Abstract
This study aims to investigate whether alpinetin prevents lipopolysaccharide/D-galactosamine (LPS/D-GalN)-induced acute liver injury (ALI) in mice by inhibiting ferroptosis, and to explore the potential molecular mechanisms involved. Thirty-six male BALB/c mice were randomly assigned to one of six groups (n = 6): the control group, the LPS/D-GalN group, the Ferrostatin-1 (Fer-1) + LPS/D-GalN group, and the alpinetin (12.5, 25, or 50 mg/kg) + LPS/D-GalN groups.The ferroptosis-specific inhibitor Fer-1 was used as a positive control to verify the involvement of ferroptosis in the injury. Following intraperitoneal (i.p.) administration of alpinetin (12.5, 25, or 50 mg/kg) for three consecutive days, acute liver injury was induced by an i.p. injection of LPS (30 µg/kg) and D-GalN (600 mg/kg). The effect of alpinetin on ALI was detected by serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and hematoxylin and eosin (H&E) staining of hepatic tissue. Ferroptosis levels were analyzed by malondialdehyde (MDA), reduced glutathione (GSH), and Fe2+. In addition, the expression levels of ferroptosis-related proteins were determined by western blot and quantitative real-time PCR (qRT-PCR). The results show that alpinetin inhibited the increase of serum ALT and AST levels and hepatic tissue pathological damage induced by LPS/D-GalN. Alpinetin also inhibited ferroptosis by reducing MDA and Fe2+ levels and enhancing GSH activity. Simultaneously, alpinetin regulates iron metabolism-related proteins by inhibiting the expression of transferrin receptor 1 (TFR1) and divalent metal transporter 1 (DMT1) while promoting the expression of ferritin heavy chain (FTH). Additionally, alpinetin increased the expression of the ferroptosis-related protein nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream target genes solute carrier family 7 member 11 (SLC7A11) and glutathione peroxidase 4 (GPX4). Alpinetin protects against LPS/D-GalN-induced ALI by inhibiting the ferroptosis pathway, and its protective effect may depend on the activation of the Nrf2/SLC7A11/GPX4 axis.
Similar content being viewed by others
Introduction
The liver is the core organ of body metabolism and detoxification, and is also an organ that is extremely vulnerable to damage1. Acute liver injury (ALI) is a disease characterized by the collapse of the liver’s defense mechanism and the induction of uncontrolled inflammation2, which can be caused by factors such as viral infection, drug toxicity, environmental toxins, and alcohol abuse3,4,5. ALI triggers extensive hepatocellular necrosis/apoptosis, steatosis, oxidative stress, and inflammatory cascade activation, with severe cases progressing to acute liver failure (ALF), cirrhosis, and hepatocellular carcinoma (HCC)6,7. In recent years, the incidence and mortality of ALI have gradually increased globally, seriously threatening human health8. Consequently, studying the pathogenesis and treatment strategies of ALI plays a vital role in the prevention and treatment of ALI.
Ferroptosis is a form of cell death driven by iron-dependent lipid peroxidation, which is regulated by iron ion accumulation, lipid metabolism disorders, and redox imbalance9. This process weakens the cellular antioxidant defense system through ferroptosis-inducing factors and triggers the accumulation of lipid peroxides, ultimately leading to oxidative cell death10. Studies have shown that ferroptosis is associated with the occurrence of many diseases such as inflammation, tumors and organ damage9,11,12. In recent years, more and more studies have confirmed that ferroptosis plays an important role in the occurrence and development of ALI13,14.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is an antioxidant transcription factor associated with inflammation and ferroptosis, and the antioxidant response it induces is closely related to the inhibition of ferroptotic cell death15. Solute Carrier Family 7 Member 11 (SLC7A11) and Glutathione Peroxidase 4 (GPX4) are central regulators of ferroptosis and downstream target genes of Nrf216. SLC7A11 is the major cysteine source for Glutathione (GSH) synthesis, and GSH promotes GPX4 to downregulate lipid peroxides, thereby protecting cells from lipid peroxidation and ferroptosis17. Studies have shown that the Nrf2/SLC7A11/GPX4 pathway plays a crucial role in the cellular oxidation/antioxidant system and the regulation of ferroptosis18.
Alpinetin (Fig. 1), a novel plant flavonoid derived from Alpinia katsumadai Hayata, possesses pharmacological properties such as anti-inflammatory and antioxidant activities19. Its antioxidant activity stems from its capacity to scavenge free radicals and boost the activity of endogenous antioxidant enzymes, such as SOD and CAT20. Meanwhile, its anti-inflammatory effects are mediated through the suppression of key signaling pathways such as NF-κB and NLRP3 inflammasome, leading to the reduced production of pro-inflammatory cytokines like TNF-α and IL−1β21,22. Currently, studies have shown that alpinetin has a protective effect on diseases such as lung injury, kidney injury and myocardial injury23,24,25, but its protective effect and mechanism of action on liver injury have not yet been clarified.
Notably, alpinetin has been shown to activate the Nrf2 signaling pathway26. Furthermore, activation of Nrf2 can upregulate the expression of its downstream target genes such as SLC7A11 and GPX4, which are core molecules inhibiting ferroptosis. Therefore, we propose the following scientific hypothesis: Alpinetin may activate the Nrf2/SLC7A11/GPX4 signaling pathway, enhance cellular antioxidant capacity, thereby inhibiting the ferroptosis process, and ultimately protecting against LPS/D-GalN-induced acute liver injury. This study aims to investigate the protective effect of Alpinetin and its underlying mechanism, to reveal how it intervenes in ferroptosis, and to provide a new candidate drug and a theoretical basis for the treatment of liver injury.
Materials and methods
Chemicals and reagents
Alpinetin was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Lipopolysaccharide (LPS) (Escherichia coli lipopolysaccharide, O55:B5), d-galactosamine hydrochloride (D-GalN) and Ferrostatin-1 (Fer-1) were acquired from Sigma-Aldrich (St. Louis, MO, USA). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), malondialdehyde (MDA) and reduced glutathione (GSH) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Iron assay kit was purchased from Solarbio (Beijing, China). RIPA lysis buffer was purchased from Beyotime (Shanghai, China). Protease inhibitor cocktail was purchased from MedChemExpress (NJ, USA). Primary antibodies against transferrin receptor 1 (TFR1) (ab214039), divalent metal transporter 1 (DMT1) (ab55735), SLC7A11 (ab307601), ferritin heavy chain (FTH) (ab183781), GPX4 (ab125066) were purchased from abcam (Cambridge, UK). Primary antibody against Nrf2 (R380773) was purchased from ZEN-BIO (Chengdu, China). Primary antibody against β-actin (66009-1-IG) was purchased from Proteintech (Rosemont, IL, USA). The secondary HRP-conjugated goat anti-rabbit IgG (H + L) (BA1054) and the HRP-conjugated goat anti-mouse IgG (H + L) (BA1050) were purchased from Boster Biological Technology (Wuhan, China). PVDF membranes were purchased from Merck Millipore (MA, USA). TRIzol reagent, a first-strand cDNA synthesis kit and SYBR Green PCR Master Mix were purchased from LABLEAD (Beijing, China).
Animals
Male BALB/c mice (6–8 weeks old, weight 20–25 g) were obtained from Jiangsu Jicui Yaokang Biotechnology Co., Ltd. All mice were housed in a specific pathogen-free (SPF) facility under controlled conditions (40–60% humidity, 22–25 °C temperature, 12:12 dark–light cycle) and acclimated for one week before experiments. The mice were housed in groups of six per cage with ad libitum access to standard chow and water. All cages were cleaned regularly, with mice remaining in their home cages until the end of the study. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The study was approved by the Animal Care and Research Ethics Committee of Binzhou Medical University (Ethics Approval No. 2022 − 420). This study followed the ARRIVE Guidelines 2.0 for reporting animal research.
Study design
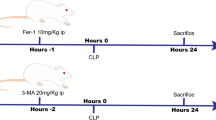
Thirty-six mice were randomly divided into six groups (n = 6): (1) Control group; (2) LPS/D-GalN group; (3) Fer-1 (5 mg/kg) + LPS/D-GalN group; (4–6) Alpinetin (12.5, 25, or 50 mg/kg) + LPS/D-GalN groups. Groups 3–6 received daily intraperitoneal (i.p.) injections of saline, Fer-1 (5 mg/kg) or Alpinetin (12.5, 25, or 50 mg/kg) for three consecutive days27,28. The ferroptosis-specific inhibitor Fer-1 was employed as a positive control to confirm the model’s responsiveness to ferroptosis inhibition and to verify the involvement of ferroptosis in the injury. One hour after the final dose, mice (except the Control group) were challenged via an intraperitoneal (i.p.) injection of LPS (30 µg/kg) and D-GalN (600 mg/kg), which were dissolved in saline, sonicated for homogeneity, and administered to induce acute liver injury29,30.
Anesthesia and euthanasia
At 6 h post-challenge, all mice were deeply anesthetized with an i.p. injection of ketamine (100 mg/kg) and xylazine (10 mg/kg)31. The absence of pedal and corneal reflexes confirmed a surgical plane of anesthesia. Euthanasia was then performed by cervical dislocation, with death verified by the absence of a heartbeat and respiration32. Blood and hepatic tissue were subsequently collected for analysis (Fig. 2).
The liver index measurement
The liver index was measured on the following equation:
liver index (%) = liver wet weight/mouse body weight × 100%.
Biochemical assessment
Serum was collected to measure ALT and AST levels. Approximately 100 mg of hepatic tissue was homogenized in 1 mL of ice-cold PBS, and the homogenate supernatant was used to quantify MDA and GSH. The levels of ALT, AST, MDA and GSH were tested by specific determination kits according to the manufacturer’s instruction. The absorbance of the resulting reaction products was measured using a microplate reader (Thermo, MA, USA).
Measurement of Fe2+ content
Serum Fe2+ content was measured using a commercial iron assay kit according to the manufacturer’s instructions. The absorbance value at 593 nm was measured with a microplate reader (Thermo, MA, USA) and the iron content of each sample was obtained based on the standard curve.
Histopathological evaluation
The hepatic tissue was collected and fixed in 4% paraformaldehyde for 24 h, dehydrated, embedded in paraffin and sliced into 4-µm sections. After hematoxylin and eosin (H&E) staining, pathological changes of the hepatic tissue were observed under a light microscope. Liver histopathological changes were scored as described previously33. 0–1: no injury, 2–3: mild, 4–6: moderate, 7–9: severe.
Western blot analysis
Approximately 50 mg of hepatic tissue was homogenized in RIPA lysis buffer supplemented with protease inhibitor cocktail to extract total protein. The protein concentration was determined by BCA method. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes. The membranes were blocked with 5% skim milk powder for 2 h at room temperature and then incubated with specific primary antibodies overnight at 4 °C. Subsequently, the membranes were washed three times with TBST and incubated with secondary antibodies for 1 h at room temperature. The membranes were washed again three times with TBST and the target protein blots were visualized by chemiluminescent substrates.
Quantitative real time PCR (qRT-PCR)
Total RNA was extracted from approximately 30 mg of hepatic tissue using TRIzol reagent according to the manufacturer’s instructions and the concentration and quality of RNA were assessed. After mRNA was subsequently reverse transcribed into cDNA using a first-strand cDNA synthesis kit, qRT-PCR was performed using SYBR Green PCR Master Mix. The results were normalized to β-actin. The primers used to amplify specific gene fragments are listed in Table 1.
Statistical analysis
Statistical analysis and graph generation were performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). All data are expressed as the mean ± SEM. The Shapiro-Wilk and Brown-Forsythe tests were employed to verify the normality of the data and the homogeneity of variances, respectively. Comparisons among groups were made with one-way analysis of variance (ANOVA) followed by Dunnett’s test. P < 0.05 was considered statistically significant.
Results
Alpinetin attenuates LPS/D-GalN-induced liver pathological alterations and the increase in liver index
H&E staining revealed the histopathological changes in hepatic tissue induced by LPS/D-GalN (Fig. 3A, B). The liver architecture in the control group appeared normal. In contrast, the LPS/D-GalN group exhibited significant pathological alterations, including structural disarray, inflammatory cell infiltration, congestion, and necrotic areas. However, pretreatment with alpinetin or Fer-1 reduced these histopathological damages. Furthermore, LPS/D-GalN challenge significantly increased the liver index compared to the control group (Fig. 3C, P < 0.001). This increase was significantly attenuated by alpinetin pretreatment when compared to the LPS/D-GalN group (12.5 mg/kg, P < 0.05; 25 mg/kg, P < 0.01; 50 mg/kg, P < 0.001), with an effect comparable to that of Fer-1 (P < 0.001).
Effect of alpinetin in mice with LPS/D-GalN-induced ALI. (A) Histopathological sections of the liver (Original magnification, 200 ×, scale bar, 100 μm). (B) Liver histopathologic score. (C) Liver index (%). All data are presented as the mean ± SEM. P### < 0.001 vs. control group; P* < 0.05, P** < 0.01 and P*** < 0.001 vs. LPS/D-GalN group.
Alpinetin protects against LPS/D-GalN-induced liver dysfunction
ALT and AST are the core serum enzyme indicators for evaluating hepatocellular damage34. Following LPS/D-GalN challenge, serum ALT and AST levels were significantly increased compared to the control group (both P < 0.001). These elevations were significantly reduced by alpinetin pretreatment. Specifically, versus the LPS/D-GalN group, alpinetin at 12.5, 25, and 50 mg/kg reduced both ALT and AST levels (P < 0.01, P < 0.001, and P < 0.001, respectively). The effect of Fer-1 was also significant (for both ALT and AST, P < 0.001). Alpinetin showed a dose-dependent decrease in ALT/AST levels (Fig. 4).
Alpinetin inhibits LPS/D-GalN-induced liver oxidative stress in ALI mice
MDA levels reflect the level of oxidative stress in the liver35. GSH content reflects the antioxidant capacity of the liver36. As shown in Fig. 5, the MDA level in the LPS/D-GalN group was significantly increased (P < 0.001), while the GSH content was significantly decreased (P < 0.001) compared to the control group. Treatment with alpinetin or Fer-1 reduced the MDA level and increased the GSH content. Alpinetin dose-dependently inhibited MDA accumulation (12.5 mg/kg, P < 0.05; 25 mg/kg, P < 0.01; 50 mg/kg, P < 0.001) and increased GSH content (12.5 mg/kg, P < 0.05; 25 mg/kg, P < 0.01; 50 mg/kg, P < 0.001), with an effect comparable to Fer-1 (for both MDA and GSH, P < 0.001).
Effects of alpinetin on MDA and GSH activities in mice after LPS/D-GalN treatment. (A) Serum levels of MDA in each group. (B) Serum levels of GSH in each group. All data are presented as the mean ± SEM (n = 3/group). P### < 0.001 vs. control group; P* < 0.05, P** < 0.01 and P*** < 0.001 vs. LPS/D-GalN group.
Alpinetin inhibits LPS/D-GalN-induced liver ferroptosis and regulates iron metabolism
Iron metabolism disorder, lipid metabolism disorder, and redox imbalance are the core pathological mechanisms of ferroptosis10. As shown in Fig. 6, the LPS/D-GalN group exhibited a significant increase in Fe2+ accumulation (P < 0.001), accompanied by altered expression of iron metabolism-related molecules (TFR1, DMT1, and FTH). Specifically, the protein and mRNA levels of TFR1 and DMT1 were significantly up-regulated (P < 0.001), whereas both protein expression and mRNA transcription of FTH were down-regulated (P < 0.001). Alpinetin treatment reversed these changes in a dose-dependent manner. Versus the LPS/D-GalN group, alpinetin at 12.5, 25, and 50 mg/kg significantly reduced Fe2+ content (P < 0.05, P < 0.001, P < 0.001), suppressed TFR1 protein (P < 0.05, P < 0.01, P < 0.001) and mRNA (P < 0.05, P < 0.001, P < 0.001), suppressed DMT1 protein (P < 0.05, P < 0.05, P < 0.01) and mRNA (P < 0.05, P < 0.001, P < 0.001), and elevated both FTH protein and mRNA levels (P < 0.05, P < 0.01, P < 0.001). This effect was consistent with that of Fer-1, which also significantly reversed Fe2+ accumulation (P < 0.001), TFR1 protein (P < 0.01) and mRNA (P < 0.001), DMT1 protein (P < 0.01) and mRNA (P < 0.001), and FTH protein and mRNA (both P < 0.001).
Effects of alpinetin on Fe2+ and iron metabolism proteins in mice after LPS/D-GalN treatment. (A) Western blot bands of TFR1, DMT1 and FTH. (B) Fe2+ content. (C-E) Relative protein expression of TFR1, DMT1 and FTH. (F-H) Relative mRNA expression of TFR1, DMT1 and FTH. All data are presented as the mean ± SEM (n = 3/group). P### < 0.001 vs. control group; P* < 0.05, P** < 0.01 and P*** < 0.001 vs. LPS/D-GalN group.
Alpinetin activates the Nrf2/SLC7A11/GPX4 axis in LPS/D-GalN-induced liver injury to inhibit ferroptosis
The Nrf2/SLC7A11/GPX4 signaling axis is one of the core pathways that regulate ferroptosis and antagonize oxidative stress18. As shown in Fig. 7, the protein expression and mRNA levels of Nrf2, SLC7A11 and GPX4 in the LPS/D-GalN group were significantly down-regulated compared with the control group (P < 0.001). Alpinetin treatment dose-dependently reversed these decreases. Versus the LPS/D-GalN group, alpinetin at 12.5, 25, and 50 mg/kg significantly elevated Nrf2 protein (P < 0.05, P < 0.01, P < 0.001) and mRNA (P < 0.05, P < 0.001, P < 0.001), SLC7A11 protein and mRNA (P < 0.05, P < 0.01, P < 0.001), as well as GPX4 protein (P < 0.001) and mRNA (P < 0.01, P < 0.001, P < 0.001). This effect was consistent with that of Fer-1, which also significantly reversed the decreases in Nrf2 protein (P < 0.01) and mRNA (P < 0.001), SLC7A11 protein and mRNA (P < 0.001), and GPX4 protein (P < 0.001) and mRNA (P < 0.01).
Effects of alpinetin on ferroptosis-related proteins in mice after LPS/D-GalN treatment. (A) Western blot bands of Nrf2, SLC7A11 and GPX4. (B-D) Relative protein expression of Nrf2, SLC7A11 and GPX4. (E-G) Relative mRNA expression of Nrf2, SLC7A11 and GPX4. All data are presented as the mean ± SEM (n = 3/group). P### < 0.001 vs. control group; P* < 0.05, P** < 0.01 and P*** < 0.001 vs. LPS/D-GalN group.
Discussion
ALI refers to a severe clinical condition triggered by diverse etiological insults, leading to rapid hepatocyte damage and liver dysfunction37. ALI is frequently complicated by multiple systemic comorbidities and exhibits a high mortality rate in the absence of targeted therapeutic interventions38. In recent years, traditional Chinese medicine (TCM) has emerged as a pivotal focus in hepatoprotective drug development due to its unique advantages, including stable efficacy, few adverse reactions, and abundant resources39. Notably, alpinetin is a new plant flavonoid derived from Alpinia katsumadai Hayata, which has anti-inflammatory and antioxidant pharmacological effects19. In this study, we confirmed the protective effects of alpinetin against LPS/D-GalN-induced ALI. Mechanistically, alpinetin may inhibit ferroptosis by activating the Nrf2/SLC7A11/GPX4 signaling axis, thereby alleviating LPS/D-GalN-induced ALI.
To evaluate the hepatoprotective effects of alpinetin, we employed the LPS/D-GalN-induced mouse model of ALI, which recapitulates key pathological features of human fulminant hepatitis40. Consistent with previous studies, this model successfully induced the characteristic ALI phenotype, as evidenced by significantly elevated serum ALT/AST levels and marked histopathological alterations41,42. Notably, alpinetin treatment effectively reversed these pathological alterations, a protective effect consistent with reports by Liu et al.33. Moving beyond the established anti-inflammatory and antioxidant properties of alpinetin, our study elucidates that its protective action is associated with the regulation of ferroptosis, a form of regulated cell death, thereby providing a new perspective on its mechanism of action.
Ferroptosis is a form of cell death driven by iron-dependent lipid peroxidation and is primarily mediated through dysregulation of iron metabolism and lipid peroxidation signaling pathways9. Its characteristic features, as demonstrated in previous studies, include the accumulation of reactive iron and MDA, depletion of GSH, and alterations in iron metabolism-related proteins (TFR1, DMT1, FTH)10,43,44. Consistent with previous studies, our study showed that LPS/D-GalN stimulation upregulates TFR1 and DMT1 expression while downregulating FTH expression. These alterations were accompanied by increased Fe2+ and MDA levels and decreased GSH activity. Importantly, these changes were reversed by Fer-1 pretreatment, which confirmed the activation of ferroptosis in this liver injury model. These findings corroborate the work of Huang et al., who similarly reported the activation of ferroptosis in an LPS/D-GalN-induced liver injury model45. Notably, alpinetin pretreatment inhibited these pathological alterations with an efficacy comparable to Fer-1. Recent work by Zou et al. demonstrated that alpinetin prevents myocardial ischemia-reperfusion injury by inhibiting ferroptosis, though its role in hepatic ferroptosis remained unexplored46. Thus, our study is the first to reveal that alpinetin protects against ALI by suppressing ferroptosis, providing novel evidence and insights into its hepatoprotective mechanisms.
Recent studies indicate that ferroptosis plays a pivotal role in the pathophysiology of ALI9. This study not only reaffirms the protective effect of alpinetin against LPS/D-GalN-induced ALI but, more importantly, reveals that its protective mechanism is closely associated with improving iron metabolism disorders and inhibiting lipid peroxidation. This suggests that alpinetin exerts its hepatoprotective effects by suppressing ferroptosis. Previous studies have indicated that the Nrf2/SLC7A11/GPX4 pathway plays a crucial role in regulating ferroptosis47,48,49. In this pathway, Nrf2 serves as the core transcription factor that upregulates SLC7A11 expression to promote GSH synthesis while enhancing GPX4 activity, thereby synergistically inhibiting lipid peroxidation16,17. Therefore, we examined the effects of alpinetin on Nrf2, SLC7A11, and GPX4 protein expression in an LPS/D-GalN-induced ALI mouse model. Results showed that LPS/D-GalN treatment significantly suppressed Nrf2, SLC7A11, and GPX4 protein expression in hepatic tissue, while alpinetin intervention dose-dependently reversed these inhibitory effects, indicating its protective role through activation of this pathway. Our findings align with those of Du et al., who confirmed APE1 regulates ferroptosis via this pathway to protect hepatocellular carcinoma cells50. Deng et al. also reported similar results, demonstrating that xanthohumol improves drug-induced hepatic ferroptosis by activating this pathway27. However, our observation of pathway suppression in the model group contrasts with findings by Liu et al. in a cerebral ischemia-reperfusion model, where SLC7A11 and Nrf2 levels were elevated in the model group compared to controls51. This discrepancy, alongside Guo et al.‘s demonstration that Maresin1 protects against sepsis-induced liver injury via this pathway, suggests that differences may stem from distinct disease models and pathophysiological mechanisms18. Specifically, early cerebral ischemia-reperfusion may induce compensatory activation of the Nrf2 pathway52. In contrast, the LPS/D-GalN fulminant liver injury model employed in this study, due to its potent toxicity, may rapidly overwhelm cellular endogenous defense systems, leading to overall pathway suppression. Notably, although previous studies have demonstrated that alpinetin protects against ALI by activating Nrf233. This study is the first to systematically elucidate its mechanism of inhibiting hepatic ferroptosis via the Nrf2/SLC7A11/GPX4 pathway in an ALI model, providing new theoretical support for understanding its hepatoprotective effects.
This study has several limitations. First, although animal models have confirmed that alpinetin can inhibit ferroptosis to protect against acute liver injury, it lacks synchronous validation at the cellular level in vitro. Future research should prioritize the establishment of relevant cell models to confirm the molecular targets of alpinetin. Second, the association between Nrf2-inhibited ferroptosis and SLC7A11 and GPX4 has not been verified. More conclusive methods (such as Nrf2-knockout mice or specific Nrf2 inhibitors) are needed to establish a causal relationship and confirm that the observed anti-ferroptosis effects are indeed achieved through the regulation of SLC7A11 and GPX4. Additionally, although the study focused on the relationship between alpinetin and the Nrf2-mediated ferroptosis pathway in LPS/D-GalN-induced acute liver injury, other potential pathways have not been explored. Given the complexity of the pathological mechanisms of acute liver injury, alpinetin may also exert protective effects by regulating other mechanisms. Future studies could use transcriptomic or proteomic sequencing technologies to reveal these potential alternative or synergistic mechanisms. Subsequent research will carry out these targeted experiments to comprehensively elucidate the mechanism of action of alpinetin.
Conclusions
In conclusion, this study demonstrates that alpinetin protects against LPS/D-GalN-induced ALI by inhibiting ferroptosis, and its protective effect may depend on the activation of the Nrf2/SLC7A11/GPX4 signaling axis, which provides a new potential strategy for the treatment of ALI.
Data availability
Data supporting this study are included within the article and/or supporting materials.
Abbreviations
- LPS/D-GalN:
-
Lipopolysaccharide/D-galactosamine
- ALI:
-
Acute liver injury
- Fer-1:
-
Ferrostatin-1
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- MDA:
-
Malondialdehyde
- GSH:
-
Glutathione
- TFR1:
-
Transferrin receptor 1
- DMT1:
-
Divalent metal transporter 1
- FTH:
-
Ferritin heavy chai
- Nrf2:
-
Erythroid 2-related factor 2
- SLC7A11:
-
Solute carrier family 7 member 11
- GPX4:
-
Glutathione peroxidase 4
References
Qian, H. et al. Autophagy in liver diseases: A review. Mol. Aspects Med. 82, 100973 (2021).
Maiwall, R., Kulkarni, A. V., Arab, J. P. & Piano, S. Acute liver failure. Lancet 404, 789–802 (2024).
Chiew, A. L. & Buckley, N. A. Acetaminophen poisoning. Crit. Care Clin. 37, 543–561 (2021).
Louvet, A. & Mathurin, P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 12, 231–242 (2015).
Chen, T., Li, R. & Chen, P. Gut microbiota and Chemical-Induced acute liver injury. Front. Physiol. 12, 688780 (2021).
Ding, K. et al. Gbp5 promotes liver injury and inflammation by inducing hepatocyte apoptosis. Faseb J. 36, e22119 (2022).
Yu, C., Chen, P., Miao, L. & Di, G. The role of the Nlrp3 inflammasome and programmed cell death in acute liver injury. Int J. Mol. Sci. 24, 3067 (2023).
Coccolini, F. et al. Liver trauma: Wses 2020 guidelines. World J. Emerg. Surg. 15, 24 (2020).
Chen, J., Li, X., Ge, C., Min, J. & Wang, F. The multifaceted role of ferroptosis in liver disease. Cell. Death Differ. 29, 467–480 (2022).
Tang, D., Chen, X., Kang, R. & Kroemer, G. Ferroptosis: molecular mechanisms and health implications. Cell. Res. 31, 107–125 (2021).
Chen, X., Kang, R., Kroemer, G. & Tang, D. Ferroptosis in Infection, Inflammation, and immunity. J Exp. Med. 218, e20210518 (2021).
Lei, G., Zhuang, L. & Gan, B. The roles of ferroptosis in cancer: tumor Suppression, tumor Microenvironment, and therapeutic interventions. Cancer Cell. 42, 513–534 (2024).
Lei, Z. Y. et al. Med1 inhibits ferroptosis and alleviates liver injury in acute liver failure via Nrf2 activation. Cell. Biosci. 14, 54 (2024).
Zhao, T. et al. Regulating Nrf2-Gpx4 axis by bicyclol can prevent ferroptosis in carbon Tetrachloride-Induced acute liver injury in mice. Cell. Death Discov. 8, 380 (2022).
Dodson, M., Castro-Portuguez, R. & Zhang, D. D. Nrf2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 23, 101107 (2019).
Chen, X., Li, J., Kang, R., Klionsky, D. J. & Tang, D. Ferroptosis: machinery and regulation. Autophagy 17, 2054–2081 (2021).
Liu, J. et al. Nrf2 and its dependent autophagy activation cooperatively counteract ferroptosis to alleviate acute liver injury. Pharmacol. Res. 187, 106563 (2023).
Guo, Y., Chen, H., Sun, J., Zhang, J. & Yin, Y. Maresin1 inhibits ferroptosis via the Nrf2/Slc7a11/Gpx4 pathway to protect against Sepsis-Induced acute liver injury. J. Inflamm. Res. 17, 11041–11053 (2024).
Zhao, G. et al. Alpinetin: A review of its Pharmacology and pharmacokinetics. Front. Pharmacol. 13, 814370 (2022).
Kongsui, R., Thongrong, S. & Jittiwat, J. In vivo neuroprotective effects of alpinetin against experimental ischemic stroke damage through antioxidant and Anti-Inflammatory mechanisms. Int J. Mol. Sci 26, (2025).
Wei, L. et al. Alpinetin ameliorates bone loss in Lps-Induced inflammation osteolysis via Ros mediated P38/Pi3K signaling pathway. Pharmacol. Res. 184, 106400 (2022).
Chen, Y. et al. Inhibiting mitochondrial inflammation through Drp1/Hk1/Nlrp3 pathway: A mechanism of alpinetin attenuated Aging-Associated cognitive impairment. Phytother Res. 37, 2454–2471 (2023).
Huo, M. et al. Traditional medicine alpinetin inhibits the inflammatory response in Raw 264.7 cells and mouse models. Int. Immunopharmacol. 12, 241–248 (2012).
Huang, Y. et al. Alpinetin inhibits Lipopolysaccharide-Induced acute kidney injury in mice. Int. Immunopharmacol. 28, 1003–1008 (2015).
Suo, C., Sun, L. & Yang, S. Alpinetin activates the ∆ receptor instead of the Κ and Μ receptor pathways to protect against rat myocardial cell apoptosis. Exp. Ther. Med. 7, 109–116 (2014).
Zhu, Z. et al. Alpinetin exerts Anti-Inflammatory, Anti-Oxidative and Anti-Angiogenic effects through activating the Nrf2 pathway and inhibiting Nlrp3 pathway in carbon Tetrachloride-Induced liver fibrosis. Int. Immunopharmacol. 96, 107660 (2021).
Deng, Y. et al. Xanthohumol ameliorates Drug-Induced hepatic ferroptosis via activating Nrf2/Xct/Gpx4 signaling pathway. Phytomedicine 126, 155458 (2024).
Zhou, Y. et al. Alpinetin improved high fat Diet-Induced Non-Alcoholic fatty liver disease (Nafld) through improving oxidative Stress, inflammatory response and lipid metabolism. Biomed. Pharmacother. 97, 1397–1408 (2018).
Li, J. et al. Role of liensinine in sensitivity of activated macrophages to ferroptosis and in acute liver injury. Cell. Death Discov. 9, 189 (2023).
Li, Z. et al. Chicoric acid ameliorate inflammation and oxidative stress in lipopolysaccharide and D-Galactosamine induced acute liver injury. J. Cell. Mol. Med. 24, 3022–3033 (2020).
Li, Z. et al. Best practices for blood collection and anaesthesia in mice: Selection, application and reporting. Br. J. Pharmacol. 182, 2337–2353 (2025).
AVMA. Avma Guidelines for the Euthanasia of Animals: 2020 Edition. (2020).
Liu, T. G. et al. Protective effects of alpinetin on Lipopolysaccharide/D-Galactosamine-Induced liver injury through inhibiting inflammatory and oxidative responses. Microb. Pathog. 126, 239–244 (2019).
McGill, M. R. The past and present of serum aminotransferases and the future of liver injury biomarkers. Excli J. 15, 817–828 (2016).
Janero, D. R. Malondialdehyde and thiobarbituric Acid-Reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol. Med. 9, 515–540 (1990).
Rushworth, G. F. & Megson, I. L. Existing and potential therapeutic uses for N-Acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 141, 150–159 (2014).
Zeng, Y. et al. Cohabitation facilitates Microbiome shifts that promote isoflavone transformation to ameliorate liver injury. Cell Host Microbe 33, 688–704 (2025).
Li, H. et al. Integrated transcriptomic and proteomic profiling reveals the Anti-Inflammatory mechanism of Dihydroartemisinin in the treatment of acute liver injury by targeting Cyba and Cybb. Biochem. Biophys. Res. Commun. 764, 151821 (2025).
Ma, J. T. et al. The Pharmacology and mechanisms of traditional Chinese medicine in promoting liver regeneration: A new therapeutic option. Phytomedicine 116, 154893 (2023).
Kashiwabara, D. et al. Structural determination of the Sheath-Forming polysaccharide of Sphaerotilus Montanus using thiopeptidoglycan lyase which recognizes the 1,4 linkage between Α-D-Galn and Β-D-Glca. Int. J. Biol. Macromol. 183, 992–1001 (2021).
Zeng, M., Feng, A., Wang, L., Li, K. & Zhou, J. Aralia saponin a isolated from achyranthes bidentata Bl. Ameliorates Lps/D-Galn induced acute liver injury via Sphk1/S1P/S1Pr1 pathway in vivo and in vitro. Int. Immunopharmacol. 124, 110912 (2023).
Gao, Z. et al. Hydroxytyrosol alleviates acute liver injury by inhibiting the Tnf-Α/Pi3K/Akt signaling pathway via targeting Tnf-Α signaling. Int J. Mol. Sci. 25, 12844 (2024).
Jiang, X., Stockwell, B. R., Conrad, M. & Ferroptosis Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell. Biol. 22, 266–282 (2021).
Li, J. et al. Ferroptosis: Past, present and future. Cell. Death Dis. 11, 88 (2020).
Huang, S. et al. Hepatic Tgfβr1 deficiency attenuates Lipopolysaccharide/D-Galactosamine-Induced acute liver failure through inhibiting Gsk3Β-Nrf2-Mediated hepatocyte apoptosis and ferroptosis. Cell. Mol. Gastroenterol. Hepatol. 13, 1649–1672 (2022).
Zou, C. et al. Alpinetin protects against myocardial Ischemia-Reperfusion injury by inhibiting ferroptosis and apoptosis via mitochondrial ferritin. Eur. J. Pharmacol. 1005, 178123 (2025).
Wei, X. et al. Dietary Limonin ameliorates heart failure with preserved ejection fraction by targeting ferroptosis via modulation of the Nrf2/Slc7a11/Gpx4 axis: an integrated transcriptomics and metabolomics analysis. Food Funct. 16, 3553–3574 (2025).
Yang, H. et al. Lycium barbarum polysaccharide alleviates ferroptosis in Sertoli cells through Nrf2/Slc7a11/Gpx4 pathway and ameliorates Dehp-Induced male reproductive damage in mice. Int. J. Biol. Macromol. 282, 137241 (2024).
Deng, X., Lin, B., Wang, F., Xu, P. & Wang, N. Mangiferin attenuates osteoporosis by inhibiting osteoblastic ferroptosis through Keap1/Nrf2/Slc7a11/Gpx4 pathway. Phytomedicine 124, 155282 (2024).
Du, Y. et al. Ape1 Inhibition enhances ferroptotic cell death and contributes to hepatocellular carcinoma therapy. Cell. Death Differ. 31, 431–446 (2024).
Liu, H. et al. Rhein attenuates cerebral Ischemia-Reperfusion injury via Inhibition of ferroptosis through Nrf2/Slc7a11/Gpx4 pathway. Exp. Neurol. 369, 114541 (2023).
Lucero, G. R. E., Villanueva, C. & Bond, R. A. Hypoxia inducible factors as central players in the pathogenesis and pathophysiology of cardiovascular diseases. Front. Cardiovasc. Med. 8, 709509 (2021).
Funding
This research was supported by the Natural Science Foundation of Shandong Province (ZR2023MH055, ZR2020MH322).
Author information
Authors and Affiliations
Contributions
Y.L.: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Writing – original draft, Writing – review and editing. J.Z.: Investigation, Methodology, Formal Analysis, Writing – original draft. W.C.: Software, Writing – original draft. T.L.: Project administration, Resources, Funding acquisition, Writing – original draft. K.S.: Project administration, Resources, Supervision, Validation, Funding acquisition, Writing – original draft, Writing – review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the Animal Care and Research Ethics Committee of Binzhou Medical University (Ethics Approval No. 2022 − 420), and the procedures were conducted following the Care and Use of Laboratory Animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Zhao, J., Cong, W. et al. Alpinetin pretreatment prevents lipopolysaccharide/D-galactosamine-induced acute liver injury in mice by inhibiting ferroptosis via the Nrf2/SLC7A11/GPX4 pathway. Sci Rep 15, 40065 (2025). https://doi.org/10.1038/s41598-025-26588-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26588-8