Abstract
Brain age gap refers to the difference between chronological and brain age based on computational models derived from various neuroimaging phenotypes. Studies in humans have reported that brain age gap is a biological measure that is sensitive to the effects of genetic, environmental and health-related variables on the pace of aging. Here, for the first time, we tested whether estimates of brain age gap could be derived from neuroimaging data obtained in chimpanzees and whether they were associated with different cognitive and motor phenotypes. Archived measures of cortical thickness and surface area were obtained from 34 brain regions in a sample of 215 chimpanzees from the National Chimpanzee Brain Resource. Brain age gap values were computed and tested for their association with individual variation in cognition and motor function. The mean absolute average age gap was ~ 6 years in chimpanzees, a value that overlaps with reports in human subjects. Chimpanzees with “older” brain ages performed more poorly on a measure of cognition compared to individuals with “younger” brains, after controlling for the sex and rearing effects. Like in humans, brain age gap can be used as a valid biomarker of brain aging in chimpanzees and is sensitive to individual differences in cognition.
Similar content being viewed by others
Introduction
All biological systems age throughout the lifespan and studies in humans have shown that different organ systems and tissues may age at different rates that deviate from an individual’s chronological age1,2. A key metric for calculating the pace of brain aging is the brain age-gap estimate or brainAGE, which is defined as the difference between an individual’s chronological age and their brain-age, where brain-age refers to the biological age of the brain as predicted from multivariate patterns of neuroimaging features1,3. By quantifying deviations from normative age- and sex-specific trajectories, brainAGE provides a sensitive index of whether an individual’s brain appears to be aging “faster” or slower than expected4,5. Unlike single imaging features such as cortical thickness or volume, brainAGE provides an integrative, multivariate index of biological brain aging that captures deviations from normative lifespan trajectories. It therefore reflects subtle and distributed effects on the pace of aging that may not be evident when examining individual brain measures in isolation. Studies in humans have shown that a range of environmental and health-related factors can impact brainAGE, including substance or alcohol abuse6,7, mental illness8,9,10,11,12 and early adversity13,14,15,16. Moreover, in humans, there is some evidence that brain aging is accelerated in some neurodegenerative disorders such as in patients with Alzheimer’s disease and related dementias17,18,19,20 and it can even predict lifespan and mortality21,22.
The impact of experiential factors on the rate of aging in other species23, notably nonhuman primates, is a growing topic of interest. Using DNA methylation from blood or other organ tissues, epigenetic clocks have now been established in several nonhuman primate species24,25,26,27,28. There is also evidence in nonhuman primates that the accumulation of adverse lifespan experiences, can impact epigenetic age, an estimate of the pace of aging derived from DNA methylation profiles. For instance, in wild baboons, males with high allostatic load (a metric of cumulative lifespan stress) were found to have higher epigenetic ages compared to males with lower allostatic load values24. In contrast to studies on epigenetic age, we know of only a single study in nonhuman primates that has attempted to calculate brain age. Franke et al.29 examined the impact of fetal nutrition on brain aging in a sample of 29 baboons estimated from measures of gray matter volume obtained from magnetic resonance imaging scans. These authors reported that the brainAGE gap in baboons that experienced fetal undernutrition was on average + 2.7 years higher than those who were not undernourished. Thus, undernourished baboons appeared to age at a faster pace than those that were not. Despite these promising early findings, the assessment of brain age in nonhuman primates and its potential association with different aspects of cognitive and motor function is unknown.
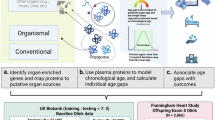
To address this gap in knowledge, we tested the applicability of brainAGE models based on large human neuroimaging datasets to compute brainAGE in chimpanzees using corresponding and analogous data obtained in these same individuals. Accordingly, the model used in this study was based on brain morphometric measures from 35,683 healthy humans and was subsequently applied to analogous brain morphometric data from chimpanzees to calculate their brainAGE. Specifically, we used neuroimaging data from a sample of captive chimpanzees to compute their brainAGE using models adapted from corresponding human neuroimaging data30. We subsequently tested whether brainAGE was a significant predictor of individual differences in cognition and motor skill in chimpanzees, two behavioral abilities reported to decline with increasing age. In humans, previous studies have reported significant associations between brainAGE and cognitive development as well as cognitive decline18,22. Although there are no previous studies on brainAGE in chimpanzees, older individuals exhibit reductions in gray matter volume and cortical thickness, which are associated with loss in cognitive functions in a sex dependent manner31,32,33. In this study, we tested whether brainAGE was associated with cognitive abilities and motor function in the chimpanzees while controlling for sex and early rearing experiences. Sex and rearing history of the chimpanzees were included as covariates because previous studies in humans have reported that sex and early rearing experience can impact brainAGE34,35.
Results
For the entire sample, brainAGE values ranged between − 18.14 and 10.27 years and the mean absolute error (MAE) was 6.23 years with a SD of 4.17 years (see Fig. 1 for frequency distribution of brainAGE values binned in intervals of 5 years). These values are similar to those reported in human brainAGE studies where MAE typically ranges between 4 and 8 years36,37.
Multiple regression analyses demonstrated that brainAGE significantly predicted cognitive performance on the unit weighted average (UWA) composite cognition score, even after accounting for sex and rearing history (mother-reared, nursery-reared or wild born apes) (see Table 1). The full model was significant, F(3, 188) = 3.97, p = .033, with an R² = 0.045. The addition of brainAGE after entering sex and rearing history significantly improved the model, F change (1, 188) = 4.95, p = .027, ∆R² = 0.025. In this model, higher brainAGE values were associated with lower cognitive performance (Fig. 2a). In contrast, when chronological age was substituted for brainAGE, the model was not significant, F(3, 188) = 1.29, p > .10, and none of the predictor variables significantly contributed to variance in UWA scores. Follow-up ANOVA using quartiles of residualized brainAGE (adjusted for sex and rearing) revealed a significant effect of brainAGE group on UWA scores, F(3, 188) = 3.32, p = .021 (Fig. 2b). Post-hoc LSD comparisons showed that chimpanzees in the youngest quartile had significantly higher UWA scores than those in the two oldest quartiles.
For tool use performance, the regression model including brainAGE was not significant F(3, 194) = 2.47, p = .063, with R² = 0.037 (see Table 1; Fig. 3a). Entry of brainAGE into the model yielded did not significantly increase the proportion of variance explained, F change(1, 194) ≈ 3.8, p ≈ .05, ∆R² = 0.02. Though not significant, interestingly, more youthful brainAGE values were associated with slower motor performance. When chronological age replaced brainAGE, the model remained non-significant, F(3, 194) = 1.77, p = .153, R² = 0.027. In follow-up ANOVA using quartiles of residualized brainAGE (adjusted for sex and rearing) the group effect was not significant, F(3, 194) = 2.09, p = .103 (Fig. 3b), though the trend echoed the regression result. Model-fitting for non-linear effects indicated that for tool use skill, both linear and quadratic terms of chronological age significantly explained variance (see Table 2), replicating earlier findings. In contrast, brainAGE was associated with tool use skill through linear, quadratic, and cubic terms, suggesting a U-shaped relationship, where both extremely low and high brainAGE values were associated with poorer performance.
To address potential confounding from imprecise birth dates in wild-born chimpanzees as well as the differences in age between when the chimpanzee were scanned and their behavioral testing took place, partial correlations were conducted in the subset of animals with confirmed birthdates, controlling for sex, rearing and the differences in age between behavioral testing and the collection of the MRI scans. BrainAGE remained significantly associated with UWA (r = − .197, df = 153, p = .033) and tool use skill (r = − .221, df = 161, p = .001) (see Table 3). Further, group differences by brainAGE quartiles for the UWA and tool use performance outcome measures remained directionally consistent in this subset of chimpanzees (Fig. 4a and b), reinforcing the consistency in findings.
Discussion
This study provides the first evidence that brainAGE models trained on large-scale human neuroimaging data can be applied to chimpanzees using homologous morphometric features, yielding plausible estimates of biological brain age. The validity of these estimates is supported by the model’s predictive accuracy, as reflected in a mean absolute error of 6.23 years, which falls within the range typically reported in human brainAGE studies. This level of performance indicates that the models generalize well across species and strengthens confidence in their application to chimpanzees. Together, these findings suggest conserved patterns of structural brain aging between humans and chimpanzees and highlight the translational potential of normative modeling frameworks across closely related species.
Crucially, we observed that brainAGE—but not chronological age—was meaningfully associated with cognitive performance in chimpanzees. Individuals with more “aged” brains, as indexed by positive brainAGE deviations, exhibited lower scores on the UWA composite derived from the Primate Cognition Test Battery (PCTB), a well-validated measure of cognition in this species32,38. These findings held after adjustment for sex and rearing history of the chimpanzees and remained robust when restricting analyses to individuals with confirmed birthdates. Thus, brainAGE appears to index interindividual variability in neurobiological aging more precisely than chronological age, echoing findings from human aging research (Biondo et al., 2022; Elliott et al., 2021; Huang et al., 2021; Yin et al., 2023).
The relationship between brainAGE and tool use skill followed a non-linear trend. Both relatively youthful and older brainAGE estimates were associated with diminished motor performance, suggesting that optimal tool use ability may lie within a mid-range of biological brain age. This U-shaped pattern aligns with prior studies in chimpanzees showing age-related fluctuations in fine motor skills39 and supports the idea that complex motor behavior is sensitive to both maturation and aging. These associations were not explained by the potential confounds of rearing history or uncertainty in age estimation for wild-born apes.
Collectively, these results position brainAGE as a biologically meaningful, species-translatable marker of brain aging. Future work should extend this approach to other cognitive domains, explore links with neuropathological burden, and evaluate modifiability through interventions or environmental factors. By capturing deviations from normative neurodevelopment, brainAGE may offer a valuable window into the mechanisms and modifiers of cognitive resilience and decline across species.
Methods
Subjects
Magnetic resonance image (MRI) scans of the brain were obtained in 215 captive chimpanzees from two different cohorts including the Yerkes National Primate Research Center (YNPRC, n = 77) and the National Center for Chimpanzee Care (NCCC, n = 138) of the University of Texas MD Anderson Cancer Center. There were 131 females and 84 males ranging from 8 to 53 years of age (Mean = 27.04 years, SD = 10.53). Within this sample, there were 110 mother-reared (MR), 66 nursery-reared (NR) and 39 wild born (WB) individuals. Except for two individuals, NR chimpanzees were separated from their mothers within the first 30 days of life, due to unresponsive care by the mothers, sickness or injury40,41. The NR chimpanzees were placed in incubators, fed standard human infant formula, and cared for by humans until they could sufficiently care for themselves. Once this occurred, they were placed with other infants of the same age until they were 3 years of age40,41. At or about 3 years of age, NR chimpanzees were integrated into larger social groups of adult and sub-adult chimpanzees. The two NR exceptions were chimpanzees born at the YNPRC, but were subsequently transferred and raised in a rich social-linguistic environment by humans as part of an initiative to investigate the linguistic skills of apes at the Language Research Center of Georgia State University42,43,44. MR chimpanzees were not separated from their mother for at least the first 2.5 years of life and were raised in nuclear family groups, ranging between 4 and 20 individuals. WB chimpanzees were individuals who had been captured in the wild and subsequently brought to research facilities within the United States prior to 1974, when the importation of chimpanzees was banned. All procedures performed with the chimpanzees were approved by the Institutional Animal Care and Use Committees at MD Anderson and Emory University and followed all recommendations by the Institute of Medicine and NIH policy for the ethical treatment of chimpanzees in research. Reporting of animal use and research design are in accordance with the ARRIVE guidelines and all methods were carried out in accordance with relevant guidelines and regulations. Data are available from the National Chimpanzee Brain Resource at https://www.chimpanzeebrain.org.
MRI image collection
Scans were obtained at the time the chimpanzees were being surveyed for their annual physical examinations beginning in 1998 and continuing until 2014. Subjects were first immobilized by ketamine (10 mg/kg) or telazol (3-5 mg/kg) and subsequently anesthetized with propofol (40–60 mg/(kg/h)) following standard procedures at the YNPRC and NCCC facilities. YNPRC subjects were then transported to the MRI facility, while NCCC subjects were wheeled to a mobile imaging unit. The subjects remained anesthetized for the duration of the scans, as well as the time needed to transport them between their home enclosure and the imaging facility (between 5 and 10 min) or mobile imaging unit (total time ~ 5 min). Chimpanzees were placed in the scanner in a supine position and imaged using the human-head coil. Scan duration ranged between 40 and 60 min as a function of brain size. Seventy-seven chimpanzees were scanned using a 3-Tesla scanner (Siemens Trio, Siemens Medical Solutions USA, Inc., Malvern, Pennsylvania, USA) at YNPRC. T1-weighted images were collected using a three-dimensional gradient echo sequence with some minor variation in sequences to accommodate different individuals (pulse repetition range = 2300–2600 ms, echo time range = 3.0 to 4.4 ms, number of signals averaged = 1 to 3, matrix size = 320 × 320 × 192 or 256 × 256 × 128). Additionally, 138 NCCC chimpanzees were scanned using a 1.5-Tesla scanner (GE, Milwaukee, WI). T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, number of signals averaged = 8, and a 256 × 256 matrix). After completing MRI procedures, the subjects were temporarily housed in a single enclosure for 6–12 h to allow the effects of the anesthesia to wear off, after which they were returned to their social group.
Image processing
A pipeline integrating FSL (version 5.0.9, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) and FreeSurfer (version 6.0, https://surfer.nmr.mgh.harvard.edu/) was performed for all chimpanzee MRI data and has been described in detail elsewhere45,46. First, the brain images were skull stripped, bias field corrected and normalized using 7 degrees of freedom transformation including 3 translations, 3 rotations and 1 uniform scaling in FSL. The normalization step registered all chimpanzee brain volume images to the standard human MNI152 template while preserving the morphology of the brain. Second, the pre-processed brains were subsequently put through the standard FreeSurfer streamline47. Specifically, a volumetric analysis was performed to label the white matter for the left and right cerebral hemispheres, respectively. Then, a triangular mesh that tightly covers each white matter component, also known as the white matter surface, was generated and deformed with respect to the intensity gradients between the white matter and gray matter. The white matter surface area further expanded along the direction of the intensity gradients between the gray matter and the cerebrospinal fluid CSF until it coincided with the gray matter surface, also referred to as the pial surface. Cortical thickness at each surface vertex was computed as the average of the closest distance in either direction between the white and pial surfaces48. The size of triangles on each subject’s gray matter surface, obtained during the initial tessellation using FreeSurfer, remained fixed, but the number of triangles varied according to brain size. Each vertex possessed an identical value for surface area calculated from the triangles surrounding it.
Extraction of regional measures of cortical thickness and surface area
The surface area and cortical thickness brain measurements were computed for 34 region-of-interests (ROIs) derived from the Desikan-Killiany atlas49 for the left and right cerebral hemispheres of individual subjects (see Fig. 5). At each ROI, the cortical thickness was computed as the mean of the values of all vertices belonging to the region, while the surface area was the sum of facets of the 3D triangular mesh surface generated for the region. The Desikan-Killiany parcellation atlas in FreeSurfer was chosen because it was shown to provide high-quality subdivision of the chimpanzee brain45,46. The regional subdivision strategy was based on the methods showing it to be less prone to the inherent bias from the parcellation atlases that were independently constructed for the left and right cerebral hemisphere. In brief, the vertex-wise inter-hemispheric correspondence was identified by co-registering both cerebral hemisphere surfaces of individual chimpanzee subjects to a symmetric registration atlas. Specially, a study-specific atlas was established based on 30 training brains (randomly selected from the 3T chimpanzee cohort) as described elsewhere46. This inter-hemispheric correspondence enabled the regional label of both cerebral hemispheres to be projected from a single atlas, i.e. either the left or the right atlas, avoiding the direct introduction of the bias between the left and right parcellation atlases.
Left panel: Photo of 47 year-old female chimpanzee. Photo taken and provided by Mary Catherine Mareno. Right panel: Lateral, medial and inferior renderings of chimpanzee brain with the Desikan-Killiany atlas maps from Freesurfer projected onto the surface of a chimpanzee brain. Image reprinted from45.
BrainAGE calculation
As there is insufficient data from non-human primates to estimate BrainAGE, we used sex-specific models developed from human whole-brain T1-weighted scans. The development and validation of the human brainAGE models has been described in detail in prior publications37,50. In brief, we selected the best-performing model after systematically examining the impact of algorithm, site harmonization, age range, and sample size on brain-age prediction in a discovery sample of brain morphometric measures from 35,683 healthy individuals (age range: 5–90 years; 53.59% female). The optimized sex-specific models showed cross-dataset generalizability in an independent sample comprising 2101 healthy individuals (age range: 8–80 years; 55.35% female) and for longitudinal consistency in a further sample comprising 377 healthy individuals (age range: 9–25 years; 49.87% female). The models were corrected by adjusting the bias of chronological age as per Beheshti et al.51 and are publicly available at (https://centilebrain.org/#/brainAGE). Model performance was assessed using the mean absolute error (MAE) which represents the absolute difference between brain-age and chronological age. The average MAE for the human models was around 5 years which is within the range of MAE values (i.e., 4–8 years) reported in other human studies of brainAGE36. For the current study, we recomputed the sex-specific brainAGE using the same approach but limited to measures of cortical thickness and surface area based on the Desikan-Killiany parcellation atlas in FreeSurfer in order to match the chimpanzee cortical parcellation data. Using these models, we computed the brainAGE of each chimpanzee. As per the human literature, positive brainAGE values indicate older appearing brain relative to chronological age, while the opposite is true for negative values.
Behavioral testing
Within the chimpanzee sample, in addition to the available neuroimaging data, there were also measures of cognition assessed using the Primate Cognition Test Battery (PCTB) originally developed by Herrmann, et al.38 to assess comparative differences in physical and social cognition between human children, chimpanzees and orangutans. Here, we used the available PCTB data from a subset of 192 individuals within the larger sample of 215 apes. The PCTB cognition data were collected in the chimpanzees following the method described in Russell, et al.52 and more recently in Hopkins et al.32. In total, there are 13 tasks within the PCTB, including 9 that assess physical cognition and 4 that assess social cognition. Physical cognition tasks include measures of spatial memory, object permanence, rotation, transposition, relative quantity discrimination, understanding of causality (2 types), tool properties and tool use. Social cognition measures include comprehension of pointing cues, initiation of pointing, the use of attention-getting behaviors and gaze following. Performance scores on each task were summed across trials and converted to standardized z-scores. The z-scores were averaged across the physical cognition and social cognition tasks to derive single unit weighted performance score (herein, UWA); higher scores indicate better performance. The average difference in age between when chimpanzees were tested on the PCTB tasks and the acquisition of their MRI scan was 1.50 years with a standard deviation of 2.66.
In addition to the PCTB tasks, motor performance ona tool use task was quantified in 201 chimpanzees in 2015 using a device that was designed to simulate termite fishing or ant dipping in wild chimpanzees53,54,55,56. Details of the testing method have been described in detail elsewhere39,57. Briefly, a PVC pipe was attached to the subject’s home cage that was blocked at one end and had a small opening on the opposite end. Food with an adhesive quality was placed inside the PVC pipe and in order to obtain the food, the chimpanzee had to insert a small lollipop stick into the hole, then extract the stick and consume the food that adhered to the stick. The latency to successfully insert the stick was recorded on 50 responses for each chimpanzee measured from the time the subject initiated an attempt to insert the tool with one hand and ended when the chimpanzee successfully inserted and removed the tool;39,57. The average latency of the 50 responses served as the outcome measure of interest. Because the two chimpanzee cohorts had different experiences with this tool use device, within the NCCC and YNPRC cohorts, the average latency scores were converted to standardized z-scores and we removed three chimpanzees from all subsequent analyses that were identified as outliers based on box plots (those with z-scores > 3.0). This included two individuals within the NCCC and one in the YNPRC cohort, thereby reducing the sample to 198 chimpanzees for all further analyses. The remaining 198 chimpanzees were between 5 and 50 years of age (Mean = 24.80, SD = 10.39) when tool use data performance was collected. The majority of the tool use data were obtained within 2 to 4 years of the acquisition of their MRI scans.
Data analysis
We conducted multiple linear regression analyses to examine the associations between brainAGE and the behavioral measures in the chimpanzees, adjusting for relevant covariates. For each behavioral outcome (UWA cognition scores and tool use motor skill), we used a hierarchical regression approach in which sex and rearing history were entered as covariates in the initial step, followed by brainAGE as the key predictor in the second step. We assessed the incremental variance explained (ΔR²) and statistical significance of each model step. To compare the predictive value of brainAGE with that of chronological age, we repeated the regression analyses replacing brainAGE with chronological age as the second-step predictor. We note here that brainAGE is defined as the residual difference between predicted brain age and chronological age, with residualization applied to remove any persistent effect of age. Because of this adjustment, including both brainAGE and chronological age in the same statistical model would introduce redundancy and collinearity; therefore, we evaluated their predictive value in parallel models.
To further characterize the relationship between brainAGE and behavioral outcomes, we performed a follow-up analysis of variance (ANOVA). We first regressed sex and rearing on brainAGE and saved the standardized residuals to isolate variance attributable to brainAGE independent of these covariates. These residuals were then divided into quartiles. One-way ANOVAs were conducted with quartile group as the independent variable and either UWA or tool use skill as the dependent variable, followed by post-hoc least squares difference (LSD) tests.
Lastly, we examined the potential non-linear relationship between brainAGE (and separately, chronological age) and tool use performance by fitting linear, quadratic, and cubic models and comparing the variance explained by each. To control for the confounding of age and rearing (particularly among wild-born chimpanzees with estimated birthdates), we conducted partial correlation analyses restricted to apes with confirmed birthdates, adjusting for sex and rearing. These multilevel and complementary analytic strategies were designed to robustly test the association between brainAGE and behavior while addressing potential confounding influences of rearing experience. For all analyses, alpha was set to p < .05.
Data availability
All brainAGE data will made available at the National Chimpanzee Brain Resource upon publication. Additional, the corresponding author can be contacted directly and brainAGE data will be made available to them.
References
Franke, K. & Gaser, C. Ten years of brainage as a neuroimaging biomarker of brain aging: What insights have we gained? Front. Neurol. 10, 789. https://doi.org/10.3389/fneur.2019.00789 (2019).
Levine, M. E. et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 10, 573–591. https://doi.org/10.18632/aging.101414 (2018).
Dular, L. & Spiclin, Z. Alzheimer’s disease neuroimaging, I. BASE: Brain age standardized evaluation. Neuroimage 285, 120469. https://doi.org/10.1016/j.neuroimage.2023.120469 (2024).
Cole, J. H. Neuroimaging-derived brain-age: An ageing biomarker? Aging (Albany NY) 9, 1861–1862. https://doi.org/10.18632/aging.101286 (2017).
Cole, J. H. & Franke, K. Predicting age using neuroimaging: Innovative brain ageing biomarkers. Trends Neurosci. 40, 681–690. https://doi.org/10.1016/j.tins.2017.10.001 (2017).
Beheshti, I. Cocaine destroys gray matter brain cells and accelerates brain aging. Biology (Basel) 12. https://doi.org/10.3390/biology12050752 (2023).
Angebrandt, A. et al. Dose-dependent relationship between social drinking and brain aging. Neurobiol. Aging 111, 71–81. https://doi.org/10.1016/j.neurobiolaging.2021.11.008 (2022).
Seitz-Holland, J., Haas, S. S., Penzel, N., Reichenberg, A. & Pasternak, O. BrainAGE, brain health, and mental disorders: A systematic review. Neurosci. Biobehav Rev. 159, 105581. https://doi.org/10.1016/j.neubiorev.2024.105581 (2024).
Ge, R., Sassi, R., Yatham, L. N. & Frangou, S. Neuroimaging profiling identifies distinct brain maturational subtypes of youth with mood and anxiety disorders. Mol. Psychiatry. 28, 1072–1078. https://doi.org/10.1038/s41380-022-01925-9 (2023).
Han, L. K. M. et al. Brain aging in major depressive disorder: Results from the ENIGMA major depressive disorder working group. Mol. Psychiatry. 26, 5124–5139. https://doi.org/10.1038/s41380-020-0754-0 (2021).
Haas, S. S. et al. Accelerated global and local brain aging differentiate cognitively impaired from cognitively spared patients with schizophrenia. Front. Psychiatry 13, 913470. https://doi.org/10.3389/fpsyt.2022.913470 (2022).
Chakrabarty, T., Frangou, S., Torres, I. J., Ge, R. & Yatham, L. N. Brain age and cognitive functioning in first-episode bipolar disorder. Psychol. Med. 53, 5127–5135. https://doi.org/10.1017/S0033291722002136 (2023).
Colich, N. L., Rosen, M. L., Williams, E. S. & McLaughlin, K. A. Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychol. Bull. 146, 721–764. https://doi.org/10.1037/bul0000270 (2020).
Miller, J. G. et al. Early life Stress, frontoamygdala Connectivity, and biological aging in adolescence: A longitudinal investigation. Cereb. Cortex. 30, 4269–4280. https://doi.org/10.1093/cercor/bhaa057 (2020).
Vidal-Pineiro, D. et al. Individual variations in ‘brain age’ relate to early-life factors more than to longitudinal brain change. Elife 10 https://doi.org/10.7554/eLife.69995 (2021).
Beck, D. et al. Dimensions of early life adversity are differentially associated with patterns of delayed and accelerated brain maturation. Biol. Psychiatry. https://doi.org/10.1016/j.biopsych.2024.07.019 (2024).
Huang, W. et al. Accelerated brain aging in amnestic mild cognitive impairment: relationships with individual cognitive Decline, risk factors for alzheimer Disease, and clinical progression. Radiol. Artif. Intell. 3, e200171. https://doi.org/10.1148/ryai.2021200171 (2021).
Elliott, M. L. et al. Brain-age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Mol. Psychiatry. 26, 3829–3838. https://doi.org/10.1038/s41380-019-0626-7 (2021).
Biondo, F. et al. Brain-age is associated with progression to dementia in memory clinic patients. Neuroimage Clin. 36, 103175. https://doi.org/10.1016/j.nicl.2022.103175 (2022).
Yin, C. et al. Anatomically interpretable deep learning of brain age captures domain-specific cognitive impairment. Proc. Natl. Acad. Sci. U S A. 120, e2214634120. https://doi.org/10.1073/pnas.2214634120 (2023).
Cole, J. H. et al. Brain age predicts mortality. Mol. Psychiatry. 23, 1385–1392. https://doi.org/10.1038/mp.2017.62 (2018).
Cole, J. H. Multimodality neuroimaging brain-age in UK biobank: relationship to biomedical, lifestyle, and cognitive factors. Neurobiol. Aging. 92, 34–42. https://doi.org/10.1016/j.neurobiolaging.2020.03.014 (2020).
Brusini, I. et al. MRI-derived brain age as a biomarker of ageing in rats: validation using a healthy lifestyle intervention. Neurobiol. Aging. 109, 204–215. https://doi.org/10.1016/j.neurobiolaging.2021.10.004 (2022).
Anderson, J. A. et al. High social status males experience accelerated epigenetic aging in wild baboons. Elife 10 https://doi.org/10.7554/eLife.66128 (2021).
Guevara, E. E. et al. Age-associated epigenetic change in chimpanzees and humans. Philos. Trans. R Soc. Lond. B Biol. Sci. 375, 20190616. https://doi.org/10.1098/rstb.2019.0616 (2020).
Ryan, C. P. Epigenetic clocks: Theory and applications in human biology. Am J Hum Biol, e23488 (2020). https://doi.org/10.1002/ajhb.23488
Neal, S. J., Whitney, S., Yi, S. & Simmons, J. H. Epigenetic and accelerated age in captive Olive baboons (Papio anubis), and relationships with walking speed and fine motor performance. Aging 17, 740–756 (2025).
Guevara, E. E. et al. Epigenetic ageing of the prefrontal cortex and cerebellum in humans and chimpanzees. Epigenetics 17, 1774–1785 (2022).
Franke, K. et al. Premature brain aging in baboons resulting from moderate fetal undernutrition. Front. Aging Neurosci. 9, 92. https://doi.org/10.3389/fnagi.2017.00092 (2017).
Ge, R. et al. Normative modeling of brain morphometry across the lifespan using centilebrain: algorithm benchmarking and model optimization. BioRxiv https://doi.org/10.1101/2023.01.30.523509 (2023).
Hopkins, W. D. et al. Age differences in cortical thickness and their association with cognition in chimpanzee (Pan troglodytes). Neurobiol. Aging. 126, 91–102. https://doi.org/10.1016/j.neurobiolaging.2023.02.008 (2023).
Hopkins, W. D. et al. Age-related changes in chimpanzee (Pan troglodytes) cognition: Cross-sectional and longitudinal analyses. Am. J. Primatol. 83, e23214. https://doi.org/10.1002/ajp.23214 (2021).
Mulholland, M. M., Sherwood, C. C., Schapiro, S. J., Raghanti, M. A. & Hopkins, W. D. Age- and cognition-related differences in the Gray matter volume of the chimpanzee brain (Pan troglodytes): A voxel-based morphometry and conjunction analysis. Am. J. Primatol. 83, e23264. https://doi.org/10.1002/ajp.23264 (2021).
Goyal, M. S. et al. Persistent metabolic youth in the aging female brain. Proc. Natl. Acad. Sci. U S A. 116, 3251–3255. https://doi.org/10.1073/pnas.1815917116 (2019).
Sanford, N. et al. Sex differences in predictors and regional patterns of brain age gap estimates. Hum. Brain Mapp. 43, 4689–4698. https://doi.org/10.1002/hbm.25983 (2022).
More, S. et al. Brain-age prediction: A systematic comparison of machine learning workflows. Neuroimage 270, 119947. https://doi.org/10.1016/j.neuroimage.2023.119947 (2023).
Yu, Y. et al. Brain-age prediction: systematic evaluation of site effects, and sample age range and size. Hum. Brain Mapp. 45, e26768. https://doi.org/10.1002/hbm.26768 (2024).
Herrmann, E., Call, J., Hernandez-Lloreda, M. V., Hare, B. & Tomasello, M. Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science 317, 1360–1366 (2007).
Hopkins, W. D., Reamer, L., Mareno, M. C. & Schapiro, S. J. Genetic basis for motor skill and hand preference for tool use in chimpanzees (Pan troglodytes). Proceedings of the Royal Society: Biological Sciences B 282 (2015).
Bard, K. A., Platzman, K. A., Lester, B. M. & Suomi, S. J. Orientation to social and nonsocial stimuli in neonatal chimpanzees and humans. Infant Behav. Dev. 15, 43–56. https://doi.org/10.1016/0163-6383(92)90005-q (1992).
Bard, K. A. Evolutionary roots of intuitive parenting: maternal competence in chimpanzees. Early Dev. Parent. 3, 19–28. https://doi.org/10.1002/edp.2430030104 (1994).
Rumbaugh, D. M. Language Learning by a Chimpanzee: the Lana Project (Academic, 1977).
Brakke, K. E. & Savage-Rumbaugh, E. S. The development of Language skills in Bonobo and chimpanzee - i. comprehension. Lang. Communication. 15, 121–148 (1995).
Brakke, K. E. & Savage-Rumbaugh, E. S. The development of Language skills in Pan - ii. production. Lang. Communication. 16, 361–380 (1996).
Hopkins, W. D., Li, X., Crow, T. & Roberts, N. Vertex- and atlas-based comparisons in measures of cortical thickness, gyrification and white matter volume between humans and chimpanzees. Brain Struct. Funct. 222, 229–245. https://doi.org/10.1007/s00429-016-1213-1 (2017).
Xiang, L., Crow, T. J., Hopkins, W. D. & Roberts, N. Comparison of surface area and cortical thickness asymmetry in the human and chimpanzee brain. Cereb. Cortex. https://doi.org/10.1093/cercor/bhaa202 (2020).
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-Based analysis I: segmentation and surface reconstruction. NeuroImage 9, 179–194 (1999).
Fischl, B. & Dale, A. M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy Science USA 97, 11050–11055 (2000). https://doi.org/10.1073/pnas.200033797
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980 (2006).
Modabbernia, A. et al. Systematic evaluation of machine learning algorithms for neuroanatomically-based age prediction in youth. Hum. Brain Mapp. 43, 5126–5140. https://doi.org/10.1002/hbm.26010 (2022).
Beheshti, I., Nugent, S., Potvin, O. & Duchesne, S. Bias-adjustment in neuroimaging-based brain age frameworks: A robust scheme. Neuroimage Clin. 24, 102063. https://doi.org/10.1016/j.nicl.2019.102063 (2019).
Russell, J. L., Lyn, H., Schaeffer, J. A. & Hopkins, W. D. The role of socio-communicative rearing environments in the development of social and physical cognition in apes. Dev. Sci. 14, 1459–1470 (2011).
Marchant, L. F. & McGrew, W. C. Ant fishing by wild chimpanzees is not lateralised. Primates 48, 22–26 (2007).
Bogart, S. L. et al. Termite fishing laterality in the fongoli savanna chimpanzees (Pan troglodytes verus): further evidence of a left hand preference. Am. J. Phys. Anthropol. 149, 591–598 (2012).
Humle, T. & Matsuzawa, T. Laterality in hand use across four tool use behaviors among the wild chimpanzees of Bossou, Guinea, West Africa. Am. J. Primatol. 71, 40–48 (2009).
Sanz, C. M., Morgan, D. B. & Hopkins, W. D. Lateralization and performance asymmetries in the termite fishing of wild chimpanzees in the Goualougo triangle, Republic of congo. Am. J. Primatol. 78, 1190–1200. https://doi.org/10.1002/ajp.22574 (2016).
Hopkins, W. D., Mareno, M. C. & Schapiro, S. J. Further evidence of left hemisphere dominance in motor skill by chimpanzees on a tool use task. J. Comp. Psychol. 133, 512–519 (2019).
Acknowledgements
This work was supported, in part, by NIH grants AG-067419 and NS-092988. Chimpanzee maintenance at the National Center for Chimpanzee Care was previously funded by NIH/NCRR U42- OD-011197. All aspects of this research conformed to existing US and NIH federal policies on the ethical use of chimpanzees in research. Reprint requests may be sent to: William D Hopkins, Department of Comparative Medicine, Keeling Center for Comparative Medicine and Research, Bastrop, Texas 78602 Email: wdhopkins@mdanderson.org.
Author information
Authors and Affiliations
Contributions
WDH = conceived of study, wrote the paper and performed statistical analyses. SF = assisted in computations of brainAGE and wrote paper. RG assisted in computations of brainAGE and wrote paper. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hopkins, W.D., Frangou, S. & Ge, R. Brain age gap is associated with cognitive abilities in captive chimpanzees. Sci Rep 15, 41984 (2025). https://doi.org/10.1038/s41598-025-26062-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26062-5