Abstract
Di-n-butyl phthalate (DBP), an environmental endocrine disruptor, can induce male reproductive dysfunction. In the study, the protective mechanism of ginsenoside Rg3 (Rg3) against DBP-induced damage to spermatogenic function was explored through the Nrf2/antioxidant response element (ARE) signaling pathway in mice. The protective effects of Rg3 were analyzed by comparing the quality of sperm, the levels of reproductive hormones and the mRNA and protein expression of Nrf2, NQO1, and StAR in the testicular tissue. The results showed that Rg3 restored the reproductive damage caused by DBP, improved the thesticular structure, enhanced the number and motility of sperm, and upregulated the mRNA expression levels of Nrf2, NQO1, StAR and increased the phosphorylation of proteins in testicular tissue. Ginsenoside Rg3 can activate the Nrf2/ARE pathway, upregulate NQO1, regulate StAR protein expression, and has a relieving effect on DBP-induced reproductive dysfunction in mice. Rg3 may become a potential therapeutic drug for male infertility due to environmental toxicants.
Similar content being viewed by others
Introduction
The infertility rate has been rising year by year in the past 50 years, from 7% to 8% in the early 1900 s to 15% now1.Globally, 724 million people of childbearing age are likely to be infertile2.Male factors account for about half.The causes include heat stress3,environmentalants4,infectious disease5, and so on.Of which, 25 to 87% of male subfertility is considered to be affected by oxidative stress6.At present, oxidative stress, a state associated with cell damage caused by oxygen and oxygen-derived free radicals, has been identified as a mediator of male infertility. As shown in the study by Li et al., endocrine disruptors act on the hypothalamic-pituitary-gonadal axis, increasing the levels of MDA and LDH activity in the hypothalamus, reducing the activity of GSH-Px and SOD, and leading to hormonal imbalance in reproduction7. Therefore, finding an appropriate therapy to improve male reproductive health is an urgent problem to be solved in the clinic. Toxicological experiments have shown that it is possible to protect the testis and epididymis of experimental animals (boars) from oxidative stress damage by the antioxidant capacity, regulating the secretion and expression of inflammatory cytokines8. Twelve small or medium-sized randomized controlled trials also suggested that antioxidant supplementation for male infertility may improve the clinical pregnancy rate of couples attending a clinic9. Therefore, the development of antioxidant substances has a certain prospect for the adjuvant treatment of reproductive system diseases.
With the rapid development of industrialization and social economy, environmental factors have become one of the most important risk factors for male reproductive impairment. Phthalates (PAEs), a class of synthetic compounds, are widely used in various consumer products, and as one of the PAEs, DBP is widely used in global industry. DBP is non-covalently bound to the plastic matrix, so it can easily leach into the environment, causing water, air, soil and packaging materials pollution and threatening human health. Epidemiological evidence shows that long-term exposure to DBP can cause serious damage on various organs of the human body, especially the male reproductive system10. Toxicological evidence indicates that DBP can inhibit key enzymes in male testosterone synthesis, leading to reproductive disorders such as apoptosis of spermatogenic cells and testicular atrophy, reduced sperm motility and sperm count, and induced defects in the sperm formation process11.
Ginsenoside Rg3 is a tetracyclic triterpenoid diol-type saponin monomer in ginseng. It is divided into two isoisomers: 20 (R) Rg3 and 20 (S) Rg3 according to the spatial position difference of hydroxyl group on the 20th carbon atom. Studies have shown that 20(R) Rg3 can reduce tissue damage by inhibiting oxidative stress and inflammation. For example, 20(R)-ginsenoside Rg3 (R-Rg3) can enhance the antioxidant capacity and reduce the inflammatory response in diabetic mice12. Meanwhile, R-Rg3 could inhibit the apoptotic pathway, improve cisplatin-induced nephrotoxicity13 and acetophen-induced hepatotoxicity14. R-Rg3 was also confirmed to inhibit D-galactose-induced damage by regulating oxidative stress15. Therefore, it is necessary to explore its action mechanism in-depth so that its pharmacological effects can be better developed as a dual therapeutic modulator for the treatment of inflammatory and oxidative stress-related diseases.
Oxidative stress damage is a key link in the dysfunction of male reproductive function16. Therefore, in addition to treating the underlying disease or correcting the cause of infertility, it is necessary to maintain the balance between oxidation and antioxidation. Nuclear factor E2-related factor 2 (Nrf2) is a transcription factor that mediates oxidative stress pathways, Activating the NFR and antioxidant response elements pathway, it can promote the expression of antioxidant protein and phase II detoxification enzyme and effectively eliminate free radicals produced by oxidative stress in vivo, thus maintaining the redox state of cells. Nrf2 deficiency reduces antioxidant enzymes in the body, exacerbates the negative effects of oxidative stress in cells, and induces oxidative stress fibrosis17,18. Nrf2 affects the physiology and pathology of testicular dysfunction, particularly in spermatogenesis, through cellular resistance to oxidative stress, inflammation, environmental toxins. Nrf2 activators may have therapeutic effects in the prevention and treatment of testicular dysfunction19. Based on the above literature research, this study will explore the mitigation effect of 20 (R) Rg3 on testicular damage from the Nrf2/ARE pathway, providing basic information for exploring new infertility biomarkers, and experimental basis for Rg3 treatment of male infertility.
Result
Analysis of body weight of mice, testis weight, testis organ coefficient in each group
There was no significant difference in the body weight of mice among the groups by statistical analysis. The testicular weight(P = 0.000) and coefficient of testicular organs(P = 0.001) in the DBP group were significantly lower than those in the control group(, and compared with the DBP group, the testicular weight(P = 0.001) and coefficient of testicular organs (P = 0.031) in the mice were significantly increased. See Table 1.
Analysis of sperm quality results of mice in each group
Compared with the control group, sperm density and sperm motility in the DBP group (400 mg/kg) were significantly decreased (P = 0.000), while sperm density and sperm motility in the Rg3 + DBP group (DBP 400 mg/kg; Rg3 20 mg/kg) were significantly increased (P = 0.000). There was no significant difference in sperm abnormality rate among the groups (P = 0.144), which can be preliminarily inferred that Rg3 has protection on testicular spermatogenesis. See Fig. 1.
Analysis of serum reproductive hormone levels in mice of each group
Compared with the control group, T value and LH value in the DBP group were significantly lower (P = 0.000), the FSH level was significantly higher (P = 0.000), and T value and LH value in the DBP group were significantly higher (P = 0.000), the FSH level was significantly lower (P = 0.000) in the DBP + Rg3 group. See Fig. 2.
Analysis of histological results of testis in mice of each group
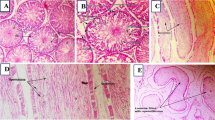
Histological results of the testes showed that the basement membrane and spermatogenic epithelium of the testes in the control group were intact, interstitial cells were evenly arranged, the lumen was filled, and the tissue structure was normal. Compared with the control group, the testicular tissue structure of mice in DBP group had obvious changes, some cells in the lumen dropped off, the number of Sertoli cells decreased obviously, and the arrangement of interstitial cells was disordered. Compared with the DBP group, the testis structure of the DBP + Rg3 group tended to be normal, interstitial cells arranged more evenly, seminiferous tubules arranged more closely and regularly, and there were no exfoliated cells in the lumen. See Fig. 3.
mRNA expression levels of StAR, Nrf2 and NQO1 in the testes of mice in each group
RT-qPCR results showed that StAR(P < 0.001), Nrf2(P < 0.001) and NQO1(P < 0.001) mRNA expression levels in testicular tissue of mice in DBP group were significantly lower than those in the control group, and StAR(P = 0.002), Nrf2(P = 0.035) and NQO1(P < 0.001) mRNA expression levels in testicular tissue of mice in the DBP + Rg3 group were significantly higher than those in the DBP group. See Fig. 4.
Expression levels of p-StAR, p-Nrf2 and p-NQO1 proteins in the testes of mice in each group
Western blotting analysis showed that compared with the control group, the expression levels of p-StAR(P = 0.001), p-Nrf2(P = 0.042) and p-NQO1(P = 0.000) proteins in testicular tissue of DBP group were significantly decreased. Compared with DBP group, the expression levels of p-StAR(P = 0.003), p-Nrf2(P = 0.014) and p-NQO1(P = 0.000) proteins in testicular tissue of DBP + Rg3 group were increased. See Fig. 5.
Electrophoregram (A) and histogram (B) of expressions of p-StAR, p-Nrf2 and p-NQO1 proteins in testicular tissue of mice in various groups. Lane 1–3: Control group; Lane 4–6: DBP group; Lane 7–9: DBP + Rg3 group; use clear delineation by group with dividing lines. *P < 0.05 compared with control group; ∆ P < 0.05 compared with DBP group.
Discussion
Male infertility is a serious global problem. Physiological factors (age, weight, body fat, etc.), pathological factors (metabolic, infection, inflammation, etc.), psychological factors (trauma, stress, etc.), lifestyle (physical exercise, smoking, alcohol, drug use, etc.), and environmental factors (exposure to toxins, heavy metals, radiation, etc.) can all contribute to reproductive dysfunction. Therefore, male infertility is a complex disease influenced by genetic, lifestyle, and environmental factors. In recent years, the role of environmental pollution in exacerbating male reproductive health problems has attracted increasing attention. These pollutants can mimic or block natural hormones, disrupt the normal functioning of the endocrine system, and may cause long-term damage to reproductive health. Endocrine disrupting chemicals (EDCs) such as DEHP can interfere with hormone regulation, leading to adverse reproductive outcomes such as a decrease in sperm count, motility, and quality20. Given the presence of these chemicals in the environment and the potential for chronic exposure, this damaging effect is of concern and alarming. The testes are responsible for sperm production and the production of sex hormones, making them key organs for reproductive health and therefore often studied in reproductive toxicology. Studies have shown that endocrine disruptors can adversely affect testicular function by disrupting hormone balance and cellular processes necessary for sperm production. Research in animal models has demonstrated that endocrine disruptors can lead to impaired testicular structure and function, including altered testosterone synthesis and increased oxidative stress. These findings underscore the importance of investigating the mechanisms by which environmental factors impact testicular health and overall fertility. The body weight, organ weight and organ coefficient of experimental animals are the biological characteristic indexes, which can not only provide basic data for clinical research, but also reflect the nutritional status and disease status of animals. The weight of the testes and the coefficient of organs can reflect the development state of reproductive organs. The results of this study on the reproductive toxicity model of male mice showed that DBP led to a decrease in testicular weight and organ coefficient, testicular tissue structure damage, cell shedding in the lumen, sparse sperm in the lumen, etc. Sperm quality assessment can reflect the final toxic effect of environmental factors on male reproductive damage. We found that DBP can reduce the number and survival ability of sperm, indicating that DBP interferes with the spermatogenic process of sperm. The levels of serum reproductive hormones can reflect the state of testicular damage. Male reproductive function is mainly controlled by the hypothalamic-pituitary-testicular axis. The hypothalamus secretes gonadotropin-releasing hormone (GnRH), which acts on the pituitary gland, and the pituitary gland secretes luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which act on the testes causing the secretion of testosterone(T) hormone. At the same time, the synthesis of testosterone depends on the catalysis of steroidogenic enzymes. The steroidogenic acute regulatory (StAR) protein is the rate-limiting enzyme in testosterone synthesis, and its expression level directly determines the amount of testosterone synthesis. This experiment shows that DBP causes a decrease in both the mRNA and protein of StAR, a decrease of LH, T and an increase of FSH in serum, which suggests that DBP indirectly damages testicular tissue through endocrine disruption.The results of this experimental animal model validated that DBP can affect the testicular structure and functional changes21, and the testes may be a direct target of DBP toxicity22.
Ginseng is a well-known and widely used herbal medicine in Asian countries and ginsenoside Rg3 is a saponin extracted from geng (C. A. Meyer), notoginseng or American ginseng (Panax notoginseng, Panax quinquefolius L.) The compound has a wide range of pharmacological properties, including antioxidant and anti-inflammatory activities23. Inflammation and oxidative stress can cause a variety of acute or chronic diseases, including pneumonia, hepatic and renal damage, cardiovascular and reproductive diseases, metabolic diseases, and tumors. Panaxoside Rg3 plays anti-inflammatory role by inhibiting NF-κB and related factors; plays antioxidant role by regulating N2 pathway and improving enzyme levels; plays anti-diabetic role by promoting insulin secretion through MAPK pathway; plays hepatoprotective role by relieving liver inflammation through inhibiting NF-κB phosphorylation; plays promoting role in wound healing by regulating TGF-β/SMAD signaling pathway; and plays regulating role by regulating immune cells and inhibiting PD-L1 glycosylation. Therefore, it has significant pharmacological effects such as antitumor, anti-inflammatory, antidiabetic, hepatoprotective, wound healing promotion, and immunomodulation, and has been developed as an adjuvant therapy in clinical practice24. In recent years, there has also been certain progress on the auxiliary therapeutic effect of ginseng in the field of reproductive medicine25. Studies have shown that Ginseng can improve sperm quality by inhibiting the apoptosis of sperm26,ginseng extract treated with pectinase (GINST) has antioxidant effects and can effectively treat heat stress-induced damage to the rat testis27, Korean red ginseng (KRG), can prevent testicular damage caused by doxorubicin and aging28. Korean ginseng can induce spermatogenesis29, and crude ginseng extract shows different effects on different layers of the hypothalamic-pituitary-testicular axis, ultimately leading to an increase in testosterone levels and a significant increase in the number and motility of patients’ sperm30。Ginsenosides are one of the compounds with pharmacological activity in ginseng, and ginsenoside Rb can act on the pituitary gonadotropin cells, promote the secretion of luteinizing hormone, increase the level of testosterone, and further promote sperm maturation31. Ginsenoside Rg3 is the most active compound among ginsenosides32. There has also been some progress in the application of Rg3 in the field of reproduction. Rg3 can improve erectile dysfunction in streptozotocin-induced diabetic rats33. Rg3 could improve endometrial lesions by promoting the apoptosis of ectopic endometrial cells34. Rg3-enriched KRG extract from ginseng can play an important role in preventing the changes in sex hormone receptors and spermatogenesis caused by heat stress35. Meanwhile, Rg3 has been shown to protect mouse stromal cells by down-regulating miR-26a and to exert its effects as a facilitator of protein-energy in the G2 phase of the spermatogenic cycle36. This study shows that after Rg3 intervention, there was a significant improvement in the structure of the testis and the number of sperm, the expression level of enzymes for testosterone synthesis was increased, and the level of serum reproductive hormones was improved to a certain extent. These results preliminarily indicate that Rg3 has a role in improving dysfunction.
Oxidative stress is a common pathogenic mechanism of the numerous etiological factors of male infertility. Due to the strong association between Oxidative stress and male infertility, Agarwal et al. coined the term “Male oxidative stress infertility (IOSI)” to emphasize the role of Oxidative stress in male infertility37. Environmental stimuli can disrupt the internal redox balance of organisms, leading to cellular stress responses6. To combat these effects, cells have evolved complex defense mechanisms and the Nrf2/ARE signaling pathway plays a crucial role38。Nrf2 is an important regulatory factor for protection in cells. When cells are subjected to oxidative stress, Nrf2 enters the cell nucleus, regulates the transcription and synthesis of various protective proteins, and promotes the expression of some antioxidant factors by activating the antioxidant response element (ARE), regulating their oxidation and antioxidant effects39. Oxidative stress plays an indispensably important role in testicular toxic damage and is one of the main mechanisms causing reproductive dysfunction40. Inhibition of Nrf2 molecule can promote oxidative stress by interfering with Nrf2 signaling pathway, resulting in the functional disorder of the testis. For example, miR-506-3p can aggravate the oxidative stress damage of DBP-treated testis both in vivo and in vitro by inhibiting the expression of ANXA5 and down-regulating Nrf2/HO-1 signaling pathway41. Nrf2 activators can protect cells from oxidative damage and activate Nrf2/Keap1 signaling, promoting its translocation to the nucleus and the function and expression of Nrf2 and its downstream antioxidant genes, including mainly superoxide dismutase (SOD), heme oxygenase-1 (HO-1), and NAD(P)H quinone reductase 1 (NQO1) protein molecules. These enzymes help to scavenge free radicals and oxidative damage to cells and tissues42,43. For example, USP15 relieved DBP-induced oxidative stress damage by inhibiting the ubiquitination and degradation of Nrf244. 1α, 25(OH)2D3 ameliorates oxidative damage of Sertoli cells induced by perfluorooctane sulfon through the Nrf2-ARE pathway45. Tadalafil up-regulated the gene expression levels of Nrf2 and HO-1 and the activity of HO-1 by activating Nrf/HO-1 pathway, inhibited oxidative stress and apoptosis, reduced the changes in testicular oxidative stress markers caused by cisplatin, alleviated the disorders of sperm number and activity, normalized the level of serum testosterone, improved the changes in epididymis and testicular weight, and restored the normal structure of testicular tissue46. Sulfoglucosamine can protect against testicular oxidative stress damage induced by DBP in male mouse offspring through Nrf2/ARE signaling47. Since plants and their extracts contain a large number of phytochemicals that exhibit antioxidant activity, toxicological experiments have shown that the administration of these antioxidants can activate the Nrf2/ARE pathway, achieving the effect of improving male reproductive function. For example, Polysaccharide from Ostrea rivularis attenuates can activate the Keap1-Nrf2/ARE signaling pathway, increase the expression level of Nrf2 and its downstream ARE gene protein in the testis, reduce the reproductive oxidative stress damage induced by cyclophosphamide, and improve the quality of sperm in the epididymis48. Curcumin protected cadmium-induced testicular damage in mice by activating the Nrf2/ARE pathway, increasing the expression of Nrf2 and the mRNA and protein expression levels of Nrf2 downstream target molecules glutathione peroxidase (GSH-Px) and γ-glutamylsteine synthetase (γ-GCS), and reducing oxidative stress49. Umbelliferone inhibits lead-induced testicular damage in rats by suppressing oxidative stress and inflammation and improving Nrf2/HO-1 signaling50. In conclusion, the activation of Nrf2 signaling could represent a valuable protective strategy against the testicular damage caused by chemical toxicants. In this study, DBP significantly down-regulated the gene expression of Nrf2 and NQO-1 in the testis, while 20 (R) Rg3 treatment obviously reversed the gene expression levels of Nrf2 and NQO-1, indicating that 20 (R) Rg3 protected males from oxidative damage caused by DBP through the activation of Nrf2/ARE pathway. Based on the literature and this study, Nrf2 activators can protect cells from oxidative damage and activate Nrf2/ARE signaling, increase the function and expression of Nrf2 and antioxidant genes of its downstream19. Therefore, Nrf2 activators can play a role in the prevention and treatment of testicular dysfunction, and the activation of Nrf2 can become a promising therapeutic target for testicular dysfunction.
In summary, ginsenoside 20(R) Rg3 can significantly promote the Nrf2/ARE signaling-mediated oxidative response in the testis, up-regulate NQO1 expression, improve testosterone levels, and promote the improvement of sperm quality, thereby alleviating male reproductive damage and achieving the protective effect of Rg3 on testicular damage. The results of this study provide experimental evidence for the application of 20 (R) Rg3 in male reproductive damage.Based on natural antioxidant, RG3 can be used as a potential candidate for male reproductive protection.
This study preliminarily revealed that ginsenoside 20 (R) Rg3 could exert a protective effect on DBP-induced reproductive damage in mice by activating the Nrf2/ARE signaling pathway, but there are still limitations: On the level of mechanism research, the upstream regulatory mechanism of Nrf2 activation (such as the expression and phosphorylation status of Kelch-like ECH- protein 1 (Keap1)) has not been extensively investigated, and there is a lack of direct evidence that Nrf2 directly binds to the promoter region of the StAR gene and regulates its expression, which cannot completely rule out the cross-interference of other signaling pathways (such as MAPK, NFκB, etc.) in this process; On the level of experimental design, this study only used 6-week-old C57BL/6 strain mice as models, and both DBP poisoning and 20 (R) Rg3 intervention used a single dose design, which did not cover different strains of mice, animal models at different stages of life, and did not set up a gradient of DBP poisoning doses and 20 (R) Rg3 intervention doses, making it difficult to clarify the dose-response relationship and safe dose range of 20 (R) Rg3 exerting reproductive protective effects. On the level of functional verification and clinical transformation connection, there is a lack of experimental verification at the cellular level (such as primary spermatogenic cells, interstitial cells), and the key indicators of oxidative stress in reproductive tissues (such as ROS level, MDA content, SOD and GSH-Px activity, etc.) were not systematically detected, which made it difficult to further prove the molecular mechanism of 20 (R) Rg3 playing a role by regulating oxidative stress, and also limited the transformation of research results into clinical applications. Based on the above limitations, future research can be carried out in the following directions: First, using immunoprecipitation (Co-IP), chromatin immunoprecipitation (ChIP), genomics, proteomics, and other technologies, systematically parse the upstream regulatory network of the Nrf2/ARE signaling pathway (such as the dissociation mechanism of the Keap1/Nrf2 complex, PI3K/Akt’s phosphorylation regulation of Nrf2) and the cross-talk mechanism with other signaling pathways, and build a molecular network of 20 (R) Rg3 regulating reproductive damage repair. The second is to carry out cell-level experiments to verify the regulatory effect of 20 (R) Rg3 on the vitality, apoptosis and oxidative stress status of reproductive cells, and to improve its bioavailability by optimizing the preparation process (such as preparing a nano-drug delivery system), and to screen specific biomarkers related to the Nrf2/ARE pathway (such as the expression level of Nrf2, HO-1, StAR), to provide theoretical basis and experimental support for the precise diagnosis and targeted treatment of male infertility.
Materials and methods
Main reagent and instrument
DBP (Santa Cruz Company, USA), 20 (R) Rg3 (Pharmaceutical College of Jilin University), HE stain (Beijing Dingguo Changsheng Company), fluorescence microscope Olympus Corporation, WLJY-9000 Color Sperm Quality Detection System (Beijing Weili Corporation), paraformaldehyde (Beijing Solaibao Technology Co., Ltd.), Eosin dye solution (Sericebio, Sigma, TECAN), Mouse Serum Luteinizing Hormone (LH), Follicle Stimulating Hormone (FSH), Testosterone (T) RIA Kit (Shanghai Jianglai Biological Company); TRIzol reagent, primer synthesis, PCR reaction system, phosphorylated Nrf2 (phosphorylated Nrf2, p-Nrf2), phosphorylated NQO1 (phosphorylated NQO1, p-NQO1), phosphorylated StAR (phosphorylated StAR, p-StAR) and BCA protein quantitative detection kit (Wuhan Sewell Biotechnology Co., Ltd.), reverse transcription reagent, QuantStudio™1Plus real-time fluorescence quantitative PCR system (ThermoFisher, USA).
Experimental animals and their grouping, administration and model Preparation
All animal experimental procedures were approved by the Animal Ethics Committee of Beihua University Jilin, China (CPBHU IACUC 2020–003), which abides by the “Guide for Care and Use of Laboratory Animals.” Animals were bred in a 12-h light/12-h dark cycle, relative humidity was 50–60% and appropriate temperature was 22–25 °C. After the mice were acclimatized in an isolation chamber for one week, thirty clean grade 6-week-old C57BL/6 male mice (provided by Experimental Animal Center of Jilin University, Animal Production License No.: SCXK (Ji) 2018-0001, Animal Use License No.: SYXK (Ji) 2018-0003) were randomly divided into 3 groups: control group, DBP group (400 mg/kg), Rg3 + DBP group (DBP 400 mg/kg; Rg3 20 mg/kg). The preparation of the mouse model of male reproductive injury and the experimental dose of ginsenoside Rg3 in this experiment were based on the results of previous experiments in our group6. DBP was administered by gavage and Rg3 was injected subcutaneously once a day for 35 consecutive days. After an overnight fast, the mice were anesthetized via intraperitoneal injection with sodium pentobarbital at a dose of 30 mg/kg body weight. The mice were sacrificed by cervical dislocation, testicular tissue and epididymis were collected after collecting blood samples from the retroorbital vein. All methods were performed in accordance with the relevant guidelines and regulations and ARRIVE guidelines.
Body weight, testicular weight and the coefficient of organs analysis
At the end of the dosing period, the body weight of the animals was measured.After stunning the animals by dislocating the spinal cord, the bilateral testes were removed and weighed, and the coefficient of the testes was calculated: coefficient of organs = organ weight/body weight × 100%.
Sperm quality analysis
Take the bilateral epididymis of mice, prepare sperm filtrate: put the epididymis into a flat dish, add 3 ml of physiological saline at 37 ℃, cut the epididymal tissue along the lumen, place it for 1–2 min, prepare a sperm suspension drop it onto a pre-warmed sperm counting plate, and use the WLJY-9000 type sperm quality detection system for sperm quality analysis. Select 4 fields of vision and complete the detection within 2 min. Detection indicators: sperm density (1 × 106/mL), spermility (%), total deformity rate (%).
Determination of serum reproductive hormone levels
Blood samples of mice were centrifuged (2500 r/min, 10 min, 4℃) and serum testosterone (T), follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were measured by radioimmunoassay.
Histological analysis of testis
One side of the mouse testis was taken and prepared into testicular tissue section, fixed (4% polyformaldehyde solution), dehydrated, embedded in paraffin, sectioned and stained with hematoxylin and eosin (HE).
Determination of mRNA expression level of StAR, Nrf2, NQO1 in the testis
Total RNA was extracted by Trizol method and reverse transcribed into single-stranded cDNA. GAPDH was used as internal reference. qRT-PCR amplification was performed (predenaturation 95℃, 2 min; 95℃, 10 s; 60℃, 30 s; 40 cycles; 72℃, 10 min). Primer sequence (5’−3’) GAPDH (F) CCTCGTCCCGTAGACAAAATG, (R) TGAGGTCAATGAAGGGGTCGT; StAR(F)GAAGGAAAGCCAGCAGGAGA, ༈R༉TCTGTCCATGGGCTGGTCTA; Nrf2 (F) GATGACCATGAGTCGCTTGC, (R) TATTGAGGGACTGGGCCTGA; NQO1(F)GTAGCGGCTCCATGTACTCTC, (R) GTAGCGGCTCCATGTACTCTC.
Western blotting was used to detect the expression of p-StAR, p-Nrf2 and p-NQO1 protein in the testis of mice in each group
Frozen mouse testicular tissue was added to 4 ℃ RIPA lysate and homogenized in a homogenizer. After centrifugation at 12,000 r/min for 10 min, the supernatant was taken and protein was quantified by the BCA method. Protein was separated by 10% SDS-PAGE, transferred to PVDF membrane, blocked with 5% BSA dissolved in TBST for 1 h, added 1∶1000 p-StAR, p-Nrf2 and p-NQO1 primary antibodies, incubated overnight at 4℃, added 1∶5000 corresponding secondary antibodies the next day, incubated at 37℃ for 1 h, washed 3 times with TBST, developed by ECL, GAPDH was used as an internal reference, and the gray values of StAR, Nrf2 and NQO1 protein bands were analyzed by gel image processing system, and their expressions were calculated.
Statistical analysis
SPSS 26.0 statistical software (SPSS Inc, Chicago, IL, USA) was used for statistical analysis and processing of experimental data.The experimental datawere expressed as mean ± standard deviation.Group variance was performed using one-way ANOVA, while pairwise comparisons between groups were conducted using the LSD-t test, Probability value < 0.05 was considered statistically signiffcant.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author Li Qu on reasonable request.
References
Levine, H. et al. Temporal trends in sperm count: a systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum. Reprod. Update. 29 (2), 157–176 (2023).
Dyer, S. J. International estimates on infertility prevalence and treatment seeking: potential need and demand for medical care. Hum. Reprod. 24 (9), 2379–2380 (2009).
Gao, Y. & Wang C,Wang, K. The effects and molecular mechanism of heat stress on spermatogenesis and the mitigation measures. Syst. Biology Reproductive Med. 68 (5–6), 331–347 (2022).
Seli, D. A. & Taylor, H. S. The impact of air pollution and endocrine disruptors on reproduction and assisted reproduction. Curr. Opin. Obstet. Gynecol. 35 (3), 210–215 (2023).
Zhou et al. Postpartum hemorrhage emerges as a key outcome of maternal SARS-CoV-2 Omicron variant infection surge across pregnancy trimesters. J. Infect. Public Health. 18 (6), 2025102733 (2025).
Dutta, S., Sengupta, P., Slama, P. & Roychoudhury, S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int. J. Mol. Sci. 22 (18), 10043 (2021).
Li, Y. et al. Combined exposure of beta-cypermethrin and Emamectin benzoate interferes with the HPO axis through oxidative stress, causing an imbalance of hormone homeostasis in female rats. Reprod. Toxicol. 123, 108502 (2024).
Tang, W. et al. Glutamate and aspartate alleviate testicular/epididymal oxidative stress by supporting antioxidant enzymes and immune defense systems in boars. Sci. China Life Sci. 63 (1), 116–124 (2020).
De Ligny, W. Et alantioxidants for male subfertility. Cochrane Database Syst. Rev. 5 (5), CD007411 (2022).
Chang, W. H., Herianto, S., Lee, C. C., Hung, H. & Chen, H. L. The effects of phthalate ester exposure on human health: A review. Sci. Total Environ. 786, 147371 (2021).
Pan, J., Liu, P., Yu, X., Zhang, Z. & Liu, J. The adverse role of endocrine disrupting chemicals in the reproductive system. Front. Endocrinol. (Lausanne). 14, 1324993 (2024).
Li, W. L. et al. 20(R)-ginsenoside Rg3 alleviates diabetic retinal injury in T2DM mice by attenuating ROS-mediated ER stress through the activation of the Nrf2/HO-1 axis. Phytomedicine 135, 156202 (2024).
Zhai, J. et al. Ginsenoside Rg3 attenuates cisplatin-induced kidney injury through Inhibition of apoptosis and autophagy-inhibited NLRP3. J. Biochem. Mol. Toxicol. 35 (11), e22896 (2021).
Gao, Y. et al. Ginsenoside Rg3 ameliorates acetaminophen-induced hepatotoxicity by suppressing inflammation and oxidative stress. J. Pharm. Pharmacol. 73 (3), 322–331 (2021).
Li, W. et al. Rare ginsenoside 20(R)-Rg3 inhibits D-Galactose-induced liver and kidney injury by regulating oxidative stress-induced apoptosis. Am. J. Chin. Med. 48 (5), 1141–1157 (2020).
Henkel, R. Oxidative stress and toxicity in reproductive biology and medicine: historical perspectives and future horizons in male fertility. Adv. Exp. Med. Biol. 1358, 1–7 (2022).
Li, X. Q. et al. The dual role of Keap1-Nrf2/ARE signaling pathway in tumors and its relationship with drug resistance. Biotechnol. Bull. 30, 827–832 (2019).
Huang, K. & Zhao, X. USP9X prevents AGEs-induced upregulation of FN and TGF-β1 through activating Nrf2-ARE pathway in rat glomerular mesangial cells. Exp. Cell. Res. 393(2), 112100 (2020).
Rotimi, D. E., Ojo, O. A., Olaolu, T. D. & Adeyemi, O. S. Exploring Nrf2 as a therapeutic target in testicular dysfunction. Cell. Tissue Res. 390 (1), 23–33 (2022).
Hong, Y. et al. Assessing male reproductive toxicity of environmental pollutant di-ethylhexyl phthalate with network toxicology and molecular Docking strategy. Reprod. Toxicol. 130, 108749 (2024).
Nelli, G. & Pamanji, S. R. Di-n-butyl phthalate prompts interruption of spermatogenesis, steroidogenesis, and fertility associated with increased testicular oxidative stress in adult male rats. Environ. Sci. Pollut Res. Int. 24 (22), 18563–18574 (2017).
Lara, N. L. M., van den Driesche, S., Macpherson, S., França, L. R. & Sharpe, R. M. Dibutyl phthalate induced testicular dysgenesis originates after seminiferous cord formation in rats. Sci. Rep. 7 (1), 2521 (2017).
Wang, J. et al. Pharmacological properties, molecular mechanisms and therapeutic potential of ginsenoside Rg3 as an antioxidant and anti-inflammatory agent. Front. Pharmacol. 13, 975784 (2022).
Xu, W. et al. Preparation and bioactivity of the rare ginsenosides Rg3 and Rh2: an updated review. Fitoterapia 167, 105514 (2023).
Ruan, L. L. et al. Metabolic landscape and pathogenic insights: a comprehensive analysis of high ovarian response in infertile women undergoing in vitro fertilization. J. Ovarian Res. 17 (1), 105 (2024).
Eskandari, M. et al. Co-administration of ginseng and Ciprofloxacin ameliorates epididymo-orchitis induced alterations in sperm quality and spermatogenic cells apoptosis following infection in rats. Andrologia 49(3), e12621 (2017).
Kim, M. K. et al. Pectinase-treated Panax ginseng protects heat stress-induced testicular damage in rats. Reproduction 153 (6), 737–747 (2017).
Kopalli, S. R. et al. Korean red ginseng extract rejuvenates testicular ineffectiveness and sperm maturation process in aged rats by regulating redox proteins and oxidative defense mechanisms. Exp. Gerontol. 9, 94e102 (2015).
Park, W. S. et al. Korean ginseng induces spermatogenesis in rats through the activation of cAMP-responsive element modulator (CREM). Fertil. Steril. 88, 1000–1002 (2007).
Salvati, G. et al. Effects of Panax ginseng C.A. Meyer saponins on male fertility. Panminerva Med. 38, 249–254 (1996).
Wang, X. Y. & Zhang, J. T. Effect of ginsenoside Rb1 on sexual function in mice and its mechanism. J. Pharm. Sci. 35, 492–495 (2000).
Su, X., Zhang, D., Zhang, H., Zhao, K. & Hou, W. Preparation and characterization of angiopep-2 functionalized Ginsenoside-Rg3 loaded nanoparticles and the effect on C6 glioma cells. Pharm. Dev. Technol. 25 (3), 385–395 (2020).
Wang, J. et al. Effect of ginsenoside Rg3 on biochemical indexes and pathology in rats with diabetic nephropathy. Adv. Mod. Biomed. 14, 7015–7018 (2014).
Chen, S. H. & Li, Z. A. Study on the mechanism of ginsenoside Rg3 promoting apoptosis in endometriosis stromal cells. Chin. J. Drug Clin. Med. 19, 715–717 (2019).
Kopalli, S. R., Cha, K. M., Hwang, S. Y., Jeong, M. S. & Kim, S. K. Korean red ginseng (Panax ginseng Meyer) with enriched Rg3 ameliorates chronic intermittent heat stress-induced testicular damage in rats via multifunctional approach. J. Ginseng Res. 43, 135–142 (2019).
Liang, H., Zhang, S. & Li, Z. Ginsenoside Rg3 protects mouse Leydig cells against triptolide by downregulation of miR-26a. Drug Des. Devel Ther. 13, 2057–2066 (2019).
Agarwal, A. et al. Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J. Mens Health. 37, 296 (2019).
Shaw, P. & Chattopadhyay, A. Nrf2-ARE signaling in cellular protection: mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 235 (4), 3119–3130 (2020).
Chen et al. Research progress of traditional Chinese medicine for activating Nrf2/are signaling pathway to reduce liver injury. World Sci. Technology-Modernization Traditional Chin. Med. 22, 1102–1107 (2020).
Xi, H. et al. FSH-inhibited autophagy protects against oxidative stress in goat Sertoli cells through p62-Nrf2 pathway. Theriogenology 195, 103–114 (2023).
Tang, M. et al. Overexpression of miR-506-3p Aggravates DBP-induced testicular oxidative stress in rats by downregulating ANXA5 via Nrf2/HO-1 signaling pathway. Oxid Med Cell Longev. :4640605(2020). (2020).
Sun, H. J., Ding, S., Guan, D. X. & Ma, L. Q. Nrf2/Keap1 pathway in countering arsenic-induced oxidative stress in mice after chronic exposure at environmentally-relevant concentrations. Chemosphere.303,135256(2022).
Kurzhagen, J. T. et al. T cell Nrf2/Keap1 gene editing using CRISPR/Cas9 and experimental kidney ischemia-reperfusion injury. Antioxid. Redox Signal. 38, 959–973 (2023).
Zhang, L. et al. USP15 participates in DBP-induced testicular oxidative stress injury through regulating the Keap1/Nrf2 signaling pathway. Sci. Total Environ. 783, 146898 (2021).
Liang, Y. et al. 1α,25(OH)2D3 alleviates perfluorooctane sulfonic acid-induced oxidative injury through the Nrf2-ARE pathway via VDR in Sertoli cells. J. Endocrinol. Invest. 48 (10), 2413–2426 (2025).
Abdel-Wahab, B. A., Alkahtani, S. A. & Elagab, E. A. M. Tadalafil alleviates cisplatin-induced reproductive toxicity through the activation of the Nrf2/HO-1 pathway and the Inhibition of oxidative stress and apoptosis in male rats. Reprod. Toxicol. 96, 165–174 (2020).
Qin, Z. et al. Protective effects of Sulforaphane on di-n-butylphthalate-induced testicular oxidative stress injury in male mice offsprings via activating Nrf2/ARE pathway. Oncotarget 8 (47), 82956–82967 (2017).
Li, S. et al. Polysaccharide from ostrea rivularis attenuates reproductive oxidative stress damage via activating Keap1-Nrf2/ARE pathway. Carbohydr. Polym. 186, 321–331 (2018).
Yang, S. H. et al. Protective role of Curcumin in cadmium-induced testicular injury in mice by attenuating oxidative stress via Nrf2/ARE pathway. Environ. Sci. Pollut Res. Int. 26 (33), 34575–34583 (2019).
Alotaibi, M. F. et al. Umbelliferone inhibits spermatogenic defects and testicular injury in lead-intoxicated rats by suppressing oxidative stress and inflammation, and improving Nrf2/HO-1 signaling. Drug Des. Devel Ther. 14, 4003–4019 (2020).
Acknowledgements
The authors thank all the animals that participated in the experiment.
Funding
This work was supported by Traditional Chinese Medicine Science and Technology Project of Jilin Province(No.2023068, No.2024156); Nature and Science Fund from Jilin Province Ministry of Education (No.JJKH20200075KJ, No.JJKH20220078KJ); the Science and Technological Project of Jilin Province in China (No.20210203187SF); Health Technological Innovation Project of Jilin Province(No.2023JC032).The authors thank for them.
Author information
Authors and Affiliations
Contributions
Conception and design: Huan Li, Li Qu. Animal experiments: Xinyan Luo, Jing Zhang, Lan Lan. Collection and assembly of data: Hongyan Wang, Xinyan Luo, Jing Zhang. Analysis and interpretation: Xinyan Luo, Jing Zhang. Promoting of article and final approval of the article: Huan Li, Li Qu.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, H., Wang, H., lan, L. et al. Protective effects of 20(R) ginsenoside Rg3 on DBP-induced reproductive injury in mice through Nrf2/ARE pathway. Sci Rep 15, 41658 (2025). https://doi.org/10.1038/s41598-025-25606-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-25606-z