Abstract
To investigate the effectiveness of 80% ethanol and 2% chlorhexidine gel in reducing Pseudomonas aeruginosa (P. aeruginosa) biofilm formation on different types of customized implant abutments, using the crystal violet staining method. Three types of implant abutments were tested: Ti-base, monoblock titanium, and zirconia hybrid abutment. A total of 84 abutments were prepared and divided into four groups for each abutment type (n = 7): negative control (no biofilm), positive control (biofilm, no disinfection), ultrasonic cleaning with 80% ethanol for 5 min, and immersion in 2% chlorhexidine gel for 10 min. All abutments were incubated with P. aeruginosa for 24 h. Biofilm formation was quantified using the crystal violet staining method, and bacterial adhesion was confirmed in the positive control group via scanning electron microscopy. Statistical analysis was performed using one-way ANOVA and Tukey’s test. There was no significant difference in bacterial retention among the different abutment designs tested (p > 0.05). The analyses revealed that both cleaning methods significantly reduced biofilm formation in all abutment types (p < 0.05) but were not superior to each other (p > 0.05). Both ethanol- and chlorhexidine-based disinfection protocols were effective in reducing Pseudomonas aeruginosa biofilm formation on different abutment designs under in-vitro conditions.
Similar content being viewed by others
Introduction
Abutments are a crucial component in dental implant procedures1, and the biological properties of their surfaces play a critical role in determining the long-term success of implant therapy2.
Osteoblasts and gingival cells located on the support surface ensure osseointegration and healthy healing of the gingival tissue around the abutment3,4. In particular biofilm formation is a parameter that affects the osseointegration process5.
Compared with the periodontal tissue surrounding natural teeth, the soft tissue surrounding an implant is composed of more hypovascular and hypocellular tissue6. Studies have shown that this tissue around the implant results in a lower degree of immunological defense against bacteria7,8. Factors such as poor oral hygiene, improper loading protocols, or misdirection of the gingiva can damage peri-implant tissues3,5.
Implant abutments can be prefabricated or customized1. Owing to their simplicity and lower cost than other types, prefabricated titanium abutments are the most frequently used9. Nevertheless, these abutments are limited to cement-retained restorations and require ideal implant placement in terms of depth, emergence profile, and diameter for the restored edentulous area10. However, in many cases, the need to individualize the emergence profile for improved biological and aesthetic outcomes, limited interocclusal distance and difficulty in removing excess cement necessitates the use of custom abutments11. Custom implant abutments can be manufactured by computer-aided design/computer-aided manufacturing (CAD/CAM) systems in addition to traditional laboratory procedures, owing to continuous advances in computer technologies and dental materials12. Today, customized abutment designs with different types and material properties can be used1. Custom abutments traditionally milled in a metal can cause unnatural bluish discoloration of the gingival mucosa, especially in patients with a thin gingival biotype in the anterior region13.
Nowadays, dentists’ requirements for suitable abutments in the aesthetic region have been largely addressed by the use of titanium-based (ti-base) abutments1. All-ceramic abutments or crowns placed over ti-base abutments are frequently preferred, particularly for their superior aesthetic performance. Several in vitro and clinical studies have shown that zirconia abutments offer better color matching with peri-implant soft tissues and result in less gingival discoloration compared to titanium abutments, especially in thin gingival biotypes14,15,16. Patient-reported outcomes have also confirmed that zirconia abutments are associated with more satisfactory aesthetic results17. In addition to their aesthetic advantages, these abutments provide the ability to restore soft tissue anatomical contours, create an optimal emergence profile, avoid visible material at the implant-abutment connection level, allow for extraoral cementation under controlled laboratory conditions, and demonstrate favorable biomechanical properties18.
Two designs are possible for restorations using ti-base. 1st Design: The hybrid abutment-crown joint design, which is produced as a single piece that is bonded directly to the ti-base abutment and then screwed to the implant. 2nd Design: The hybrid abutment acts as a mesostructure bonded to the ti-base abutment and screwed to the implant, followed by a separate all-ceramic crown cemented on top19. With a digital workflow, hybrid supports provide multiple advantages, including enhanced mechanical properties, ease of use, and time efficiency12.
In in-vitro studies, various types of debris have been detected on the surfaces of titanium and zirconia CAD/CAM milled customized implant supports prepared by various manufacturers20. Roughening, micro-etching debris, organic, and inorganic residues related to the manufacturing process were observed on all the prepared abutments. Machining residues, coolants, or chemical washing procedures following milling can lead to debris accumulation as a byproduct of surface treatment during production21.
Different abutment materials result in significant differences in cell adhesion depending on their surface properties and microstructural structures22. Materials such as metal and zirconia differ from each other in terms of surface roughness, chemical composition, hydrophilic/hydrophobic properties and microtopography. These factors affect how cells attach to the support surface and to what extent these cells colonize the abutment surface7.
During the construction of the final implant prosthesis, the abutment and superstructure are repeatedly transported back and forward between the laboratory and the clinic. This has been found to cause contamination of the support surface with various microorganisms and debris23. Microorganisms and their endotoxins increase osteoclastic activity and damage implant stability24. For this reason, abutments received from dental laboratories should be decontaminated before bonding with the implant. Additionally, studies have demonstrated that decontaminating implant abutments enhances soft tissue attachment and helps preserve marginal bone around the implants8,20,25.
The best approach to prevent biofilm formation on implants is to use antibacterial biomaterials or surface modifications26. The International Organisation for Standardisation (ISO 17664:2021) has approved cleaning and disinfection procedures for semi-critical medical devices such as CAD/CAM milling implant abutments27. These procedures stimulate either ultrasonic cleaning with approved disinfectants or sterilization of components at 134 ˚C28,29,30,31,32. However, there is no fully consistent statement in the literature concerning the sterilization and disinfection of implant abutments12,21,33.
Various disinfection strategies have been proposed for implant abutments. These include ultrasonic cleaning, chlorhexidine application, autoclaving, as well as more advanced methods such as plasma, UV light, ozone, and laser decontamination21,34. However, advanced technologies may not always be readily available or practical in routine clinical settings. In contrast, simpler protocols such as ultrasonic cleaning with ethanol and chlorhexidine gel application are widely accessible and commonly used35. Despite this, the scientific evidence supporting their effectiveness in reducing biofilm formation on different abutment designs remains limited. When microbial studies on implant abutments are analyzed in the literature, microbial cleaning effects have been investigated by using different oral pathogens on the implant-abutment junction area34,36 or on material surfaces of various compositions prepared in disc form37,38. Studies investigating the biofilm formation of the entire surface in the form of abutments with different cleaning methods are very limited20,39.
This study aimed to comparatively investigate the effectiveness of various cleaning procedures that can be easily applied in clinical practice on the biofilm formation of different materials and types of custom abutments. The first null hypothesis of the study is that different types of abutments will not affect biofilm formation, and the second null hypothesis is that various cleaning procedures will have not effect on biofilm formation.
Materials and methods
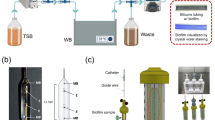
In this study, the effects of three different abutment types (ti-base, titanium and zirconia hybrid abutment) and two different cleaning procedures (ultrasonication with ethanol and soaking in 2% chlorhexidine gel) on P. aeruginosa biofilm formation was investigated. The minimum sample size was determined as n = 7 per subgroup using G*Power software (effect size = 0.50, α = 0.05, power = 80%). Considering 3 abutment types and 4 experimental groups (including negative and positive controls), the total sample size was N = 84. The abutment materials used in the study and their contents are shown in Table 1. The flow of this study is presented in Fig. 1.
An 11.5 mm long, 4.5 mm diameter Zimmer Tapered Screw-Vent implant (Zimmer Dental Inc., Carlsbad, CA, USA) was embedded in an autopolymerized acrylic resin block at a 90˚ angle. For the production of ti-base zirconia hybrid abutments, after the ti-base abutment was screwed onto the implant model, the scan piece was placed. Then, three-dimensional (3D) models were obtained by scanning optically with the Cerec 3 CAD/CAM system (Sirona Dental System Gmbh, Bensheim, Germany). In these models, a zirconia coping hybrid abutment part was designed with a ridge-type chamfer, ridge width, and gingival height of 1 mm. The zirconia coping design was manufactured from partially sintered inCoris ZI meso L blocks (Sirona Dental System Gmbh, Bensheim, Germany) in a Cerec milling machine (inLab MC XL Sirona Dental System Gmbh, Bensheim, Germany). The samples were subsequently sintered in an oven (inFire HTC Speed Sirona Dental System Gmbh, Bensheim, Germany) at 1580 ˚C for 2 h as recommended by the manufacturer.
Dual-cure resin cement (Bifix QM, VOCO GmbH, Germany) was used to bond the zirconia coping to the prefabricated ti-base. The internal surface of the zirconia coping was air‑abraded with 50 μm alumina particles at approximately 2 bar pressure for 10 s from a distance of about 10 mm. After cleaning, a ceramic primer provided in the Bifix QM system was applied and allowed to react for 60 s before gentle air‑drying. The Ti‑base surface was cleaned with alcohol and air‑dried. Dual‑cure resin cement (Bifix QM, VOCO GmbH) was then applied to the intaglio surface of the zirconia coping. The coping was seated on the ti‑base under uniform finger pressure, excess cement was removed, and the assembly was light cured from four directions (20 s each) using an LED curing light. All procedures were performed by the manufacturer’s instructions to ensure optimal bonding and clinical relevance. This protocol was consistently applied to all zirconia hybrid abutments to maintain standardization and minimize surface variability.
Since the Sirona Cerec 3 system cannot mill titanium blocks, the data of the ti-base zirconia samples designed with the Cerec 3 CAD/CAM system (Sirona Dental System Gmbh, Bensheim, Germany) were transferred to the Avamill Chrome CAD/CAM system (Lansing, MI, USA) in standard tessellation language (STL) to ensure standardization. Monoblock titanium abutments were manufactured on a five-axis Avamill Chrome milling machine using Kera Ti 5 titanium discs (Eisenbacher Dentalwaren ED GmbH, Wörth am Main, Germany).
All the obtained abutments were sterilized in an autoclave (Strong 23 LT, Strong) at 134 °C (200 kPa) for 20 min following ISO 17664:2021 standards21,40 to prevent contamination and ensure standardization before microbiological testing. Each abutment type was then randomly divided into subgroups according to the cleaning procedures to be performed (Table 2).
Pseudomonas aeruginosa [P. aeruginosa (ATCC 27853)] was used for the incubation of the test samples. P. aeruginosa was incubated in 5% sheep blood agar for 24 h at 37 °C. Following incubation, a bacterial suspension equivalent to the 0.5 McFarland standard (10⁸ cfu/mL) was prepared in Tryptic Soy Broth (TSB) with 1% glucose. Test samples were placed on well plates for incubation. In the wells containing the abutments in the experimental groups (EOH and CHX groups) and the positive control group (Group PC), 250 µl of the prepared bacterial suspension was distributed. In the wells containing the abutments in the negative control groups (NC group), only 250 µl of TSB medium was added. The prepared plates were incubated for 24 h at 37 °C and 100 rpm in a shaking oven (FinePCR BAE07, Korea). After incubation, the media in the plates were removed. The Group EOH test samples were then immersed in a solution containing 80% ethanol and kept in an ultrasonication device (Alsonic, Turkey) for 5 min8,41. For the group CHX test samples, 2% chlorhexidine gel (Best, Turkey) was added, and the samples were incubated for 10 min39,42. Following the disinfection procedures, all abutments were thoroughly rinsed twice with ample volumes of phosphate-buffered saline (PBS) to ensure the complete removal of chlorhexidine gel residues, and subsequently dried at room temperature. All test samples were fixed with 95% methanol. The crystal violet staining method was used to evaluate the biofilm formation of the test samples43. For this purpose, 0.1% crystal violet was added to each well, and the samples were incubated for 30 min. After staining, the wells were washed twice with PBS. The stain bound to the biofilm layer was dissolved by adding 200 µL of 33% acetic acid to the wells. The optical density (OD) of the dissolved crystal violet stain was measured with a microplate reader (Biotek Synergy H1, USA) at a wavelength of 550 nm43,44. Biofilm formation was determined according to the negative control absorbance value (ODNC) according to the following formula44,45.
To obtain scanning electron microscopy (SEM) images of biofilm formation according to the cleaning procedures, 3 samples from each subgroup (Group PC, Group CHX, Group EOH) were additionally prepared. These samples were subjected to the same microbial test procedure and cleaning procedures appropriate for their subgroups, fixed in 2.5% glutaraldehyde for 1 h to ensure bacterial adhesion and fixation, dehydrated in ethanol washes, and then dried at 37 °C in a bacteriological incubator. The test samples were then coated with gold spray in a rotary vacuum apparatus (K550X, EMITECH, UK) and analyzed by scanning electron microscopy (EVO 40, ZEISS, Germany) at different magnifications (x64-20,000) to confirm the adhesion of P. aeruginosa bacteria and to examine the effects of the cleaning procedures.
The statistical data collected were analyzed using the IBM SPSS 22.0 software package. The Shapiro-Wilk test was applied to assess the normality of the data, and group comparisons were conducted using one-way analysis of variance (ANOVA). Tukey‘s test was performed to identify the specific groups with significant differences. (α = 0.05).
Results
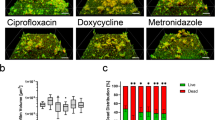
The distribution of biofilm formation among the test groups is shown in Table 3. The comparison of biofilm formation percentages according to abutment type is shown in Fig. 2. The highest value was observed for Group ti-base (51.8%). No statistically significant difference was observed among the groups (p>0.05).
The percent biofilm formation rates were compared according to the cleaning procedures, and both cleaning procedures were found to be successful. These values were significantly lower than those of the positive control group (p < 0.05). However, the CHX group and the EOH group did not significantly differ (p > 0.05) (Fig. 3).
The SEM images revealed that P. aeruginosa biofilms formed in the positive control groups according to the abutment types of all the test groups. More intense bacterial adhesion and colonization were observed on all the surfaces of the monoblock titanium and prefabricated ti-base abutments (Figs. 4 and 5) than on the hybrid zirconia abutment surfaces (Fig. 6). The amount of bacteria adhering to all abutment types was very low in both cleaning procedures (Figs. 4, 5 and 6). This finding was consistent with the biofilm quantification values obtained via the crystal violet staining technique.
Discussion
In this study, the effectiveness of various cleaning protocols that can be easily applied in clinical and laboratory practice on biofilm adhesion of P. aeruginosa, one of the periimplantitis pathogens, to different types of customized implant abutments was investigated. The first null hypothesis of the study, that different abutment types would not affect biofilm formation, was accepted since there was no significant difference between the abutment types. The second null hypothesis, which states that various cleaning procedures do not affect biofilm formation, was also accepted, as there was no significant difference among the cleaning procedures. However, regardless of the cleaning procedure used, P. aeruginosa biofilm adhesion was significantly lower than that in the positive control group.
The adhesion of microorganisms present in the oral cavity, especially those associated with periodontal diseases, to the implant surface and the resulting plaque formation are considered among the most important factors affecting the primary stability of implants36,37. This plaque formation is often associated with a lack of proper oral hygiene as well as the properties of the implant components used in the restoration37. Several studies have reported that the penetration of bacteria through the implant-support interface can constitute a potential risk of supporting tissue inflammation that, if uncontrolled, can compromise the long-term success of implant-supported restorations37,46. An improper fit between these two components can lead to colonization by bacteria, which can ultimately lead to inflammatory reactions with bone resorption47. Therefore, in our study, we examined different types of zirconia and titanium materials, which are frequently used in current implant practices, and different types of customized abutment components in terms of biofilm formation.
Considering that both ti-base and monoblock titanium abutments are made from titanium grade 5, their differences lie primarily in design and surface characteristics. Ti-base abutments are prefabricated components designed as bonding bases for ceramic superstructures, featuring standardized and smoother surfaces optimized for cementation48. On the other hand, monoblock titanium abutments are custom milled as single-piece components with distinct surface topographies and marginal adaptations49. These variations can influence bacterial adhesion and biofilm formation.
In particular, customized two-piece hybrid abutments may have higher contamination levels after reprocessing than one-piece abutments33. Nascimento et al., in their study evaluating bacterial adhesion on two different titanium (milled and cast) and one ceramic (milled) abutment surface, asked their participants to use an individually prepared intraoral splint containing the tested materials for 24 h. They then used the DNA hybridization method (checkerboard) to identify and quantify 38 different bacterial species, including P. aeruginosa, colonizing the biofilms formed on the surfaces. All three materials tested showed bacterial colonization after 24 h of oral cavity exposure. Compared with those in the other groups, the mean number and species of titanium abutments obtained by casting were greater. The zirconia abutment group presented the lowest amount of bacteria37. Similarly, Grobner-Schreiber et al.50 and Dantas et al.51 reported higher total bacterial colonization rates on titanium surfaces than on zirconia surfaces. In our study, although zirconia supports had the lowest bacterial retention, there was no statistically significant difference between the types of abutments used. These differences may be due to microbial methodology. Similarly, Lee et al. examined bacterial adhesion on three different materials (resin, titanium, and zirconia) under the same conditions and reported no significant difference between titanium and zirconia specimens. They found the highest sensitivity to bacterial adhesion in resin samples52.
In-vitro studies have identified substances that can contaminate the surfaces of titanium and zirconia CAD/CAM customized implant abutments12,20. These agents may also originate from dental technicians and physicians during the manufacturing, transport, and packaging stages up to the delivery of the restoration to the patient, including the proofing stages21,39,53. If these contamination agents are not removed from the surface, they may induce inflammatory responses in peri-implant tissues21,54. This is because the soft tissues surrounding the implants have a much lower immunological capacity than the periodontal tissues around the teeth55. Accordingly, a comprehensive evaluation of various disinfection and sterilization protocols for implant abutments is warranted.
According to the European Health Regulations and the guidelines of the American Dental Association (ADA), only sterilization or high-level ultrasonic disinfection procedures are approved for the decontamination of semi-critical medical devices, such as implant abutments27. Autoclave sterilization, a physical method, employs a specific combination of heat and pressure to eradicate all viable microorganisms, including highly resistant bacterial spores21. Owing due to their heat resistance, titanium implant abutments can undergo autoclave sterilization without adversely affecting their structural or material integrity56. Hybrid zirconia supports, on the other hand, can be sterilized under moist heat and pressure, while the crystalline structure may be damaged, thus increasing the risk of fracture18. In addition, there are conflicting results regarding the effect of autoclaving on the bond strength of the resin cement in hybrid abutments. In their meta-analysis, Özcan and Bernasconi expressed some concerns about hydrothermal aging resistance during autoclaving and reported that it may have a damaging effect on bond strength57. Nonetheless, other studies have indicated that autoclave sterilization does not adversely affect the structural integrity or the bond strength between zirconia and ti-base abutments48,58.
Moreover, while sterilization is effective in eliminating microbial surface contamination to ensure aseptic and sterile conditions, it is insufficient on its own to remove particulate residues present on CAD/CAM milled abutments21. However, ultrasonic cleaning utilizing ultra-high frequency waves in combination with disinfectants has been shown to be effective in both mechanically and chemically eliminating foreign particles and microbial contaminants from the surface of implant abutments12,59. Despite the presence of established regulatory guidelines regarding the proper sterilization and disinfection of implant abutments, the literature does not reflect a fully consistent approach12,21,33.
Alresheedi and Alazmi conducted a randomized trial comparing photodynamic therapy and chlorhexidine gel immediately before prosthesis delivery and found similar radiographic and clinical outcomes at one year clinical follow-up35. Alsahhaf et al. extended this approach to include argon plasma treatment alongside chlorhexidine gel and steam sterilization, reporting favorable long-term peri-implant health at five years60. Asbi et al. demonstrated that a single application of chlorhexidine gel significantly reduced peri-implant inflammation and interleukin‑1β levels following one-stage implant placement61. Hofmann et al. compared various cleaning protocols (steam, argon–oxygen plasma, and simple ultrasonic cleaning) applied to customized abutments using artificial intelligence-assisted SEM/EDS analysis in in-vitro studies and confirmed that there were significant differences in surface cleaning depending on the method used53. Al‑Aali et al. investigated Nd: YAG and Er, Cr: YSGG laser treatment alongside chlorhexidine and steam cleanup and reported significant improvements in bacterial parameters post-treatment34. More recently, Morin et al. studied a graphene nanocoating on titanium surfaces and found preserved antibiofilm properties even after sterilization procedures62. Also, Yang et al. assessed triple-modified PEEK surfaces combining plasma treatment, polydopamine coating, and chlorhexidine application, observing enhanced antibacterial and biocompatible performance63.
In this study, routine autoclave sterilization, as recommended by current guidelines, was performed before incubation in order to standardize bacterial adhesion across different abutment types. Instead of advanced or technique-sensitive disinfection methods, two simple and accessible cleaning procedures were deliberately selected: immersion in 2% chlorhexidine gel and ultrasonic cleaning with 80% ethanol. These protocols were chosen based on their practicality, cost-effectiveness, and ease of application, as they can be carried out by non-clinical personnel under typical clinical or laboratory conditions. These protocols were chosen based on their practicality, cost-effectiveness, and ease of application, as they can be implemented by non-clinical personnel under typical clinical or laboratory conditions. The results demonstrated a significant reduction in P. aeruginosa biofilm formation for all abutment types following either cleaning procedure, compared to positive controls. Although no statistically significant difference was found between the two disinfection protocols in terms of their effectiveness, the outcomes suggest that even simple interventions can meaningfully reduce bacterial adhesion. This reinforces the clinical relevance of implementing standardized, easily applicable cleaning steps before final prosthesis delivery, particularly in scenarios where more advanced techniques may not be available.
P. aeruginosa, which is among the pathogenic microorganisms in periimplantitis lesions, is known to colonize the implant surface relatively well64. P. aeruginosa is a gram-negative, aerobic or facultative anaerobic, rod-shaped bacterium with unipolar motility and is approximately 0.5 to 1 μm in size. It is recognized as an opportunistic pathogen in humans37,65. In addition, this bacterium, which can be found in water, soil, and dental water lines66, has also been found around failed implants and in peri-implant tissues in some studies64,67. Colombo et al. reported that P. aeruginosa levels in the oral epithelial cells of individuals with periodontitis were significantly greater than those in healthy individuals68. In this study, P. aeruginosa was preferred as the causative bacterium because of these characteristics.
Biofilms are dynamic microbial communities embedded within a polymeric matrix that is secreted by microorganisms69. Biofilm formation can be measured using both direct and indirect quantification techniques. The Colony Forming Unit (CFU) method, a direct quantification method, is difficult to apply, is time-consuming and has difficulty obtaining reproducible results. Therefore, this method may not be preferable in certain cases70. One of the indirect methods for quantifying biofilm formation is the crystal violet assay, which is simple to perform, reproducible, and enables the simultaneous evaluation of multiple samples26,70. In this study, the aim was to inhibit biofilm formation with the application of the cleaning procedures. For this reason, the crystal violet method, which is frequently used in microbiological analyses, was preferred.
In-vitro studies can replicate only limited aspects of biofilm formation. A key limitation of this study is the use of a single-species biofilm model with P.aeruginosa. Although this bacterium is commonly found in peri-implant infections and has strong surface adhesion properties, it does not fully represent the polymicrobial nature of oral biofilms. Additionally, the 24-hour incubation period reflects only the early stage of biofilm formation, rather than mature or chronic biofilms typically found in-vivo. Furthermore, the study investigated only two cleaning protocols: 2% chlorhexidine gel and ultrasonic cleaning with ethanol. These techniques were selected based on their clinical practicality and common usage. However, the omission of other advanced decontamination methods may limit the comprehensiveness of the findings. Future research should aim to model in-vivo conditions more realistically by including multi-species biofilms, longer incubation periods, and a broader range of cleaning techniques. Using complementary microbiological methods may also provide a more detailed understanding of biofilm reduction and surface decontamination.
Conclusion
Within the limitations of this in-vitro study, no statistically significant difference was observed in biofilm formation among the different abutment materials tested. Both ultrasonic ethanol cleaning and immersion in 2% chlorhexidine gel proved effective in significantly reducing P. aeruginosa adhesion on all abutment types, although their comparative effectiveness did not differ.
These findings highlight that even simple, accessible disinfection protocols can substantially reduce microbial contamination and may support peri-implant tissue health.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ersöz, E., Özer, N. E. & Çiçekci, G. Titanium base abutment. In Karaağaçlıoğlu L, ed. Current Information on Implant Supported Prostheses. 1st ed. Ankara: Turkiye Klinikleri J Med Sci. 8–15. (2024).
Mavrogenis, A. F., Dimitriou, R., Parvizi, J. & Babis, G. C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 9 (2), 61–71 (2009).
Bhat, P. R., Thakur, S. L. & Kulkarni, S. S. The influence of soft tissue biotype on the marginal bone changes around dental implants: A 1-year prospective clinico-radiological study. J. Indian Soc. Periodontol. 19 (6), 640–644 (2015).
Bosshardt, D. D., Chappuis, V. & Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: current knowledge and open questions. Periodontol 2000. 73 (1), 22–40 (2017).
Albrektsson, T., Buser, D. & Sennerby, L. Crestal bone loss and oral implants. Clin. Implant Dent. Relat. Res. 14 (6), 783–791 (2012).
Sculean, A., Gruber, R. & Bosshardt, D. D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 41 (Suppl 15), S6–22 (2014).
Tang, K. et al. The integration of peri-implant soft tissues around zirconia abutments: challenges and strategies. Bioact Mater. 27, 348–361 (2023).
Wiedenmann, F., Liebermann, A., Spintzyk, S., Eichberger, M. & Stawarczyk, B. Influence of different cleaning procedures on tensile bond strength between zirconia abutment and titanium base. Int. J. Oral Maxillofac. Implants. 34 (6), 1318–1327 (2019).
Yi, Y., Heo, S. J., Koak, J. Y. & Kim, S. K. Comparison of CAD/CAM abutment and prefabricated abutment in Morse taper internal type implant after Cyclic loading: axial displacement, removal torque, and tensile removal force. J. Adv. Prosthodont. 11 (6), 305–312 (2019).
Rodriguez, L. C., Saba, J. N., Chung, K. H., Wadhwani, C. & Rodrigues, D. C. In vitro effects of dental cements on hard and soft tissues associated with dental implants. J. Prosthet. Dent. 118 (1), 31–35 (2017).
Al-Thobity, A. M. Titanium base abutments in implant prosthodontics: A literature review. Eur. J. Dent. 16 (1), 49–55 (2022).
Gehrke, P. et al. Qualitative and Semi-Quantitative assessment of Processing-Related surface contamination of One- and Two-Piece CAD/CAM abutments before and after ultrasonic cleaning. Materials 13 (14), 3225 (2020).
Elsayed, S. & Elbanna, K. Effect of different fabrication materials and techniques on the retention of implant meso-structures to Ti-base abutments. Egypt. Dent. J. 67 (3), 2567–2585 (2021).
Jirajariyavej, B., Wanapirom, P. & Anunmana, C. Influence of implant abutment material and ceramic thickness on optical properties. J. Prosthet. Dent. 119 (5), 819–825 (2018).
Cosgarea, R. et al. Peri-implant soft tissue colour around titanium and zirconia abutments: a prospective randomized controlled clinical study. Clin. Oral Implants Res. 26 (5), 537–544 (2015).
Bittencourt, T. C., Souza Picorelli Assis, N. M., Ribeiro, C. G., Ferreira, C. F. & Sotto-Maior, B. S. Evaluation of the peri-implant tissues in the esthetic zone with prefabricated titanium or zirconia abutments: A randomized controlled clinical trial with a minimum follow-up of 7 years. J. Prosthet. Dent. 129 (4), 573–581 (2023).
Thakare, V., Chaware, S., Kakatkar, V. & Darekar, A. An insight performance of zirconia implant abutment: A systematic review and meta-analysis of randomized controlled clinical trial. Indian J. Dent. Res. Off Publ Indian Soc. Dent. Res. 34 (1), 80–86 (2023).
Lang, R., Hiller, K. A., Kienböck, L., Friedl, K. & Friedl, K. H. Influence of autoclave sterilization on bond strength between zirconia frameworks and Ti-base abutments using different resin cements. J. Prosth. Dent. 127(4), 617.e1–617.e6 (2022).
Nouh, I. et al. Mechanical behavior of posterior all-ceramic hybrid-abutment-crowns versus hybrid-abutments with separate crowns-A laboratory study. Clin. Oral Implants Res. 30 (1), 90–98 (2019).
Canullo, L., Micarelli, C., Lembo-Fazio, L., Iannello, G. & Clementini, M. Microscopical and microbiologic characterization of customized titanium abutments after different cleaning procedures. Clin. Oral Implants Res. 25 (3), 328–336 (2014).
Gehrke, P. et al. Microbiological cleaning and disinfection efficacy of a three-stage ultrasonic processing protocol for CAD-CAM implant abutments. J. Adv. Prosthodont. 14 (5), 273–284 (2022).
Tzimas, K., Rahiotis, C. & Pappa, E. Biofilm formation on Hybrid, Resin-Based CAD/CAM materials for indirect restorations: A comprehensive review. Mater. Basel Switz. 17 (7), 1474 (2024).
Tamizifar, A. et al. Microflora of Laboratory-Customized dental implant abutments. J. Int. Acad. Periodontol. 20 (3), 86–93 (2018).
Ujiie, Y., Todescan, R. & Davies, J. E. Peri-Implant crestal bone loss: A putative mechanism. Int. J. Dent. 2012, 742439 (2012).
Homayouni, A. et al. Effect of 5 popular disinfection methods on microflora of laboratory: customized implant abutments. Implant Dent. 28 (5), 437–446 (2019).
Doll, K., Jongsthaphongpun, K. L., Stumpp, N. S., Winkel, A. & Stiesch, M. Quantifying implant-associated biofilms: comparison of microscopic, microbiologic and biochemical methods. J. Microbiol. Methods. 130, 61–68 (2016).
ISO. 17664-1. Processing of Health Care products - Information To Be Provided by the Medical Device Manufacturer for the Processing of Medical Devices – Part 1: Critical and semi-critical Medical Devices (International Standard Organization (ISO); Geneva, 2021).
Flinn, B. D., deGroot, D. A., Mancl, L. A. & Raigrodski, A. J. Accelerated aging characteristics of three yttria-stabilized tetragonal zirconia polycrystalline dental materials. J. Prosthet. Dent. 108 (4), 223–230 (2012).
Hallmann, L. et al. The influence of grain size on low-temperature degradation of dental zirconia. J. Biomed. Mater. Res. B Appl. Biomater. 100 (2), 447–456 (2012).
Kim, J. W., Covel, N. S., Guess, P. C., Rekow, E. D. & Zhang, Y. Concerns of hydrothermal degradation in CAD/CAM zirconia. J. Dent. Res. 89 (1), 91–95 (2010).
Lee, T. H., Lee, S. H., Her, S. B., Chang, W. G. & Lim, B. S. Effects of surface treatments on the susceptibilities of low temperature degradation by autoclaving in zirconia. J. Biomed. Mater. Res. B Appl. Biomater. 100 (5), 1334–1343 (2012).
Li, K. C. et al. Effect of autoclave induced low-temperature degradation on the adhesion energy between yttria-stabilized zirconia veneered with porcelain. Dent. Mater. Off Publ Acad. Dent. Mater. 29 (11), e263–270 (2013).
Canullo, L. et al. Plasma of argon cleaning treatment on implant abutments in periodontally healthy patients: six years postloading results of a randomized controlled trial. Int. J. Periodontics Restor. Dent. 37 (5), 683–690 (2017).
Al-Aali, K. A., Alzaid, A. A., Alsaloum, M., Alanazi, K. K. & Almujel, S. H. Clinical, Bacterial, and prosthodontic parameters after implant abutment disinfection using Nd:YAG, Er,Cr:YSGG, Chlorhexidine, and conventional steam before prosthesis delivery. Photobiomodulation Photomed. Laser Surg. 41 (12), 703–709 (2023).
Alresheedi, B. & Alazmi, S. Disinfection of implant abutment connection using antimicrobial photodynamic therapy and 0.2% chlorhexidine gel applications immediately before prosthesis delivery: clinical and radiographic status at 1-year of follow-up. Photodiagnosis Photodyn Ther. 38, 102790 (2022).
D’Ercole, S. et al. Implants with internal hexagon and conical implant-abutment connections: an in vitro study of the bacterial contamination. J. Oral Implantol. 40 (1), 30–36 (2014).
do Nascimento, C. et al. Bacterial adhesion on the titanium and zirconia abutment surfaces. Clin. Oral Implants Res. 25 (3), 337–343 (2014).
Yamane, K. et al. Bacterial adhesion affinities of various implant abutment materials. Clin. Oral Implants Res. 24 (12), 1310–1315 (2013).
Sharon, E. et al. Surface Morphology, and bacterial load of dental implant abutments following decontamination protocols: an In-Vitro study. Mater. Basel Switz. 16 (11), 4080 (2023).
Stacchi, C., Berton, F., Porrelli, D. & Lombardi, T. Reuse of implant healing abutments: comparative evaluation of the efficacy of two cleaning procedures. Int. J. Prosthodont. 31 (2), 161–162 (2018).
Gehrke, P. et al. The influence of an ultrasonic cleaning protocol for CAD/CAM abutment surfaces on cell viability and inflammatory response in vitro. Vivo Athens Greece. 33 (3), 689–698 (2019).
Asli, H. N., Saberi, B. V. & Fatemi, A. S. In vitro effect of chlorhexidine gel on torque and detorque values of implant abutment screw. Indian J. Dent. Res. Off Publ Indian Soc. Dent. Res. 28 (3), 314–319 (2017).
Christensen, G. D. et al. Adherence of coagulase-negative Staphylococci to plastic tissue culture plates: a quantitative model for the adherence of Staphylococci to medical devices. J. Clin. Microbiol. 22 (6), 996–1006 (1985).
Çali, A. & Çelik, C. Determination of in vitro synergy and antibiofilm activities of antimicrobials and essential oil components. Biofouling 40 (8), 483–498 (2024).
Chusri, S., Phatthalung, P. N. & Voravuthikunchai, S. P. Anti-biofilm activity of Quercus infectoria G. Olivier against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 54 (6), 511–517 (2012).
Quirynen, M. et al. Microbiological and clinical outcomes and patient satisfaction for two treatment options in the edentulous lower jaw after 10 years of function. Clin. Oral Implants Res. 16 (3), 277–287 (2005).
do Nascimento, C. et al. Leakage of saliva through the implant-abutment interface: in vitro evaluation of three different implant connections under unloaded and loaded conditions. Int. J. Oral Maxillofac. Implants. 27 (3), 551–560 (2012).
Bergamo, T. P., Zahoui, E. & Luri Amorin Ikejiri, A. Retention of zirconia crowns to Ti-base abutments: effect of Luting protocol, abutment treatment and autoclave sterilization. J. Prosthodont. Res. 65 (2), 171–175 (2021).
Gonzalo, E. et al. Evaluation of milled titanium versus laser sintered Co-Cr abutments on the marginal misfit in internal Implant-Abutment connection. Mater. Basel Switz. 13 (21), 4873 (2020).
Grössner-Schreiber, B. et al. Modified implant surfaces show different biofilm compositions under in vivo conditions. Clin. Oral Implants Res. 20 (8), 817–826 (2009).
Dantas, T. et al. Bacteria co-culture adhesion on different texturized zirconia surfaces. J. Mech. Behav. Biomed. Mater. 123, 104786 (2021).
Lee, B. C., Jung, G. Y., Kim, D. J. & Han, J. S. Initial bacterial adhesion on resin, titanium and zirconia in vitro. J. Adv. Prosthodont. 3 (2), 81–84 (2011).
Hofmann, P., Kunz, A., Schmidt, F., Beuer, F. & Duddeck, D. Influence of exposure of customized dental implant abutments to different cleaning procedures: an in vitro study using AI-assisted SEM/EDS analysis. Int. J. Implant Dent. 9 (1), 33 (2023).
Nakajima, K. et al. Effects of cleaning methods for custom abutment surfaces on gene expression of human gingival fibroblasts. J. Oral Sci. 59 (4), 533–539 (2017).
Piattelli, A., Pontes, A. E. F., Degidi, M. & Iezzi, G. Histologic studies on osseointegration: soft tissues response to implant surfaces and components. A review. Dent. Mater. Off Publ Acad. Dent. Mater. 27 (1), 53–60 (2011).
Park, J. H. et al. Effect of cleaning and sterilization on titanium implant surface properties and cellular response. Acta Biomater. 8 (5), 1966–1975 (2012).
Özcan, M. & Bernasconi, M. Adhesion to zirconia used for dental restorations: a systematic review and meta-analysis. J. Adhes. Dent. 17 (1), 7–26 (2015).
Pils, D., Baeppler, R. J., Junker, R., Kielbassa, A. M. & Nothdurft, F. P. Application of a standard autoclaving protocol does not harm structural integrity of two-piece zirconia abutments under detachment force testing. Clin. Oral Investig. 23 (7), 3133–3137 (2019).
Jatzwauk, L., Schöne, H. & Pietsch, H. How to improve instrument disinfection by ultrasound. J. Hosp. Infect. 48 (Suppl A), S80–83 (2001).
Alsahhaf, A., Alrabiah, M., Ali, K., Vohra, F. & Abduljabbar, T. Implant abutment disinfection using plasma of argon and 0.2% and chlorhexidine gel applications immediately before prosthesis delivery: clinical and radiographic status at 5-years of follow-up. Eur. Rev. Med. Pharmacol. Sci. 27 (1), 116–121 (2023).
Asbi, T. et al. A single application of chlorhexidine gel reduces gingival inflammation and Interleukin 1-β following one-stage implant placement: A randomized controlled study. Clin. Implant Dent. Relat. Res. 23 (5), 726–734 (2021).
Morin, J. L. P. et al. Graphene Nanocoating on titanium maintains structural and antibiofilm properties post-sterilization. Dent. Mater. Off Publ Acad. Dent. Mater. 41 (1), 7–15 (2025).
Yang, T., Zhang, Y., Gao, Z. & Li, B. Triple-modified PEEK surface via plasma treatment, polydopamine coating and chlorhexidine: assessment of biocompatibility and antibacterial properties. Dent. Mater. Off Publ Acad. Dent. Mater. 41 (6), 730–744 (2025).
Alagl, A. S., Madi, M., Bedi, S., Al Onaizan, F. & Al-Aql, Z. S. The effect of Er,Cr:YSGG and diode laser applications on dental implant surfaces contaminated with acinetobacter baumannii and Pseudomonas aeruginosa. Mater. Basel Switz. 12 (13), 2073 (2019).
Bergamo, A. Z. N. et al. Microbial complexes levels in conventional and self-ligating brackets. Clin. Oral Investig. 21 (4), 1037–1046 (2017).
Lyczak, J. B., Cannon, C. L. & Pier, G. B. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2 (9), 1051–1060 (2000).
Canullo, L., Rossetti, P. H. O. & Penarrocha, D. Identification of Enterococcus faecalis and Pseudomonas aeruginosa on and in implants in individuals with Peri-implant disease: A Cross-Sectional study. Int. J. Oral Maxillofac. Implants. 30 (3), 583–587 (2015).
Colombo, A. V. et al. Quantitative detection of Staphylococcus aureus, Enterococcus faecalis and Pseudomonas aeruginosa in human oral epithelial cells from subjects with periodontitis and periodontal health. J. Med. Microbiol. 62 (Pt 10), 1592–1600 (2013).
Sahin, Z., Ozer, N. E. & Calı, A. Biofilm Inhibition of denture cleaning tablets and carvacrol on denture bases produced with different techniques. Clin. Oral Investig. 28 (7), 413 (2024).
Wilson, C. et al. Quantitative and qualitative assessment methods for biofilm growth: A Mini-review. Res. Rev. J. Eng. Technol. ;6(4) (2017).
Acknowledgements
The authors represent gratitude to Dr Elif Yildiz from the Ankara University Institute of Nuclear Sciences for the SEM analysis.
Author information
Authors and Affiliations
Contributions
NEO (First author): Conceptualization, methodology, validation, investigation, resources, data analysis, writing of the manuscript, and visualization. BN: Conceptualization, methodology, validation, investigation, resources, writing of the manuscript. AC: Methodology, validation, investigation, resources, data analysis, and writing of the manuscript. ZS: Conceptualization, methodology, writing of the manuscript, and visualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Özer, N.E., Niran, B., Çalı, A. et al. In-vitro evaluation of the effectiveness of various disinfection procedures in reducing biofilm formation on customized implant abutments of different designs. Sci Rep 16, 2846 (2026). https://doi.org/10.1038/s41598-025-26007-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26007-y