Abstract
Due to the limitations of analytical methodologies, the geochemical significance of C5 alkylated benzenes has largely been overlooked. Thirty-four light oils and condensates were collected from the Tarim Basin and Beibuwan Basin for analysis using comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GC×GC-TOFMS). The concentrations of twelve C5 alkylated benzenes exhibit distinct distribution patterns in light oils and condensates originating from varying sedimentary environments and organic matter sources. The concentrations of 1-E-3,4,5-TMB, 1-E-2,3,4-TMB, and 1-E-2,4,5-TMB are likely influenced by the source of organic matter, whereas those of 1-E-2,4,6-TMB, 1-E-2,3,5-TMB, and 1,2,3,4,5-PMB are potentially governed by the sedimentary environment. Based on the differential sensitivity of C5 alkylated benzenes to sedimentary environments and organic matter sources, the l-ethyl (E)-2,4,6-trimethylbenzene (TMB)/1-E-2,3,6-TMB (C5-R1) and 1-E-2,4,6-TMB/1-E-3,4,5-TMB (C5-R2) have been proposed as geochemical indicators for distinguishing the sedimentary environments and organic matter sources of crude oils from their respective source rocks. Oils derived from their respective source rocks that formed in oxic/disyoxic sedimentary environments are characterized by relatively high C5-R1 values (greater than 0.5), while those formed under reduced sedimentary conditions exhibit relatively low C5-R1 values (less than 0.5). Oils originating from terrestrial higher plants are characterized by relatively high C5-R2 values (greater than 1.0), whereas those derived from a mixed input of lower aquatic organisms and terrestrial higher plants display relatively low C5-R2 values (less than 1.0). The C5-R1 and C5-R2 ratios are likely to remain unaffected or only minimally influenced by secondary alteration processes (evaporative fractionation, biodegradation, and thermal maturity). The C5-R1 and C5-R2 ratios can serve as supplementary parameters for identifying sedimentary environments and organic matter sources, particularly in light oils and condensates where conventional biomarkers are significantly depleted.
Similar content being viewed by others
Introduction
Due to the high degree of thermal evolution of characteristic light oils and condensates, their composition is dominated by light hydrocarbons, which constitute more than 90%1,2. Alkylbenzenes (monocyclic aromatic hydrocarbons) are an important component of light hydrocarbons, accounting for more than 2–6 wt.%3. Alkylbenzenes are believed to be generated through homolytic cleavage of the carbon-carbon bonds in alkyl side chains attached to macromolecular structure (such as kerogen)4. Alkylbenzene compounds are utilized as indicators for assessing the source5, sedimentary environment6 and maturity7 of ancient organic matter.
There are many previous reports on the geochemical data of C0-C4 alkylbenzenes, mainly including the biological sources of 1,2,3-trimethylbenzene (TMB), 1,3-+1,4-dimethylbenzene (DMB), 1,2,3,4-tetramethylbenzene (TeMB)4,8,9,10,11,12,13. The ratios of isoproyltoluene (iPT) isomers were proposed to indicate the origin of organic matter5. 1,2,3,5-/1,2,3,4-TeMB (TeMBr) and 1,2,3,5-TeMB concentrations were used to indicate the sedimentary environments6. 1,2,4,5-/1,2,3,4-TeMB and 1,2,4,5-TeMB concentration can be used to distinguish the source and depositional environment of ancient organic matter14. A series of alkylbenzene parameters have been employed to assess the maturity of sedimentary organic matter,6,7,15,16 while the Tol/nC7 has been used to distinguish secondary alterations17,18.
Compared with C0-C4 alkylbenzenes, the research of C5 alkylbenzenes have been studied relatively limited, and the current research mainly includes the distribution characteristics of C5 alkylbenzenes and the possible biological sources of individual compounds. The C5-alkylated benzenes are predominantly composed of 1-ethyl-2,3,6-trimethylbenzene4. 1-Ethyl-2,3,6-trimethylbenzene and 1-ethyl-3,4,5-trimethylbenzene are likely derived from the aromatic carotenoids possessing the 2,3,6- and 3,4,5- substitution pattern incorporated into the kerogen4. In addition, 1-ethyl-2,3,6-trimethylbenzene derived from Chlorobiaceae can be significantly enriched in 13C19,20.
Due to the relatively low concentration of C5 alkylbenzenes in crude oils and their tendency to co-elute with other compounds during gas chromatography (GC) analysis, as reported by Jia et al.12, these compounds have received limited research attention. Consequently, a significant amount of geochemical information related to C5 alkylbenzenes remains underexplored due to limitations in current analytical methodologies. Fortunately, comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GC×GC–TOFMS) can separate compounds based on their boiling point differences in the first dimension and their polarity difference in the second dimension, respectively. This method provides excellent resolution in compound separation, thereby offering a powerful tool for the study of C5 alkylbenzenes6,14.
In this paper, we report the results of detailed study of twelve C5 alkylated benzenes distributions in thirty-four light oils and condensates from diverse sedimentary environments and geological ages. It primarily involves the analysis of similarities and differences in C5 alkylbenzene distributions between light oils and condensates, and discusses potential geochemical indicators. The C5 alkylbenzenes, which has a specific biological origin and formation environment, are of great geochemical significance for light oils and condensates where biomarkers are scarce.
Samples and methods
Samples
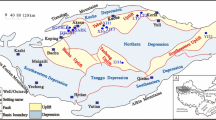
A total of thirty-four light oil and condensate samples were collected from the Tarim Basin (25 samples) and Beibuwan Basin (9 samples). The detailed characteristics of crude oil samples, including sample naming, source age and sedimentary environment, is shown in Table 1.
Tarim marine and swamp oils
A total of twenty-five light oil and condensate samples were collected from the Tarim Basin, of which fifteen oils were from the Cambrian-Ordovician marine source rocks in the Halahatang Depression, five oils were derived from Jurassic coal-measure source rocks in the Luntai Sub-uplift, and five oils were originated from Jurassic coal-measure source rocks in the Kuqa Depression (Fig. 1a). The light oils and condensates from Cambrian-Ordovician marine source rocks are derived from lower aquatic organisms and formed in a reduced sedimentary environment22,23. The light oils and condensates derived from the Jurassic coal-measure source rocks originated from terrigenous higher plants and were formed in an oxidizing sedimentary environment24,25,26,27. The Tarim marine oils are at a mature stage of thermal evolution25. The swamp oils are at a mature to high-maturity stage of thermal evolution24,26,27,28.
Beibuwan lacustrine oils
A total of nine light oil and condensate samples were collected from the Fushan depression, Beibuwan Basin (Fig. 1b). The crude oils are in the stage of high maturity and their sterane and hopane biomarkers are depleted29. These light oils and condensates are mainly from black mudstone and shale of the Liushagang Formation (E2l), which are typical lacustrine oils30. The source rocks of the Liushagang Formation (E2l) were primarily deposited in an oxidizing sedimentary environment and are predominantly composed of terrigenous organic matter31,32.
Methods
The saturated hydrocarbons and aromatic hydrocarbons were separated from light oil samples by column chromatography. Crude oil samples were analyzed mainly by GC and GC×GC-TOFMS. The saturated hydrocarbons and aromatic hydrocarbons were analyzed by GC-MS.
The GC model is Agilent 6890 A with a fused silica HP-PONA column (50 m×0.2 mm×0.5 μm). The program temperature setting is: the initial oven temperature is 35℃, keep for 5 min, heated at 3 °C /min to 70 °C, and then heated at 4.5 °C to 300 °C, held for 25 min. The GC-MS model is Agilent 6890 A GC-5979i MS, with is an HP-5MS column (60 m×0.25 μm×0.25 μm). The GC program temperature setting is: for saturated hydrocarbons, the initial oven temperature is 50 °C, held for 1 min, heated at 20 °C/min to 100 °C, and then heated at 3 °C/min to 310 °C for 10 min. For aromatic hydrocarbons, the initial oven temperature is 80 °C for 1 min, and the temperature is raised to 300 °C at 3 °C/min for 20 min.
Before crude oils were analyzed, internal standard (1-hexene) was added into the crude oils of known weight. The GC×GC-TOFMS mode is Agilent 7890 GC-Pegasus 4D TOFMS with non-polar capillary HP-PONA column (50 m×0.20 mm×0.50 μm) in the first dimension and polar capillary Rxi-17 column (1.7 m×0.1 mm×0.1 μm) in the second dimensions. The GC program temperature setting is: for the first dimensional column, the initial temperature is 35 °C, held for 5 min, and the temperature is raised at 2 °C/min to 295 °C, held for 30 min. For the second dimensional column, the initial temperature is 40 °C for 5 min, and the temperature was raised to 280 °C at 2 °C/min for 30 min. The modulation period is 1.2 s hot pulse and 4.8 s cold pulse. The detector and ion source voltages are 1350 V and 70 eV, respectively. Helium is used as a carrier. The above experimental analysis was done in the National Key Laboratory of Petroleum Resources and Engineering, China University of Petroleum (Beijing).
Results
Identification of C5 alkylated benzenes in light oils
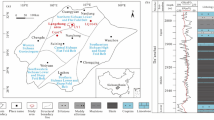
As shown in Fig. 2, mass chromatogram of m/z 148 was used to present C5 alkylated benzenes, which presents an echelon distribution. The separation of alkylbenzene compounds on the chromatogram is mainly based on the boiling point of the compounds in the first dimension and by their polarity in the second dimension. The twelve C5 alkylated benzenes were detected in the Tarim marine and swamp oils, and Beibuwan lacustrine oils using GC×GC-TOFMS and were identified by comparison with Nist Standard Reference Database version 2.0 and published literature4 (Table 2). 1-Methyl-2-tert-butylbenzene (1-M-2-t-BB), 1-methyl-4-tert-butylbenzene (1-M-4-t-BB), 2-methylbutylbenzene (2-MBB), 3-methylbutylbenzene (3-MBB) were identified by Nist Standard Reference Database version 2.0. The remaining eight C5 alkylated benzenes were identified with the aid of Hartgers et al.4. The molecular structures of twelve C5 alkylated benzenes are presented in Fig. 2.
The m/z 148 mass chromatogram of oil YM4 (a) and DB2 (b) from the Tarim Basin, oil H2 (c) from the Beibuwan Basin show the distribution of C5 alkylated benzenes in GC×GC–TOFMS. The peak numbers correspond to the compound names listed in Table 2.
The first dimension and second dimension retention times, retention indices, molecular masses, base peaks, and compound abbreviations are shown in Table 2. All C5 alkylbenzenes exhibit a parent ion at m/z 148 and possess the molecular formula of C11H16. The mass spectrum of both 2-MBB and 3-MBB exhibit a base peak at m/z 92. The mass spectrum of n-pentylbenzene (n-PB) exhibits a base peak at m/z 91. The base peak of the remaining C5 alkylbenzenes is at m/z 133. A higher peak at m/z 105 occurs in the mass spectrum of both 1-M-2-t-BB and 1-M-4-t-BB. The mass spectrum of both 2-MBB and 3-MBB exhibit a more intense peak at m/z 91, whereas the mass spectrum of n-PB shows a more intense peak at m/z 92.
Distribution of C5 alkylated benzenes in light oils
Figure 3 shows the concentration distribution of twelve C5 alkylated benzenes in all analyzed oil samples from the Tarim marine and swamp oils, and Beibuwan lacustrine oils. Based on the distribution characteristics of twelve C5 alkylated benzenes in the Tarim marine and swamp oils, and Beibuwan lacustrine oils, these oils can be classified into four categories. (1) 1-M-2-t-BB, 1-M-4-t-BB and 3-MBB are enriched in the Tarim swamp oils (0.19–0.75 (average 0.46), 0.50–1.99 (average 1.28), 0.05–0.68 (average 0.22) mg/g whole oil) (Table 3), and are below the detection limit or depleted in the Tarim marine oils and Beibuwan lacustrine oils. (2) The concentration of l-ethyl-3,4,5-trimethylbenzene (1-E-3,4,5-TMB), 1-E-2,3,4-TMB, and 1-E-2,4,5-TMB in the Tarim marine oils (0.14–0.40 (average 0.25), 0.07–0.26 (average 0.14), 0-0.31 (average 0.18) mg/g whole oil) and Beibuwan lacustrine oils (0.30–0.75 (average 0.46), 0.13–0.41 (average 0.25), 0.40–1.85 (average 0.89) mg/g whole oil) are higher than those in the Tarim swamp oils (0.01–0.15 (average 0.07), 0.03–0.16 (average 0.09), 0.03–0.34 (average 0.15) mg/g whole oil) (Table 3). (3) The concentration of 1-E-2,4,6-TMB, 1-E-2,3,5-TMB and 1,2,3,4,5-PMB in the Beibuwan lacustrine oils (0.12–0.37 (average 0.22), 0.22–0.84 (average 0.58), 0.39–1.48 (average 0.71) mg/g whole oil) are higher than those in the Tarim marine oils (0.03–0.08 (average 0.05), 0.12–0.46 (average 0.25), 0-0.40 (average 0.12) mg/g whole oil) and Tarim swamp oils (0.03–0.19 (average 0.10), 0.03–0.39 (average 0.16), 0-0.41 (average 0.18) mg/g whole oil) (Table 3). (4) There was no significant difference in the contents of 2-MBB, n-PB and 1-E-2,3,6-TMB in all studied oils (Table 3).
The concentrations of C5 alkylated benzenes in light oils and condensates. (a) 1-M-2-t-BB; (b) 1-M-4-t-BB; (c) 3-MBB; (d) 1-E-3,4,5-TMB; (e) 1-E-2,3,4-TMB; (f) 1-E-2,4,5-TMB; (g)1-E-2,4,6-TMB; (h) 1-E-2,3,5-TMB; (i) 1,2,3,4,5-PMB; (j) 2-MBB; (k) n-PB; (l) 1-E-2,3,6-TMB. The concentration unit is mg/g of whole oil.
Discussion
Origins of C5 alkylated benzenes
Alkylbenzenes are mainly generated via α-, β-, γ- and δ-cleavages of the side chain by which the aromatic moiety with specific substitution patterns attached to macromolecular structures (e.g., kerogen)4. For example, 1-E-2,3,6-TMB is likely generated γ-cleavage of macromolecular-bound aromatic carotenoids with a 2,3,6-trimethyl substitution pattern4. 1-E-2,3,6-TMB is indeed derived from isorenieratene biosynthesized by Chlorobiaceae33. Similar to the origin of 2,3,6-trimethylbenzene moieties in pyrolysis products, Hartgers et al.33 proposed a diaromatic carotenoid with an unprecedented substitution pattern to explain the presence of 3,4,5-trimethylbenzene moieties. 1-E-3,4,5-TMB are derived from an organism that lived in the same habitat as the Chlorobiaceae33. Other C5 alkylated benzenes may be also generated via γ-cleavages of the side chain by which the aromatic moieties with specific substitution patterns linked to kerogen.
The high contents of 1-M-2-t-BB, 1-M-4-t-BB and 3-MBB in the Tarim swamp oils indicate these compouds were likely formed in sub-oxidized environment and are derived from terrestrial higher plants. The contents of 1-E-3,4,5-TMB, 1-E-2,3,4-TMB and 1-E-2,4,5-TMB in the Tarim marine oils and Beibuwan lacustrine oils are higher than those in the Tarim swamp oils, indicating that they may not be easily formed in sub-oxidized environment and derived from the terrestrial higher plants. The contents of 1-E-2,4,6-TMB, 1-E-2,3,5-TMB and 1,2,3,4,5-PMB in the Tarim marine and swamp oils are lower than those in the Beibuwan lacustrine oils, indicating they may be formed in oxidized sedimentary environment. Based on the sensitivity of different C5 alkylated benzenes to sedimentary environments and organic matter sources, the ratios of 1-E-2,4,6-TMB to 1-E-2,3,6-TMB (C5-R1) and 1-E-2,4,6-TMB to 1-E-3,4,5-TMB (C5-R2) have been proposed to distinguish the sedimentary environments and organic matter sources of crude oils from their respective source rocks.
C5-R1 as an indicator of depositional environments
Pristane/phytane (Pr/Ph)34, 2-methyl-3-ethylheptane/2,6-dimethyloctane (MTR)28, 2-methyl-3-ethylheptane/3-methylnonane (MT1) and 1,1,2,3-tetramethylcyclohexane/propylcyclohexane (MT2) were proposed to distinguish the redox conditions of sedimentary organic matter35. In Fig. 4, all studied oils can be divided into two groups based on Pr/Ph, MTR, MT1, MT2 linked with C5-R1. The Tarim marine oils having relatively low Pr/Ph, C5-R1 values, and high MTR, MT1, and MT2 values (Table 4), indicate oils derived from the source rocks formed in anoxic sedimentary environments. The Tarim swamp oils and Beibuwan lacustrine oils relatively high Pr/Ph, C5-R1 values, and low MTR, MT1, and MT2 values (Table 4), indicate oils derived from the source rocks formed in oxic/dysoxic depositional environment.
1,2,4,5-/1,2,3,4-TeMB14 and 1,2,4,5-/1,2,3,4-TeMB (TeMBr)6 were proposed as indicators to infer the sedimentary environments of the crude oils derived from their respective source rocks. In Fig. 5, the Tarim marine oils with relatively low 1,2,4,5-/1,2,3,4-TeMB, TeMBr, and C5-R1 values (Table 4), indicate oils derived from the source rocks deposited under reducing/anoxic conditions. The Beibuwan lacustrine oils and the Tarim swamp oils with higher 1,2,4,5-/1,2,3,4-TeMB, TeMBr, and C5-R1 values (Table 4), suggest oils derived from source rocks formed under oxic/dysoxic sedimentary environments. In addition, the dibenzothiophene/phenanthrene (DBT/P) ratio and the C5-R1 ratio can also well distinguish crude oil from different sedimentary environments (Table 4).
In general, there is a good correspondence between the C5-R1 values and the sedimentary environment indicators (Pr/Ph, MTR, MT1, MT2, 1,2,4,5-/1,2,3,4-TeMB and TeMBr). The higher C5-R1 value (> 0.5) indicates that the oils originated from source rocks formed in oxidizing sedimentary environments, while a lower C5-R1 value (< 0.5) indicates that the oils were derived from source rocks formed in reducing sedimentary environments.
C5-R2 as an indicator of organic matter sources
1-Methyl-3-isopropylbenzene (1-M-3-iPB)/1-methyl-2-isopropylbenzene (1-M-2-iPB) (iPTr1), 1-methyl-4-isopropylbenzene (1-M-4-iPB)/1-M-2-iPB (iPTr2), and (1-M-3-iPB + 1-M-4-iPB)/1-M-2-iPB (iPTr) were proposed by Meng et al.5 to distinguish the organic matter origin of the crude oils from their respective source rocks. In Fig. 6, all studied oils can be divided into two groups based on iPTr1, iPTr2, iPTr linked to C5-R2 values. The Tarim marine oils and Beibuwan lacustrine oils with relatively high iPTr1, iPTr2, iPTr values, and low C5-R2 values (Table 4), indicate that the organic matter in their respective source rocks was derived from a mixture of lower aquatic organisms and terrestrial higher plants. The Tarim swamp oils with relatively low iPTr1, iPTr2, iPTr values, and high C5-R2 values (Table 4), suggest that the organic matter in their respective source rocks was predominantly derived from terrestrial higher plants.
Relatively high cadinane/(cadinane + 8β(H)-drimane), 2,2,4a,7,8-pentamethyl-decalin/(2,2,4a,7,8-pentamethyl-decalin + 8β(H)-drimane), 1,2,2,5,5-pentamethyl-trans-decalin/8β(H)-drimane, 1,1,2,5,5-pentamethyl-trans-decalin/8β(H)-drimane values indicate terrestrial organic matter input36,37. In Fig. 7, the Tarim marine oils with relatively low cadinane/(cadinane + 8β(H)-drimane), bicyclic sesquiterpenes compounds ratios, and C5-R2 values (Table 4), indicate that the organic matter in their respective source rocks was predominantly derived from lower aquatic organisms. The Tarim swamp oils with relatively high cadinane/(cadinane + 8β(H)-drimane), bicyclic sesquiterpenes compounds ratios, and C5-R2 values (Table 4), suggest that the organic matter in their respective source rocks was predominantly derived from the terrestrial higher plants. The Beibuwan lacustrine oils with relatively high cadinane/(cadinane + 8β(H)-drimane), bicyclic sesquiterpenes compounds ratios, and low C5-R2 values (Table 4), suggest that the organic matter in their respective source rocks was derived from a mixture of lower aquatic organisms and terrestrial higher plants.
Cross-plots of C5-R2 vs. cadinane/(cadinane + 8β(H)-drimane) (a), 1,2,2,5,5-pentamethyl-trans-decalin/(1,2,2,5,5-pentamethyl-trans-decalin + 8β(H)-drimane) (b), 2,2,4a,7,8-pentamethyl-decalin/(2,2,4a,7,8-pentamethyl-decalin + 8β(H)-drimane) (c), 1,1,2,5,5-pentamethyl-trans-decalin/(1,1,2,5,5-pentamethyl-trans-decalin + 8β(H)-drimane) (d) in all studied oils.
Relatively high nC7/MCyC6 ratio indicates marine organic matter input, and relatively high CyC5 − 7/nC5 − 7 ratio indicates the terrestrial organic matter input38,39,40. In Fig. 8, the Tarim marine oils with relatively high nC7/MCyC6, low CyC5 − 7/nC5 − 7, and C5-R2 values (Table 4), indicate that the organic matter in their respective source rocks was predominantly derived from lower aquatic organisms. The Tarim swamp oils with relatively low nC7/MCyC6, high CyC5 − 7/nC5 − 7, and C5-R2 values (Table 4), indicate that the organic matter in their respective source rocks was predominantly derived from the terrestrial higher plants. The Beibuwan lacustrine oils with relatively low nC7/MCyC6, high CyC5 − 7/nC5 − 7, and low C5-R2 values (Table 4), suggest that the organic matter in their respective source rocks was derived from a mixture of lower aquatic organisms and terrestrial higher plants.
In conclusion, the relatively high C5-R2 value (> 1.0) suggests the oils originate from source rocks associated with terrestrial higher plants, while the relatively low C5-R2 value (< 1.0) indicates the oils are derived from source rocks containing a mixture of lower aquatic organisms and terrestrial higher plants.
Secondary alteration influence on C5-R1 and C5-R2 values
Evaporative fractionation
The low to medium molecular weight hydrocarbons in light oils and condensates are easily changed into vapor phase by evaporative fractionation, and then the primary composition of crude oil compounds is changed17,18. Thompson (1988) proposed the intersection chart of nC7/MCyC6 (F) and Tol/nC7 (B) values to evaluate whether evaporative fractionation occurs in crude oils. If the crude oils undergo evaporative fractionation, it will deviate from the normal oils, which is characterized by the decrease of F value (< 0.5) and the increase of B value (> 1.0)17. The Tarim marine oils with relatively high F value (1.64–2.04) and low B value (0.08–0.31) indicate these oils did not undergo the effect of evaporative fractionation. The Tarim swamp oils and Beibuwan lacustrine oils exhibit relatively low F values (0.19–0.69 and 0.18–0.53) and high B values (0.49–3.55 and 0.61–2.66). The F value is controlled by thermal maturity and organic matter type, and the low F values of Beibuwan lacustrine oils and Tarim swamp oils may mainly reflect significant the input of terrestrial organic matter. The toluene content in terrigenous organic matter is usually higher than that in marine organic matter41,42. Therefore, the Tarim swamp oils and Beibuwan lacustrine oils with relatively low F values and high B values may reflect terrestrial organic matter input. In addition, the compound into the vapor phase depends mainly on the vapor pressure of the compound, which depends on the molecular structure and molecular weight of the compound. Thus, the ratios of 1-E-2,4,6-TMB to 1-E-2,3,6-TMB (C5-R1) and 1-E-2,4,6-TMB/1-E-3,4,5-TMB (C5-R2) may not or slight be affected by evaporative fractionation due to 1-E-2,4,6-TMB, 1-E-2,3,6-TMB and 1-E-3,4,5-TMB with similar molecular structure and same molecular weight.
Biodegradation
Biodegradation can change the original composition of crude oil and has a great impact on the use of light hydrocarbon parameters, so it is of great significance to determine the impact of biodegradation on newly established parameters. The 25-norhopanes are usually effective indicator of biodegradation of crude oils43,44,45. There is no obvious 25-norhopanes detected in the Tarim swamp oils and Beibuwan lacustrine oils, indicating these oils did not undergo biodegradation. Although 25-norhopanes were detected in the Tarim marine oils, the light hydrocarbons in the Tarim marine oils were late charging components, and they did not occur biodegradation22,46. For aromatic compounds, the biodegradation rate is inversely proportional to the number of aromatic rings and directly proportional to the number of alkyl substituents. Due to 1-E-2,4,6-TMB, 1-E-2,3,6-TMB and 1-E-3,4,5-TMB with similar molecular structure and same molecular weight, even if the crude oils is biodegraded, the selective degradation of 1-E-2,4,6-TMB, 1-E-2,3,6-TMB and 1-E-3,4,5-TMB is not possible. Therefore, C5-R1 and C5-R2 ratios may not be affected by biodegradation.
Thermal maturity
The ratios of methyldibenzothiophene (MDBT), ethyldibenzothiophene (EDBT), and dimethyldibenzothiophene (DMDBT) compounds are used to indicate the thermal maturity of sedimentary organic matter based on the difference in thermal stability of compounds with different substituents of dibenzothiophene47,48. In Fig. 9, there is a good positive correlation between MDR with MDR’, EDR and EDR’ in all studied oils. There is obvious difference in thermal maturity in the Tarim marine oils, Tarim swamp oils, and Beibuwan lacustrine oils. Based on the different thermal stability of methylphenanthrene (MP) substituents, F1 and F2 have been proposed to indicate the thermal maturity of sedimentary organic matter49. The Tarim marine oils and the Beibuwan lacustrine oils are in the mature evolution stage, while the Tarim swamp oils is in the mature to high mature evolution stage (Fig. 10). The thermal maturity of the Tarim marine oils is similar to that in Beibuwan lacustrine oils, but the values of C5-R1 and C5-R2 in the Tarim marine oils are lower than those in Beibuwan lacustrine oils. Therefore, the C5-R1 and C5-R2 parameters are largely unaffected by thermal maturity.
Conclusions
The contents of twelve C5 alkylated benzenes in thirty-four light oils were quantitatively analyzed by GC×GC-TOFMS. 1-M-2-t-BB, 1-M-4-t-BB and 3-MBB are enriched in the Tarim swamp oils but are below the detection limit or depleted in the Tarim marine oils and Beibuwan lacustrine oils. The concentration of 1-E-3,4,5-TMB, 1-E-2,3,4-TMB, and 1-E-2,4,5-TMB in the Tarim marine oils and Beibuwan lacustrine oils are higher than those in the Tarim swamp oils. The concentrations of 1-E-2,4,6-TMB, 1-E-2,3,5-TMB and 1,2,3,4,5-PMB in the Beibuwan lacustrine oils are higher than those in the Tarim marine oils and Tarim swamp oils. There were no significant differences in the contents of 2-MBB, n-PB and 1-E-2,3,6-TMB among all studied oils. Based on the sensitivity of different C5 alkylated benzenes to sedimentary environment and organic matter sources, the C5-R1 and C5-R2 were proposed to distinguish the sedimentary environments and organic matter sources of crude oils derived from their respective source rocks. A higher C5-R1 value (> 0.5) indicates that the oils derived from their respective source rocks were formed in oxidizing sedimentary environments, while a lower C5-R1 value (< 0.5) indicates that the oils originated from source rocks formed in reducing sedimentary environments. A relatively high C5-R2 value (> 1.0) suggests that the oils derived from their respective source rocks was predominantly originated from terrestrial higher plants, while a relatively low C5-R2 value (< 1.0) indicates that the oils were derived from a mixture of lower aquatic organisms and terrestrial higher plants. The C5-R1 and C5-R2 ratios are unlikely to be significantly affected by evaporative fractionation, biodegradation, and thermal maturity.
Data availability
All data generated or analysed during this study are included in this published article.
References
Hunt, J. M., Huc, A. Y. & Whelan, J. K. Generation of light hydrocarbons in sedimentary rocks. Nature 288 (5792), 688–690. https://doi.org/10.1038/288688a0 (1980).
Song, D. F., Wang, T. G., Deng, W. & Shi, S. Application of light hydrocarbons (C5–C7) in paleozoic marine petroleum of the Tarim Basin, NW China. J. Petrol. Sci. Eng. 140, 57–63 (2016).
Ostroukhov, S. B. et al. C12-C30 n-alkylbenzenes in crude oils. Petrol. Chem. U S S R. 23, 1–12. https://doi.org/10.1016/S0031-6458(83)80090-2 (1983).
Hartgers, W. A., Sinninghe Damsté, J. S. & de Leeuw, J. W. Geochemical significance of alkylbenzene distributions in flash pyrolysates of kerogens, coals, and asphaltenes. Geochem. Cosmochim. Acta. 58, 1759–1775. https://doi.org/10.1016/0016-7037(94)90535-5 (1994).
Meng, B. K. et al. Origin, distribution and geochemical significance of isopropyltoluene isomers in crude oil. J. Earth Sci. 33, 215–228. https://doi.org/10.1007/s12583-020-1348-0 (2022).
Cheng, B. et al. Ratios of low molecular weight alkylbenzenes (C0-C4) in Chinese crude oils as indicators of maturity and depositional environment. Org. Geochem. 88, 78 – 90. https://doi.org/10.1016/j.orggeochem.2015.08.008 (2015).
Zhang, S. C. et al. Geochemistry of alkylbenzenes in the paleozoic oils from the Tarim Basin, NW China. Org. Geochem. 77, 126–139. https://doi.org/10.1016/j.orggeochem.2014.10.003 (2014).
Requejo, A. G. et al. Aryl isoprenoids and diaromatic carotenoids in paleozoic source rocks and oils from the Western Canada and Williston basins. Org. Geochem. 19 (1–3), 245–264. https://doi.org/10.1016/0146-6380(92)90041-U (1992).
Hartgers, W. A., Sinninghe Damsté, J. S. & de Leeuw, J. W. Identification of C2-C4 alkylated benzenes in flash pyrolysates of kerogens, coals and asphaltenes. J. Chromatogr. A. 606, 211–220. https://doi.org/10.1016/0021-9673(92)87027-6 (1992).
Hoefs, M. J. L. et al. Alternative biological sources for 1,2,3,4-tetramethylbenzene in flash pyrolysates of kerogen. Org. Geochem. 23, 975–979. https://doi.org/10.1016/0146-6380(95)00078-X (1995).
Pedentchouk, N. et al. Sources of alkylbenzenes in lower cretaceous lacustrine source rocks, West African rift basins. Org. Geochem. 35, 33–45. https://doi.org/10.1016/j.orggeochem.2003.04.001 (2004).
Jia, W. L., Peng, P. A., Yu, C. L. & Xiao, Z. Y. Source of 1,2,3,4-tetramethylbenzene in asphaltenes from the Tarim basin. J. Asian Earth Sci. 30, 591–598. https://doi.org/10.1016/j.jseaes.2006.09.003 (2007).
Jia, W. L., Peng, P. A. & Xiao, Z. Y. Carbon isotopic compositions of 1,2,3,4-tetramethylbenzene in marine oil asphaltenes from the Tarim basin: evidence for the source formed in a strongly reducing environment. Sci. China. 51, 509–514. https://doi.org/10.1007/s11430-008-0030-7 (2008).
Meng, B. K., Song, D. F., Chen, Y. & Shi, S. B. Distribution and geochemical significance of alkylbenzenes for crude oil with different depositional environments and thermal maturities. Petrol. Sci. 21, 777–790. https://doi.org/10.1016/j.petsci.2023.10.030 (2024).
Hill, R. J. et al. C4-benzene and C4-naphthalene thermal maturity indicators for pyrolysates, oils and condensates. In The Geochemical Society Special Publications (Vol. 9, pp. 303 – 319). Elsevier. https://doi.org/10.1016/S1873-9881(04)80022-1(2024).
Lis, G. P., Mastalerz, M. & Schimmelmann, A. Increasing maturity of kerogen type II reflected by alkylbenzene distribution from pyrolysis-gas chromatography-mass spectrometry. Org. Geochem. 39, 440–449. https://doi.org/10.1016/j.orggeochem.2008.01.007 (2008).
Thompson, K. F. M. Fractionated aromatic petroleums and the generation of gas-condensates. Org. Geochem. 11, 573–590. https://doi.org/10.1016/0146-6380(87)90011-8 (1987).
Thompson, K. F. M. Gas-condensate migration and oil fractionation in deltaic systems. Mar. Petrol. Geol. 5, 237–246. https://doi.org/10.1016/0264-8172(88)90004-9 (1988).
Summons, R. E. & Powell, T. G. Identification of Aryl isoprenoids in source rocks and crude oils: biological markers for the green sulphur bacteria. Geochem. Cosmochim. Acta. 51, 557–566. https://doi.org/10.1016/0016-7037(87)90069-X (1987).
Hartgers, W. A. et al. A molecular and carbon isotopic study towards the origin and diagenetic fate of diaromatic carotenoids. Org. Geochem. 22, 703 – 725. https://doi.org/10.1016/0146-6380(94)90134-1 (1994).
Zhang, S. C. & Huang, H. P. Geochemistry of paleozoic marine petroleum from the Tarim Basin, NW China, part 1. Oil family classification. Org. Geochem. 36 (8), 1204–1214. https://doi.org/10.1016/j.orggeochem.2005.01.013 (2005).
Chang, X. C. et al. Geochemistry and possible origin of petroleum in palaeozoic reservoirs from Halahatang depression. J. Asian Earth Sci. 74, 129–141. https://doi.org/10.1016/j.jseaes.2013.03.024 (2013).
Song, D. F., Wang, T. G. & Li, H. B. Geochemical characteristics and origin of the crude oils and condensates from Yakela Faulted-Uplift, Tarim basin. J. Petrol. Sci. Eng. 133, 602–611. https://doi.org/10.1016/j.petrol.2015.07.007 (2015).
Fan, M., Huan, J. & Chen, Z. Thermal simulating experiment of source rock and Gas-Source correlation in the Kuqa depression of the Tarim basin. Petroleum Geol. Exp. 31, 518–521 (2009).
Liang, D. G. et al. Organic geochemistry of oil and gas in the Kuqa depression, Tarim Basin, NW China. Org. Geochem. 34 (7), 873–888. https://doi.org/10.1016/s0146-6380(03)00029-9 (2003).
Zhu, G. et al. The geological feature and origin of Dina 2 large gas field in Kuqa Depression, Tarim basin. Acta Pet. Sin. 28, 2479–2492 (2012).
Zhang, S. C. et al. Geochemical evidence for coal-derived hydrocarbons and their charge history in the Dabei gas Field, Kuqa thrust Belt, Tarim Basin, NW China. Mar. Petrol. Geol. 28 (7), 1364–1375. https://doi.org/10.1016/j.marpetgeo.2011.02.006 (2011).
Cheng, B., Wang, T. G., Huang, H. & Wang, G. L. Application of the monoterpane ratio (MTR) to distinguish marine oils from terrigenous origin and infer depositional environment in Northern Tarim Basin, China. Org. Geochem. 85, 1–10. https://doi.org/10.1016/j.orggeochem.2015.05.001 (2015).
Li, M. J. et al. Total alkyl Dibenzothiophenes content tracing the filling pathway of condensate reservoir in the Fushan Depression, South China sea. Sci. China. 51, 138 – 145. https://doi.org/10.1007/s11430-008-6025-6 (2008).
Li, M. J. et al. Occurrence and origin of carbon dioxide in the Fushan depression, Beibuwan Basin, South China sea. Mar. Petrol. Geol. 28, 500–513. https://doi.org/10.1016/j.marpetgeo.2007.07.007 (2008).
Li, M. J. et al. Biomarker 17α(H)-diahopane: A geochemical tool to study the petroleum system of a tertiary lacustrine basin, Northern South China sea. Appl. Geochem. 24, 172–183. https://doi.org/10.1016/j.apgeochem.2008.09.016 (2009).
Li, M. J. et al. Characteristics of oil and gas accumulation in Yong’an-Meitai area of the Fushan Depression, Beibuwan Basin, South China sea. Petrol. Sci. 4, 23–33. https://doi.org/10.1007/BF03187452 (2007).
Hartgers, W. A. et al. Evidence for only minor contributions from bacteria to sedimentary organic carbon. Nature 369, 224. https://doi.org/10.1038/369224a0 (1994).
Didyk, B. M., Simoneit, B. R. T., Brassell, S. C. & Eglinton, G. Organic geochemical indicators of palaeoenvironmental conditions of sedimentation. Nature 272 (5650), 216–222. https://doi.org/10.1038/272216a0 (1978).
Wang, G. L. et al. Monoterpanes as molecular indicators to diagnose depositional environments for source rocks of crude oils and condensates. Org. Geochem. 72, 59–68. https://doi.org/10.1016/j.orggeochem.2014.05.004 (2014).
Nytoft, H. P. et al. Novel C15 sesquiterpanes in Niger delta oils: structural identification and potential application as new markers of angiosperm input in light oils. Org. Geochem. 40, 595 – 603. https://doi.org/10.1016/S0031-6458(83)80090-2 (2009).
van Aarssen, B. G. K., Hessels, J. K. C., Abbink, O. A. & de Leeuw, J. W. The occurrence of polycyclic sesqui-, tri-, and oligoterpenoids derived from a resinous polymeric Cadinene in crude oils from Southeast Asia. Geochem. Cosmochim. Acta. 56, 1231–1246. https://doi.org/10.1016/0016-7037(92)90059-R (1992).
Odden, W., Patience, R. L. & Van Graas, G. W. Application of light hydrocarbons (C4-C13) to oil/source rock correlations: a study of the light hydrocarbon compositions of source rocks and test fluids from offshore Mid-Norway. Org. Geochem. 28 (12), 823–847. https://doi.org/10.1016/S0146-6380(98)00039-4 (1998).
Song, D. F. et al. Geochemistry and possible origin of crude oils from Bashituo oil field, Tarim Basin. AAPG Bull. 103 (4), 973–995. https://doi.org/10.1306/10031817403 (2019).
ten Haven, H. L. Applications and limitations of mango’s light hydrocarbon parameters in petroleum correlation studies. Org. Geochem. 24 (10–11), 957–976. https://doi.org/10.1016/S0146-6380(96)00091-5 (1996).
Leythaeuser, D., Schaefer, R. G., Cornford, C. & Weiner, B. Generation and migration of light hydrocarbons (C2-C7) in sedimentary basins. Org. Geochem. 1 (4), 191–204. https://doi.org/10.1016/0146-6380(79)90022-6 (1979).
Leythaeuser, D., Schaefer, R. G. & Weiner, B. Generation of low molecular weight hydrocarbons from organic matter in source beds as a function of temperature and facies. Chem. Geol. 25(1 – 2), 95 – 108. https://doi.org/10.1016/0009-2541(79)90086-X (1979).
Connan, J. Biodegradation of crude oils in reservoirs. In: (ed Welte, D.) Advances in Petroleum Geochemistry. Academic, London, 299–335. (1984).
Evans, C. R., Rogers, M. A. & Bailey, N. J. L. Evolution and alteration of petroleum in Western Canada. Chem. Geol. 8, 147 – 170. https://doi.org/10.1016/0009-2541(71)90002-7 (1971).
Peters, K. E. & Fowler, M. G. Applications of petroleum geochemistry to exploration and reservoir management. Org. Geochem. 33, 5–36. https://doi.org/10.1016/S0146-6380(01)00125-5 (2002).
Zhang, S. C. et al. Geochemistry of paleozoic marine oils from the Tarim Basin, NW China. Part 4: paleobiodegradation and oil charge mixing. Org. Geochem. 67, 41–57. https://doi.org/10.1016/j.orggeochem.2013.12.008 (2014).
Radke, M. & Willsch, H. Extractable Alkyldibenzothiophenes in posidonia shale (Toarcian) source rocks: relationship of yields to petroleum formation and expulsion. Geochem. Cosmochim. Acta. 58, 5223–5244. https://doi.org/10.1016/0016-7037(94)90307-7 (1994).
Santamarı́A-Orozco, D., Horsfield, B., Di Primio, R. & Welte, D. H. Influence of maturity on distributions of benzo- and Dibenzothiophenes in tithonian source rocks and crude oils, Sonda de Campeche, Mexico. Org. Geochem. 28, 423–439. https://doi.org/10.1016/S0146-6380(98)00009-6 (1998).
Kvalheim, O. M., Christy, A. A., Telnæs, N. & Bjørseth, A. Maturity determination of organic matter in coals using the Methylphenanthrene distribution. Geochem. Cosmochim. Acta. 51 (7), 1883–1888. https://doi.org/10.1016/0016-7037(87)90179-7 (1987).
Acknowledgements
This study was supported by Doctor’s Scientific Research Initiation Project of Yan’an University (YAU202213093) and National Nature Science Foundation of China (Grant No. 41503029).
Author information
Authors and Affiliations
Contributions
M.B.K: Contributed to processing data, researching literature, producing charts and writing manuscript. W.K.F: Contributed to data processing. M.F.M: Contributed to graphic processing. L.M.J: Contributed to the advancement of literature research. D.Y.G: Collected and analyzed samples. X.L.Y: Interpreted and analyzed the data. L.J.T: Prepared Figs. 1, 2, 3, 4 and 5. S.L: Prepared Figs. 6, 7, 8, 9 and 10. S.D.F: Contributed to provide financial support and developing the concept; C.Y: Contributed to literature research and experimental analysis; S.S.B: Contributed to providing guidance on experimental analysis and processing of data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meng, B., Wen, K., Meng, F. et al. Distribution and geochemical significance of C5 alkylated benzenes in light oils and condensates from the Tarim Basin and Beibuwan Basin. Sci Rep 15, 42368 (2025). https://doi.org/10.1038/s41598-025-26255-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26255-y