Abstract
Claudin 18.2 (CLDN18.2) is a novel treatment target for patients with unresectable or stage IV gastric cancer. However, it remains unclear whether the expression of CLDN18.2 affects survival outcomes. In total, 586 patients with GC were enrolled in this study. CLDN18.2 expression in cancer cells was analyzed by immunohistochemistry. Correlations between CLDN18.2 expression and several clinicopathological factors and survival outcomes were investigated. We also performed a systematic review and a meta-analysis. CLDN18.2 expression was mainly observed in the cell membrane. The CLDN18.2 expression was not significantly correlated with any clinicopathological factor. In all patients, CLDN18.2 did not significantly affect OS. In patients with the diffuse type, the overall survival of patients with CLDN18.2-high expression was worse than that of patients with CLDN18.2-low expression, although the difference was not significant (p = 0.092). Meta-analyses revealed that CLDN18.2 was not significant prognostic factor in resected cases, although CLDN18.2 negative cases showed a trend for worse survival. In, conclusion, CLDN18.2 was not a significant prognostic factor in general, although CLDN18.2 negative cases showed a trend for worse survival. We revealed that patients with CLDN18.2 high expression showed worse survival outcomes especially in the diffuse type.
Similar content being viewed by others
Introduction
Gastric Cancer (GC) is one of the most common cancers, accounting for over one million new cases worldwide1. Despite several new approaches, including endoscopic submucosal resection, minimally invasive surgery, and immune checkpoint inhibitors, which have improved the survival outcomes of GC2,3,4, the prognosis of patients with advanced GC, remains dismal. Hence, there is a continuous need to understand disease characteristics, including biomarkers.
Claudin-18 (CLDN18) is a member of the claudin family and a component of tight junctions. Claudin 18 isoform 2 (CLDN18.2) is present in the gastric mucosa and buried within tight junctions. In malignant transformation, CLDN18.2 is known to become exposed because of the loss of polarity. Thus, CLDN18.2 is considered a promising treatment target. Recently, two pivotal studies (SPOTLIGHT and GLOW trials) revealed that CLDN-targeted therapy significantly improved survival outcomes for patients with HER2 negative Stage IV GC5,6.
In addition to CLDN-targeted therapy, immune checkpoint inhibitors targeting the programmed death (PD)−1/PD-L1 axis have been widely used for HER2 negative Stage IV GC in clinical practice since 2021 in Japan2,7. However, it remains uncertain which treatment is better for GC patients with both PD-L1 and CLDN18.2 expression. Therefore, we attempted to clarify the role and impact of CLDN18.2 for finding a clue to an optimal treatment strategy.
In this report, we focused on the prognostic significance of CLDN18.2 in GC patients. In addition to our original analysis, we thoroughly searched the literature on this issue and performed meta-analyses. GC has been histologically classified into intestinal and diffuse types by Lauren8; however, most previous reports did not focus on the difference in GC histological types9. Thus, this study aimed to clarify the clinical significance of CLDN18.2 expression in both histological types by Lauren classification.
Methods
Patients
This study included 586 patients who provided informed consent and underwent primary GC resection between September 2000 and December 2006. The last follow-up date in this dataset was March 2009. Tissue microarrays (TMAs) were generated for immunohistochemical (IHC) staining. These patients were diagnosed and classified according to the UICC TNM classification of malignant tumors 7th edition10. The study protocol conforms to the ethical guidelines of the Declaration of Helsinki. The Osaka Metropolitan University ethics committee approved this study in 2022 [approval number (approval date): 2022–077 (2022/08/10)]. Informed consent was obtained in the form of opt-out.
IHC determination of CLDN18.2
All 586 GC cases were examined by IHC staining. Slides were deparaffinized and then heated for 10 min at 105 °C in an autoclave in Target Retrieval Solution (Dako, Carpinteria, CA, USA). After blocking the endogenous peroxidase activity, we incubated the specimens with CLDN18.2 antibody (1:200; ERP19202, abcam) for 1 h at room temperature, followed by the biotinylated goat anti-rabbit IgG for 10 min. The slides were treated with streptavidin-peroxidase reagent and then counterstained with Mayer’s hematoxylin. Slides were scanned using Leica Aperio CS2. Subsequently, using the QuPath software, we evaluated the CLDN18.2 expression in every tumor cell. The percentage of CLDN18.2-positive cells among the total tumor cells was calculated automatically using the threshold value. We diagnosed a case of CLDN18.2 high when the percentage of positive tumor cells was > 25%, as our aim was to evaluate outcomes in populations with significantly elevated CLDN18.2 expression levels. In addition to this cut-off value, we also analyzed data using the median (19.1%) and third quartile (11.1%) values, which are presented in the supplementary materials.
Meta-analyses
A systematic review was performed in accordance with the preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for reporting systematic reviews11. The flow chart of the literature search is shown in Fig. 1. First, we searched for literature using “Claudin” and “Gastric Cancer” as keywords through PubMed, resulting in 404 articles. From these, we selected 30 articles by reviewing the titles and abstracts, focusing on the role of CLDN18.2 expression in patients with gastric cancer. Next, we performed a full-text assessment and selected articles that examined the correlation between CLDN18.2 and survival outcomes. Finally, we selected five articles for meta-analysis, in which the HR and 95% CI were described in the manuscripts. These procedures were conducted by two-researchers independently. This review was not registered.
Meta-analyses were performed using metafor packages in R (version 4.4.0; R Core Team)12. The calculated effect sizes were the hazard ratio and 95% CI for overall survival (OS). In order to account for heterogeneity among studies, random-effects meta-analysis was conducted. The threshold for statistical significance was set at two-sided 0.05 for the tests of overall effect and 0.10 for the tests of heterogeneity. The I2 statistic, Tau2, and Q statistic were also used to quantify the degree of heterogeneity across the studies.
Statistical analysis
Associations between CLDN18.2 expression and clinicopathological findings were analyzed using the chi-square test. OS was defined as the time from surgery to death from any cause. OS curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Additionally, a subgroup analysis was conducted based on histological types. Univariate and multivariate analyses were performed using the Cox regression model. Statistical data were analyzed using the SPSS statistical software (version 29.0; IBM). A two-sided probability (p) value less than 0.05 was considered statistically significant.
Results
Relationship between CLDN18.2 expression and clinicopathological factors in GC.
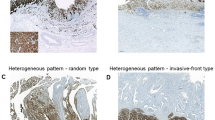
CLDN18.2 expression was mainly observed in the membrane of cancer cells (Fig. 2). Of the 586 patients, 87 were classified as having high CLDN18.2 expression in the cancer cells. Table 1 shows the correlation between CLDN18.2 expression and the clinicopathological factors. Although there was a trend where the percentage of CLDN18.2 expression was higher in female patients than in male patients, the difference was not significant. There was no significant association between CLDN18.2 expression and other clinical factors.
Survival
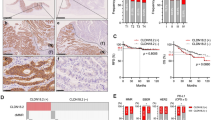
The OS curves according to the Kaplan–Meier method depending on CLDN18.2 expression are shown in Fig. 3. The 5-year OS rates of patients with high and low CLDN18.2 expression levels in cancer cells were 66.1% and 69.1%, respectively, showing no statistically significant difference in the OS outcomes (log-rank; p = 0.669).
Figure 4 shows the analysis of each histological type. As for those with intestinal cases, patients with CLDN18.2 high expression showed better survival outcomes, although the difference was not statistically significant (p = 0.082). Conversely, among those with diffuse type GC, the OS of patients with high CLDN18.2 expression was worse than that of patients with low CLDN18.2 expression (p = 0.092).
The survival analyses using different cutoff values are presented in Supplementary Figs. 1–4. When using the third quartile value as the cutoff, the survival analyses yielded results similar to those described above (Supplementary Figs. 1–2). Conversely, when the median value was used as the cutoff, survival outcomes were nearly identical between high and low CLDN18.2 expressions (Supplementary Figs. 3–4).
Table2 shows the univariate and multivariate analyses of overall survival. pStage was significantly associated with OS in both univariate and multivariate analyses, while CLDN18.2 expression was not. Supplementary Table 1 presents the univariate and multivariate analyses of overall survival in patients with diffuse and intestinal types. A trend is observed where high CLDN18.2 expression is associated with worse survival in diffuse-type patients, while high CLDN18.2 expression is linked to better survival in intestinal-type patients, even after adjusting for pStage.
Literatures and meta-analyses
Table3 summarizes the literature regarding CLDN18.2 and prognosis in patients with GC. Among 20 studies, ten, six, and three reports included resected cases, metastatic cases, and both resected and metastatic cases, respectively. 17 papers reported that CLDN18.2 expression was not significantly associated with survival outcomes, although the rest of three papers showed statistical significance. Regarding reports of the metastatic cases, only one study included the essential information, so we were not able to perform meta-analysis. On the other hand, five studies in addition to our original analysis regarding resected cases that showed the hazard ratio and 95% confidence interval (CI) were used in the meta-analysis (Fig. 5). As a result, there is no significant association about survival outcomes depending on CLDN18.2 expression (HR = 0.892, 95% CI: 0.706–1.126, reference: Low/negative CLDN expression, p = 0.335), although CLDN18.2 low expression cases showed a trend for worse survival.
Discussion
This study demonstrated no correlation between CLDN18.2 positivity and survival, although the impact on survival differed depending on histological type.
Several previous reports have shown a significant correlation between signet cell carcinoma and CLDN18.2 high expression. In our analysis, the percentage of CLDN18.2 high expression in the diffuse type was higher than that in the intestinal type. Additionally, patients with macroscopic type 4, the so-called scirrhous type, showed a higher percentage of CLDN18.2. Although our results described above did not show statistical significance, CLDN18.2 expression seems to be closely related to diffuse-type histology and scirrhous type.
We analyzed the prognostic significance of CLDN18.2, and CLDN18.2 expression did not significantly affect survival outcomes. In addition to our original analyses, a meta-analysis of previous reports strengthened our findings. On the other hand, this is the first report in which the prognostic significance of CLDN18.2 was evaluated either in intestinal or diffuse type. Interestingly, the impact on survival of CLDN18.2 was opposite between histologic types.
Our results imply that CLDN18.2 played dichotomous roles depending on histologic types. According to the previous reports, CLDN 18 knockout mice show spontaneous tumor development, suggesting its tumor suppressor function in tumor initiation. Oshima et al. reported the negative correlation between CLDN 18.2 and Ki-67 labelling index in submucosal GC. In addition, they used intestinal-type GC cell line, MKN74, and showed CLDN 18.2 knockdown by siRNA induced cell proliferation. The activated proliferation by reduced expression of CLDN18.2 can be explained by Hippo pathway. CLDN18.2 forms a complex with LATS1/2, and suppresses YAP/TAZ. Accordingly, the loss of CLDN18.2 increased the translocation of TAP/TAZ into the nucleus, which increased the proliferation in intestinal type GC cells. Thus, these data supports our survival data about intestinal type.
On the other hand, high expression of CLDN18.2 suppresses YAP/TAZ activity, which decreases the down-stream molecules, such as CTGF. We have reported that low CTGF expression is significantly associated with worse survival in diffuse type GC. CTGF was reported to be a ligand of integrin, so low CTGF in the stroma can be favorable condition for the invasion. Therefore, the worse survival of patients with high CLDN18.2 expression can be also explained by low expression of CTGF in the diffuse type GC.
In our study, the cutoff value for high CLDN18.2 expression was chosen to capture a distinct subset of patients with significantly elevated expression levels. This approach resulted in identifying 87 out of 586 patients (14.8%) for analysis. However, it is worth noting that a previous clinical trial defined high CLDN18.2 expression as > 75%5,6, a criterion met by only nine patients (1.5%) in our dataset. This discrepancy can likely be attributed to differences in the antibody used, variations in the IHC protocol, and the evaluation method applied. While these methodological differences highlight the need for standardization in biomarker assessment, our findings provide valuable insights into the characteristics of CLDN18.2 high expression, contributing to a better understanding of this biomarker in diverse populations.
In clinical practice, the optimal treatment for patients with high PD-L1 and CLDN18.2 expression remains uncertain, although ICI and CLDN18.2 targeted therapy can be used. Since PD-L1 expression is known to be an unfavorable prognostic factor, patients with PD-L1 and CLDN18.2 high expression in the diffuse type could be biologically worse than the other types. Thus, it could be better to develop the stronger chemotherapy regimen for diffuse type GC patients with PD-L1 and CLDN18.2 high expression in future.
One of the limitations of our original study is that TMA was used for the analysis; thus, the analyzed tissue area was not broad. However, we attempted to create the TMA using a representative core to justify the results. A representative core should include a greater number of tumor cells and exhibit more typical morphology compared to other areas. Nevertheless, tumor area heterogeneity may persist, and the heterogeneity of CLDN18.2 expression warrants investigation in future studies. Furthermore, we used automatic software in the analyses, making the data quite objective and less biased. For the meta-analysis, the included studies used different antibodies and cutoff values. Nevertheless, the results regarding survival outcomes were similar, and the meta-analyses supported our results. Thus, our reports should be the most reliable about this issue.
In conclusion, CLDN18.2 was not a significant prognostic factor in general, although patients with CLDN18.2 high expression showed worse survival outcomes in the diffuse type.
Data availability
Data availability statement is provided as "Original data can be provided if appropriately requested to Yuichiro Miki (y_miki@omu.ac.jp)".
References
Song, Y., Liu, X., Cheng, W., Li, H. & Zhang, D. The global, regional and national burden of stomach cancer and its attributable risk factors from 1990 to 2019. Sci. Rep. 12, 11542 (2022).
Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 398, 27–40 (2021).
Ono, H. et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig. Endosc. 28, 3–15 (2016).
Etoh, T. et al. Five-Year survival outcomes of laparoscopy-assisted vs open distal gastrectomy for advanced gastric cancer: The JLSSG0901 randomized clinical trial. JAMA Surg. 158, 445–454 (2023).
Shah, M. A. et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: The randomized, phase 3 GLOW trial. Nat. Med. 29, 2133–2141 (2023).
Shitara, K. et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet 401, 1655–1668 (2023).
Kang, Y.-K. et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 23, 234–247 (2022).
Jarvi, O. & Lauren, P. On the pathogenesis of gastric cancer. Acta - Unio Int. Contra Cancrum 8, 393–394 (1952).
Chen, C. N. et al. Connective tissue growth factor inhibits gastric cancer peritoneal metastasis by blocking integrin alpha3beta1-dependent adhesion. Gastric Cancer: Official J. Int. Gastric Cancer Assoc. Japanese Gastric Cancer Assoc. 18, 504–515 (2015).
TNM-Classification of Malignant Tumours. 7th edn (Wiley-Blackwell, 2009).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Team, R. C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Jun, K. H., Kim, J. H., Jung, J. H., Choi, H. J. & Chin, H. M. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int. J. Surg. 12, 156–162 (2014).
Dottermusch, M., Kruger, S., Behrens, H. M., Halske, C. & Rocken, C. Expression of the potential therapeutic target claudin-18.2 is frequently decreased in gastric cancer: results from a large Caucasian cohort study. Virchows Arch. 475, 563–571 (2019).
Baek, J. H. et al. Clinical implications of Claudin18.2 expression in patients with gastric cancer. Anticancer Res. 39, 6973–6979 (2019).
Xu, B. et al. Highly expressed Claudin18.2 as a potential therapeutic target in advanced gastric signet-ring cell carcinoma (SRCC). J. Gastrointest. Oncol. 11, 1431–1439 (2020).
Hong, J. Y. et al. Claudin 18.2 expression in various tumor types and its role as a potential target in advanced gastric cancer. Transl. Cancer Res. 9, 3367–3374 (2020).
Arnold, A. et al. Prognostic impact of Claudin 18.2 in gastric and esophageal adenocarcinomas. Clin. Transl. Oncol. 22, 2357–2363 (2020).
Lu, Y. et al. Correlation between Claudin-18 expression and clinicopathological features and prognosis in patients with gastric cancer. J. Gastrointest. Oncol. 11, 1253–1260 (2020).
Kim, S. R. et al. Clinical significance of CLDN18.2 expression in metastatic diffuse-type gastric Cancer. J. Gastric Cancer 20, 408–420 (2020).
Pellino, A. et al. Association of CLDN18 protein expression with clinicopathological features and prognosis in advanced gastric and gastroesophageal junction adenocarcinomas. J. Pers. Med. 11, 1095 (2021).
Weng, W. et al. Decreased expression of claudin-18.2 in alpha-fetoprotein-producing gastric cancer compared to conventional gastric cancer. J. Gastrointest. Oncol. 13, 1035–1045 (2022).
Pereira, M. A. et al. RhoA, Claudin 18, and c-MET in gastric cancer: Clinicopathological characteristics and prognostic significance in curative resected patients. Med. Sci. (Basel) 10, 4 (2021).
Kayikcioglu, E., Yuceer, R. O., Cetin, B., Yuceer, K. & Karahan, N. Prognostic value of claudin 18.2 expression in gastric adenocarcinoma. World J. Gastrointest. Oncol. 15, 343–351 (2023).
Kubota, Y. et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO Open. 8, 100762 (2023).
Kim, H. D. et al. Clinicopathologic features and prognostic value of claudin 18.2 overexpression in patients with resectable gastric cancer. Sci. Rep. 13, 20047 (2023).
Wang, C. et al. CLDN18.2 expression and its impact on prognosis and the immune microenvironment in gastric cancer. BMC Gastroenterol. 23, 283 (2023).
Qi, C. et al. Clinicopathological significance and immunotherapeutic outcome of claudin 18.2 expression in advanced gastric cancer: A retrospective study. Chin. J. Cancer Res. 36, 78–89 (2024).
Waters, R. et al. Retrospective study of Claudin 18 Isoform 2 prevalence and prognostic association in gastric and gastroesophageal junction adenocarcinoma. JCO Precis. Oncol. 8, e2300543 (2024).
Kwak, Y. et al. Clinicopathologic and molecular characterization of stages II-IV gastric cancer with Claudin 18.2 expression. Oncologist 30, oyae238 (2024).
Kim, T. Y. et al. Clinicopathological analysis of claudin 18.2 focusing on intratumoral heterogeneity and survival in patients with metastatic or unresectable gastric cancer. ESMO Open 9, 104000 (2024).
Kim, M., Kang, B. W., Park, J., Baek, J. H. & Kim, J. G. Expression of claudin 18.2 in poorly cohesive carcinoma and its association with clinicopathologic parameters in East Asian patients. Pathol. Res. Pract. 263, 155628 (2024).
Author information
Authors and Affiliations
Contributions
Y.M. wrote the main manuscript text. Y.M. and R.M performed experiments. T.B. prepared Figs. 5. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Miki, Y., Yoshii, M., Miyauchi, R. et al. Prognostic significance of Claudin18.2 expression in patients with gastric cancer. Sci Rep 15, 39072 (2025). https://doi.org/10.1038/s41598-025-26388-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26388-0