden Index (Optimal True Positive–Negative rate); 18 measurements were tested for optimal separation values between behaviors (Table 6).
Relationships between three kinds of behavior were studied using R (Supplementary File 1, 2): locomotor components (Sitting, Hovering), arm postures (Curled, Raised, others), and Disruptive chromatic pattern (Yes, No). Contingency tables were constructed based on the pairwise behaviors (locomotor, arms, Disruptive). Chi Squared with Yate’s correction was tested to examine the relationship among the behaviors. Finally, it was followed by standard residual observations to examine the relationship between corresponding behaviors (Tables 4,5).
Results
Situational camouflage
Situational components represent the relation of the squid to its surroundings, allowing and enhancing the motionless camouflage to the substrate. We have observed two main categories of situational camouflage (Figs. 1, 2 and 3; Table 1; Supplementary File 1).
Hovering (Fig. 1j, N = 22, see 10)
The animal uses water flow from its funnel and fin movements to stabilize itself against lateral and longitudinal movement. In this study, Hovering refers to an altitude of no more than roughly the height of the animal (several centimeters) above the substrate. 10% of recorded events of situational motionless camouflage to substrate behavior were Hovering.
Sitting (Fig. 1k, N = 194, see 10)
The animal is sitting directly in full contact with the substrate with little to no movement of its fins or readily detectable movement of the body. 89.8% of all recorded events were Sitting (216 recorded events were recorded in total). The body can be either flattened or contracted with fins either extended or tucked (Fig. 1 M,N).
Interaction with the habitat
Objects (rocks, tree branches, concrete blocks, large underwater surveillance cameras, etc.) were placed in the tanks primarily for environmental enrichment purposes. During the observation period, squids showed preferences (N = 197, 91% next to object) for sitting or hovering next to, touching, inside of, or under an object, as opposed to a site more than half a mantle length far from any object.
Open space (N = 19)
An animal is sitting or hovering in an open space away from any object in 9% of all recorded cases of motionless camouflage to the substrate behavior.
Next to object (N = 197)
Because the highly mobile squid’s natural habitat is a vast ocean, all objects placed in the tanks are close to the squid. For this study, we don’t consider the tank walls as objects. To isolate and describe the object relationship, we decided that the animal needed to be less than half its mantle length away to qualify as "next to an object." In many observed cases, animals had direct physical contact with the object. During the observation period, we noted a striking masquerade behavior on numerous occasions, especially when the white-squid were Sitting next to an object (Fig. 1,2, Supplementary Video 2).
Postural components
While camouflaging to the surrounding structures, the animals displayed four distinctive postures, which are newly observed in the laboratory and described in this species.
Curled arms (N = 94)
The I-IV arms are fanned out asymmetrically to either side of the head or upwards, creating an S-shaped curl. Curled is the most common arm posture displayed by the white-squid during situational motionless camouflage to substrate, comprising 48.5% of all recorded sitting arm postures (194 recorded events).
Raised arms (N = 7)
Posture similar to the “upward pointing” or “upward curl” previously described in the related species S. sepioidea48. Here, all arms are gathered together and point upwards. This posture was observed when an animal was sitting next to an object so that its silhouette fused with the three-dimensional contour of the object. This posture accounted for 3.0% of recorded Sitting postures.
Straight arms (N = 50)
All arms together form a triangle shape pointing forward. If an animal hovers, the arms tend to point slightly downward, presumably allowing water flow from the funnel to provide lift. This accounted for 23.1% of recorded postures.
Spread arms (N = 35)
At least arm III is spread to form a V-shape while the other arms often stay together at the body’s center axis, creating an isosceles triangle. This position accounted for 16% of all Sitting arm postures.
Tucked arms (N = 22)
Arms are bended down and under the head, usually resting on the substrate. The posture is similar to “downward curl” previously described in the related species S. sepioidea48 but the position of arms is affected by touching the substrate. Also, the arms are rather relaxed in this posture.
Chromatic body pattern displays
Using images collected at the OIST Marine Science Station (MSS), we identified the following chromatic patterns displayed by white-squid for situational motionless camouflage: 1) Uniform Pale; 2) Uniform Dark; 3) Disruptive; and 4) Mottled (including—Light, Medium, and Dark).
Uniform Pale (N = 15)
The animal appears pale to transparent on both sides of the mantle, head, and arms, with no detectable expansion of dark chromatophores. This coloration often comprises chromatic components such as a clear head, pale arms and fins; glittering spots; and gold and green eyes. Sometimes, a faint bluish tint and various other complex colorations (yellow, pink, brown, or gray) are visible because the animal is transparent aside from the beak and other internal organs. Shortly after feeding, a squid’s stomach contents may also be visible. We recorded only 15 Uniform Pale events in total, all in combination with Hovering (Table 1).
Uniform dark (N = 47)
The animal’s whole body is dark brown amber to dark reddish brown. At first glance, it appears homogeneously brown in color. Still, upon closer examination high-definition photographs, it is possible to discern glittering spots on its mantle and green eyebrows (markings around the eye.) The dark chromatophores, however, generally obscure these markings. The dark coloration pattern is observed when an animal sits on the substrate near and next to an object in the tank (78.7%).
Mottled (N = 131, see 10)
Mottled patterns have varying degrees of dark brown and reddish-brown speckles distributed across the head, arms, mantle, and fins. Clusters of dark chromatophores produce these speckles. These speckles’ brightness, clarity, and location vary by individual and situation. The overall appearance of Mottled depends on the initial chromatophore activity, which can range from transparent pale to dark brown: the speckles are less apparent against a darker base color than against a lighter base color. We subclassify Mottled further based on the overall visual characteristics: Mottled Light body pattern (62 events), Mottled Medium body patterns (59 events), and Mottled Dark body pattern (10 events; Figs. 3, 4). The light-mottled body pattern has little to no dark chromatic components expressed on the fins, and small darker blotches can be seen on the mantle. The Medium mottled body pattern has more blotches on the fins and dark chromatophores are expanded in the disruptive zone. The Dark mottled body pattern is dark overall reducing the contrast of transverse bars (Figs. 3 and 4).
Disruptive (N = 23, see 10)
The body pattern Disruptive consists of broad, dark brown to black, transverse bars across the mantle. In white-squid, transverse bars appear on the dorsal and ventral sides of the mantle. Transverse bars on the dorsal side extend continuously to the edge of the fins. In addition to transverse bars, posterior bars, and anterior bars are found at both ends of the mantle, and a dark head bar is also expressed. The bars divide the whole animal into ten contrasting sections, alternating light to dark. White-squid expresses “blotchy” bars like those of S. sepioidea74. Disruptive is often difficult to distinguish from the mottled pattern. We define Disruptive as having a clear and high-contrast presence of all five bars across the entire animal width and classifying other, more amorphous expressions as mottled (Fig. 3). For the purpose of describing the difference between Disruptive and Mottled, we have created a template of a fully disruptive body pattern consisting of two transverse mantle bars, anterior and posterior mantle bars, and a head bar (Fig. 4). When these components separate the body into ten areas with high contrast and full coverage of the entire width of the animal, we scored it as disruptive based on subjective visual inspection (N = 15) and we then corrected our observation to N = 23 following the statistical examination below.
Disruptive body principal color (RGB) individual component analysis
This analysis was performed on subset of 75 completely visible squid body images that contained all 15 images identified as disruptive based on visual inspection described above. The principal color (RGB) individual component values showed that Red had the narrowest range (min = 3; max = 176), followed by Green (4; 214) and Blue (5; 243). The grayscale brightness (Brightness) ranges between 6.06 and 256. On the other hand, when observing from the percentage ratio standpoint, Red (min = 5.36%; max = 65.98%) has the largest percentage range, followed by Blue (7.22%; 60.34%) and Green (23.91%; 48.21%) has the narrowest percentage range. Ternary plot (Fig. 5, Supplementary File 1) of the subject zones (a-c) showed that darker grayscale (low Brightness) points were mostly associated with higher Red color ratio, while lighter grayscale (high Brightness) were associated with higher Blue ratio. Green was not the main driver of color differences among the measured points.
Looking into the color and Brightness based on measured zones (Table 2, Fig. 6) showed there were significant differences (Friedman: X^2^ > 42.067, p < 0.001) among the zones for all the dependent variables (Red, Green, Blue, grayscale Brightness). Pairwise comparisons (Table 3) of adjacent zones (a-b, and b-c), indicated all the zones showed distinct Brightness. The pairwise comparison demonstrated that zone a and c are disruptive dark zones, while zone b is the light zone (see also Fig. 7).
Boxplot representing the range and median values of Red (R), Green (G), Blue (B), and Grayscale brightness (Brightness) among the zones (zone a-c). The dark central line of the box represents median values based on the groupings. The extended line represents the line between the Interquartile Range. The points represent outlier/extreme values based on each grouping.
Boxplot representing the range and median values of Red (R), Green (G), Blue (B), and Grayscale brightness (Brightness) among the zones (zone a-c) separated by Non-disruptive and Disruptive subjects. The dark central line of the box represents median values based on the groupings. The extended line represents the line between the Interquartile Range. The points represent outlier/extreme values based on each grouping.
Identification between disruptive and non-disruptive
Based on the visual observation described above, there were 15 individuals identified as fully disruptive, while 60 individuals were considered non-disruptive. The disruptive individuals have significantly lower Blue (Difference = 24; p = 0.037); but no difference with Red (Difference = 3; p = 0.610), and Green (Difference = 8; p = 0.199) and Brightness (Difference = 9; p = 0.098). Examination of Fig. 8 shows that Disruptive individuals have more distinct zone color contrast as compared to non-disruptive individuals.
Scatter plot of Brightness ratio between zone a and b for Disruptive (blue) and non-disruptive (red) chromatic patterns. Threshold (1.36) is the value of cutoff point between the behaviors based on Receiver operating characteristic (AUC = 0.963, Specificity = 86.67%, Sensitivity = 100.00%), validated with Youden’s index (86.67%).
Threshold value for color differences
Eighteen measurements (Table 6) were examined for the accuracy of separating the Disruptive and non-disruptive patterns using ROC. Brightness ratio between zone a and b had highest area under curve (AUC = 0.963), sensitivity (True Positive = 100%), and Youden Index (86.67%). On the other hand, the empirical and percentage values for zone a-b Blue color had the highest specificity (True Negative = 91.67%). Nevertheless, Brightness ratio between zone a and b had the best efficiency in the discrimination threshold (1.360), that caught all the True Positive, albeit with slightly higher false positive (13.33%) identification. This translates to one of the highest overall performances (Youden Index = 86.67%).
Relation between Disruptive chromatic pattern, locomotor components and arm postures The locomotor components (Hovering, Sitting) were significantly (Chi-square: X^2^ = 18.987, df = 2, p < 0.001) related to arm postures (Curled, Raised, others). Curled arms were more related to Sitting subjects (Table 4).
On the other hand, the Disruptive chromatic pattern (Table 5) was not significantly related to a locomotor component (Chi-square: X^2^ = 1.806, df = 1, p = 0.179) nor the arms behavior (Chi-square: X^2^ = 0.949, df = 2, p < 0.622).
Discussion
White-squid often camouflage to substrate31. During adolescence, white-squid start showing the newly described specific behavior: Sitting on the substrate and adjusting their color and shape to the surrounding environment. This Sitting on substrate behavior is very common in all captive adult white-squid of any origin. Over the study period, we have never observed the Sitting on substrate behavior in immature white-squid younger than 60 days with total mantle length under 6 cm and all 194 recorded events of Sitting behaviors examined in this study are shown by white-squid older than 60 days and exceeding 8 cm total mantle length. White-squid in the Ryukyu Archipelago lay their eggs in seagrass meadows at less than 3-m depths39, often in the intertidal zone. The white-squid camouflage-to-substrate behavior is a potentially effective strategy to avoid detection from underwater and aerial predators in shallow water. The appearance of the Sitting behavior could correspond with the return of subadult and adult white-squid from deeper waters to shallower waters of coral lagoons and seagrass beds for reproduction. In this habitat, countershading would be less effective than the camouflage-to-substrate capability of white-squid observed, especially in combination with the Sitting behavior. Conducting further field observations in a comparative study would be meaningful in understanding such behavior and its function better.
Disruptive coloration is a form of camouflage that doesn’t necessarily match the surroundings but still offers a high level of protection by responding to the 3D environment75,76. Unlike distractive markings, disruptive patterning visually disrupts the outline and hinder the true shape of the animal17,77. In the white-squid, the transverse bars are not as cleanly articulated as in the more commonly studied cuttlefish, such as S. officinalis10 and S. pharaonis14. We eliminated the subjective plasticity of visual observation by quantification of the Disruptive chromatic pattern in white-squid.
The cephalopod camouflage behavior is effective against very diverse predators with different, acute vision (often colorblind) in highly variable visual environments of the ocean. Thus, we needed to develop a robust and simple method that identifies and uses a key feature of the Disruptive pattern. We recorded the behaviors occasionally using various consumer-quality products under variable lighting conditions that introduce variation in brightness and light level of each image, without referencing to an RGB calibration standard. First, we described dark and light chromatic components on the white-squid body plan (Fig. 4). We found that the complexity of the Disruptive pattern can be reduced to a contrast (difference in grayscale Brightness) between two specific chromatic components, which is caused by a simultaneous expansion /contraction of brown and red chromatophores on their fin in the central area of their mantle (zones a-b; Table 6, Fig. 7) and identified the Disruptive contrast cutoff point (1.36; Fig. 8). This method can become useful also in future studies of disruptive and distractive patterning.
White-squid effectively camouflages to the substrate using a broad repertoire of body patterns while Sitting. Although the Disruptive patterning is more frequent in the Sitting than in the Hovering position (Table 5), this relationship doesn’t appear statistically significant in our data. However, the locomotor behaviors were significantly (Chi-square: X^2^ = 18.987, df = 2, p < 0.001) related to arm postures.
Similarly to sympatric cuttlefish S. pharaonis14, white-squid frequently displays asymmetrical body patterns, which likely further increases the effectiveness of their camouflage to substrate. Strikingly, all squids in Fig. 2 show asymmetrically Curled arms, which is a newly described postural component (Fig. 1) significantly (p < 0.001) associated with Sitting. Although the chromatic components might often seem rather symmetrical, the white and dark spots within chromatic components of disruptive and mottled patterns are asymmetrically distributed (for examples see Fig. 2A, C, D, E, G; Supplementary Videos 1, 2) which might possibly result from the hyperdisordered growth of white-squid skin78. It is well established that symmetrically patterned camouflaged targets generally have lower survival rates than asymmetrical ones79,80. This is because most substrates are asymmetrical at the spatial scale of the animal, making symmetry a salient Gestalt cue to a predator80,81. Visual and brain lateralizations have been recently discovered in the white-squid57 and their relationships with asymmetrical body patterning remain to be evaluated.
“Masquerade” refers to defense tactics among animals in which an organism adopts features of an inedible or inanimate object to hide from or deter predators3. In cephalopods, such behavior has been documented in cuttlefish and octopuses’ species10,17,82,83,84. White-squid are usually masquerading while Sitting next to an object that they are trying to resemble (Fig. 2G, Supplementary Video 2).
Squids face many predators during their ontogeny, and their defensive strategies change accordingly85. Our study shows that subadult and adult white-squid can camouflage to substrate in various situations. Combined with the smaller number of chromatophores per body surface and the possibility to breed the subjects in captivity, they could be the key to better understanding the amazing and unique system of camouflage in cephalopods. White-squid can be a model animal to study visual perception and neural control of the peripheral muscles trough the central nervous system. Detailed knowledge of squid behavior, in general, enables more accurate monitoring and tracking of multiple species of squid, which represent a vital part of the marine food chain, one of growing importance also for human consumption86,8788. The rare combination of semitransparency with the ability to change body color (metachrosis) found in white-squid makes it an attractive model organism for further research on camouflage to substrate and background matching.
Data availability
All data generated and analysed during this study are included in this published article [and its supplementary information files].
Code availability
Code is included in the Supplementary file 1.
References
Josef, N. & Shashar, N. Camouflage in benthic cephalopods: What does it teach us? In Cephalopod cognition (eds Darmaillacq, A.-S. et al.) (Cambridge University Press, Cambridge, 2014).
Endler, J. A. Interactions between predators and prey. In behavioural ecology: An evolutionary approach 3rd edn (eds Krebs, J. R. & Davies, N. B.) 169–196 (Blackwell, Oklahoma, 1991).
Stevens, M. & Merilaita, S. Animal camouflage: Current issues and new perspectives. Philos. Trans. R. Soc. B 364, 423–427. https://doi.org/10.1098/rstb.2008.0217 (2009).
Stevens, M. & Merilaita, S. Animal camouflage: function and mechanisms. In Animal camouflage: mechanisms and function (eds Stevens, M. & Merilaita, S.) 1–17 (Cambridge University Press, Cambridge, 2011).
Reiter, S. & Laurent, G. Visual perception and cuttlefish camouflage. Curr. Opin. Neurobiol. 60, 47–54. https://doi.org/10.1016/j.conb.2019.10.010 (2020).
Hill, A. V. & Solandt, D. Y. Myograms from the chromatophores of Sepia. J. Physiol. 83, 13P-14P (1935).
Hanlon, R. T. Cephalopod dynamic camouflage. Curr. Biol. 17, R400–R404. https://doi.org/10.1016/j.cub.2007.03.034 (2007).
Barbosa, A. et al. Disruptive coloration in cuttlefish: A visual perception mechanism that regulates ontogenetic adjustment of skin patterning. J. Exp. Biol. 210, 1139–1147. https://doi.org/10.1242/jeb.03341 (2007).
Williams, T. L. et al. Dynamic pigmentary and structural coloration within cephalopod chromatophore organs. Nat. Commun. 10, 1004. https://doi.org/10.1038/s41467-019-08891-x (2019).
Hanlon, R. T. & Messenger, J. B. Adaptive coloration in young cuttlefish (Sepia officinalis L.): The morphology and development of body patterns and their relation to behavior. Philos. Trans. R. Soc. Lond. B 320, 437–487. https://doi.org/10.1098/rstb.1988.0087 (1988).
Ferguson, G., Messenger, J. B. & Budelmann, B. Gravity and light influence the countershading reflexes of the cuttlefish Sepia officinalis. J. Exp. Biol. 191, 247–256. https://doi.org/10.1242/jeb.191.1.247 (1994).
Shohet, A. J., Baddeley, R. J., Anderson, J. C., Kelman, E. J. & Osorio, D. Cuttlefish responses to the visual orientation of substrates, water flow and a model of motion camouflage. J. Exp. Biol. 209, 4717–4723. https://doi.org/10.1242/jeb.02580 (2006).
Chiao, C.-C., Chubb, C. & Hanlon, R. T. Interactive effects of size, contrast, intensity and configuration of background objects in evoking disruptive camouflage in cuttlefish. Vision Res. 47, 2223–2235. https://doi.org/10.1016/j.visres.2007.05.001 (2007).
Nakajima, R. & Ikeda, Y. A catalog of the chromatic, postural, and locomotor behaviors of the pharaoh cuttlefish (Sepia pharaonis) from Okinawa Island. Japan. Mar. Biodivers. 47, 735–753. https://doi.org/10.1007/s12526-016-0523-0 (2017).
Caldwell, R. L., Ross, R., Rodaniche, A. F. & Huffard, C. L. Behavior and body patterns of the larger Pacific striped octopus. PLoS ONE 10, e0134152. https://doi.org/10.1371/journal.pone.0134152 (2015).
Josef, N., Amodio, P., Fiorito, G. & Shashar, N. Camouflaging in a complex environment—octopuses use specific features of their surroundings for background matching. PLoS ONE 7, e37579. https://doi.org/10.1371/journal.pone.0037579 (2012).
Hanlon, R. T. & Messenger, J. B. Cephalopod behaviour 2nd edn. (Cambridge University Press, Cambridge, 2018). https://doi.org/10.1017/9780511843600.
Gutnick, T., Zullo, L., Hochner, B. & Kuba, M. J. The motor and the brake of the squid chromatophore organ. FASEB J. 30, 675 (2016).
Messenger, J. B. Cephalopod chromatophores: Neurobiology and natural history. Biol. Rev. 76, 473–528. https://doi.org/10.1017/S1464793101005772 (2001).
Hanlon, R. T. et al. Rapid adaptive camouflage in cephalopods. In Animal camouflage: Mechanisms and functions (eds Stevens, M. & Merilaita, S.) 145–163 (Cambridge University Press, Cambridge, 2011). https://doi.org/10.1017/CBO9780511852053.008.
Suzuki, M., Kimura, T., Ogawa, H., Hotta, K. & Oka, K. Chromatophore activity during natural pattern expression by the squid Sepioteuthis lessoniana: Contributions of miniature oscillation. PLoS ONE 6, e18244. https://doi.org/10.1371/journal.pone.0018244 (2011).
Reiter, S. et al. Elucidating the control and development of skin patterning in cuttlefish. Nature 562, 361–366. https://doi.org/10.1038/s41586-018-0591-3 (2018).
Woo, T. et al. The dynamics of pattern matching in camouflaging cuttlefish. Nature 619, 122–128. https://doi.org/10.1038/s41586-023-06259-2 (2023).
Mäthger, L. M. & Hanlon, R. T. Malleable skin coloration in cephalopods: Selective reflectance, transmission and absorbance of light by chromatophores and iridophores. Cell Tissue Res. 329, 179–186. https://doi.org/10.1007/s00441-007-0384-8 (2007).
Ghoshal, A., DeMartini, D. G., Eck, E. & Morse, D. E. Optical parameters of the tunable Bragg reflectors in squid. J. R. Soc. Interface 10, 20130386. https://doi.org/10.1098/rsif.2013.0386 (2013).
Cloney, R. & Brocco, S. Chromatophore organs, reflector cells, iridocytes, and leucophores. Am. Zool. 23, 581–592. https://doi.org/10.1093/icb/23.3.581 (1983).
Mäthger, L. M., Denton, E. J., Marshall, N. J. & Hanlon, R. T. Mechanisms and behavioural functions of structural coloration in cephalopods. J. R. Soc. Interface 6, S149–S163. https://doi.org/10.1098/rsif.2008.0366.focus (2009).
Mäthger, L. M. et al. Bright white-scattering from protein spheres in color changing, flexible cuttlefish skin. Adv. Funct. Mater. 23, 3980–3989. https://doi.org/10.1002/adfm.201203705 (2013).
Josef, N., Berenshtein, I., Fiorito, G., Sykes, A. V. & Shashar, N. Camouflage during movement in the European cuttlefish (Sepia officinalis). J. Exp. Biol. 218, 3391–3398. https://doi.org/10.1242/jeb.118950 (2015).
Josef, N. et al. Size matters: observed and modeled camouflage response of European cuttlefish (Sepia officinalis) to different substrate patch sizes during movement. Front. Physiol. 7, 671. https://doi.org/10.3389/fphys.2016.00671 (2017).
Nakajima, R. et al. Squid adjust their body color according to substrate. Sci. Rep. 12, 5227. https://doi.org/10.1038/s41598-022-09209-6 (2022).
Zylinski, S. & Johnsen, S. Mesopelagic cephalopods switch between transparency and pigmentation to optimize camouflage in the deep. Curr. Biol. 21, 1937–1941. https://doi.org/10.1016/j.cub.2011.10.014 (2011).
Staudinger, M. D., Hanlon, R. T. & Juanes, F. Primary and secondary defences of squid to cruising and ambush fish predators: Variable tactics and their survival value. Anim. Behav. 81, 585–594. https://doi.org/10.1016/j.anbehav.2010.12.002 (2011).
Anderson, F. E. Phylogeny and historical biogeography of the loliginid squids (Mollusca: Cephalopoda) based on mitochondrial DNA sequence data. Mol. Phylogenet. Evol. 15, 191–214. https://doi.org/10.1006/mpev.1999.0753 (2000).
Salman, A. New report of the loliginid squid Sepioteuthis lessoniana Lesson, 1830 in the Mediterranean. Isr. J. Zool. 48, 249–250. https://doi.org/10.1560/X4K9-7D1H-17QX-6Y3Q (2002).
Mienis, H. K. New data concerning the presence of Lessepsian and other Indo-Pacific migrants among the mollusks in the Mediterranean Sea with emphasis on the situation in Israel. Turk. J. Aquat. Life 2, 117–131 (2004).
Lefkaditou, E., Corsini-Foka, M. & Kondilatos, G. Description of the first Lessepsian squid migrant, Sepioteuthis lessoniana (Cephalopoda: Loliginidae), in the Aegean Sea (Eastern Mediterranean). Mediterr. Mar. Sci. 10, 87–98. https://doi.org/10.12681/mms.112 (2009).
Jereb, P. & Roper, C. F. E. Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Volume 2. Myopsid and Oegopsid Squids. (FAO, 2010).
Izuka, T., Segawa, S., Okutani, T. & Numachi, K. Evidence on the existence of three species in the oval squid Sepioteuthis lessoniana complex in Ishigaki Island, Okinawa, southwestern Japan, by isozyme analyses. Venus 53, 217–228 (1994).
Izuka, T. Biochemical study of the population heterogeneity and distribution of the oval squid Sepioteuthis lessoniana complex in southwestern Japan. Am. Malacol. Bull. 12, 129–135 (1996).
Cheng, S. H. et al. Molecular evidence for co-occurring cryptic lineages within the Sepioteuthis cf. lessoniana species complex in the Indian and Indo-West Pacific Oceans. Hydrobiologia 725, 165–188. https://doi.org/10.1007/s10750-013-1778-0 (2014).
Okutani, K. Cuttlefishes and squids of the world (Tōkai Daigaku Shuppanbu, 2015). (In Japanese).
Sugimoto, C., Yanagisawa, R., Nakajima, R. & Ikeda, Y. Observations of schooling behaviour in the oval squid Sepioteuthis lessoniana in coastal waters of Okinawa Island. Mar. Biodivers. Rec. 6, e34. https://doi.org/10.1017/S1755267212001159 (2013).
Sugimoto, C. & Ikeda, Y. Ontogeny of schooling behavior in the oval squid Sepioteuthis lessoniana. Fish. Sci. 78, 287–294. https://doi.org/10.1007/s12562-012-0463-y (2012).
Lin, C.-Y., Tsai, Y.-C. & Chiao, C.-C. Quantitative analysis of dynamic body patterning reveals the grammar of visual signals during the reproductive behavior of the oval squid Sepioteuthis lessoniana. Front. Ecol. Evol. 5, 30. https://doi.org/10.3389/fevo.2017.00030 (2017).
Jantzen, T. M. & Havenhand, J. N. Reproductive behavior in the squid Sepioteuthis australis from South Australia: Interactions on the spawning grounds. Biol. Bull. 204, 305–317. https://doi.org/10.2307/1543603 (2003).
Moynihan, M. & Rodaniche, A. F. Communication, crypsis, and mimicry among cephalopods. In How animals communicate (ed. Sebeok, T. A.) 293–302 (Indiana University Press, Bloomington, 1977).
Moynihan, M. & Rodaniche, A. F. The Behavior and natural history of the caribbean reef squid Sepioteuthis sepioidea. With a consideration of social, signal and defensive patterns for difficult and dangerous environments. Verlag Paul Parey, 1982.
Byrne, R. A., Griebel, U., Wood, J. B. & Mather, J. A. Squid say it with skin: a graphic model for skin displays in Caribbean reef squid (Sepioteuthis sepioidea). In Coleoid cephalopods through time (eds Warnke, K. et al.) 29–35 (Berliner Paläobiol. Abh, Berlin, 2003).
Borrelli, L., Gherardi, F. & Fiorito, G. A catalogue of body patterning in cephalopoda (Firenze University Press, Firenze, 2006).
Mather, J. A., Griebel, U. & Byrne, R. A. Squid dances: An ethogram of postures and actions of Sepioteuthis sepioidea squid with a muscular hydrostatic system. Mar. Freshw. Behav. Physiol. 43, 45–61. https://doi.org/10.1080/10236241003660737 (2010).
Baldwin, J. Correlations between enzyme profiles in cephalopod muscle and swimming behavior. Pac. Sci. 36, 349–356 (1982).
Boal, J. G. & Gonzales, S. A. The social behaviour of individual oval squids (Cephalopoda, Teuthoidea, Loliginidae, Sepioteuthis lessoniana) within a captive school. Ethology 104, 161–178. https://doi.org/10.1111/j.1439-0310.1998.tb00059.x (1998).
Wada, T., Takegaki, T., Mori, T. & Natsukari, Y. Alternative male mating behaviors dependent on relative body size in captive oval squid Sepioteuthis lessoniana (Cephalopoda, Loliginidae). Zool. Sci. 22, 645–651. https://doi.org/10.2108/zsj.22.645 (2005).
Sugimoto, C. & Ikeda, Y. Comparison of the ontogeny of hunting behavior in pharaoh cuttlefish (Sepia pharaonis) and oval squid (Sepioteuthis lessoniana). Biol. Bull. 225, 50–59. https://doi.org/10.1086/BBLv225n1p50 (2013).
Barbosa, A., Allen, J. J., Mäthger, L. M. & Hanlon, R. T. Cuttlefish use visual cues to determine arm postures for camouflage. Proc. R. Soc. B 279, 84–90. https://doi.org/10.1098/rspb.2011.0196 (2012).
Sakurai, Y. & Ikeda, Y. Visual and brain lateralization during the posthatching phase in squid under solitary and group conditions. Anim. Behav. 183, 13–28. https://doi.org/10.1016/j.anbehav.2021.10.015 (2022).
Clark, C. W. Marine reserves and the precautionary management of fisheries. Ecol. Appl. 6, 369–370. https://doi.org/10.2307/2269380 (1996).
Mather, J. A. Vigilance and antipredator responses of Caribbean reef squid. Mar. Freshw. Behav. Physiol. 43, 357–370. https://doi.org/10.1080/10236244.2010.526669 (2010).
Wood, J. B., Pennoyer, K. E. & Derby, C. D. Ink is a conspecific alarm cue in the Caribbean reef squid, Sepioteuthis sepioidea. J. Exp. Mar. Bio. Ecol. 367, 11–16. https://doi.org/10.1016/j.jembe.2008.08.004 (2008).
Packard, A. Cephalopods and fish: the limits of convergence. Biol. Rev. 47, 241–307. https://doi.org/10.1111/j.1469-185X.1972.tb00975.x (1972).
Hanlon, R. T. & Messenger, J. B. Cephalopod behaviour (Cambridge University Press, Cambridge, 1996).
Hanlon, R. T. et al. An ethogram of body patterning behavior in the biomedically and commercially valuable squid Loligo pealei off Cape Cod. Massachusetts. Biol. Bull. 197, 49–62. https://doi.org/10.2307/1542996 (1999).
Huffard, C. L. Locomotion by Abdopus aculeatus (Cephalopoda: Octopodidae): Walking the line between primary and secondary defenses. J. Exp. Biol. 209, 3697–3707. https://doi.org/10.1242/jeb.02435 (2006).
Bush, S. L. & Robison, B. H. Ink utilization by mesopelagic squid. Mar. Biol. 152, 485–494. https://doi.org/10.1007/s00227-007-0704-2 (2007).
Harrop, J., Vecchione, M. & Felley, J. D. In situ observations on behaviour of the ommastrephid squid genus Illex (Cephalopoda: Ommastrephidae) in the northwestern Atlantic. J. Nat. Hist. 48, 2501–2516. https://doi.org/10.1080/00222933.2014.937365 (2014).
Sakurai, Y. et al. Todarodes pacificus, Japanese common squid. In Advances in squid biology ecology and fisheries. Part II - Oegopsid Squids (eds Rosa, R. et al.) 249–272 (Nova Science Publishers, Hauppauge, 2013).
Lee, P. G., Turk, P. E., Yang, W. T. & Hanlon, R. T. Biological characteristics and biomedical applications of the squid Sepioteuthis lessoniana cultured through multiple generations. Biol. Bull. 186, 328–341. https://doi.org/10.2307/1542279 (1994).
Walsh, L. S., Turk, P. E., Forsythe, J. W. & Lee, P. G. Mariculture of the loliginid squid Sepioteuthis lessoniana through seven successive generations. Aquaculture 212, 245–262. https://doi.org/10.1016/S0044-8486(02)00027-1 (2002).
Zoral, M. A., Lajbner, Z., Zifcakova, L. & Miller, J. Peracetic acid treatment of squid eggs infected with parasitic copepod (Ikanecator primus gen. et sp. Nov.). Sci. Rep. 14, 14513. https://doi.org/10.1038/s41598-024-65452-z (2024).
Packard, A. & Sanders, G. D. Body patterns of Octopus vulgaris and maturation of the response to disturbance. Anim. Behav. 19, 780–790. https://doi.org/10.1016/S0003-3472(71)80181-9 (1971).
Saravanan, C. Color image to grayscale image conversion. In: 2010 Second International Conference on Computer Engineering and Applications vol. 2 196–199 (IEEE, 2010). https://doi.org/10.1109/ICCEA.2010.197
Robin, X. et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinf 12, 77. https://doi.org/10.1186/1471-2105-12-77 (2011).
Moynihan, M. Conservatism of displays and comparable stereotyped patterns among cephalopods. In Function and evolution in behaviour (eds Baerends, G. et al.) 276–291 (Clarendon Press, Clarendon, 1975).
Merilaita, S. & Lind, J. Background-matching and disruptive coloration, and the evolution of cryptic coloration. Proc. R. Soc. B 272, 665–670. https://doi.org/10.1098/rspb.2004.3000 (2005).
El Nagar, A., Osorio, D., Zylinski, S. & Sait, S. M. Visual perception and camouflage response to 3D backgrounds and cast shadows in the European cuttlefish Sepia officinalis. J. Exp. Biol. 224, jeb238717. https://doi.org/10.1242/jeb.238717 (2021).
Stevens, M. & Merilaita, S. Defining disruptive coloration and distinguishing its functions. Philos. Trans. R. Soc. B 364, 481–488. https://doi.org/10.1098/rstb.2008.0216 (2009).
Ross, R. J. et al. Hyperdisordered cell packing on a growing surface. Phys. Rev. X 15, 021064. https://doi.org/10.1103/PhysRevX.15.021064 (2025).
Cuthill, I. C., Hiby, E. & Lloyd, E. The predation costs of symmetrical cryptic colouration. Proc. R. Soc. B 273, 1267–1271. https://doi.org/10.1098/rspb.2005.3438 (2006).
Wainwright, J. B., Scott-Samuel, N. E. & Cuthill, I. C. Overcoming the detectability costs of symmetrical coloration. Proc. R. Soc. B 287, 20192664. https://doi.org/10.1098/rspb.2019.2664 (2020).
Cuthill, I. C. & Troscianko, T. S. Animal camouflage: Biology meets psychology, computer science and art. Int. J. Des. Nat. Ecodynamics 4, 183–202. https://doi.org/10.2495/DNE-V4-N3-183-202 (2009).
Buresch, K. C. et al. The use of background matching vs. masquerade for camouflage in cuttlefish Sepia officinalis. Vision Res. 51, 2362–2368. https://doi.org/10.1016/j.visres.2011.09.009 (2011).
Hanlon, R. T. et al. Cephalopod dynamic camouflage: Bridging the continuum between background matching and disruptive coloration. Philos. Trans. R. Soc. B 364, 429–437. https://doi.org/10.1098/rstb.2008.0270 (2009).
Panetta, D., Buresch, K. & Hanlon, R. T. Dynamic masquerade with morphing three-dimensional skin in cuttlefish. Biol. Lett. 13, 20170070. https://doi.org/10.1098/rsbl.2017.0070 (2017).
York, C. A. & Bartol, I. K. Anti-predator behavior of squid throughout ontogeny. J. Exp. Mar. Bio. Ecol. 480, 26–35. https://doi.org/10.1016/j.jembe.2016.03.011 (2016).
Arkhipkin, A. I. et al. World squid fisheries. Rev. Fish. Sci. Aquac. 23, 92–252. https://doi.org/10.1080/23308249.2015.1026226 (2015).
Vincent, P. J. P. Innovations in coastal management from unconventional origins: in the pursuit of exploring and managing coastal zones, unconventional approaches and methodologies can act as catalysts for fostering research and innovation. In Constraints and adaptations to global change at the land-sea interface: for a shared ecological and energy transition COAST 2023 (eds Bennis, A. C. et al.) (Springer, Cham, 2025). https://doi.org/10.1007/978-3-031-90050-1_10
Zoral, M. A. et al. Infection of two cestode larvae, Nybelinia enterika sp. nov. and Phoreiobothrium sp. in oval squid Sepioteuthis lessoniana species complex. J. Invertebr. Pathol. 211, 108329. https://doi.org/10.1016/j.jip.2025.108329 (2025).
Acknowledgements
We thank all OIST, especially Physics and Biology Unit, members, present and past, who helped in the initial stage of the squid breeding program. We thank the Marine Science Section staff for their dedicated support, namely Koichi Toda, Nobuo Ueda, and Kosuke Mori. We also thank the Onna Village Fishery Association for their generous help and continuous support, namely Shuichi Mekaru. The Physics and Biology Unit of the Okinawa Institute of Science and Technology Graduate University, OIST Animal Resources Section, and OIST Marine Science Section supported this work.
Funding
The Physics and Biology Unit and the Marine Science Section of the Okinawa Institute of Science and Technology Graduate University supported this work.
Author information
Authors and Affiliations
Contributions
RN, ZL, HBW, JM, TG, and MJK contributed to the conception and design of the study; RN and ZL collected the data and organized the database; TG, KA, TN, and TLI contributed to the data collection; HBW, RN, and ZL performed statistical analyses; RN, HBW, and ZL drew figures; RN and ZL wrote the first draft of the manuscript; HBW, MJK, JM and TG wrote sections of the manuscript. All authors contributed to the manuscript revision and read and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
No competing interests were declared.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Supplementary Video 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nakajima, R., Lajbner, Z., Boo, W.H. et al. Situational motionless camouflage of a loliginid squid. Sci Rep 15, 41203 (2025). https://doi.org/10.1038/s41598-025-25212-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-25212-z