Abstract
In this work, Bismuth oxide nanoparticles (Bi2O3 NPs) were prepared by green synthesis method as cost effective and ecofriendly method using olive leaves extract. The successful preparation of nanoparticles was confirmed using IR and XRD. The Bi2O3 NPs were used in preparation of unsaturated polyester nanocomposites with different ratios (1, 5, 10, and 20 wt%). The shielding properties of these composites were studied against gamma radiation with different energies emitted from different radioactive sources (59.5, 661.7, (1173 and 1333) keV from Am-241, Cs-137, and Co-60 in series) since different parameters were measured. The results showed that LAC and RSE% increased and HVL and TVL decreased with increase of Bi2O3 NPs ratio. The 20 wt% nanocomposite sample has highest LAC values among all samples where it has LAC values of 1.668, 0.134, 0.094, and 0.088 cm− 1 at 59, 661, 1173, and 1333 keV, respectively. On other hand, pure unsaturated polyester has lowest values where it has LAC values of 0.250, 0.105, 0.078, 0.075 cm− 1 at 59, 661, 1173, and 1333 keV, respectively. Also, addition of Bi2O3 NPs improves thermal properties of the composites where the 20 wt% sample shows 10% weight loss only after 326 °C compared to 247 °C for pure polyester. On other hand, the mechanical properties of unsaturated polyester were negatively affected where compressive strength decreased from 10 to 4.94 MPa with 20 wt% addition percent.
Similar content being viewed by others
Introduction
Gamma rays and X-rays are utilized in several applications such as scientific research, industrial applications, medicine, and nuclear power plants1,2,3,4. However, undesired exposure to ionizing radiation has harmful effects on humans and the environment. It may result in cell alterations, damage to organs, and other health issues5,6.
Reduction of absorbed dose can be achieved by control in duration of exposure and remoteness from the radiation source and presence of shields3,6. Reinforcement of polymers with high atomic number micro and nano metal oxides enhances its radiation attenuation properties and makes it suitable for use as shields2,5,7,8. In addition, polymer composites are flexible and have light weight and good mechanical strength compared to other materials used in shielding7,9,10.
One of the metal oxides that is used is bismuth oxide (Bi2O3) in nano size. Bismuth oxide has a high density and a large atomic number for bismuth (Z = 83). Also, it is non-toxic compared to lead11. Many researchers used bismuth oxide nanoparticles in the fabrication of polymer composites for shielding applications. El Sharkawy et al. studied the shielding properties of recycled PVC doped with different percentages of Bi2O3 NPs at different energies emitted from the Eu-152 source11. Also, Yassene et al. prepared HDPE composite reinforced with Bi2O3 NPs and studied its shielding properties12. Elsafi et al. used Bi2O3 NPs and waste marble with unsaturated polyester and studied the attenuation characteristics for these composites at various energies13. Also, Elsafi et al. prepared epoxy composites using Bi2O3 NPs for shielding applications14. Cao et al. improved the shielding properties of PMMA by adding bismuth oxide, and other studies used bismuth oxide with different polymers5,7,15.
Unsaturated polyester is a type of thermoset polymer that is used in many applications such as automotive, military products, aircraft, construction, and the marine industry. It is characterized by its low cost, ease of processing, and good mechanical properties16,17,18,19. Many researchers used unsaturated polyester as a matrix in the composites for radiation shielding applications. Ozdogan et al. added different ratios of PbO to unsaturated polyester and measured its shielding properties10. More et al. studied attenuation properties against gamma rays and neutrons for TiO2 NPs filled unsaturated polyester composite20. Hemily et al. prepared unsaturated polyester composites reinforced with waste marble and WO3 NPs for gamma shielding applications21. Also, more et al. added SnO2 NPs to unsaturated polyester and studied its shielding properties9. As well, different studies focused on unsaturated polyester composites for shielding applications1,22,23,24.
Bi2O3 NPs can be synthesized by various methods such as sol-gel method, co-precipitation, and hydrothermal method, but these methods use toxic chemicals, so it is hazardous to humans and environment. On the other hand, green synthesis is cost-effective, non-toxic, and eco-friendly, as it uses microorganisms and plant parts instead of toxic chemicals. The different plant parts contain a lot of phytochemicals such as flavonoids, phenolic compounds, alkaloids, proteins, and terpenoids which act as reducing agents and stabilizing agents in nanoparticles synthesis25,26,27. Many studies reported the preparation of Bi2O3 NPs using different plant parts such as leaves of (A) indica plant28, (B) multifida plant29, leaves of Datura inoxia plant30, roots of ginger plant31, leaves of Mexican mint plant32, S. persica plant33, and leaves of Ficus benghalensis plant26.
In the present work, Bi2O3 NPs were prepared by the green synthesis method using olive leaves extract. Then, it used in the fabrication of unsaturated polyester nanocomposites with different filler percentages. The attenuation properties of these composites were studied at different energies in addition to its thermal and mechanical properties.
Materials and methods
Materials
Olive leaves were purchased from local market. Bismuth nitrate pentahydrate Bi(NO3)3 .5H2O were obtained from universal fine chemicals Ltd. Glacial acetic acid CH3COOH was supplied from fisher scientific. Pre-accelerated unsaturated polyester resin was supplied from SUPIC Co. Methyl ethyl ketone peroxide (MEKP) was supplied from AKPA chemicals.
Preparation of Olive leaves extract
First, olive leaves were cut into small pieces. Then, 40 gm of chopped leaves were boiled in 500 ml of distilled water at 95 °C for 60 min. After that, the extract was cooled in air, filtered and stored in the refrigerator for further utilization.
Green synthesis of Bi2O3 NPs
Initially, 50 ml of 0.1 M Bi(NO3)3 solution was prepared by dissolution of 2.425 gm Bi(NO3)3.5H2O in 5 ml of acetic acid at 60 °C. Then, it was completed to 50 ml with distilled water. This solution was added dropwise to 400 ml of olive leaves extract at 60 °C under vigorous stirring. The mixture was stirred for 3 h under the same conditions. After this period, the resultant precipitate was filtered, washed several times with distilled water, then dried in an oven at 70 °C for 4 h and subsequently calcinated in a muffle furnace at 600 °C for 2 h in ambient air.
Preparation of unsaturated polyester nanocomposites
The Bi2O3 NPs with different wt % (0, 1, 5, 10, and 20) were mixed with unsaturated polyester resin followed by sonication for 30 min. Thereafter, the mixture was stirred for 30 min. Then, 1% of MEKP was added to the mixture to initiate curing reaction. Finally, the mixture was poured into cylindrical molds and allowed to solidify. Table 1; Fig. 1 show the composite samples and its composition and density. The mass per volume law was used to practically estimate the samples’ density. A digital balance with a ± 1 mg precision was used to measure the mass, and the (x πr2) formula was used to calculate the volume, where x and r stand for the sample’s thickness and radius, respectively.
Characterization
The size and shape of the Bi2O3 NPs was measured by transmission electron microscope (JEOL JEM-1400 plus). IR spectra of the nanoparticles and nanocomposites were measured by a PerkinElmer spectrum two FT-IR spectrometer in the wave number range of 4000–450 cm− 1. XRD patterns were measured by a Bruker XRD D2 phaser diffractometer using Cu Kα radiation (λ = 1.54 Å). Thermal stability of nanocomposites was evaluated with heating rate 10 C°/min using Q600 thermogravimetric analyzer, TA instruments. Scanning electron microscope (JEOL JSM IT200) was utilized to study fracture surface of nanocomposites and to make EDX analysis for Bi2O3 NPs. In addition to, compression test was conducted for nanocomposite samples using universal testing machine (5ST, Tinius Olsen).
Measurement of attenuation properties
The attenuation characteristics of the composites were examined using a high purity germanium (HPGe) detector with a relative efficiency of 24% and an energy resolution of 1.96 keV at 1333 keV and several radioactive point sources emit gamma rays with different energies (Am-241 emits at 59 keV, Cs-137 emits at 661 keV, and Co-60 emits at 1173 and 1333 keV) with an initial activity of 40.3 kBq in 1998. The composites samples were placed between the detector and the radioactive source as shown in Fig. 2. The peak intensity was measured in absence and presence of the samples and the linear attenuation coefficient (LAC) was determined at each energy according to Eq. (1)
Where, I0, I, and x represent peak intensity in absence of sample, peak intensity in presence of sample, and thickness of sample, respectively. From LAC values, other parameters of shielding such as half value layer (HVL), tenth value layer (TVL), and radiation shielding efficiency (RSE%) were be measured using Eqs. (2), (3), and (4)6,34.
Results and discussion
TEM and EDX analysis of Bi2O3 NPs
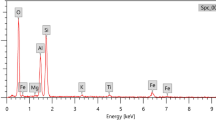
According to illustrated TEM image of Bi2O3 NPs (Fig. 3), it can be seen that Bi2O3 NPs have spherical shape with an average particle size of 15 nm. On other hand, EDX analysis (Fig. 4) shows presence of bismuth and oxygen as major elements which confirms the formation of Bi2O3 with other characterization results in addition to presence of minor amounts of carbon and phosphor which may be residue from calcination step and impurities in starting precursor, respectively.
FT-IR spectroscopy
Figure 5 shows FT-IR spectra of Bi2O3 NPs, pure UP, and 20 wt% Bi2O3 nanocomposite. As seen in Fig. 5a, the peak at 546 cm− 1 is assigned to metal-oxygen bond (Bi-O-Bi) vibration in Bi2O325,27. The peaks 937 and 963 cm− 1 may be related to Bi-O bond stretching vibrations25,27,35. Also, the peak at 1074 cm− 1 may be assigned to vibration of Bi-O bond28,35. Figure 5b represents the spectrum of pure UP. In which the peak at 3463 cm− 1 corresponding to O-H stretching vibration, the peak at 3028 cm− 1 is attributed to aromatic C-H bond vibration, and the peak at 2954 cm− 1 is related to aliphatic C-H bond stretching. While, the peaks at 1728 and 1453 cm− 1 are related to C = O bond stretching vibration and C-H bond bending vibration, respectively. The peaks at 1283, 1130, and 1072 cm− 1 are related to C-O bond vibrations and the observed peaks at 745 and 702 cm− 1 are related to aromatic C-H bending vibrations36,37. In comparing to the spectrum of pure UP (Fig. 5b), it is noticed the presence of new peaks in the spectrum of Bi2O3/UP composite (Fig. 5c) at 545 and 937 cm− 1 related to Bi-O bond which indicates successful addition of Bi2O3 NPs to unsaturated polyester. Also, it is obvious that there is no shift on the peaks of unsaturated polyester in the composite spectrum which indicates that there is no chemical interaction between the nanoparticles and the matrix37,38,39.
X-ray diffraction (XRD)
Figure 6 Show the XRD patterns of Bi2O3 NPs, pure UP, and 20% wt Bi2O3 nanocomposite. As seen in Fig. 6a, there are several peaks in the XRD pattern of Bi2O3 NPs. The peaks at 2θ values of 24.99°, 26.89°, 27.76°, 33.64°, 41.40°, 42.04°, 44.16°, 46.83°, 55.42°, and 58.91° which corresponding to (102), (111), (120), (200), (131), (122), (040), (041), (241), and (024) diffraction planes which can be attributed to monoclinic Bi2O3 (JCPDS card 41-1449) with the following lattice parameters: a = 5.849 Å, b = 8.169 Å, c = 7.512 Å, α = γ = 90°, and β = 112.96°25,30,31,33,35,40,41. While the peaks at 2θ values of 28.28°, 30.53°, 31.84°, 32.30°, 45.75°, 47.54°, 54.13°, 55.96°, and 57.46° corresponding to (201), (211), (002), (220), (222), (400), (203), (421), and (402) diffraction planes of tetragonal Bi2O3 (JCPDS cards 27–0050) with the following lattice parameters: a = b = 7.73 Å, c = 5.64 Å, and α = β = γ = 90°25,35,42,43. On other hand, the peak at 2θ value of 29.16° may be attributed to non-stoichiometric phase of Bi2O344. Also, the peak at 2θ value of 51.01° can be assigned to (023) diffraction plane in BiPO4 which confirm with presence of phosphor element in EDX analysis45. The average crystallite size of Bi2O3 NPs is calculated and it is found to be 12.98 nm using Scherrer’s Eqs.25,29 :
Where D is the crystallite size, K is the shape factor (taken as 0.9), λ is the X-ray wavelength (1.54 Å for Cu Kα radiation), β is the full width at half maximum (FWHM) of the diffraction peak, and θ is the Bragg’s diffraction angle. Table 2 shows FWHM values and other XRD parameters for each peak.
It is noticed in Fig. 6b. that there are broad peaks at 2θ values of 19.33°, 28.74°, and 40.64° which indicates the amorphous nature of unsaturated polyester. On other hand, XRD pattern of the nanocomposite (Fig. 6c.) show the decrease in intensity of broad peaks and appearance of sharp peaks at 2θ values of 26.73°, 28.14°, 31.49°, 47.22°, and 55.30° related to Bi2O3 NPs which reflects the slight increase in crystallinity with addition of Bi2O3 NPs20,46,47,48.
Thermal gravimetric analysis (TGA)
Figure 7 Shows the TGA curve of Bi2O3 NPs, pure UP, and 20% wt Bi2O3 nanocomposite. It is noticed that Bi2O3 NPs have high thermal stability up to 800 °C where it lost about 1.33% from its weight only. This very small weight loss may be because of the evaporation of adsorbed water on the surface of Bi2O3 NPs11. Also, it is seen that pure UP lost 10% of its weight at 247 °C and lost 50% of its weight at 357 °C. On other hand, the 20% wt nanocomposite degraded at higher temperatures where it lost 10% of its weight at 326 °C and lost 50% of its weight at 395 °C which indicates that the addition of Bi2O3 NPs retards the thermal decomposition of unsaturated polyester and improves its thermal stability49.
Scanning electron microscope (SEM)
Figure 8 Shows SEM images of fracture surfaces of pure UP, 5% wt nanocomposite, and 20% wt nanocomposite. Figure 8a. shows a smooth fracture surface for pure UP which indicates the brittle fracture of unsaturated polyester. While, Fig. 8b and c show rough fracture surfaces for Bi5 and Bi20 nanocomposites which indicate that the nanocomposites have some toughness. Also, at higher loading, it is clear there is good dispersion of Bi2O3 nanoparticles in polymer matrix. However, it is noticed the presence of some agglomerations of nanoparticles in both samples and it increases with the increase of Bi2O3 NPs ratio36,49. Despite the good dispersion of nanoparticles, these agglomerations reduce distribution homogeneity of Bi2O3 NPs in UP matrix which may affect mechanical properties of the composites as discussed below.
Compressive strength test
The mechanical properties of nanocomposites were evaluated through compressive strength test. Figure 9 Shows values of compressive strength for UP and nanocomposites with different percentages of Bi2O3 NPs. It is noticed the slight increase in compressive strength value for 1% wt nanocomposite which may be due to good dispersion of nanoparticles in composite. Then, the compressive strength decreases with increasing of Bi2O3 NPs ratio which may be related to agglomeration of nanoparticles and negative effect of nanoparticles on crosslinking reaction during curing process of composite49,50.
Radiation attenuation properties
Io / I ratio was plotted against the thickness (x) for each sample as shown in Fig. 10 and the slope of the curve represents the value of LAC. Table 3 shows the relative deviation between experimental and theoretical values of LAC where the relative deviation was calculated according to the following equation:
It is noticed that relative deviation for all samples doesn’t exceed 6% which indicates compatibility between experimental and theoretical results51. The theoretical results were calculated using XCOM software where it is an online software and is used in calculation of attenuation coefficient by entering chemical composition of samples.
Figure 11 shows values of LAC for unsaturated polyester the different composites at different energies. It is observed that LAC has higher values at lower energy (59 keV) and decreases with increase of energy where photoelectric effect is more prevalent at lower energy while Compton scattering and pair production are prevalent at higher energies7,51,52. Also, it is noticed the increase of LAC with increase of Bi2O3 NPs ratio which largely increases from 0.250 cm− 1 for pure unsaturated polyester to 1.668 cm− 1 for 20 wt% nanocomposite at 59 keV and slightly increases at higher energies due to dominance of photoelectric effect at lower energy.
As it is expected, Fig. 12 shows increase of HVL values with increase of energy where HVL is inversely proportional to LAC and the more thickness is required to prevent higher energy photons from penetrating. It also shows the improvement of HVL values with addition of Bi2O3 NPs where HVL value of unsaturated polyester improves from 2.77 cm to 0.41 cm at 59 keV with addition of 20% Bi2O3 NPs. Therefore, a thin layer is enough to absorb half of photons since the photoelectric effect is the dominant at low energy. On other hand, the same addition slightly decreases the HVL value from 9.25 cm to 7.88 cm at 1333 keV where the photoelectric effect becomes less dominant and Compton scattering and pairs production are more dominant at higher energies. As shown in Fig. 13, TVL values have the same trends as HVL values. Also, it is observed that TVL values are greater than HVL values since the more thickness is needed to reduce radiation intensity to tenth of its value. The addition of 20 wt% of Bi2O3 NPs to unsaturated polyester enhances its TVL value from 9.22 cm to 1.38 cm at 59 keV while it is improved from 30.74 cm to 26.21 cm at 1333 keV which indicates that low thickness of 20 wt% nanocomposites is useful in attenuation of low energy photons. This kind of performance implies that these nanocomposites could be especially helpful for real-world shielding applications. These include lightweight protection panels used in mammography centers, dental radiography offices, and diagnostic X-ray rooms. These composites can also be used in laboratory-scale shielding, where low-energy isotopes are commonly used, and in the creation of building materials for radiation-safe walls and partitions. Their suitability for architectural and biomedical applications is strengthened by the fact that they are lead-free. Additionally, using a green synthesis method lowers its manufacturing cost. Another parameter was be measured is radiation shielding efficiency. Figure 14 shows shielding efficiency at different energies for different thickness. It shows decrease of shielding efficiency with increase of photon energy which is attributed to dominance of photoelectric effect at lower energies as explained in previously discussed parameters. Also, it is observed that 20 wt% nanocomposite has the highest shielding efficiency at 59 keV (81.14% and 91.81% at 1 and 1.5 cm thickness respectively) which reveal its good ability in attenuation of low energy photons.
Finally, our results were compared with different previously reported unsaturated polyester composites. Figure 15 Shows LAC values of different unsaturated polyester composites containing Bi2(WO4)31, BiClO53, CdTe2, SnO54, and PbO49 and our composites. It shows the agreement of our results with other published results. In addition, it is observed that LAC value of 20 wt% Bi2O3 nanocomposite is greater than LAC value of the composites containing other bismuth compounds. Also, when 20 wt% Bi2O3 nanocomposite is compared with PbO composites, it shows greater LAC value which indicates importance of Bi2O3 as the safe relative for lead compounds in shielding composites.
Conclusion
In this study, shielding characteristics of unsaturated polyester nanocomposites filled with different ratios of Bi2O3 NPs were studied. Bi2O3 NPs were synthesized using olive leaves extract with average particle size of 15 nm as confirmed by TEM image. Also, the results of IR and XRD revealed the good preparation of Bi2O3 NPs. The LAC, HVL, TVL, and RSE% of the nanocomposites were measured at different energies (59, 661, 1173, and 1333 keV). In general, attenuation properties decreased with increase of energy for all samples. For example, RSE% of unsaturated polyester decreased from 22.09% to 7.22% with increasing of energy from 59 to 1333 keV at 1 cm thickness. On other hand, attenuation properties were improved with increase of Bi2O3 NPs ratio. The 20 wt% nanocomposite had the best results especially at 59 keV where it has highest LAC value of 1.668 cm− 1 and lowest HVL value of 0.415 cm which revealed that it is appropriate choice for shielding applications at lower energies. In summary, the prepared nanocomposites are more suitable for practical low-energy shielding applications rather than high-energy radiation. Also, the addition of Bi2O3 NPs improves resistance of unsaturated polyester for thermal degradation but, it decreases compressive strength of the composites.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Yılmaz, M. & Akman, F. Gamma radiation shielding properties for polymer composites reinforced with bismuth tungstate in different proportions. Appl. Radiat. Isot. 200, 110994. https://doi.org/10.1016/J.APRADISO.2023.110994 (Oct. 2023).
Akman, F. et al. Gamma Attenuation characteristics of CdTe-Doped polyester composites. Prog. Nucl. Energy. 131, 103608. https://doi.org/10.1016/J.PNUCENE.2020.103608 (Jan. 2021).
Gouda, M. M., Obeid, A., Awad, R. & Badawi, M. S. Gamma-ray Attenuation parameters of HDPE filled with different nano-size and bulk WO3. Appl. Radiat. Isot. 197, 110790. https://doi.org/10.1016/J.APRADISO.2023.110790 (Jul. 2023).
Elsafi, M., Abdel-Gawad, E. H., El-Nahal, M. A. & Sayyed, M. I. Effect of Tin oxide particle size on epoxy resin to form new composites against gamma radiation. Sci. Rep. 14 (1), 27901. https://doi.org/10.1038/s41598-024-78608-8 (Nov. 2024).
Cao, D., Yang, G., Bourham, M. & Moneghan, D. Gamma radiation shielding properties of poly (methyl methacrylate) / Bi2O3 composites, Nuclear Engineering and Technology, vol. 52, no. 11, pp. 2613–2619, Nov. (2020). https://doi.org/10.1016/J.NET.2020.04.026
Elsafi, M., Hedaya, A. M. & Sayyed, M. I. Study the impact of MgO, Al2O3, MoO3, BaO, ZnO and PbO on the radiation shielding performance of borate glasses, Nuclear Engineering and Technology, vol. 57, no. 7, p. 103475, Jul. (2025). https://doi.org/10.1016/j.net.2025.103475
Ghule, P. G. et al. Gamma radiation shielding properties of unsaturated polyester /Bi2O3 composites: an experimental, theoretical and simulation approach. Radiat. Phys. Chem. 216, 111452. https://doi.org/10.1016/J.RADPHYSCHEM.2023.111452 (Mar. 2024).
Alorain, D. A., Almuqrin, A. H., Sayyed, M. I. & Elsafi, M. Impact of WO 3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites, e-Polymers, vol. 23, no. 1, Aug. (2023). https://doi.org/10.1515/epoly-2023-0037
More, C. V. et al. Radiation shielding efficacy of unsaturated polyester composites for gamma and neutron attenuation-enhanced with SnO2. Radiat. Phys. Chem. 229, 112484. https://doi.org/10.1016/J.RADPHYSCHEM.2024.112484 (Apr. 2025).
Özdoğan, H., Üncü, Y. A., Akman, F., Polat, H. & Kaçal, M. R. Investigation of gamma ray shielding characteristics of binary composites containing polyester resin and lead oxide. Polym. (Basel). 16 (23), 3324. https://doi.org/10.3390/polym16233324 (Nov. 2024).
El-Sharkawy, R. M., Abdou, F. S., Gizawy, M. A., Allam, E. A. & Mahmoud, M. E. Bismuth oxide nanoparticles (Bi2O3 NPs) embedded into recycled- Poly(vinyl chloride) plastic sheets as a promising shielding material for gamma radiation. Radiat. Phys. Chem. 208, 110838. https://doi.org/10.1016/J.RADPHYSCHEM.2023.110838 (Jul. 2023).
Yassene, A. A. M., Abd, N. R., Elwahab & Eyssa, H. M. Development of < scp > HDPE /natural fiberboard nanocomposite reinforced < scp > Bi 2 O 3 / BaO and < scp > H 3 BO 3 : A sustainable approach for gamma and neutron shielding application, Polym Eng Sci, vol. 64, no. 7, pp. 3271–3288, Jul. https://doi.org/10.1002/pen.26768. (2024).
Alsafi, K. et al. Utilization of waste marble and Bi2O3-NPs as a sustainable replacement for lead materials for radiation shielding applications. Ceramics 7 (2), 639–651. https://doi.org/10.3390/ceramics7020042 (May 2024).
Hedaya, A., Elsafi, M., Al-Saleh, W. M. & Saleh, I. H. Effect of Bi2O3 particle size on the radiation-Shielding performance of Free-Lead epoxide materials against ionizing radiation. Polym. (Basel). 16 (15), 2125. https://doi.org/10.3390/polym16152125 (Jul. 2024).
Elsafi, M. et al. Development of gloves and protective jackets from ionizing radiation made of silicone rubber using nano-bismuth and tin oxides, Silicon, vol. 15, no. 18, pp. 7885–7892, Dec. (2023). https://doi.org/10.1007/s12633-023-02637-7
Gañán, P. et al. The evolution and future trends of unsaturated polyester biocomposites: A bibliometric analysis. Polym. (Basel). 15, 2970. https://doi.org/10.3390/polym15132970 (Jul. 2023).
Al-Mufti, S. M. S., Almontasser, A. & Rizvi, S. J. A. Unsaturated Polyester Resin Filled with Cementitious Materials: A Comprehensive Study of Filler Loading Impact on Mechanical Properties, Microstructure, and Water Absorption, ACS Omega, vol. 8, no. 23, pp. 20389–20403, Jun. (2023). https://doi.org/10.1021/acsomega.3c00353
Nusyirwan, Z. K. & Vachanidyo, X. Study of the Flexural Resistance of Unsaturated Polyester Composite Reinforced by Finely Ground Sugarcane Bagasse Fiber for Light Construction, JOURNAL OF MECHANICAL ENGINEERING MANUFACTURES MATERIALS AND ENERGY, vol. 8, no. 2, pp. 210–225, Nov. (2024). https://doi.org/10.31289/jmemme.v8i2.11482
Islam, S. & Arbab, M. N. Celite and Unsaturated Polyester Resin Composite for High-Voltage Automotive Ignition Coil Insulation-Based Applications, Transactions of the Indian Institute of Metals, vol. 76, no. 8, pp. 2161–2175, Aug. (2023). https://doi.org/10.1007/s12666-023-02914-4
More, C. V., Botewad, S. N., Akman, F., Agar, O. & Pawar, P. P. UPR/Titanium dioxide nanocomposite: Preparation, characterization and application in photon/neutron shielding. Appl. Radiat. Isot. 194, 110688. https://doi.org/10.1016/J.APRADISO.2023.110688 (Apr. 2023).
Hanaa, M. et al. Radiation shielding enhancement of polyester adding artificial marble materials and WO3 nanoparticles. Sustainability 14, 13355. https://doi.org/10.3390/su142013355 (Oct. 2022).
Oğul, H. et al. Gamma and neutron shielding parameters of Polyester-based composites reinforced with Boron and Tin nanopowders. Radiat. Phys. Chem. 201, 110474. https://doi.org/10.1016/j.radphyschem.2022.110474 (Nov. 2022).
Almuqrin, A. H., Yasmin, S., Abualsayed, M. I. & Elsafi, M. An experimental investigation into the radiation-shielding performance of newly developed polyester containing recycled waste marble and bismuth oxide. Appl. Rheology. 33 (1). https://doi.org/10.1515/arh-2022-0153 (May 2023).
Elsafi, M., Sayyed, M. I. & Almuqrin, A. H. New polyester composites synthesized with additions of different sized ZnO to study their shielding efficiency, Nuclear Engineering and Technology, vol. 56, no. 7, pp. 2821–2827, Jul. (2024). https://doi.org/10.1016/j.net.2024.02.044
Meena, P. L., Surela, A. K., Saini, J. K. & Chhachhia, L. K. Millettia pinnata plant pod extract-mediated synthesis of Bi2O3 for degradation of water pollutants, Environmental Science and Pollution Research, vol. 29, no. 52, pp. 79253–79271, Nov. (2022). https://doi.org/10.1007/s11356-022-21435-z
Rao, R. P., Mishra, S., Tripathi, R. M. & Jain, S. K. Bismuth oxide nanorods: phytochemical mediated one-pot synthesis and growth mechanism, Inorganic and Nano-Metal Chemistry, vol. 53, no. 11, pp. 1203–1210, Nov. (2023). https://doi.org/10.1080/24701556.2021.1980037
Meena, P. L., Surela, A. K., Poswal, K., Saini, J. K. & Chhachhia, L. K. Biogenic synthesis of Bi2O3 nanoparticles using Cassia fistula plant pod extract for the effective degradation of organic dyes in aqueous medium, Biomass Convers Biorefin, vol. 14, no. 3, pp. 3793–3809, Feb. (2024). https://doi.org/10.1007/s13399-022-02605-y
Choudhary, S., Rani, M., Keshu & Shanker, U. Green biosynthesized N-doped Bi2O3@SnO2 nanocomposite for efficient remediation of endocrine disrupting pesticides. Environ. Nanotechnol Monit. Manag. 18, 100746. https://doi.org/10.1016/j.enmm.2022.100746 (Dec. 2022).
Sarani, M. et al. Biosynthesis of ZnO, Bi 2 O 3 and ZnO – Bi 2 O 3 bimetallic nanoparticles and their cytotoxic and antibacterial effects. ChemistryOpen 13 (4). https://doi.org/10.1002/open.202300176 (Apr. 2024).
Sunil Kumar, K. C., Chandra, S., Lakshmi Ranganatha, V., Shivaganga, G. S. & Mallikarjunaswamy, C. Facile green synthesis of α-Bi2O3 nanoparticles: its photocatalytic and electrochemical sensing of lead and ascorbic acid. Nano-Structures Nano-Objects. 38, 101159. https://doi.org/10.1016/j.nanoso.2024.101159 (May 2024).
Zhou, Y., Zhang, H., Cheng, Z. & Wang, H. Regulation of the PI3K/AKT/mTOR signaling pathway with synthesized bismuth oxide nanoparticles from ginger (Zingiber officinale) extract: mitigating the proliferation of colorectal cancer cells. Arab. J. Chem. 15 (2), 103607. https://doi.org/10.1016/j.arabjc.2021.103607 (Feb. 2022).
Alam, M. W. et al. Facile green synthesis of α-Bismuth oxide nanoparticles: its photocatalytic and electrochemical sensing of glucose and uric acid in an acidic medium. J. Compos. Sci. 8 (2, p. 47, ). https://doi.org/10.3390/jcs8020047 (Jan. 2024).
Sarani, M. et al. Jul., Study of in vitro cytotoxic performance of biosynthesized α-Bi2O3 NPs, Mn-doped and Zn-doped Bi2O3 NPs against MCF-7 and HUVEC cell lines, Journal of Materials Research and Technology, vol. 19, pp. 140–150, (2022). https://doi.org/10.1016/j.jmrt.2022.05.002
Hannachi, E., Sayyed, M. I., Slimani, Y. & Elsafi, M. Experimental study of yttrium-based ceramic systems containing nanomaterials for gamma radiation protecting applications. Appl. Phys. A. 128 (10), 859. https://doi.org/10.1007/s00339-022-05996-x (Oct. 2022).
Bera, K. K., Majumdar, R., Chakraborty, M. & Bhattacharya, S. K. Phase control synthesis of α, β and α/β Bi2O3 hetero-junction with enhanced and synergistic photocatalytic activity on degradation of toxic dye, Rhodamine-B under natural sunlight, J Hazard Mater, vol. 352, pp. 182–191, Jun. (2018). https://doi.org/10.1016/j.jhazmat.2018.03.029
Embirsh, H. S. A. et al. Sep., Synthesis, Characterization and Application of Biobased Unsaturated Polyester Resin Reinforced with Unmodified/Modified Biosilica Nanoparticles, Polymers (Basel), vol. 15, no. 18, p. 3756, (2023). https://doi.org/10.3390/polym15183756
Md., F. et al. Effects of inorganic materials on the waste chicken feather fiber reinforced unsaturated polyester resin-based composite: an approach to environmental sustainability. Compos. Part. C: Open. Access. 9, 100320. https://doi.org/10.1016/j.jcomc.2022.100320 (Oct. 2022).
Ambika, M. R. et al. Preparation and characterisation of Isophthalic-Bi2O3 polymer composite gamma radiation shields. Radiat. Phys. Chem. 130, 351–358. https://doi.org/10.1016/j.radphyschem.2016.09.022 (Jan. 2017).
Salemane, M. G., Baruwa, A. D. & Makhatha, M. E. Investigating the chemical stability and thermal functionality of DMPT promoted TiO2 nanoparticles on unsaturated polyester resin. Results Eng. 22, 102116. https://doi.org/10.1016/j.rineng.2024.102116 (Jun. 2024).
Sarani, M. et al. Green synthesis of ag and Cu-doped bismuth oxide nanoparticles: revealing synergistic antimicrobial and selective cytotoxic potentials for biomedical advancements. J. Trace Elem. Med Biol. 81, 127325. https://doi.org/10.1016/j.jtemb.2023.127325 (Jan. 2024).
Abu-Dief, A. M. & Mohamed, W. S. α -Bi 2 O 3 nanorods: synthesis, characterization and UV-photocatalytic activity. Mater. Res. Express. 4 (3), 035039. https://doi.org/10.1088/2053-1591/aa6712 (Mar. 2017).
Zeng, H. et al. Ni-doped β-Bi2O3 microspheres cooperated with amorphous carbon nitride (ACN) with three coordinate nitrogen vacancies to construct heterojunction for enhanced pollutants degradation and photocatalytic H2 production. J. Solid State Chem. 313, 123252. https://doi.org/10.1016/j.jssc.2022.123252 (Sep. 2022).
Indriyani, A. et al. One-pot green fabrication of BiFeO3 nanoparticles via Abelmoschus esculentus L. leaves extracts for photocatalytic dye degradation, Appl Surf Sci, vol. 563, p. 150113, Oct. (2021). https://doi.org/10.1016/j.apsusc.2021.150113
Shirzadi, H., Nezamzadeh-Ejhieh, A. & Kolahdoozan, M. Synthesis of Bi2O3 NPs with polyethylene glycol and investigation of photodegradation of phenazopyridine in aqueous solution. Inorg. Chem. Commun. 150, 110464. https://doi.org/10.1016/j.inoche.2023.110464 (Apr. 2023).
Yetiman, S. et al. Rational Integration of ZIF-8 and BiPO 4 for Energy Storage and Environmental Applications, ACS Omega, vol. 7, no. 49, pp. 44878–44891, Dec. (2022). https://doi.org/10.1021/acsomega.2c04835
Nguyen, S. A. et al. Boosting the ultraviolet shielding and thermal retardancy properties of unsaturated polyester resin by employing electrochemically exfoliated e-GO nanosheets. RSC Adv. 13 (37), 25762–25777. https://doi.org/10.1039/D3RA03762B (2023).
Shoaib, M. et al. Room temperature synthesized TiO2 nanoparticles for Two-Folds enhanced mechanical properties of unsaturated polyester. Polym. (Basel). 15 (4), 934. https://doi.org/10.3390/polym15040934 (Feb. 2023).
Pradhan, P. & Satapathy, A. Physico-mechanical characterization and thermal property evaluation of polyester composites filled with walnut shell powder. Polym. Polym. Compos. 30 https://doi.org/10.1177/09673911221077808 (Jan. 2022).
Bagheri, K., Razavi, S. M., Ahmadi, S. J., Kosari, M. & Abolghasemi, H. Thermal resistance, tensile properties, and gamma radiation shielding performance of unsaturated polyester/nanoclay/PbO composites. Radiat. Phys. Chem. 146, 5–10. https://doi.org/10.1016/J.RADPHYSCHEM.2017.12.024 (May 2018).
Gamea, E. G., Anwar, A., Ezzat, A. A. & El-Rafey, M. E. Utilization of electric arc furnace dust as a filler for unsaturated polyester resin, Process Safety and Environmental Protection, vol. 159, pp. 1194–1202, Mar. (2022). https://doi.org/10.1016/j.psep.2022.01.079
Abdel-Hameed, R. et al. Effect of ZnO micro and nano ratios on the capability of ZnO-doped epoxy ceramics as gamma-ray shielding materials. Ceram Int Mar. https://doi.org/10.1016/j.ceramint.2025.03.232 (2025).
Mahmoud, M. E. et al. Fabrication, characterization and gamma rays shielding properties of nano and micro lead oxide-dispersed-high density polyethylene composites, Radiation Physics and Chemistry, vol. 145, pp. 160–173, Apr. (2018). https://doi.org/10.1016/J.RADPHYSCHEM.2017.10.017
Sharma, A. et al. Photon-shielding performance of bismuth oxychloride-filled polyester concretes. Mater. Chem. Phys. 241, 122330. https://doi.org/10.1016/J.MATCHEMPHYS.2019.122330 (Feb. 2020).
Al-Saleh, W. M., Almutairi, H. M., Alsafi, K., Nabil, I. M. & Elsafi, M. Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study, e-Polymers, vol. 24, no. 1, Jun. (2024). https://doi.org/10.1515/epoly-2024-0039
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding.
Author information
Authors and Affiliations
Contributions
Mohamed A. Hamada: conceptualization; methodology; investigation; writing – original draft, Olfat M. Sadek: visualization; resources; validation; writing – review & editing, W.k. Mekhamer: conceptualization; supervision; writing – review & editing, Ibrahim H. Saleh: supervision; writing – review & editing, M. Elsafi: conceptualization; methodology; writing – original draft; writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamada, M.A., Sadek, O.M., Mekhamer, W.K. et al. Green synthesis and characterization of Bi2O3 nanoparticles as outstanding filler in polyester for radiation shielding performance. Sci Rep 15, 40352 (2025). https://doi.org/10.1038/s41598-025-26415-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26415-0