Abstract
Baicalin (BA) is an active component of the natural plant Scutellaria baicalensis, which has been used over many years to treat inflammation and respiratory infections. In this study, the sepsis-induced acute lung injury (ALI) mouse model, network pharmacology and molecular docking were used to predict and explore the potential targets and signaling pathways of BA against ALI. The results showed that BA inhibited the major characteristics of ALI, including pathological changes, alveolar-capillary barrier dysfunction, increased inflammatory response and M1 macrophage polarization. Based on network pharmacology and molecular docking analyses, the PI3K/AKT/NF-κB pathway might be an important mechanism by which BA mitigated sepsis-induced ALI. Moreover, BA attenuated the phosphorylation of PI3K, AKT, IκB and NF-κB in the lung tissues of the ALI mice. In conclusion, this study demonstrated that BA ameliorated lung injury in mice, and depressed M1 macrophage polarization through inhibition of the PI3K/AKT/NF-κB pathway.
Similar content being viewed by others
Introduction
Sepsis is a devastative systemic disease resulting from a dysregulated host response to severe infection, and may progress to multi-organ dysfunction syndrome1. The lungs are particularly vulnerable to sepsis, and acute lung injury (ALI) secondary to sepsis is one of the leading causes of death in patients with sepsis2. Although supportive care, anti-inflammatory and antioxidant agents, and antibiotics have been used to treat sepsis-induced ALI, their effectiveness in reducing mortality is limited3,4. Therefore, developing safe and effective drugs or therapeutic strategies to address sepsis-induced ALI is urgently necessary.
Despite numerous studies, the pathogenic mechanism of septic ALI remains incompletely understood5. However, dysregulated immune responses and imbalanced inflammation are considered to be crucial inducers of septic ALI6,7. As critical cells of the innate immune system, macrophages play a crucial role in the development of sepsis-induced ALI by modulating lung inflammation7. Once activated, M1 macrophages release pro-inflammatory mediators and chemokines that promote the infiltration of monocytes and neutrophils into the lungs, amplifying the inflammatory response, which in turn causes tissue damage and leads to the development of ALI8. Therefore, strategies to suppress M1 macrophage polarization are emerging as potential approaches for treatment or prevention of ALI.
Baicalin (BA, Fig. 1A) is an active component of the natural plant Scutellaria baicalensis, which has been used over many years to treat inflammation and respiratory infections9. As its key active flavonoid component, BA has been reported to have various biological properties, mediating anti-inflammatory, antioxidation, anti-tumor, anti-viral, antibacterial, and neuroprotective effects10,11,12,13,14,15. Based on its anti-inflammatory and antioxidation activity, BA is commonly used in the treatment of several lung diseases16,17. For example, BA halts the development of pediatric asthma by upregulating miR-103 and inhibiting the Toll-like receptor (TLR) 4/Nuclear factor-kappa B (NF-κB) axis18. BA inhibits inflammation and oxidative stress in rats with chronic obstructive pulmonary disease via the TLR2/Myeloid differentiation factor 88/NF-κB axis inhibition19. BA mitigates bleomycin-induced early pulmonary fibrosis in mice by activating the mitochondrial ATP-sensitive potassium signaling pathway20. Moreover, BA has also been shown to attenuate lipopolysaccharide-induced ALI in mice by inhibiting the NF-κB and mitogen-activated protein kinase pathways21, activating the Nuclear factor erythroid-related factor 2/Heme oxygenase-1 pathway22. Considering that the protective effect of BA on cecal ligation and puncture (CLP) induced septic ALI has not been studied from the perspective of macrophage polarization, especially how BA regulates macrophage polarization to protect lung injury has not been thoroughly studied. Therefore, the role and mechanism of BA for protection against sepsis-induced ALI deserve further in-depth study.
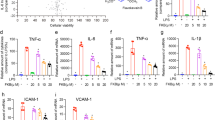
Baicalin (BA) alleviated sepsis-induced lung damage in cecal ligation and puncture (CLP) mice. (A) The chemical structure of BA. (B-E) Representative images of hematoxylin and eosin stained lung sections from four experimental groups. (B) Control group; (C) BA group; (D) CLP group; (E) BA + CLP group. (F) Lung injury score (n = 6). (G) Lung wet/dry weight ratio (n = 6). (H) Bronchoalveolar lavage fluid protein concentration (n = 6). (I) Total cell number in BALF (n = 6). (J-K) RT-PCR analysis of TNF-α and IL-6 mRNA expression in lung tissues (n = 6). (L-N) The protein levels of TNF-α and IL-6 was detected by western blot assay (n = 3). Data are means ± standard errors. ##P < 0.01 versus the control group, *P < 0.05, **P < 0.01 versus CLP group.
In this study, the sepsis-induced ALI mouse model, network pharmacology and molecular docking were used to predict and explore the potential targets and signaling pathways of BA against ALI, so as to provide the novel insights of BA in the treatment of sepsis-induced ALI.
Results
BA alleviated sepsis-induced lung damage in CLP mice
To assess the protective impact of BA against sepsis-induced lung tissue damage, hematoxylin and eosin (HE) staining was performed on mice lung tissues. The outcomes presented clear alveolar structure with little histological change in the sham group and BA groups, while in the CLP group, lung structure was damaged with inflammatory cell infiltration, thickened alveolar septa, alveolar congestion and hemorrhage (Fig. 1B-D). In contrast, pretreatment with BA markedly attenuated lung histopathological damage caused by sepsis (Fig. 1E). Consistently, the increased lung injury score was dramatically decreased by BA pretreatment (Fig. 1F). In addition, the lung wet/dry (W/D) ratio and bronchoalveolar lavage fluid (BALF) protein concentration were evidently increased in septic mice (Fig. 1G-H), whereas BA reduced both measures, suggesting an attenuation of lung edema and alveolar protein leakage and an improvement in alveolar-capillary barrier dysfunction.
BA ameliorated inflammation in CLP mice
Production of high levels of inflammatory factors is closely associated with the development of septic lung injury. Therefore, the effect of BA on inflammatory response in CLP mice was investigated. Cell counts in BALF demonstrated a significant increase in inflammatory cells in the CLP group (Fig. 1I). In contrast, BA pretreatment significantly reduced the number of inflammatory cells. As shown in Fig. 1J-K, mice in the BA + CLP group exhibited lower mRNA expressions of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in lung tissue than those in the CLP group. Moreover, the results of TNF-α and IL-6 expression in lung tissues detected by western blot were consistent with RT-qPCR (Fig. 1L-N), which suggested that BA inhibited CLP-induced generation of inflammatory factors in lung tissues.
BA inhibited M1 macrophage polarization in CLP mice
Considering the important role of M1 macrophage-mediated inflammation in the development of ALI, we next explored the effect of BA on macrophage M1 polarization in vivo. As shown in Fig. 2A-B, the mRNA expression levels of inducible nitric oxide synthase (iNOS) and CD86 were considerably higher in the CLP group than in the sham group. However, pretreatment with BA significantly reduced CLP-induced mRNA levels of iNOS and CD86. Moreover, the results of iNOS and CD86 expression in lung tissues detected by western blot and immunofluorescence (IF) assay were consistent with RT-qPCR (Fig. 2C-I), which suggested that BA suppressed macrophage polarization toward M1.
Baicalin inhibited inflammatory responses and macrophages M1 polarization in cecal ligation and puncture (CLP) mice. (A-B) RT-PCR analysis of iNOS and CD86 mRNA expression in lung tissues (n = 6). (C-E) The protein levels of iNOS and CD86 were detected by western blot assay (n = 3). (F-I) Immunofluorescence analysis of the expression of iNOS and CD86 (n = 3). Data are means ± standard errors. ##P < 0.01 versus the control group, *P < 0.05, **P < 0.01 versus CLP group.
Network pharmacology and molecular docking analysis of BA
To investigate the mechanism of action of BA in sepsis-induced ALI, network pharmacology and molecular docking methods were employed to predict and explore potential targets and signaling pathways. In the present study, 298 drug-related targets of BA were retrieved from three drug databases (TCMSP, PharmMapper, Swiss Target Prediction), 1574 sepsis-related targets were obtained from three disease-related databases (GeneCards, OMIM, DisGeNET) and 1533 ALI-related targets were detected from three disease-related databases (GeneCards, OMIM, DisGeNET). A total of 66 potential targets of BA in the treatment of ALI were identified by Venn analysis (Fig. 3A). Subsequently, by visualization and analysis of these 66 intersecting genes, a BA-ALI-related network was generated and the top 5 core genes were screened: AKT1, SRC, TNF, CASP3 and MMP9 (Fig. 3B-C). The gene ontology (GO) biological process enrichment analysis of the intersection targets revealed that BA might impact protein kinase B signaling (Fig. 3D). The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis revealed that BA might regulate phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway (Fig. 3E). These results suggested that BA could alleviate lung inflammation by modulating the PI3K/AKT pathway.
Network pharmacologic analysis of Baicalin (BA) in the treatment of sepsis-induced acute lung injury (ALI). (A) Venn diagram of common targets of BA and sepsis-induced ALI. (B) PPI network analysis of common targets of BA and sepsis-induced ALI. (C) The hub targets of BA against sepsis-induced ALI. (D) The top 10 enriched GO (BP, CC, and MF) items. (E) KEGG sankey diagram of the common targets of BA and sepsis-induced ALI.
In addition, studies have shown that inhibition of PI3K/AKT/NF-κB pathway could inhibit M1 macrophage polarization and reduce inflammation23,24,25. Interestingly, molecular docking results revealed that BA had good binding affinity with targets PI3K, AKT, inhibitor of kappa B (IκB) and NF-κB. Among them, BA binds to PI3K through two hydrogen bonds (amino acid residues SER-774, VAL-851) with the average binding energy of −9.0 kcal/mol (Fig. 4A-C), BA binds to AKT through two hydrogen bonds (amino acid residues THR-82, TYR-272) with the average binding energy of −10.4 kcal/mol (Fig. 4D-F), BA binds to IκB through five hydrogen bonds (amino acid residues THR-168, THR-169, HIS-173, SER-204, LEU-205) with the average binding energy of −6.0 kcal/mol (Fig. 4G-I), BA can also bind to NF-κB through four hydrogen bonds (amino acid residues GLU-49, ASP-53, ILE-224, GLU-225) with the average binding energy of −8.3 kcal/mol (Fig. 4J-L). So, BA is a potential drug candidate which could act on PI3K/AKT/NF-κB signaling pathway. Therefore, we selected the PI3K/AKT/NF-κB pathway for detailed mechanistic analysis.
Molecular docking and interaction analysis. (A) Docking of Baicalin (BA) with PI3K (PDB ID: 8EXL). (B-C) 3D and 2D docking pattern and molecular interactions of BA and PI3K. (D) Docking of BA with AKT (PDB ID: 3O96). (E-F) 3D and 2D docking pattern and molecular interactions of BA and AKT. (G) Docking of BA with IκB (PDB ID: 1NFI). (H-I) 3D and 2D docking pattern and molecular interactions of BA and IκB. (J) Docking of BA with NF-κB (PDB ID: 1IKN). (K-L) 3D and 2D docking pattern and molecular interactions of BA and NF-κB.
BA inhibited PI3K/AKT/NF-κB signaling pathway in vivo
To illuminate the protective mechanism of BA on sepsis-induced ALI, western blotting was used to examine the expression of PI3K/AKT/NF-κB signaling pathway-related proteins in mouse lung tissues. As depicted in Fig. 5A-F, the expression of p-PI3K, p-AKT, p-IκB, and p-NF-κB was markedly increased in the CLP group compared with the sham group; however, these effects were remarkably reversed by BA. Moreover, the results of p-NF-κB expression in lung tissues detected by IF assay were consistent with western blot (Fig. 5G-H). These results indicated that BA could regulate macrophage M1 polarization by inhibiting the PI3K/AKT/NF-κB pathway, thereby attenuating the inflammatory response in septic mice in vivo.
Baicalin (BA) inhibited PI3K/AKT/NF-κB signaling pathway in the lungs of cecal ligation and puncture (CLP) mice. (A-F) The protein levels of p-PI3K, p-AKT, p-IκB, and p-NF-κB were detected by western blot assay. (G-H) Immunofluorescence analysis of the expression of p-NF-κB. Data are means ± standard errors (n = 3). ##P < 0.01 versus the control group, *P < 0.05, **P < 0.01 versus CLP group.
Discussion
Sepsis is the third major contributor to hospital deaths and ALI is a leading cause of death in septic patients26. In recent years, the application of natural active ingredients from medicinal plants in the treatment of ALI caused by sepsis has attracted wide attention27. Previous studies have shown that BA exerts a protective effect against experimental ALI21,28. However, the particular mechanism remains unclear. Thus, elucidating the beneficial effects and the underlying mechanisms of BA on alleviating septic ALI would promote the discovery of novel therapeutic strategies for ALI.
In this study, the sepsis-induced ALI mouse model was constructed to explore the protective effects of BA against ALI and its potential effects on M1 macrophage polarization. Network pharmacology and molecular docking were then used to excavate the key therapeutic targets of BA, as well as the potential signaling pathways through which BA regulates M1 macrophage polarization to alleviate lung injury. Subsequently, experimental validation verified speculation on the underlying mechanism of BA in the treatment of ALI. The experimental results showed that pretreatment with BA inhibited the major characteristics of ALI, including pathological changes, alveolar-capillary barrier dysfunction, increased inflammatory response and M1 macrophage polarization. Further mechanistic studies indicated that BA might regulate macrophage polarization through the PI3K/AKT/NF-κB signaling pathway in CLP-induced ALI.
Notably, the time point selected for evaluating lung injury and inflammation is 24 h post-CLP, which is an essential aspect of validating BA’s protective effects. This selection was determined by the pathophysiology of ALI. Specifically, this time point corresponds to the peak of the exudative phase, which is characterized by robust neutrophil infiltration and inflammatory cytokine release29. Furthermore, previous studies in CLP-induced ALI models have shown that both inflammatory mediator levels and histopathological alterations are prominently present at 24 h30,31. Consequently, this time point was chosen for our study.
Macrophages are highly plastic and are competent to respond to the microenvironment in ALI by converting to a classically activated (M1) state32. Accumulating evidence indicates that overactivation of M1 macrophages is engaged in the pathogenesis of ALI33,34,35. In this study, M1 polarization and the associated inflammatory response were evaluated using four specific markers. The rationale for selecting these specific markers is as follows: iNOS is a key enzyme in M1 macrophages that produces nitric oxide (NO) from L-arginine and is highly expressed in the pro-inflammatory M1 phenotype36. In contrast, M2 macrophages metabolize arginine to ornithine and urea via arginase37. This difference in arginine metabolism pathways is a classic phenotypic feature distinguishing M1 from M2. CD86 is constitutively expressed on antigen-presenting cells such as M1 macrophages and is rapidly induced during infection38. This upregulation is associated with the early phase of immunity38. Its elevated expression on M1 macrophages reflects their role in initiating and amplifying immune reactions, a key feature of M1 polarization39. Thus, iNOS and CD86 were selected to accurately define the M1 phenotype by targeting two key facets: metabolic function (NO production) and immune activation (antigen presentation). As prototypical pro-inflammatory cytokines, TNF-α and IL-6 are key mediators in initiating, amplifying, and maintaining the inflammatory response during ALI. These cytokines are elevated in the BALF of acute respiratory distress syndrome (ARDS) patients and are associated with poor clinical outcomes. Notably, IL-6 and TNF-α also emerge as promising molecular biomarkers for predicting the morbidity and mortality of ALI/ARDS. Therefore, the mRNA and protein expression levels of TNF-α, IL-6, iNOS, and CD86 were determined in lung tissue. In this study, pretreatment with BA significantly decreased the mRNA and protein levels of pro-inflammatory cytokines (TNF-α and IL-6) and M1 macrophage markers (iNOS and CD86). These findings suggested that BA protected against sepsis-induced ALI by inhibiting M1 macrophage polarization and reducing inflammatory cytokines production.
Network pharmacology is an innovative and effective approach to predict potential targets and mechanisms of action of BA in the context of sepsis-induced ALI from a macroscopic perspective. Through protein-protein interaction (PPI) construction and analysis, 5 core targets, namely AKT1, SRC, TNF, CASP3, and MMP9, were screened out to be closely relevant to BA against ALI. Among them, AKT1 is the most relevant target of BA and a core element of PI3K/AKT signaling pathway. AKT is known as protein kinase B, and activated AKT is closely associated with different biological activities such as metabolism, growth, and proliferation40. Interestingly, GO analysis revealed that AKT signaling played an important role in the biological processes of BA-ALI network. Following KEGG pathway enrichment analyses illustrated that PI3K/AKT pathway probably played a crucial role in BA protection against sepsis-induced ALI. Moreover, the molecular docking result showed that BA forms hydrogen bonds with two amino acid residues of AKT and they have a strong binding interaction. Therefore, we hypothesized that BA could exert a protective effect against ALI by regulating the PI3K/AKT pathway.
PI3K/AKT pathway plays an important role in mitigating ALI by attenuating lung inflammation41,42. As we know, NF-κB pathway is not only a classical inflammatory pathway but also one of the important downstream pathways of PI3K/AKT pathway43. Excitation of the PI3K/AKT pathway speeds up IκB degradation and contributes to the phosphorylation of NF-κB p65, thereby promoting the transcriptional activity of NF-κB and increasing the release of inflammatory factors24,44. Previous research has indicated that NF-κB pathway could regulate the activation and polarization of macrophages, thereby playing a crucial role in macrophage-mediated inflammatory responses45,46. Recent study has also revealed that PI3K/AKT/NF-κB pathway was involved in the regulation of M1 macrophage polarization. Wang et al. found that Shenhuang plaster alleviated postoperative ileus inflammation by inhibiting M1 macrophage polarization through the PI3K/AKT/NF-κB pathway23. In addition, increasing studies indicated that drugs could attenuate ALI by inhibiting the PI3K/AKT/NF-κB pathway42,47. Therefore, it was hypothesized that BA might regulate PI3K/AKT/NF-κB, thereby inhibiting M1 macrophage polarization, ameliorating the inflammatory response and contributing to the recovery of damaged lung tissue after CLP surgery. To further validate the findings from the network pharmacology and molecular docking, we measured the expression of PI3K/AKT/NF-κB pathway-related proteins in lung tissues. As expected, the experimental results showed that pretreatment with BA significantly inhibited the phosphorylation levels of the PI3K, AKT, and NF-κB proteins. Notably, BA also exhibited inhibition of M1 macrophage polarization and suppression of inflammatory factor production. Therefore, the current study revealed that BA pretreatment could inhibit M1 macrophage polarization by regulating the PI3K/AKT/NF-κB signaling pathway, thereby alleviating sepsis-induced lung injury.
Our study has several limitations. First, in addition to the PI3K/AKT/NF-κB pathway, other signaling pathways may also be involved in the mechanism of action of BA in regulating macrophage M1 polarization, which needs to be further investigated. Second, it remains unknown whether BA can promote the repair of damaged lung tissue by regulating macrophage M2 polarization in lung tissue. Third, in this study, we investigated the effect and mechanism of BA in protecting against sepsis-induced ALI in mice; however, we did not explore the therapeutic effect of BA on sepsis-induced ALI. Fourth, the sample size per group was relatively small. This decision was based on the effect sizes observed in preliminary experiments and resource constraints. Nevertheless, the statistically significant differences observed in key outcomes provide preliminary evidence supporting our conclusions.
In conclusion, as illustrated in Fig. 6, this study demonstrated that BA ameliorated lung injury in mice, and depressed M1 macrophage polarization through inhibition of the PI3K/AKT/NF-κB pathway. These findings provide strong support for the potential application of BA in preventing sepsis-induced ALI. Furthermore, our findings suggest that future research could explore the correlation between the proportion of M1 macrophages in the BALF of patients with sepsis-associated ALI and disease severity, and also provide a direction for developing new drugs that protect against lung injury by targeting the PI3K/AKT/NF-κB pathway and macrophage polarization.
Materials and methods
Reagents
BA (purity > 98%) was supplied by Biopurify Phytochemicals Ltd. (Chengdu, China). NovoStart®SYBR qPCR SuperMix Plus and NovoScript®Plus All-in-one1st Strand cDNA Synthesis SuperMix Kit were obtained from novoprotein (Suzhou, China). Antibodies against iNOS, CD86, PI3K, p-PI3K, AKT, p-AKT, NF-κB, p-NF-κB, IκB and p-IκB were acquired from Affinity Biosciences (Liyang, China). Antibodies against TNF-α, IL-6 and AKT were provided by Proteintech (Wuhan, China). Anti-β-actin and BCA protein assay kit were purchased from Abbkine Scientific Co., Ltd. (Wuhan, China). HRP-conjugated secondary antibodies were purchased from Bioworld Biotechnology (Nanjing, China).
Animals
Male C57BL/6J mice (6–7 weeks, weighing 18–20 g) were obtained from Henan Skobes Biotechnology Co., Ltd. (Anyang, China) and maintained under specific pathogen-free conditions at controlled conditions (22–26 °C, at 50–60% humidity) with a 12-hour dark-light cycle. All experimental procedures were approved by the Animal Experimental Ethics Committee of the First Affiliated Hospital of Xinxiang Medical University (No. EC-024–309), which strictly followed the Guide for Care and Use of Laboratory Animals.
Experimental procedures
After a week of adaptive feeding, C57BL/6J mice were randomly divided into 4 groups (n = 6): sham, BA, CLP, CLP + BA, using a random number table, by an investigator not involved in subsequent procedures. CLP was performed to construct a septic ALI mice model, as previously described48. C57BL/6J mice were anesthetized with isoflurane. A midline abdominal incision (1.5–2 cm) was made under sterile conditions. The cecum was exposed and ligated at the distal 1/3 with 4 − 0 silk thread, and punctured with a 21-gauge needle through the blind end. A small quantity of fecal contents was squeezed out, and the cecum was repositioned. Mice in the sham and BA groups underwent laparotomy with exposure of the cecum only, without ligation and puncture.
For treatment administration: BA and vehicle solutions were prepared by an experimenter blinded to group assignments (solutions labeled with coded labels), and injections were administered by another researcher who only knew the solution codes and followed a pre-determined dosing schedule. Mice in the BA and BA + CLP groups were intravenously administered BA (100 mg/kg) once a day for 3 consecutive days. The last administration of BA was 1 h before CLP surgery. The dosage and dosing regimen of BA in our study were determined based on previously published literature49 and our own preliminary experiments. Twenty-four hours after surgery, mice were euthanized by inhaling an overdose of isoflurane, and then lung tissues and BALF were obtained. For outcome assessment: lung tissue histopathological evaluation was conducted by two independent pathologists blinded to group assignments; for biochemical and molecular analyses, samples were coded prior to analysis, with data collected by researchers blinded to group assignments. All blinding codes were unblinded only after data collection and statistical analyses were completed.
Histological analysis
For histological analysis, the left lung was immersed in 4% formaldehyde solution overnight at room temperature, dehydrated in a graded series of alcohol concentrations, embedded in paraffin, and then paraffin sections (5 μm) were prepared. The slices were stained with HE and visualized using a microscope. Lung injury scores were calculated based on previous literature50.
Lung W/D weight ratio
To assess the severity of lung edema, the W/D ratio was computed. The excised mice lungs were washed with saline and excess moisture was absorbed with filter paper. The wet lungs were weighed immediately and then dried at 80 °C for 48 h to yield dry lungs. After weighing again, the W/D ratio of lungs was determined.
BALF acquisition and analysis
Twenty-four hours after surgery, bronchoalveolar lavage was performed with saline to obtain BALF51. A cervical incision was made with surgical scissors to expose the trachea. A 22-gauge intravenous indwelling needle was used for tracheal intubation, which was then ligated with silk suture. The bronchoalveolar lumen was lavaged three times with 1 ml of normal saline, and the lavage fluid was recovered. The recovered BALF was centrifuged at 500×g for 10 min at 4 °C to separate the supernatant, and then protein concentration in BALF was quantified using a BCA protein assay kit (Abbkine Scientific Co., Ltd., China). Meanwhile, the cell pellets were harvested and resuspended in saline, and the cells were counted.
RNA isolation and real-time PCR
Collect total RNA from lung tissues using TriQuick Reagent (Solarbio Science & Technology Co., Ltd., China). Thereafter, cDNA was synthesized using NovoScript®Plus All-in-one1st Strand cDNA Synthesis SuperMix Kit. Quantitative real-time PCR analysis for iNOS (specific primers: forward 5’-ACTCAGCCAAGCCCTCACCTAC-3’, reverse 5’-TCCAATCTCTGCCTATCCGTCTCG-3’), CD86 (specific primers: forward 5’-AGGCAGCACGGACTTGAACAAC-3’, reverse 5’-GTCTCCACGGAAACAGCATCTGAG-3’), TNF-α (specific primers: forward 5’-GGACTAGCCAGGAGGGAGAACAG-3’, reverse 5’-GCCAGTGAGTGAAAGGGACAGAAC-3’), IL-6 (specific primers: forward 5’-GAAACCGCTATGAAGTTCCTCTCTG-3’, reverse 5’-GTATCCTCTGTGAAGTCTCCTCTCC-3’) and β-actin (specific primers: forward 5’-TATGCTCTCCCTCACGCCATCC-3’, reverse 5’-GTCACGCACGATTTCCCTCTCAG-3’) were conducted using NovoStart®SYBR qPCR SuperMix Plus on a QuantStudioTM Dx Real-Time System (Applied Biosystems, USA). Relative mRNA expression of iNOS, CD86, TNF-α and IL-6 were calculated with the 2−ΔΔCT calculation method, using the β-actin to normalize values.
Immunofluorescence
After dewaxing and rehydrating the paraffin slices of lung tissue, the antigen was repaired with citrate buffer. Following culturing with 3% hydrogen peroxide for 10 min, lung tissue slices were treated with 0.3% TritonX-100 for 10 min. After that, these sections were blocked in 5% BSA solution for 1 h and incubated with anti-CD86 antibody (1:300) or anti-iNOS antibody (1:300) overnight at 4 °C. Before nuclear staining, the fluorescent-labelled secondary antibody was added and incubated for 1 h. Images were acquired using a fluorescence microscope and subsequently analyzed using Image-Pro Plus 6.0 software.
Western blot analysis
The total protein from mice lung was extracted and lysed in a protease and phosphatase inhibitor cocktail-contained RIPA buffer, and protein concentration was determined by BCA assay. The same amount of protein was loaded onto SDS-PAGE gel and blotted onto polyvinylidene fluoride membrane. Membranes underwent a blocking process with 5% nonfat milk for 2 h, and then incubated with primary antibodies against (TNF-α (1:2000), IL-6 (1:1000), iNOS (1:2000), CD86 (1:1000), p-PI3K (1:1000), PI3K (1:1000), p-AKT (1:1000), AKT (1:5000), p-IκB (1:1000), IκB (1:1000), p-NF-κB (1:1000), NF-κB (1:1000), β-actin (1:10000)) overnight at 4 ℃. The blots were subjected to 4 washes with TBST and further incubated with horseradish peroxidase-linked secondary antibody for 1 h at room temperature. After chemiluminescence, the grayscale values of protein bands were determined using ImageJ software (Version 1.52 a).
Network pharmacology
Targets associated with BA were retrieved from Traditional Chinese Medicine Systems Pharmacology (TCMSP, https://old.tcmsp-e.com/tcmsp.php), PharmMapper Server (http://www.lilab-ecust.cn/pharmmapper/) and Swiss Target Prediction databases (http://www.swisstargetprediction.ch/). Additionally, DisGeNET (https://www.disgenet.org/), GeneCards (https://www.genecards.org/) and OMIM (https://omim.org/) databases, with “sepsis” and “acute lung injury” as key words, were used to collect disease-associated targets. Subsequently, Venn diagram analysis was performed to identify the intersection of drug-related targets and disease-related targets. Overlapping targets were considered to be potential targets of BA for septic ALI treatment. Then, PPI analysis on these overlapping targets was obtained from STRING platform (https://string-db.org/), and Cytoscape 3.7.2 was used for PPI network construction and core target screening. The overlapping targets of BA and sepsis-induced ALI were imported into David online software (https://david.ncifcrf.gov/tools.jsp) for GO and KEGG pathway analysis52. Subsequently, the results were imported into bioinformatics (http://bioinformatics.com.cn/) for visual analysis.
Molecular docking
The crystal structures of the PI3K (Protein Data Bank [PDB]: 8EXL), AKT1 (PDB: 3O96), IκB (PDB: 1NFI) and NF-κB (PDB: 1IKN) that are involved in the regulation of inflammatory responses and macrophage polarization were retrieved from the PDB database (https://www.rcsb.org/). Water molecules and ligands in the target proteins were eliminated using the PyMOL 2.4 (https://pymol.org/2/). The molecular structure of BA was downloaded through the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Subsequently, BA and target proteins were repaired using the AutoDock Tools 1.5.7. Docking was executed using AutoDock Vina 1.2.1, and visualization was performed with PyMOL 2.4 and discovery studio 2021 software.
Statistical analysis
Data were shown as means ± standard errors. SPSS 25.0 software was used for processing and analyzing data. Statistical analyses were conducted using one-way analysis of variance followed by Tukey’s post-hoc test or Kruskal-Wallis test followed by Mann-Whitney U test. P < 0.05 indicates a statistical difference.
Ethics approval
The study was carried out in compliance with the ARRIVE guidelines. The study was approved by the Ethics Committee of the First Affiliated Hospital of Xinxiang Medical University (No. EC-024–309). All the methods used in this study were performed in accordance with relevant guidelines and regulations.
Data availability
All data supporting the results of this study are openly accessible and available upon reasonable request from the corresponding author (Z.J.D.).
References
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315, 801–810. https://doi.org/10.1001/jama.2016.0287 (2016).
Fujishima, S. et al. Infection site is predictive of outcome in acute lung injury associated with severe sepsis and septic shock. Respirology 21, 898–904. https://doi.org/10.1111/resp.12769 (2016).
Chen, Y. et al. Mechanism of exosomes from adipose-derived mesenchymal stem cells on sepsis-induced acute lung injury by promoting TGF-β secretion in macrophages. Surgery 174, 1208–1219. https://doi.org/10.1016/j.surg.2023.06.017 (2023).
Meyer, N. J., Gattinoni, L. & Calfee, C. S. Acute respiratory distress syndrome. Lancet 398, 622–637. https://doi.org/10.1016/s0140-6736(21)00439-6 (2021).
Tian, J. et al. Effects of the PI3K/Akt/HO-1 pathway on autophagy in a sepsis-induced acute lung injury mouse model. Int. Immunopharmacol. 124, 111063. https://doi.org/10.1016/j.intimp.2023.111063 (2023).
Lelubre, C. & Vincent, J. L. Mechanisms and treatment of organ failure in sepsis. Nat. Rev. Nephrol. 14, 417–427. https://doi.org/10.1038/s41581-018-0005-7 (2018).
Liao, Q. et al. Activation of toll-like receptor 4/nuclear factor-kappa B signaling by triggering a receptor expressed on myeloid cells 1 promotes alveolar macrophage M1 polarization and exacerbates septic acute lung injury. J. Gene Med. 26, e3650. https://doi.org/10.1002/jgm.3650 (2024).
Qiao, X. et al. Grape seed Proanthocyanidin ameliorates LPS-induced acute lung injury by modulating M2a macrophage polarization via the TREM2/PI3K/AKT pathway. Inflammation 46, 2147–2164. https://doi.org/10.1007/s10753-023-01868-5 (2023).
Zhao, Q., Chen, X. Y. & Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. (Beijing). 61, 1391–1398. https://doi.org/10.1007/s11434-016-1136-5 (2016).
Lu, Q. et al. Baicalin attenuates lipopolysaccharide-induced intestinal inflammatory injury via suppressing PARP1-mediated NF-κB and NLRP3 signalling pathway. Toxicon 239, 107612. https://doi.org/10.1016/j.toxicon.2024.107612 (2024).
Wang, H., Ma, J., Li, X., Peng, Y. & Wang, M. FDA compound library screening Baicalin upregulates TREM2 for the treatment of cerebral ischemia-reperfusion injury. Eur. J. Pharmacol. 969, 176427. https://doi.org/10.1016/j.ejphar.2024.176427 (2024).
Wen, R. J. et al. Baicalin induces ferroptosis in osteosarcomas through a novel Nrf2/xCT/GPX4 regulatory axis. Phytomedicine 116, 154881. https://doi.org/10.1016/j.phymed.2023.154881 (2023).
Feng, H. et al. Baicalin protects broilers against avian coronavirus infection via regulating respiratory tract microbiota and amino acid metabolism. Int. J. Mol. Sci. 25, 2109. https://doi.org/10.3390/ijms25042109 (2024).
Cui, X. D. et al. Synergistic antibacterial activity of Baicalin and EDTA in combination with colistin against colistin-resistant Salmonella. Poult. Sci. 102, 102346. https://doi.org/10.1016/j.psj.2022.102346 (2023).
Zheng, Y. et al. Upregulation of Nrf2 signaling: a key molecular mechanism of baicalin’s neuroprotective action against diabetes-induced cognitive impairment. Biomed. Pharmacother. 174, 116579. https://doi.org/10.1016/j.biopha.2024.116579 (2024).
Sharawi, Z. W. et al. Baicalin and lung diseases. Naunyn Schmiedebergs Arch. Pharmacol. 397, 1405–1419. https://doi.org/10.1007/s00210-023-02704-1 (2024).
Wang, D. & Li, Y. Pharmacological effects of Baicalin in lung diseases. Front. Pharmacol. 14, 1188202. https://doi.org/10.3389/fphar.2023.1188202 (2023).
Zhai, C. & Wang, D. Baicalin regulates the development of pediatric asthma via upregulating microRNA-103 and mediating the TLR4/NF-κB pathway. J. Recept Signal. Transduct. Res. 42, 230–240. https://doi.org/10.1080/10799893.2021.1900865 (2022).
Ju, J., Li, Z. & Shi, Q. Baicalin inhibits inflammation in rats with chronic obstructive pulmonary disease by the TLR2/MYD88/NF-κBp65 signaling pathway. Evid. Based Complement. Alternat. Med. 2022, 7273387. https://doi.org/10.1155/2022/7273387 (2022).
Han, M. et al. Baicalin alleviates bleomycin-induced early pulmonary fibrosis in mice via the MitoKATP signaling pathway. Toxicology 497–498, 153638. https://doi.org/10.1016/j.tox.2023.153638 (2023).
Shen, B. et al. Baicalin relieves LPS-induced lung inflammation via the NF-κB and MAPK pathways. Molecules 28, 1873. https://doi.org/10.3390/molecules28041873 (2023).
Meng, X., Hu, L. & Li, W. Baicalin ameliorates lipopolysaccharide-induced acute lung injury in mice by suppressing oxidative stress and inflammation via the activation of the Nrf2-mediated HO-1 signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 392, 1421–1433. https://doi.org/10.1007/s00210-019-01680-9 (2019).
Liu, T. et al. Shenhuang plaster ameliorates the inflammation of postoperative ileus through inhibiting PI3K/Akt/NF-κB pathway. Biomed. Pharmacother. 156, 113922. https://doi.org/10.1016/j.biopha.2022.113922 (2022).
Wang, J. et al. FAM76B regulates PI3K/Akt/NF-κB-mediated M1 macrophage polarization by influencing the stability of PIK3CD mRNA. Cell. Mol. Life Sci. 81, 107. https://doi.org/10.1007/s00018-024-05133-2 (2024).
Deng, S. et al. Lactobacillus acidophilus and its metabolite ursodeoxycholic acid ameliorate ulcerative colitis by promoting Treg differentiation and inhibiting M1 macrophage polarization. Front. Microbiol. 15, 1302998. https://doi.org/10.3389/fmicb.2024.1302998 (2024).
Yang, R., Zheng, T., Xiang, H., Liu, M. & Hu, K. Lung single-cell RNA profiling reveals response of pulmonary capillary to sepsis-induced acute lung injury. Front. Immunol. 15, 1308915. https://doi.org/10.3389/fimmu.2024.1308915 (2024).
Gao, M. et al. Kaempferol mitigates sepsis-induced acute lung injury by modulating the SphK1/S1P/S1PR1/MLC2 signaling pathway to restore the integrity of the pulmonary endothelial cell barrier. Chem. Biol. Interact. 398, 111085. https://doi.org/10.1016/j.cbi.2024.111085 (2024).
Bai, C. et al. Protective effect of Baicalin against severe burn–induced remote acute lung injury in rats. Mol. Med. Rep. 17, 2689–2694. https://doi.org/10.3892/mmr.2017.8120 (2018).
Mendez, J. L. & Hubmayr, R. D. New insights into the pathology of acute respiratory failure. Curr. Opin. Crit. Care. 11, 29–36. https://doi.org/10.1097/00075198-200502000-00005 (2005).
Hu, M. D. et al. Pretreatment with anti-flagellin serum delays acute lung injury in rats with sepsis. Inflamm. Res. 61, 837–844. https://doi.org/10.1007/s00011-012-0475-1 (2012).
Fang, W. et al. Modulation of mitogen–activated protein kinase attenuates sepsis–induced acute lung injury in acute respiratory distress syndrome rats. Mol. Med. Rep. 16, 9652–9658. https://doi.org/10.3892/mmr.2017.7811 (2017).
Yan, Z. et al. Reyanning mixture inhibits M1 macrophage polarization through the glycogen synthesis pathway to improve lipopolysaccharide-induced acute lung injury. J. Ethnopharmacol. 328, 118005. https://doi.org/10.1016/j.jep.2024.118005 (2024).
Chen, X. et al. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm. Res. 69, 883–895. https://doi.org/10.1007/s00011-020-01378-2 (2020).
Meng, Y., Kong, K. W., Chang, Y. Q., Deng, X. M. & Yang, T. Histone methyltransferase SETD2 inhibits M1 macrophage polarization and Glycolysis by suppressing HIF-1α in sepsis-induced acute lung injury. Med. Microbiol. Immunol. 212, 369–379. https://doi.org/10.1007/s00430-023-00778-5 (2023).
He, S. et al. Nicotinamide mononucleotide alleviates endotoxin-induced acute lung injury by modulating macrophage polarization via the SIRT1/NF-κB pathway. Pharm. Biol. 62, 22–32. https://doi.org/10.1080/13880209.2023.2292256 (2024).
Wang, X. et al. HMGN2 regulates non-tuberculous mycobacteria survival via modulation of M1 macrophage polarization. J. Cell. Mol. Med. 23, 7985–7998. https://doi.org/10.1111/jcmm.14599 (2019).
Deng, Z., Kim, H. K. W., Hernandez, P. A. & Ren, Y. Fat phagocytosis promotes anti-inflammatory responses of macrophages in a mouse model of osteonecrosis. Cells 13, 1227. https://doi.org/10.3390/cells13141227 (2024).
Newton, S. et al. Sepsis-induced changes in macrophage co-stimulatory molecule expression: CD86 as a regulator of anti-inflammatory IL-10 response. Surg Infect. (Larchmt). 5, 375–383. https://doi.org/10.1089/sur.2004.5.375 (2004).
Moreno-Fierros, L. et al. Cry1Ac protoxin from Bacillus Thuringiensis promotes macrophage activation by upregulating CD80 and CD86 and by inducing IL-6, MCP-1 and TNF-α cytokines. Int. Immunopharmacol. 17, 1051–1066. https://doi.org/10.1016/j.intimp.2013.10.005 (2013).
Hemmings, B. A. & Restuccia, D. F. The PI3K-PKB/Akt pathway. Cold Spring Harb Perspect. Biol. 7, a026609. https://doi.org/10.1101/cshperspect.a026609 (2015).
Jiang, L. et al. Elucidating the role of Rhodiola rosea L. in sepsis-induced acute lung injury via network pharmacology: emphasis on inflammatory response, oxidative stress, and the PI3K-AKT pathway. Pharm. Biol. 62, 272–284. https://doi.org/10.1080/13880209.2024.2319117 (2024).
Wang, L., Jiang, S., Li, X., Lin, T. & Qin, T. Astringin protects LPS-induced toxicity by suppressing oxidative stress and inflammation via suppression of PI3K/AKT/NF-κB pathway for pediatric acute lung injury. Naunyn Schmiedebergs Arch. Pharmacol. 396, 2369–2377. https://doi.org/10.1007/s00210-023-02439-z (2023).
Liu, S. et al. Magnolia officinalis alcohol extract alleviates the intestinal injury induced by Polygala tenuifolia through regulating the PI3K/AKT/NF-κB signaling pathway and intestinal flora. Drug Des. Devel Ther. 18, 1695–1710. https://doi.org/10.2147/dddt.S461152 (2024).
Jia, X. et al. Quercetin attenuates Pseudomonas aeruginosa-induced acute lung inflammation by inhibiting PI3K/AKT/NF-κB signaling pathway. Inflammopharmacology 32, 1059–1076. https://doi.org/10.1007/s10787-023-01416-5 (2024).
Capece, D. et al. NF-κB: blending metabolism, immunity, and inflammation. Trends Immunol. 43, 757–775. https://doi.org/10.1016/j.it.2022.07.004 (2022).
Yang, Z. et al. Dihydromyricetin inhibits M1 macrophage polarization in atherosclerosis by modulating miR-9-mediated SIRT1/NF-κB signaling pathway. Mediators Inflamm. 2023, 2547588. https://doi.org/10.1155/2023/2547588 (2023).
He, X. et al. Quassinoids from Brucea Javanica and attenuates lipopolysaccharide-induced acute lung injury by inhibiting PI3K/Akt/NF-κB pathways. Fitoterapia 153, 104980. https://doi.org/10.1016/j.fitote.2021.104980 (2021).
Zhou, W. et al. Chromofungin, a chromogranin A-derived peptide, protects against sepsis-induced acute lung injury by inhibiting LBP/TLR4-dependent inflammatory signaling. Eur. J. Pharmacol. 958, 176043. https://doi.org/10.1016/j.ejphar.2023.176043 (2023).
Long, Y. et al. Baicalin Liposome Alleviates Lipopolysaccharide-Induced Acute Lung Injury in Mice via Inhibiting TLR4/JNK/ERK/NF-κB Pathway. Mediators Inflamm. 2020, 8414062. https://doi.org/10.1155/2020/8414062 (2020).
Barreto, T. R. et al. Repeated Domperidone treatment modulates pulmonary cytokines in LPS-induced acute lung injury in mice. Int. Immunopharmacol. 56, 43–50. https://doi.org/10.1016/j.intimp.2018.01.009 (2018).
Kan, W. et al. Glycyrrhiza uralensis polysaccharides ameliorate acute lung injury by inhibiting the activation of multiple inflammasomes. J. Funct. Food. 100, 105386. https://doi.org/10.1016/j.jff.2022.105386 (2023).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–D677. https://doi.org/10.1093/nar/gkae909 (2025).
Acknowledgements
The first author would like to express gratitude to his friend, Zeyun Li at the First Affiliated Hospital of Zhengzhou University for proofreading the paper. Moreover, we thank the editors and reviewers for their guidance.
Funding
This research was funded by the Medical Science and Technology Research Project of Henan Province, grant number LHGJ20240476.
Author information
Authors and Affiliations
Contributions
Conceptualization, P.W.W., T.T.Z., X.H.Z. and Z.J.D.; Project administration, P.W.W. and Z.J.D.; Data curation, P.W.W., R.Z., M.M.L. and J.L.G.; Formal analysis, P.W.W. and R.Z.; Investigation, P.W.W., R.Z., M.M.L., Y.H.Z., X.H.L., C.X.L., Y.Y.Z., T.T.Z., X.H.Z. and Z.J.D.; Methodology P.W.W. and M.M.L.; Validation, P.W.W.; Software, P.W.W., R.Z. and M.M.L.; Writing – original draft, P.W.W., J.L.G. and Y.H.Z.; Writing – review & editing, P.W.W., T.T.Z., X.H.Z. and Z.J.D.; Supervision, T.T.Z., X.H.Z. and Z.J.D. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, P., Zhang, R., Liu, M. et al. Exploring the effect and mechanism of baicalin on sepsis-induced acute lung injury based on network pharmacology and experimental verification. Sci Rep 15, 41959 (2025). https://doi.org/10.1038/s41598-025-25943-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-25943-z