Abstract
Platelet concentrate transfusion remains a major therapeutic care for patient with hematological disorders. However, optimal therapeutic effect has been a major challenge. Blood donor characteristics and processing procedure remain major areas of research. Hence, this research assessed the platelet functional parameters, beta-thromboglobulin and Platelets factor IV in donors and platelet concentrate. The study was a cross-sectional descriptive study among prospective blood donors for platelet concentrate. Seventy-one prospective blood donors were recruited for this study with mean age of 34.8. Whole blood and aliquot from concentrate were collected for platelet functional parameters using Semi-automated Haematology analyzer, pH using potentiometric method while Beta-thromboglobulin and Platelet factor IV using ELISA-based assays. There was high quality prospective blood donors recruited for platelet concentrate for the current study. Furthermore, there was significant increase (P-value < 0.00) in platelet count, plateletcrit (PCT), β-thromboglobulin (BTG), Platelets factor IV (PF4) and platelet large cell concentration (PLCC) in the first 12 h of harvest and running through 5 days of storage as pre-transfusion relative to the baseline values. Conversely, there was significant decrease in Mean platelet volume (MPV), platelet distribution width coefficient of variation (PDW-CV) and Platelet large cell ratio (P-LCR). Interestingly, fall in pH for the first 12 h was significant compared to 5 days longitudinal drop in pH. These findings indicate a slight but steady increase in platelet activation markers as storage time progresses. Platelets in Platelet concentrates were of low therapeutic quality due to significant platelet activation however, transfusion within 2 h of harvest and not more than 6 h post-harvest can guarantee better patient outcome. The study further established significance of including pH in evaluating platelet concentrates pre-transfusion to optimize transfusion outcome if transfusion is delayed beyond 6 h post-harvest.
Similar content being viewed by others
Introduction
Transfusion science since 17th century continues to evolve, with significant scientific and clinical advances from transfusing whole blood to utilizing only its components such as packed red blood cells (RBC), platelets, rarely white blood cells (WBC), fresh frozen plasma, and plasma-derived products for selective indications1. Platelet is a scarce resource, partly because of their short shelf life of 5 days, and it is classified in the World Health Organization’s (WHO) list of “Essential Medicine”2. Platelet concentrate are complex multi-component products to treat thrombocytopenia or as prophylaxis for those at serious risk of bleeding3. Furthermore, Platelets concentrates (PC) are widely used to support patients with severe thrombocytopenia in patients with hematologic malignancy, bone marrow failure or other immune and non-immune causes of platelet destruction, though rarely cases could warrant transfusion in patients with normal platelet counts4. Platelet transfusion is a common practice in thrombocytopenic patients for preventing or treating hemorrhages. About 230,000 platelet component transfusions are given in Spain, and approximately two million of platelet components are transfused in the United States annually5. More than 50% of platelets concentrate are transfused to patients diagnosed of oncohaematological diseases and/or undergoing hematopoietic stem cell transplantation with the aim of helping physicians to take the most accurate decisions on platelet transfusion. Some guidelines have been developed based on the current-scientific evidence6. Platelet performs the function of optimization of haemostasis and minimization of blood loss by initiating coagulation cascade7. The manufacturing and storing of concentrated platelet components need to pay attention to standards and quality control because during storage period, changes in the structure and function of platelets can occur, which may result in damage to the components of concentrated platelet8. In vitro storage of platelet can stimulate changes in biochemical and platelet function called platelet storage lesion9. Although technical advances have been made in the field of transfusion medicine to maintain the viability and the shelf-life of platelets during preparation and storage, still a lot of work remains uncovered4. Platelets are often transfused without respecting the ABO compatibility due to the limited stock availability, however, influence of this practice on platelet transfusion outcome is not well established10. However, there are some controversial issues and available scientific evidence is still not enough to solve them. There is little information about which is the best platelet product to be transfused: random platelets or single donor apheresis platelets, and plasma-suspended or additive solution-suspended platelets11 There is currently no routine testing of platelet quality such as percentage of active platelets and quality of therapeutic outcome that is negatively affected by prolonged storage times12. Platelet quantity (platelet functional parameters) is determined by assessing certain parameters such as MPV, PDW, PT, INR, volume of platelet, platelet count and pH8. Currently, from literature search, little or no research has been conducted on Beta-thromboglobulin and PF4 more specifically in prospective blood donors recruited for platelet concentrate. Against this background, our study wishes to evaluate the platelet product chain of processes from donor characteristics to the point of release for final transfusion. Platelet contains several bioactive proteins in the granules such as (Von Willebrand Factor, P-selectin, beta-thromboglobulin, Platelet Factor 4, fibrinogen). These factors can be used to assess platelet quality for therapeutic efficacy12. Beta-thromboglobulin are specific platelet al.pha granule that are released upon platelet activation and they are useful markers of platelet activation10. It has been observed from previous studies that, platelets undergo various storage changes starting from collection, processing to storage and the underlying conditions within the patients, which may affect the therapeutic benefit to the recipient13. Over time, changes in both Platelets and their storage medium occur, with an accumulation of bio reactive substances10. Extension of platelets storage duration may expose patients to potential decreases in platelets transfusion efficacy as well as possible increases in adverse events in addition to transfusion-associated sepsis, such as inflammation and/or immune-mediated events2. Platelet storage lesions develop during platelets concentrates preparation and storage. Mechanical and biochemical forces during platelets concentrates preparation induce platelet activation which persist throughout storage duration16. There is currently no routine testing of platelet quality and as changes in structure and function of platelet can occur both from the donor and the preparation process thus, affecting platelet concentrate negatively16. The demand for platelet transfusion appears to be increasing, and there has been little attention paid to therapeutic efficacy of platelet transfusion particularly in the areas of donor quality and process, thereby resulting in non-uniform transfusion outcomes. Premised on these, this study was designed to evaluate PF4, pH, Beta-thromboglobulin, and platelets functional parameters as essential biomarkers for platelet vitality and therapeutic quality.
Materials and methods
This study is descriptive experimental research conducted at University of Ilorin Teaching Hospital (UITH). A total of 71 subjects were recruited as prospective blood donors for platelet concentrate and were assessed pre-donation, post-harvest and pre-transfusion for the quality of platelet concentrate. Ethical clearance was obtained from the Health Research Ethics Committee, Ministry of Health, Ilorin and Ethical committee, University of Ilorin Teaching Hospital, Ilorin (NHREC 02/05/2010). Informed consent was obtained from all subjects on sample collection and data usage in compliance with the institutional ethical committee approval condition.
Study design
Donors were assessed at pre-processing, and platelet concentrates were assessed post-harvest and pre-transfusion to determine the quality and quantity of platelets to validate quality of donors, effects of processing and storage on platelet concentrates in order to guarantee effective platelet transfusion to recipient. The major inclusion criteria for the prospective donors include: no systemic illness; seronegative for Transfusion Transmitted Infections (TTIs); normotensive; non-febrile, and no history of previous hospital admission in the last six (6) months. The exclusion criteria were as follows: hypertensive; coagulation defects; history of recurrent signs of systemic infection, and the use of medications affecting platelet functions such as aspirin, acetylsalicylic acid. The following data were collected from donors: age, gender, marital status, occupation, sleep duration, education, religion, ethnicity (tribe), daily stress, cigarette consumption, alcohol consumption, donor category (first time donor, second, third or regular donor), frequency of blood donation, reasons for donation, and use of analgesics and hematinics.
Blood samples analyses
Blood analyses including the assessment of platelet functional parameters were carried out using a 5-part Haematology Autoanalyzer Swelab Lumi Analyser (Spanga, Sweden), and the parameters were recorded as follow: Platelet count (PLT) count, 150–400 × 10e9/L; Plateletcrit (PCT), 37–52%; Mean Platelet Volume (MPV), 7.4–10.4 fl.; Platelet Distribution Width (PDW); PDW-CV = Platelet Distribution Width as Coefficient of Variation; PDW-SD = Platelet Distribution Width as Standard Deviation; P-LCC = Platelet-Large Cell Concentration; and P-LCR = Platelet-Large Cell Ratio; and for platelet activation biomarkers: pH; Beta-thromboglobulin (BTG); and Platelet Factor 4 (PF4) were evaluated.
Procedure for preparation of platelet concentrate
Whole blood from donor was bled into triple blood bag containing CPD-A anticoagulant and platelet concentrate harvested by two centrifugation steps (soft spin and hard spin) using Biobase BKC-BB6 JINAN Biobase Biotech Blood Bag Cold centrifuge. The triple blood bag was spun in a cold centrifuge at 2000 g for 7 min at 22 °C to obtain platelet-rich plasma. Plasma extractor was used to transfer platelet-rich plasma into the first satellite blood bag, then spun at 4000 g for 10 min. Plasma extractor was used to transfer the supernatant plasma back to the third satellite blood bag leaving about 65-70mls of the sediment which forms the platelet concentrate. Platelet concentrate was placed on agitator at 22 °C in order to avoid clumping and platelet activation.
Sample collection and processing
A volume of 4.5mL whole blood was collected from the prospective blood donors (Pre-donation) and dispensed into EDTA sample bottle for platelet functional parameters assay. The concentrate in main bags with varying yield between 65mL and 70 mL was stored at 22 °C and an aliquot of 10mL was transferred into another empty satellite blood bags where 2mL was used each day for assays consecutively for 5 days while the remaining volume were used for transfusion purpose. All methods were performed in accordance with the relevant guidelines and regulations with strict compliance to standard operating procedure (SOP).
Procedure for beta thromboglobulin assay
A volume of 100uL of standard and sample was added to each well and was incubated for 90 min at 37℃. Liquid from each well was decanted and immediately 100uL of biotinylated Ag/Ab detection was added and incubated for 1 h at 37℃. Solution from each well was decanted and 350uL of wash buffer was added and washed 3 times. A volume of 100ul of HRP conjugate was added to each well and incubated for 30 min at 37℃. It was aspirated and washed 5 times. A volume of 90uL of substrate reagent was added and incubated for 15 min at 37℃. A volume of 50uL of stop solution was added to each well and optical density of each well was determined at once with a micro plate reader set at 450 nm.
Calculation of result
To obtain averaged curve, the duplicate readings for each standard and samples calculated and then subtract the average zero standard optical density. A Plot of a four parameters logistic curve on log-log graph paper with standard concentration on the X-axis and OD on Y-axis. If the OD of the sample surpasses the upper limit of the standard curve, it was re-tested with appropriate dilution. The actual concentration is the calculated concentration multiplied by the dilution factor.
Estimation of factor IV (ELABSCIENCE, 2024)
Platelets Factor IV was performed by Enzyme Linked Immunosorbent Assay (ELISA) according to the manufacturer’s instructions, (Elabscience® Human PF4 (Platelet Factor 4) ELISA Kit, USA). This kit uses the sandwich-ELISA principle. Briefly, the principle is based on the simultaneous binding of the PF4 to two monoclonal antibodies, one immobilized on microwell plates and the other conjugated with horseradish peroxidase (HRP). After incubation, the separation bound fraction reacts with the substrate and substrate develops a color that changes when the Stop Solution was added. The optical density (OD) was measured spectrophotometrically at a wavelength of 450 ± 2 nm. The color intensity (OD) reported as proportional to the concentration of PF4 in the sample.
Statistical methods
The Data was entered into and analyzed by the Statistical package for social sciences (SPSS), version 18.0 soଅware (SPSS Inc., Chicago, IL, USA). Variables were assessed for normality, expressed as the mean ± standard deviation of normally distributed numerical variables and differences in the means of variables were determined by student ‘t’ test and F-test as appropriate. Continuous variables were compared using the appropriate tests (paired t-test for parametric data, Mann-Whitney U test for non-parametric data. Differences were considered to be statistically significant when the p-value is < 0.05.
Results
A total of 71 adult males (95.8%) and female (4.2%) prospective blood donors for platelet concentrate were recruited with mean age of 34.8; 40.8% were between the age range of 31–40 and 33.8% were ages below 30 years. High percentage of the participants were married (59.2%); unemployed (52.2%); acquired Tertiary education (64.8%); Christianity by faith (95.8%) and Yoruba by tribe (91.5%). Proportionally, 62.0% rarely experienced` stress in their daily activities; 95.8% non-cigarette smokers; 85.9% non-alcohol drinkers and usually with sleep pattern of more than 6 h per day (73.2%) on routine basis (Table 1).
According to the analysis of clinico-demographic characteristics of donation pattern: 47.9% regularly donate platelet concentrate; 29.6% donate for the first time; 18.3% donate for the second time, and 4.2% donate for the third time. Most donors donate at most twice a year (81.7%); relatives donate (59.2%); occasionally use analgesics (95.4%) and regularly use hematinics (81.3%) (Table 2).
PLT, PDW-SD, PCT &P-LCC were found to increase from pre-donation to post harvest (Concentrate). However, the increase was only statistically significant for PLT & PCT. Conversely, MPV, PDW-CV & P-LCR were found to decrease from pre-donation to post-harvest, the decrease was statistically significant with P-value of (0.001) respectively. CHANGE TO: According to t-test PLT, PDW-SD, PCT &P-LCC were found to increase from pre-donation to post harvest (Concentrate). However, the increase was only statistically significant for PLT & PCT. Conversely, MPV, PDW-CV & P-LCR were found to decrease from pre-donation to post-harvest, the decrease was statistically significant with P-value of (0.001) respectively (Table 3).
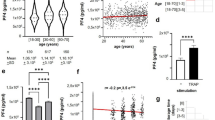
According to F-test pH and PF4 decreased from day 0 (Base-line) to 5th day (Post-harvest), however the decrease was not statistically significant for pH but significant for PF4. Conversely, beta-thromboglobulin increases from day 0 (Base-line) to 5th day (Post-harvest) and this increase was statistically significant at P-value of 0.001 (Table 4).
According to F-test Beta-thromboglobulin and PF4 concentrations increased progressively as hours of harvest increases proportionately with statistically nonsignificant P-value (Table 4). Contrariwise, there was a progressive decrease in pH crescively as hours of harvest time increases with no statistically significant mean difference (P-value˂ 0.05) (Table 5).
According to the F-test, pH was found to decrease progressively as days of storage increases over the period of 5 days pre-transfusion but the decrease was not statistically significant (P-value = 0.050). Inversely, Beta-thromboglobulin and PF4 were found to increase progressively as days advances but with no statistically significant p-value. (Table 6).
According to t-test, pH decreased from day 0 (Base-line) progressively to 5th day (Pre-transfusion). However, the decrease was not statistically significant, while Beta-thromboglobulin and PF4 increased from day 0 (Base-line) progressively to 5th day (Pre-transfusion) and this increase was statistically significant (p = 0.001) (Table 7).
Discussion
Platelet transfusion is a critical component of modern healthcare, particularly in the management of patients with hematological disorders, cancer, and those undergoing surgical procedures. The quality of platelet concentrates is important for ensuring effective transfusion outcomes and minimizing adverse reactions. It is of great interest that the study assessed platelet functional parameters, Beta-thromboglobulin levels, pH and platelet factor 4 in platelet concentrates stored at room temperature on constant agitation for 5 days pre-transfusion at the University of Ilorin Teaching Hospital’s Blood Bank and as well the socio-demographic factors of prospective blood donors for the concentrate. Recruited donors in this current study were apparently healthy youth, well-educated, non-cigarette smokers, non-alcohol drinkers and voluntary non-remunerated regular blood donors. Furthermore, most donors are relative non-remunerated donors with donation interval of at most twice a year (81.7%) who occasionally use analgesics but regularly use hematinics (Tables 1 and 2). The findings revealed high quality socio-demographic characteristics of the recruited prospective blood donors for platelet concentrates in this current study. Additionally, the baseline platelet functional parameters were within the normal threshold of an apparently healthy individual however, when compared with values post-harvest, the platelet count, plateletcrit (PCT), Platelet-large cell concentration (P-LCC), and platelet distribution width-standard deviation (PDW-SD) were higher relative to baseline values. This is consistent with the report of Maurer et al.7, Sahoo et al.17, and Nayak et al.18 stating that the increase in platelet count in the post-harvest phase is likely due to the efficiency of the harvest technique, which selectively collects platelets while returning other blood components in the satellite blood bag. Conversely, there was a significant decrease in mean platelet volume (MPV) and platelet distribution width coefficient of variation (PDW-CV) as recorded in this study which equally in agreement with existing studies reported independently by Nayak et al.18 and Wang et al.19. Antithetically, the study by Zhao et al.20, was at variance to our current finding which reported minimal changes in P-LCR after platelet donation. Justification for this incongruous could be equipment or technical standardization used in different studies, as well as potential differences in donor characteristics. This study further assessed biomarkers of platelet activation and platelet storage lesions under the heading: pH, beta-thromboglobulin (BTG) and platelet factor 4 levels in platelet concentrate at three key stages: pre-donation, post-harvest, and at 5th day storage. The pH level was found to longitudinally declined significantly at interval of three (3) hours in three consecutive clusters (the first 12 h) of preparation but when compared with daily pH drop recorded over 5 days, the difference in the fall was not statistically significant. This implies rapid fall in the pH potentiating early onset of platelet lesion during processing. This finding contradicts the outcome in previous research which reported stability of pH in stored platelet concentrates as reported by Mokhtar et al.21. As regards other platelet activation biomarkers, a non-significant progressive increase in BTG level and PF4 was recorded in the initial first twelve (12) hours of storage but the strength of increase in BTG level and PF4 over the five (5) days storage was statistically significant. This provided insights into the metabolic and activation changes that occur in platelets during storage and preparation for transfusion. This study aligned with the report previous investigators10,21,25, that platelet activation markers, such as BTG, increase during storage, primarily due to the stress exerted on platelets during the harvesting process and subsequent storage. This is also supported by the findings of Chaudhary et al.22, that platelet activation tends to accumulate over time during storage, leading to the progressive release of activation markers such as BTG. This progressive activation can impair the functional quality of platelets and potentially lead to poorer clinical outcomes following transfusion10,14,23,24,25. It has been observed that, certain contents of platelet concentrate like platelets factor IV are also altered during preparation which affect the estimation of these and quality of platelets concentrates which maybe as a result of platelets activation processes15.
Conclusion
This study revealed significant platelet activation during processing and storage. Hence, assessment of platelet functional parameters should be considered a priority in platelet concentrate and more attention should be given to platelet concentrate processes and storage in order to enhance positive clinical outcome and reducing adverse reactions.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request. All data generated or analyzed during this current study are included in this submitted article for publication.
References
Freireich, E. J. Origins of platelet transfusion therapy. Transfus. Med. Rev. 25 (3), 252–256. https://doi.org/10.1016/j.tmrv.2011.01.003 (2011).
Solves Alcaina, P. Platelet transfusion: and update on challenges and outcomes. J. Blood Med. 11, 19–26. https://doi.org/10.2147/JBM.S234374 (2020).
Blumberg, N. et al. Management of platelet disorders and platelet transfusions in ICU patients. Transfus. Med. Rev. 31 (4), 252–257. https://doi.org/10.1016/j.tmrv.2017.04.002 (2017).
Kaushansky, K. Historical review: megakaryopoiesis and thrombopoiesis. Blood 111 (3), 981–986. https://doi.org/10.1182/blood-2007-05-088500 (2008).
Ellingson, K. D. et al. Continued decline in blood collection and transfusion in the united States-2015. Transfusion 57 (Suppl 2(Suppl 2), 1588–1598. https://doi.org/10.1111/trf.14165 (2017).
Schiffer, C. A. et al. Platelet transfusion for patients with cancer: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 36 (3), 283–299. https://doi.org/10.1200/JCO.2017.76.1734 (2018).
Berti, P. et al. Impact of platelet transfusion at different doses in oncohematology pediatric inpatients and outpatients: A retrospective study. Pediatr. Blood Cancer. 72 (4), e31550. https://doi.org/10.1002/pbc.31550 (2025).
Stanworth, S. J., Hyde, C., Brunskill, S. & Murphy, M. F. Platelet transfusion prophylaxis for patients with haematological malignancies: where to now? Br. J. Haematol. 131 (5), 588–595. https://doi.org/10.1111/j.1365-2141.2005.05769.x (2005).
Andreu, G. et al. Platelet additive solutions and pathogen reduction impact on transfusion Safety, patient management and platelet supply. Transfus. Med. Rev. 39 (1), 150875. https://doi.org/10.1016/j.tmrv.2025.150875 (2025).
Vit, G., Klüter, H. & Wuchter, P. Platelet storage and functional integrity. J. Lab. Med. 44 (5), 285–293. https://doi.org/10.1515/labmed-2020-0067 (2020).
Heemskerk, J. W. M. & West, J. Emerging technologies for Understanding platelet diversity. Arterioscler. Thromb. Vasc Biol. 42 (5), 540–552. https://doi.org/10.1161/ATVBAHA.121.317092 (2022).
Goodnough, L. T. et al. Prophylactic platelet transfusions from healthy apheresis platelet donors undergoing treatment with thrombopoietin. Blood 98 (5), 1346–1351. https://doi.org/10.1182/blood.v98.5.1346 (2001).
Lozano, M. & Cid, J. Cryopreserved platelets: a narrative review of its current role in transfusion therapy. Ann. Blood. 7, 40. https://doi.org/10.21037/aob-21-31 (2022).
Yoshida, T., Prudent, M. & D’alessandro, A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 17 (1), 27–52. https://doi.org/10.2450/2019.0217-18 (2019).
Müller, M. M. et al. Aufklärung und Nebenwirkungen. Platelet Concentrates - Indication, informed Consent, transfusion and adverse events. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 60 (1), 35–51. https://doi.org/10.1055/a-2234-1341 (2025). German.
Slichter, S. J. et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl. J. Med. 362 (7), 600–613. https://doi.org/10.1056/NEJMoa0904084 (2010).
Sahoo, D., Mahapatra, S., Parida, P. & Panigrahi, R. Various aspects of plateletpheresis: its impact on donor and patients. Glob J. Transfus. Med. 2 (2), 149–154. https://doi.org/10.4103/GJTM.GJTM_24_17 (2017).
Nayak, S., Coshic, P., Pandey, R. M. & Chatterjee, K. Frequent plateletpheresis donations & its effect on haematological parameters: an observational study. Indian J. Med. Res. 150 (5), 468–476. https://doi.org/10.4103/ijmr.IJMR_512_18 (2019).
Wang, X. et al. Thrombocytopenia in pregnancy with different diagnoses: differential clinical features, treatments, and outcomes. Med. (Baltim). 96 (29), e7561. https://doi.org/10.1097/MD.0000000000007561 (2017).
Chen, J. et al. Association of longitudinal platelet count trajectory with ICU mortality: A multi-cohort study. Front. Immunol. 13, 936662. https://doi.org/10.3389/fimmu.2022.936662 (2022).
Mokhtar, M. B., Hashim, H. B. & Joshi, S. R. Assessment of quality of platelets preserved in plasma and platelet additive solution: A Malaysian experience. Asian J. Transfus. Sci. 2016 Jan-Jun ;10(1):84–87. https://doi.org/10.4103/0973-6247.172177
Chaudhary, P. K., Kim, S. & Kim, S. An insight into recent advances on platelet function in health and disease. Int. J. Mol. Sci. 23 (11), 6022. https://doi.org/10.3390/ijms23116022 (2022).
Jamal, L. et al. Emerging approaches to pre-hospital hemorrhage control: a narrative review. Ann. Transl Med. 9 (14), 1192. https://doi.org/10.21037/atm-20-5452 (2021).
Heddle, N. M. et al. Methodologic issues in the use of bleeding as an outcome in transfusion medicine studies. Transfusion 43 (6), 742–752. https://doi.org/10.1046/j.1537-2995.2003.00418.x (2003).
Weaver, A. J. Jr et al. Evaluating the effects of hypoxic storage on platelet function and health using a novel storage system. Transfusion 64 (4), 693–704. https://doi.org/10.1111/trf.17784 (2024).
Acknowledgements
We acknowledge the immense technical support of the staff of Blood Transfusion Science Unit of the department of Haematology, University of Ilorin Teaching Hospital, Ilorin to mention but not limited to Mr Lambe Rasheed, Mrs Njoku, Mr Sylvester, Mr Kinsley, Mrs Adeniyi and the Director of Medical Laboratory Services in person of Deacon Alabi Sunday.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: Olatunbosun, L. O, Muhibi M. A, and Oke O. T; Methodology: Olatunbosun, L. O, Uthman R. D, and Idris A. O; Validation: Olatunbosun, L. O, Muhibi, M. A, and Oke, O. T; Investigation: Olatunbosun, L. O, Uthman R. D, Idris A. O; Resources: Ademosun A. A, Adebayo, F. K, Olalere, F. D, Kareem, M. A, Azeez, R. T, and Adebayo, F. K; Writing—Original draft preparation: Olatunbosun, L. O, Babatunde, S. A, Ademosun, A.A, Adebayo, F. K, Olalere, F. D, Kareem, M.A, and Emmanuel S. O; Writing—Review and Editing : Olatunbosun, L. O, Sanni, E. O and Babatunde, S. A; Yusuf M. A; Supervision : Muhibi, M. A, and Oke, O. T.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Olatunbosun, L.O., Muhibi, M.A., Oke, O.T. et al. Assessment of platelet indices, beta-thromboglobulin and platelet factor IV in platelet concentrate at University of Ilorin Teaching Hospital, Nigeria. Sci Rep 15, 42155 (2025). https://doi.org/10.1038/s41598-025-26247-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26247-y